Abstract
Corticotropin-releasing factor binds with high affinity to CRF receptor 1 (CRFR1) and is implicated in stress-related mood disorders such as anxiety and depression. Using a validated CRFR1-green fluorescent protein (GFP) reporter mouse, our laboratory recently discovered a nucleus of CRFR1 expressing cells that is prominent in the female rostral anteroventral periventricular nucleus (AVPV/PeN), but largely absent in males. This sex difference is present in the early postnatal period and remains dimorphic into adulthood. The present investigation sought to characterize the chemical composition and gonadal hormone regulation of these sexually dimorphic CRFR1 cells using immunohistochemical procedures. We report that CRFR1-GFP-ir cells within the female AVPV/PeN are largely distinct from other dimorphic cell populations (kisspeptin, tyrosine hydroxylase). However, CRFR1-GFP-ir cells within the AVPV/PeN highly co-express estrogen receptor alpha as well as glucocorticoid receptor. A single injection of testosterone propionate or estradiol benzoate on the day of birth completely eliminates the AVPV/PeN sex difference, while adult gonadectomy has no effect on CRFR1GFP cell number. These results indicate that the AVPV/PeN CRFR1 is regulated by perinatal but not adult gonadal hormones. Finally, female AVPV/PeN CRFR1-GFP-ir cells are activated following an acute 30-min restraint stress, as assessed by co-localization of CRFR1-GFP cells with phosphorylated (p) CREB. CRFR1-GFP/pCREB cells were largely absent in the male AVPV/PeN. Together, these data indicate a stress and gonadal hormone responsive nucleus that is unique to females and may contribute to sex-specific stress responses.
Keywords: Corticotropin-releasing factor receptor 1, gonadal hormones, sexual differentiation, anteroventral periventricular nucleus, estrogen receptor alpha, glucocorticoid receptor, RRID: AB_631470, RRID: AB_300798, RRID: AB_2155786, RRID: AB_2296529, RRID: AB_2561044, RRID: AB_390204
Graphical abstract
1. Introduction
There is a clear discrepancy in the prevalence of stress-related mood disorders such as anxiety and depression, where women are twice as likely as men to get a diagnosis (Nolen-Hoeksma, 1987). Together, anxiety and depression affect approximately 20% of the U.S. population (Greenberg, Fournier, Sisitsky, Pike, & Kessler, 1987; Kessler et al., 1987) and incur $210 billion in annual costs. The neural mechanisms that underlie the sexually dimorphic prevalence of these disorders are unclear. Cells that produce corticotropin releasing factor (CRF), and those that express the primary receptor for CRF, the GS-coupled cognate receptor, CRF receptor 1 (CRFR1), represent intriguing cell phenotypes that might regulate these sex differences, given their known role in regulating anxiety, depression, and stress hormone secretion (Chrousos, 2009; Da Silva et al., 2016; Holly et al., 2016; Schussler et al., 2016; Garcia-Carmona JA, Baroja-Mazo, Milanes, & Laorden, 2015).
CRF signaling via CRFR1 regulates hormonal and behavioral stress responses (Heinrichs, Menzaghi, Merlo Pich, Britton, & Koob, 1993; Perrin, Donaldson, Chen, Lewis, & Vale, 1993; Smith et al., 1998; Subbannayya et al., 2013). Global knockout of CRFR1 and pharmacological antagonism of CRFR1 decrease anxiety- and depressive-like behaviors and suppress adrenocorticotropin hormone and glucocorticoid responses to stress (Contarino et al., 1999; Smith et al., 1998; Tran, Schulkin, & Meerveld, 2014; Webster et al., 1996; Zhao et al., 2007). On the contrary, CRF binding to CRFR2 generally produces anxiety/depression reducing effects (Bale et al., 2003; Bale & Vale, 2003; Zhao et al., 2007). Interestingly, a global knockout of both CRFR1 and R2 in mice results in sex-specific impacts on anxiety-like behavior (Bale et al., 2002), which suggests an important role for CRF receptor signaling in regulating sex differences in stress-associated behaviors.
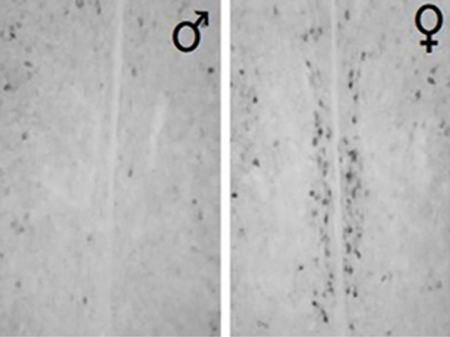
Sex differences in brain circuitry are generally believed to underlie sexually dimorphic patterns reported for stress-related behaviors and activation of the HPA axis (Handa & Weiser, 2014). In particular, sex differences in CRF and CRFR1 are potential candidates for regulating sexually dimorphic stress functions (Bangasser, Wiersielis, & Khantsis, 2016; Waters et al., 2015). Sex differences in the distribution of CRF, as well as both receptor subtypes, CRFR1 and R2 have been reported. There are more CRF expressing neurons within the preoptic area (POA) (Funabashi, Kawaguchi, Furuta, Fukushima, & Kimura, 2004; McDonald, Mascagni, & Wilson, 1994) and dorsal bed nucleus of the stria terminalis (BNST) of female rats (Funabashi, Kawaguchi, Furuta, Fukushima, & Kimura, 2004), while male rats have a greater number of CRF expressing neurons in the central amygdala (Karanikas, Lu, & Richardson, 2013). CRFR1immunoreactivity (-ir) is reported to be greater in female rats within the nucleus accumbens, olfactory tubercle, piriform cortex, anterior cingulate (Wealthington, Hamki, & Cooke, 2014). Furthermore, our laboratory recently discovered a dense cluster of CRFR1 expressing cells within the rostral anteroventral periventricular nucleus (AVPV/PeN) that is prominent in female mice but largely absent in males (Rosinger, Jacobskind, Park, Justice, & Zuloaga, 2017). This sex difference in CRFR1 is present by birth and persists through postnatal development.
Development of sex differences in the rodent brain has largely been attributed to perinatal androgen exposure that occurs only in males. Specifically, males are exposed to a prenatal surge at approximately gestation day (GD) 18 followed by a second surge that occurs on the day of birth (McCarthy & Nugent, 2015; Zuloaga, Jordan, & Breedlove, 2010). These androgen pulses serve to either increase or decrease the number of cells expressing specific phenotypes in males (Morris, Jordan, & Breedlove, 2004). In addition, sexually dimorphic nuclei can also be regulated/maintained by adult circulating gonadal hormones as has been identified for select sexually dimorphic nuclei (Morris, Jordan, & Breedlove, 2004; Zuloaga, Jordan, & Breedlove, 2010). Corresponding with alterations in brain morphology, gonadal hormone exposure during both the neonatal period and adulthood have been shown to regulate many sexually dimorphic behavioral/hormonal responses, including those associated with stress (Morris, Jordan, & Breedlove, 2004; Zuloaga, Jordan, & Breedlove, 2011). Numerous studies indicate that perinatal testosterone exposure induces lasting effects on anxiety behaviors and the HPA axis (Bingham, Wang, Innala, & Viau, 2012; Seale, Wood, Atkinson, Harbuz, & Lightman, 2005; Seale, Wood, Atkinson, Lightman, & Harbuz, 2005; Zuloaga, Jordan, & Breedlove, 2011). Furthermore, adult testosterone and estrogens dynamically regulate anxiety and depressive-like behaviors as well as glucocorticoid release via actions at their cognate receptors (Borrow & Handa, 2017; Lund, Hinds, & Handa, 2006; Lund, Rovis, Chung, & Handa, 2005; Weiser & Handa, 2009).
The specific brain regions and cell phenotypes involved in gonadal hormone regulation of stress-associated functions are largely unknown (Handa & Weiser, 2014). Therefore, our present study sought to further investigate one potential candidate region (sexually dimorphic CRFR1 in the AVPV/PeN). The described experiments specifically aimed to characterize the phenotypic profile of CRFR1-GFP-ir cells in the AVPV/PeN and determine the influence of neonatal and adult gonadal hormones on the development and maintenance of the nucleus. Due to the absence of selective antibodies for CRFR1, all experiments were performed using a validated CRFR1 reporter mouse line (bacterial artificial chromosome identified by green fluorescent protein (BAC CRFR1-GFP)) (Justice, Yuan, Sawchenko, & Vale, 2008).
2. Material and Methods
2.1. Animals:
Subjects were male and female BAC CRFR1-GFP mice on a C57BL/6J background. Mice used in the study were adult (60–110 days) or neonates (day of birth). Mice were maintained under a 12/12 L/D cycle (lights on at 0700), with food and water available ad libitum. All procedures in this study have been approved by the University at Albany Institutional Animal Care and Use Committee (IACUC) and are in accord with National Institutes of Health (NIH) guidelines.
2.2. P0 Hormone Supplement:
Experiments were performed to assess the role of neonatal gonadal hormones on CRFR1-GFP cell number in the AVPV/PeN. Male and female CRFR1GFP mice were paired and each morning throughout the study, the animal vivarium was checked for new litters. On mornings in which there were new litters, offspring were given sc injection (25 µl) of either testosterone propionate (TP; 100µg dissolved in sesame oil, N = 6 female, 7 male), estradiol benzoate (EB; 20µg dissolved in sesame oil, N = 6 female, 6 male), or sesame oil (VEH; N = 7 female, 6 male) as controls. A hypodermic needle (27 gauge) was inserted sc. at the lower portion of the pup’s back, and the needle was run until it could be seen roughly between the shoulder blades, where it was then injected, and slowly withdrawn. Animals were then returned to their home cage and left undisturbed until euthanized via rapid cervical dislocation and decapitation at P21. P21 (pre-puberty) was chosen for assessment of neonatal effects in order to avoid any confound of P0 hormone treatments on gonadal hormone production later in life. Brain tissue was extracted for histological analysis of AVPV/PeN CRFR1GFP-ir cells.
2.3. Adult Gonadectomy (GDX):
Experiments were performed to assess the role of adult gonadal hormones on CRFR1 cell number. Adult male and female mice (P60-P70; N= 32, 8 per treatment per sex) were gonadectomized or sham operated. Briefly, adult male mice were anesthetized with isoflurane and the incision areas were cleaned with ethanol and betadine. The testes were externalized via bilateral incisions made in the scrotum. The vas deferens were tied off with silk suture prior to cutting. Incisions were closed with Vetbond (3M) surgical glue. For removal of ovaries, female mice were anesthetized with isoflurane and the incision areas were cleaned with ethanol and betadine. Small bilateral incisions were made through the skin and muscle wall overlying the ovaries. The ovaries were retracted using forceps, ligated between the ovary and uterine horn, and removed using surgical scissors. Muscle walls were closed using dissolvable sutures and skin was closed using surgical staples. The analgesic carprofen (5 mg/kg) was injected sc. post operatively. Sham surgery involves only anesthesia and incision in the skin (females) or scrotum (males), post-operative procedures are identical to gonadectomized mice. Brains were collected from mice 6 weeks after gonadectomy or sham surgery for assessment of CRFR1-GFP. Estrus stage was not assessed in intact mice. This time point was chosen based on a previous study that reported a significant decrease in AVPV kisspeptin at 6 weeks in ovariectomized compared to intact freely cycling mice (Brock & Bakker, 2013).
2.4. Restraint Stress:
To assess whether AVPV/PeN CRFR1 cells are activated during acute psychological stress, gonadal intact adult mice (P60-P80; N= 5 male, 7 female) were placed into a restraint tube (L: 6–4/5”, W: 3–9/10”, H: 2–3/5”) and left inside their home cage for 30 minutes, after which they were removed from the tube and left undisturbed in their home cage until sacrificed (2hr from onset of restraint). This time point was chosen based on a previous study which performed a time course and indicated that the pCREB protein response in mice peaks at 2 hours following restraint in the paraventricular nucleus and arcuate nucleus (Kwon et al., 2006). The restraint tube has been validated in our laboratory to induce a robust corticosterone response (~400–500 ng/ml, data not shown).
2.5. Fluorogold; Neurosecretory Cells:
To determine if any CRFR1-GFP-ir neurons in the AVPV/PeN were neurosecretory, gonadal intact adult CRFR1-GFP female mice (P60–65; N=3) were sc. injected with 50µL of 5% fluorogold (Fluorochrome, Denver, CO) in saline (0.9%), based on previous approaches (Kriegsfeld, Korets, & Silver, 2003; Oyola, Thompson, Handa, & Handa, 2017). Animals were perfused with 4% paraformaldehyde 5 days later and used for assessment of immunohistochemical co-labeling of Fluorogold and CRFR1-GFP positive cells (listed within dual-label immunohistochemistry methods section).
2.6. Tissue collection for immunohistochemistry:
All adult animals underwent cervical dislocation and rapid decapitation, except those used for Fluorogold analysis which were perfused. Brains from P0 mice used for phenotypic characterization were collected following rapid decapitation. All brains were excised from the skull and immersed in 4% paraformaldehyde, then stored overnight at 4° C. The following day, brains were transferred into a 30% sucrose cryoprotectant solution, where they remained at 4° C until sectioned. For immunohistochemistry, brains were coronally sectioned at 40 μm, into 3 series using a cryostat (Microm HM505E, MICROM international GmbH, Walldorf, Germany). Tissues were placed in cryopreservative and stored at 4° C until immunohistochemistry was performed.
2.7. Immunohistochemistry:
To visualize CRFR1-GFP-ir, sections were rinsed in phosphate-buffered saline (PBS; pH 7.6), incubated in 1% hydrogen peroxide and 0.4% Triton-X in PBS (PBS-TX) for 10 minutes. Next, tissue was rinsed in PBS and incubated in 4% normal goat serum (NGS) with PBS-TX for 1 hour. Tissue was incubated overnight in primary GFP antisera (rabbit, Life Technologies, AB221570; 1:7500). The next morning, tissue was rinsed in PBS, and incubated in biotinylated goat anti-rabbit antisera with PBS-TX (Vector Laboratories; 1:500) for 1 hour. After, tissue was rinsed in PBS and then placed in avidin-biotin complex (ABC Elite kit, Vector Laboratories; 1:1000). Tissue was rinsed again in tris-buffered saline (TBS) and placed for 10 minutes in diaminobenzidine as the chromagen to visualize CRFR1-GFP-expressing cells. Sections from wild type brains were utilized as negative control to assess CRFR1-GFP-ir; no labeling was found in these sections.
2.8. Dual Label Immunohistochemistry:
For further phenotypic characterization of CRFR1GFP+ cells, we performed several dual-label fluorescence IHC studies to determine CRFR1GFP-ir co-localization patterns. Sections were thoroughly rinsed in phosphate-buffered saline (PBS; pH 7.6), then incubated in 4% normal donkey serum (4% NDS) and 0.4% Triton-X in PBS (PBS-TX) for 1 hour. Immediately following incubation, tissue was placed into the primary antisera (phosphorylated CREB (pCREB; Cell Signaling; anti-rabbit; AB2561044; 1:500), tyrosine hydroxylase (Millipore; anti-rabbit; AB390204; 1:500), kisspeptin (Millipore; anti-rabbit; AB2296529; 1:500), estrogen receptor alpha (Santa Cruz; anti-rabbit; AB631470; 1:250), or glucocorticoid receptor (Santa Cruz; anti-rabbit; AB2155786; 1:250)) and incubated at room temperature overnight. The following day, tissue was rinsed in PBS and then placed into secondary antisera (anti-rabbit, 594; 1:500) in 4% NDS and PBS-TX for 2.5 hours. Following rinses, tissue was then transferred to the second primary antisera (chicken, GFP; Abcam; AB300798; 1:1000 in 4% NDS and PBS-TX at room temperature overnight. On the third day, tissue was rinsed in PBS, then transferred to the second secondary antisera (donkey anti-chicken, 488; 1:1000) for 2.5 hours. After which, tissue was rinsed again in PBS. Immediately following the final rinse, tissue was mounted and coverslipped with Santa Cruz hard set mounting media with DAPI when dry.
2.9. Antibody characterization
See Table 1 for description of antibodies.
Table 1.
Primary Antibodies
| Target | Antigen | Source | Working dilution | Reference |
| ERα | ERα C-term (580–599) | Santa Cruz sc-542 | 1:250 | Quesada et al. (2007) Omoto et al. (2005) RRID: AB_631470 |
| GFP | Full length recombinant GFP protein | Abcam ab13970 | 1:1000 | Milosevic et al. (2017) RRID: AB_300798 |
| GR | N-terminus of GRα (5–24) | Santa Cruz sc-1004 | 1:250 | Sarabdjitsingh et al. (2010) Damjanovic et al.(2012) RRID: AB_2155786 |
| KISS | Mouse kisspeptin 10 peptide | Millipore AB9754 | 1:500 | Brock and Bakker (2013) RRID: AB_2296529 |
| pCREB | synthetic phosphopeptide that corresponds to residues that surround Ser133 of human CREB (129–137) | Cell Signaling 9198 | 1:500 | Dimitrov and Usdin (2010) Uribe-Marino et al. (2016) RRID: AB_2561044 |
| TH | Denatured tyrosine hydroxylase from rat pheochromocytoma | Millipore AB152 | 1:500 | Scott et al. (2015) RRID: AB_390204 |
2.9.1. Phosphorylated CREB (pCREB)
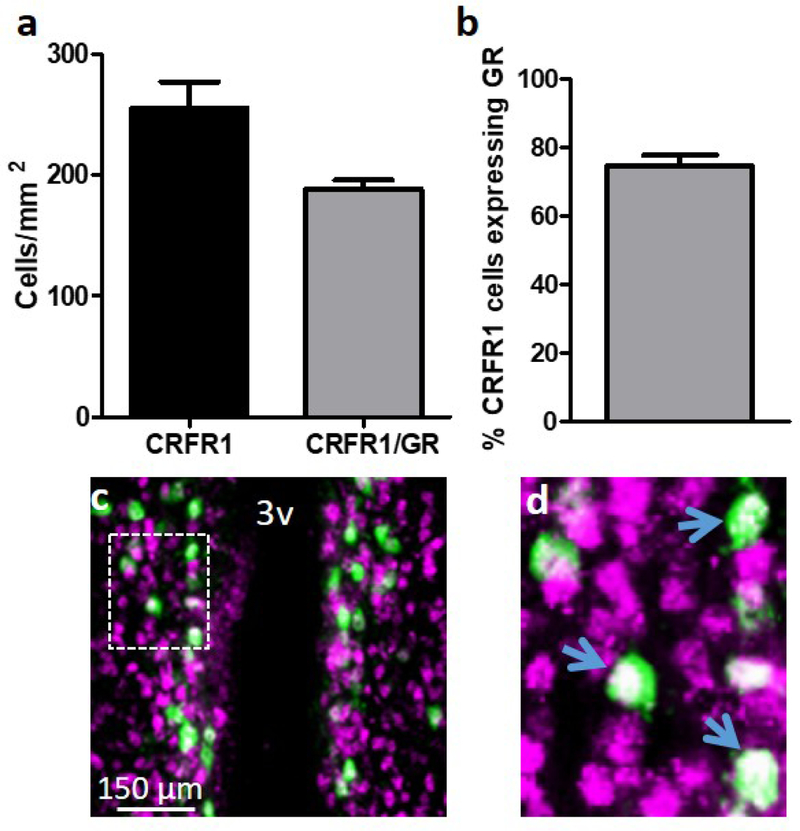
The pCREB antibody (RRID: AB_2561044) was generated by immunizing rabbits with a synthetic phosphopeptide that corresponds to residues that surround Ser133 of human CREB (129–137). Using Western blotting, this antibody recognizes a 43 kDa band selective for pCREB (manufacturer information). This antibody has been utilized to detect neural activation within CRFR1-GFP labeled cells using the CRFR1-BAC mouse line (Uribe-Marino et al., 2016). Our studies also indicate that little pCREB is present in the hypothalamus of resting mice while it is abundant following psychogenic stress (Figure 8c,d).
Figure 8.
Dual-label fluorescent immunohistochemistry showing the number of (a) CRFR1-GFP and CRFR1-GFP/GR co-expressing cells, and (b) the percentage of female AVPV/PeN CRFR1GFP+ cells that express GR (~75%). (c) Representative image of CRFR1 (green) and GR (magenta) co-expression (white), and (d) high magnification image of co-localized cells in the AVPV/PeN. Inset box in (c) indicates region further magnified in (d). Arrows indicate examples of co-localized cells. N= 4 female mice. 3V, third ventricle.
2.9.2. Estrogen Receptor Alpha (ERα)
This ERα antibody (RRID: AB_631470) was generated against a peptide at the C-terminus of mouse ERα which corresponds to amino acids 580–599 (manufacturer information). Peptide preabsorption studies have been used to confirm specificity of this antibody (Quesada, Romeo, & Micevych, 2007). An absence of ERα signal has also been confirmed in ERα knockout mice (Omoto, Imamov, Warner, & Gustafsson, 2005). We also confirmed regional ERα distribution patterns in our own tissue to known distributions in mice and found high concordance (Allen Institute ISH data, Merchenthaler, Lane, Numan, & Dellovade, 2004).
2.9.3. Green fluorescent protein (GFP)
The GFP antibody (RRID: AB_300798) was generated against the full length GFP protein. Using Western blotting, this antibody recognizes a 27–30 kDa band selective for GFP (manufacturer information). Studies in our laboratory using this antibody confirm no labeling in brain tissue from wild type mice.
2.9.4. Tyrosine hydroxylase (TH)
This polyclonal antibody (RRID: AB_390204) is generated from denatured tyrosine hydroxylase from rat pheochromocytoma with rabbit as host species. Western blotting shows it selectively labels a single 62-kDa band in PC12 cell lysate (manufacturer’s information). Specificity was confirmed in our laboratory based on specific distributions of known TH-positive cells based on previous work (Scott, Prigge, Yizhar, & Kimchi, 2015) and the Allen Institute ISH data. Specifically, high levels of TH expressing cell bodies are found in the AVPV, arcuate nucleus and locus coeruleus.
2.9.5. Kisspeptin (KISS)
This polyclonal antibody (RRID: AB_2296529) is generated using the mouse kisspeptin 10 peptide with rabbit as host species. Specificity for the KISS antibody was confirmed in our laboratory again by comparing our regional labeling distributions against published IHC (Poling & Kaufmann, 2013) and ISH (Allen Institute ISH data). Similar to previous work, we found labeled cell bodies in the AVPV/PeN and arcuate nucleus and few labeled cells elsewhere in the hypothalamus.
2.9.6. Glucocorticoid receptor (GR)
The GR polyclonal antibody (RRID: AB_2155786) was generated against a peptide at the N-terminus of GRα of mouse origin (5–24) (manufacturer’s information). Western blotting shows a specifically labeled band at 110 kDa in adrenal tissue (Damjanovic et al., 2012). Peptide preabsorption studies have been used to confirm specificity of this antibody (Damjanovic et al., 2012). Brain distributions of GR in our labeled tissue also match those reported in Allen Institute ISH data, with highest levels found in the paraventricular hypothalamus, central amygdala, and hippocampus.
2.10. Microscopic analysis:
Analysis of CRFR1-GFP-ir distribution was conducted on a Nikon 80i microscope equipped with a digital camera. The AVPV/PeN was identified using the Allen institute mouse brain reference atlas for adults and an atlas of the developing mouse brain for analysis of neonatal tissue (Paxinos, Halliday, Watson, Koutcherov, & Wang, 2007). Images were collected using a 20X objective. Following image collection, cells were bilaterally quantified within two atlas-matched sections using rectangular ROIs placed around the AVPV/PeN (P0, 138000µm2; P60, 166000µm2). Dual-labeled cells were quantified using image J and considered co-localized when green (CRFR1-GFP-ir) and pseudo-colored magenta cells (secondary labels) overlapped, appearing white. Nuclear proteins (ERα, pCREB, GR) were counted as co-localized if they appeared within the nuclear region of CRFR1 cells. Cell numbers are shown as immunoreactive cells per mm2.
2.11. Statistical Analyses:
Analysis of CRFR1-GFP-ir and co-localization patterns within the AVPV/PeN was performed using 2-way ANOVA, 1-Way ANOVA, or Student’s t-Tests as appropriate. All analysis was performed using GraphPad Prizm v.4. For neonatal hormone experiments we hypothesized treatments would affect females but not males. Therefore, we separated these analyses by sex and performed 1-way ANOVAs. Significant main effects/interactions were further analyzed using Bonferroni corrected T-tests; significance level was set at p≤0.05 and data are shown as means ± SEM.
3. Results
3.1. Phenotypic Characterization of CRFR1-GFP-ir Cells in the AVPV/PeN
3.1.1. Tyrosine Hydroxylase (TH).
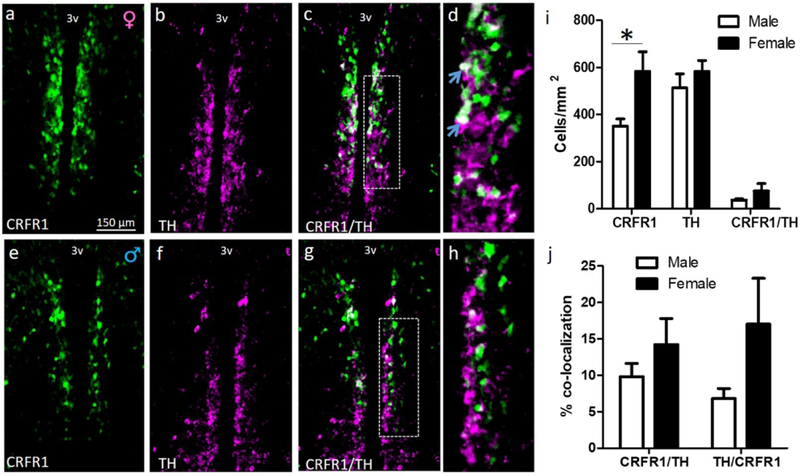
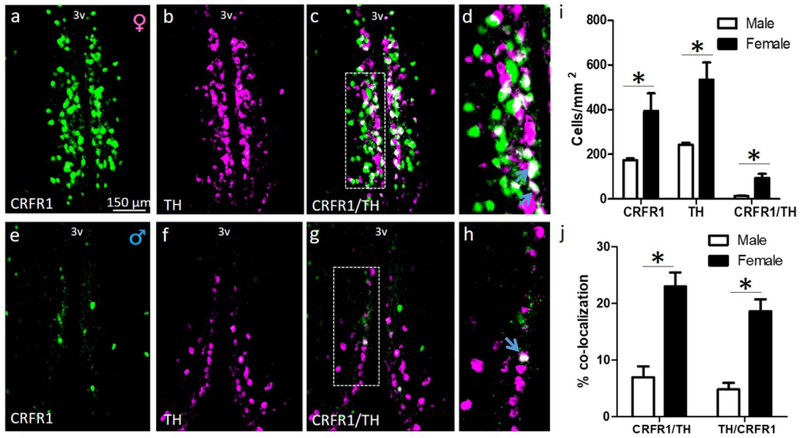
Numbers of CRFR1-GFP, TH, and CRFR1-GFP/TH-ir cells were assessed in male and female mice at P0 and P60. As previously reported we found a sex difference in CRFR1-GFP-ir cells at P0 (females > males; (t(8)=2.329, p≤.05; Figure 1). No sex difference was found in the number of TH cells at P0 (Figure 1). Neither the percentage of CRFR1 cells that co-express TH (~12%) nor the percentage of TH cells that co-express CRFR1 (~17%) differed by sex at P0 (Figure 1). At P60, CRFR1 (t(8)=2.787, p≤.05), TH (t(8)=3.755, p≤.01), and CRFR1/TH (t(8)=4.165, p≤.01) cells were more abundant in females than males (Figure 2). The percentage of CRFR1 cells that co-express TH (t(8)=5.210, p≤.001) and the percentage of TH cells that co-express CRFR1 (t(8)=5.802, p≤.001) were also greater in females than males (Figure 2).
Figure 1.
Dual-label fluorescent immunohistochemistry comparing female (a-d) and male (e-h) AVPV/PeN CRFR1-GFP, tyrosine hydroxylase (TH), and co-localization of CRFR1/TH on the day of birth (d, h; high-magnification display of images c, g respectively). (i) Comparison of male versus female CRFR1-GFP- and TH-expressing cells (green, magenta, respectively), and co-expression of TH/CRFR1-GFP (white) illustrating a significant sex difference in P0 CRFR1 (F>M), but no observed differences in TH-ir. (j) Female versus male comparison of the percentage of CRFR1 cells that co-localize TH, and percentage of TH cells that co-express CRFR1. * indicates statistical significance (p≤0.05), data reported as mean ± SEM. Inset boxes indicate regions that were further magnified. Arrows indicate examples of co-localized cells. N=5 per sex. 3V, third ventricle.
Figure 2.
Dual-label fluorescent immunohistochemistry comparing the adult (P60) female (a-d) and male (e-h) AVPV/PeN CRFR1-GFP, tyrosine hydroxylase (TH), and co-localization of CRFR1/TH (d, h; high-magnification display of images c, g respectively). (i) Comparison of female versus male CRFR1-GFP- (green), TH-expressing cells (magenta), and co-expression of TH in CRFR1-GFP cells (white) illustrating significant sex differences in P60 CRFR1-GFP-ir, TH-ir, and co-labeled cells (all F>M). (j) Female versus male comparison of the percentage of CRFR1 cells that co-localize TH, and percentage of TH cells that co-express CRFR1 (both F>M). * indicates statistical significance (p≤0.05), data are reported as mean ± SEM. Inset boxes indicate regions that were further magnified. Arrows indicate examples of co-localized cells. N=5 per sex. 3V, third ventricle.
3.1.2. Kisspeptin (KISS).
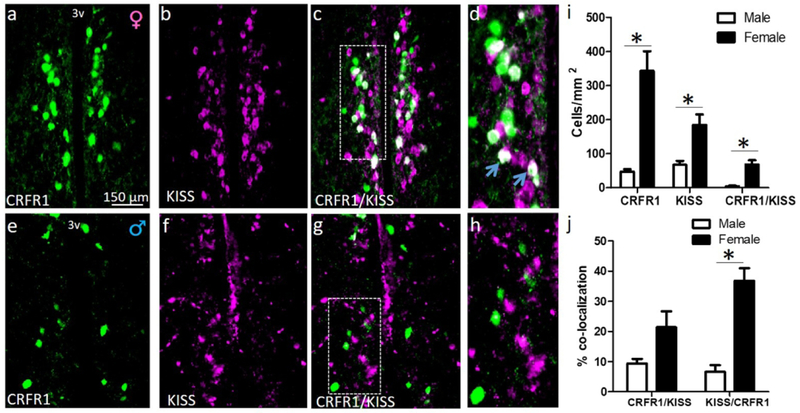
Numbers of CRFR1-GFP, KISS, and CRFR1-GFP/KISS-ir cells were assessed at P60 in male and female mice. CRFR1 (t(8)=5.048, p≤.01), KISS (t(8)=3.285, p≤.05), and CRFR1/KISS (t(8)=4.435, p≤.001) cells were more abundant in females than males (Figure 3). There was a trend toward a greater percentage of CRFR1 cells that co-express KISS in females compared to males (t(8)=2.168, p=.07). The percentage of KISS cells that coexpress CRFR1 was significantly greater in females than males (t(8)=6.458, p≤.001; Figure 3).
Figure 3.
Dual-label fluorescent immunohistochemistry comparing adult (P60) female (a-d) and male (e-h) AVPV/PeN CRFR1-GFP (a, e), kisspeptin (KISS) (b, f), and co-expression (c, g). (d, h) high magnification display of images c, g respectively. (i) Comparison of female and male CRFR1-GFP-ir and KISS-ir, with significant differences in the number of CRFR1-GFP-ir (F>M), KISS-ir (F>M), and co-localized cells (F>M). (j) Female versus male comparison of the percentage of CRFR1 cells that co-localize KISS, and percentage of KISS cells that co-express CRFR1 (both F>M). * indicates statistical significance (p≤0.05), data are reported as mean ± SEM. Inset boxes indicate regions that were further magnified. Arrows indicate examples of colocalized cells. N=5 per sex. 3V, third ventricle.
3.1.3. Estrogen Receptor Alpha (ERα).
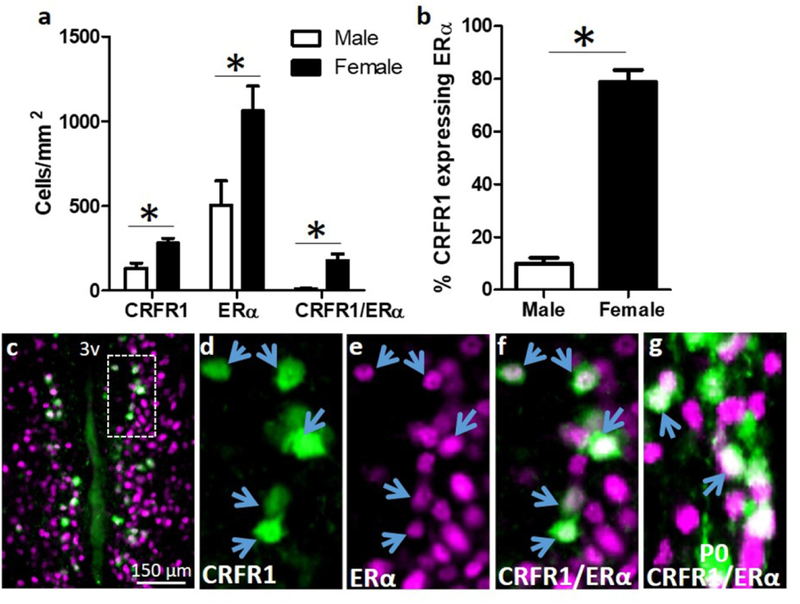
Quantification of dual label CRFR1-GFP-ir/ERα colocalization showed that females had significantly more CRFR1-GFP-ir/ERα co-localized cells than males (Age P60–70; Figure 4a). In addition, the male AVPV/PeN had significantly fewer ERα expressing cells (t(8)=2.691, p≤.05), few of which co-express CRFR1, as indicated by a significantly higher percentage of CRFR1-GFP-ir cells that co-localize ERα in the female AVPV/PeN (t(8)=5.775, p≤.001; Figure 4b). Co-localization of CRFR1 and ERα is also found in the AVPV/PeN of P0 female mice (Figure 4f).
Figure 4.
(a,b) Co-localization of CRFR1-GFP and ERα. Dual-label fluorescent immunohistochemistry showing that females have significantly more CRFR1-GFP, ERα, CRFR1-GFP/ERα co-localized, and a higher percentage of CRFR1-GFP cells that co-express ERα compared to males. (c-f) Representative images from a female AVPV/PeN showing a low magnification image of co-localized cells (c), CRFR1-GFP (d; green), ERα (e; magenta), and coexpression of CRFR1/ERα (f; white). (g) Image showing co-expression of CRFR1 and ERα on the day of birth (P0) in the female mouse AVPV/PeN. Inset box indicates region that was further magnified. Arrows indicate examples of co-localized cells. * Indicates statistical significance (p≤0.05), data are reported as mean ± SEM. N=5 per sex. 3V, third ventricle.
3.2. Neonatal Hormone Treatment on CRFR1 Expression Pattern in the P21 Female and Male AVPV/PeN
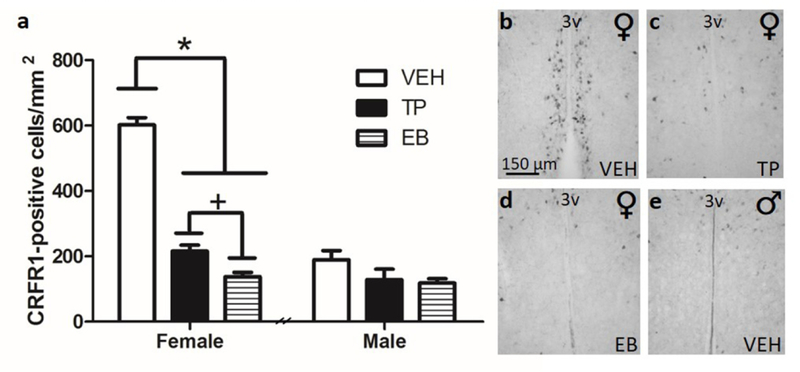
One-way ANOVA of CRFR1-GFP-ir cells within the female AVPV/PeN revealed a main effect of hormone supplementation on CRFR1-GFP expression ((F(2,14)=188, p≤.001; Figure 5) where TP and EB injection significantly reduced CRFR1-GFP cells compared with female vehicle-injected animals (ps≤.001). Post-hoc analysis further revealed a significant difference between treatments, where EB reduced CRFR1 cell number to a greater extent than TP (p≤.05). One-way ANOVA of CRFR1-GFP-ir cells within the male AVPV/PeN revealed no significant effect of treatment on CRFR1-GFP-ir cell number (Figure 5).
Figure 5.
Neonatal (P0) hormone supplement influence on CRFR1-GFP+ cell number in (a) female versus male mice treated with sesame oil (VEH), testosterone propionate (TP), or estradiol benzoate (EB). P0 treatment with either TP or EB significantly reduced the cell number in female mice, while having no impact on the male AVPV/PeN CRFR1-GFP+ cells. Treatment with EB reduced CRFR1-GFP to a greater extent than TP. Representative images from females treated with VEH (b), TP (c), EB (d), and a male treated with VEH (e). * Indicates statistical significance (p≤0.001), + indicates p≤.05. Data are reported as mean ± SEM. N=6–7 per sex/treatment. 3V, third ventricle.
3.3. Adult (P60) Gonadectomy (GDX) on CRFR1 Expression in the AVPV/PeN
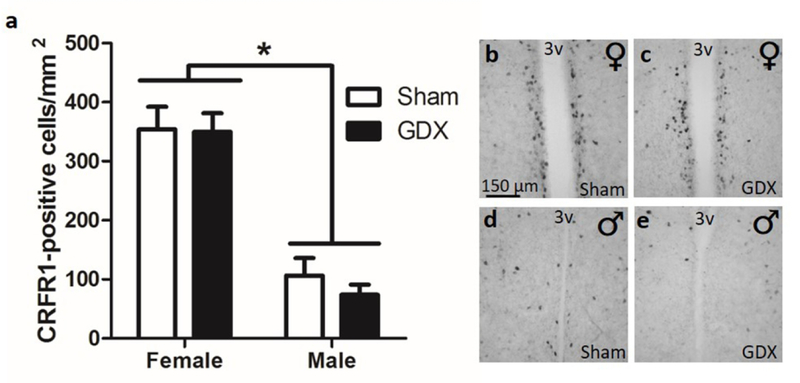
A two-way ANOVA revealed a significant sex difference in AVPV/PeN CRFR1 cells ((F(1,26)=75.50, p≤0.001) matching our previous data. However, gonadectomy had no effect on CRFR1 cell number in either sex (Figure 6).
Figure 6.
Adult gonadectomy effects on CRFR1-GFP+ cells. (a) Gonadectomy did not alter CRFR1-GFP+ cell number in either males or females. Representative images from female (b) and male (d) sham surgery compared with female (c) and male (e) gonadectomy, showing that removal of adult gonadal hormones does not change the expression pattern. * Indicates significant sex difference (p≤0.001), data are reported as mean ± SEM. N=8 per sex/treatment. 3V, third ventricle.
3.4. AVPV/PeN CRFR1-GFP-ir activation (pCREB co-expression) following a 30-minute restraint stress and CRFR1-GFP-ir co-localization with glucocorticoid receptor (GR)
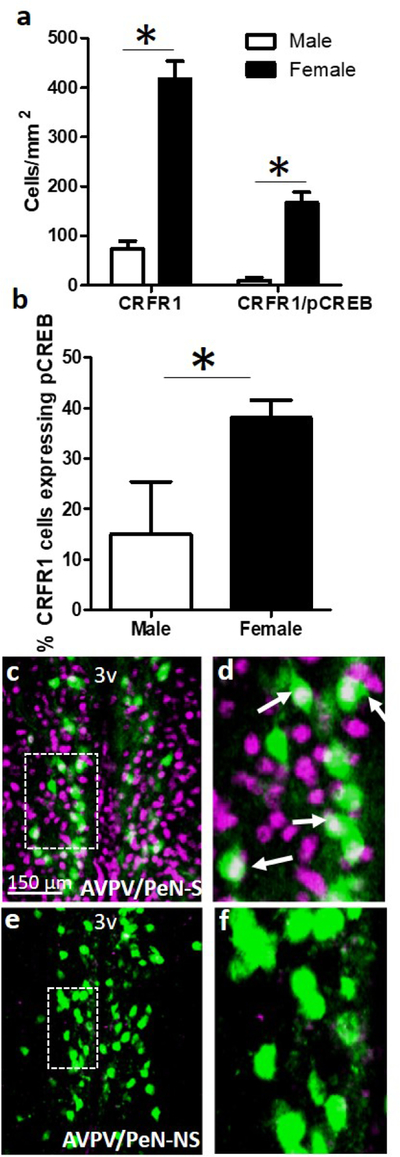
To determine whether acute psychogenic stress (30min restraint) would elicit activation of CRFR1-GFP-ir cells in the AVPV/PeN, we assessed the co-expression of CRFR1 and the transcription/neural activation marker phosphorylated (p) CREB. This analysis revealed a greater number of CRFR1-GFP/pCREB co-localized cells in females compared to males (t(10)=5.994, p≤.001; Figure 7). The percentage of AVPV/PeN CRFR1 cells co-expressing pCREB was also greater in females (t(10)=2.241, p≤.05; Figure 7). Quantification of dual-label CRFR1-GFP/GR indicated 75% co-localization in the female AVPV/PeN (Figure 8).
Figure 7.
Dual-label fluorescent immunohistochemistry showing (a) female versus male expression levels of CRFR1-GFP (F>M), and CRFR1-GFP/pCREB colocalization (F>M). (b) Comparison between female and male co-expression, showing a sex difference in the percentage of activated CRFR1-GFP+ cells (pCREB co-expression) following a 30-minute restraint stress (F>M). Low and high magnification representative images of a female AVPV/PeN from a (c-d) s; stressed and (e-f) ns; non-stressed mouse. * Indicates statistical significance (p≤0.05), data are reported as mean ± SEM. Inset boxes indicate regions that were further magnified. Arrows indicate examples of co-localized cells. N= 5 male, 7 female mice. 3V, third ventricle.
3.5. AVPV/PeN CRFR1 co-localization with fluorogold.
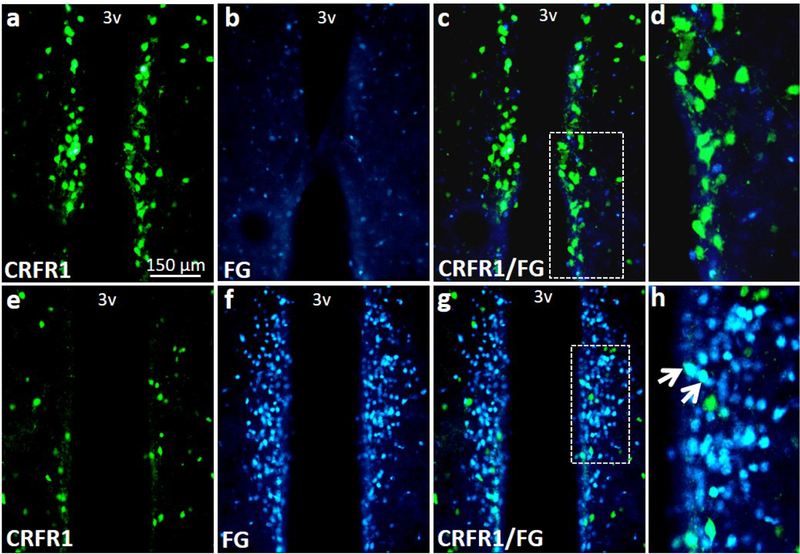
Quantification of dual label CRFR1GFP-ir/FG co-localization for adult (P60–65) mice indicated that none of the AVPV/PeN CRFR1GFP expressing cells are neurosecretory (See Figure 9). Little fluorogold was found near the sexually dimorphic CRFR1 AVPV/PeN cluster (Figure 9a-d), although abundant fluorogold and CRFR1/fluorogold co-labeling was found in more caudal periventricular sections (Bregma −0.40; Figure 9e-h).
Figure 9.
Images showing fluorogold and CRFR1-GFP labeling. Few neurosecretory (fluorogold) were found near the sexually dimorphic CRFR1 AVPV/PeN nucleus and no CRFR1GFP/fluorogold co-labeled cells were found in this region (a-d). However, high levels of fluorogold and CRFR1/fluorogold co-labeling was found in further caudal periventricular sections (e-h). Inset boxes indicate regions that were further magnified. Arrows indicate examples of colocalized cells. 3V, third ventricle.
4. Discussion
4.1. Chemical composition of AVPV/PeN CRFR1-GFP-ir cells
The current study characterized a novel cluster of CRFR1-ir cells that previous work from our lab has shown to be sexually dimorphic from P0-P21 mice (Rosinger, Jacobskind, Park, Justice, & Zuloaga, 2017). Other AVPV/PeN cell phenotypes such as TH and KISS are known to be more abundant in female than male rodents (Poling & Kauffman, 2013; Semaan et al., 2010; Scott, Prigge, Yizhar, & Kimchi, 2015). These findings prompted us to investigate whether these CRFR1 cells represent the same or distinct cell populations. We found that the majority of CRFR1-GFP+ cells in the female AVPV/PeN do not co-express TH (~22% co-expression), and conversely approximately 18% of TH+ cells co-express CRFR1-GFP. Similar low percentages of CRFR1/TH co-localization are found at P0. TH-expressing cells in the rodent AVPV have been directly implicated in regulation of maternal behaviors and TH cells are dynamically affected by maternal experience (Scott, Prigge, Yizhar, & Kimchi, 2015). The presence of CRFR1 on a subpopulation of these cells indicates that CRF signaling can potentially influence dopamine transmission and associated functions such as maternal behaviors.
A greater percentage of KISS+ cells in the AVPV/PeN co-express CRFR1-GFP (~35%), which opens the possibility for CRF signaling within the AVPV/PeN to disrupt KISS function, potentially contributing to stress-induced alterations in reproduction. CRF signaling in the medial preoptic area and the arcuate nucleus has been shown to inhibit KISS signaling to gonadotropin releasing hormone neurons, thereby affecting luteinizing hormone pulsatile secretion (Kinsey-Jones et al., 2009). In addition, intracerebroventricular administration of CRF can delay the onset of puberty in female rats (Kinsey-Jones et al., 2010), therefore CRF binding to AVPV/PeN CRFR1 cells may be key in this process. Our data also show that the majority of CRFR1-GFP-ir cells in the AVPV/PeN do not co-express KISS (~21%). In general, CRFR1 cells appear to be more abundant than KISS cells in this region which suggests that CRFR1 within the AVPV/PeN likely serves multiple functions. However, even in instances where these cells are not colocalized, the close physical proximity of CRFR1-GFP-ir cells to both TH+ and KISS+, may suggest communication between these cells, though further investigation is needed. Rodent AVPV cells project to several forebrain and hindbrain structures involved in reproductive behaviors, stress-related behaviors, and hormone release (e.g., vascular organ of the lamina terminalis, medial preoptic area, ventral lateral septum, arcuate nucleus, paraventricular hypothalamus, and periaqueductal gray; Gu & Simerly, 1997; Scott, Prigge, Yizhar, & Kimchi, 2015), further supporting the potential involvement of the AVPV/PeN in an array of sexually dimorphic responses. Whether AVPV/PeN CRFR1 cells project to these same brain regions is currently unknown, though our do data involving fluorogold injections indicate that these cells are not neurosecretory, and therefore do not directly project outside of the brain.
4.2. Gonadal hormone regulation of AVPV/PeN CRFR1-GFP-ir cells
We next investigated whether the male-typical perinatal androgen surge was responsible for sexual differentiation of the CRFR1-GFP-ir nucleus. Previous research has shown that perinatal androgen secretions are responsible for masculinizing a number of hypothalamic and other brain structures (Morris, Jordan, & Breedlove, 2004; Raisman & Field, 1971). Our findings show that P0 treatment with testosterone, or its aromatized metabolite 17β-estradiol, results in a defeminized AVPV/PeN CRFR1-GFP-ir nucleus in P21 females (Figure 6). Females treated with testosterone or 17β-estradiol were indistinguishable from their male counterparts, while having significantly fewer CRFR1-GFP cells than vehicle-treated female controls (Figure 6B-E). Furthermore, 17β-estradiol treatment in P0 females caused a slightly greater reduction in CRFR1-GFP cells than did testosterone. Although estrogens can potentially act at both ERα and β subtypes to induce these effects, ERα is the likely mechanism. We demonstrate that CRFR1 cells at P0 co-express ERα, while previous work has shown that the P0 AVPV/PeN is largely devoid of ERβ (Zuloaga, Zuloaga, Hinds, Carbone, & Handa, 2014). In addition, androgen receptor (AR) levels are barely detectable at P0 (Juntti et al., 2010; Kanaya et al., 2014). Apoptosis is potentially responsible for the observed sex difference in the AVPV as others have shown that perinatal androgen secretions trigger cell death in the developing AVPV (Forger et al., 2004; Kelly, Varnum, Krentzel, Krug, & Forger, 2013). However, whether CRFR1 cells specifically undergo apoptosis is unknown. Interestingly, the dimorphic pattern of CRFR1GFP+ cells within the AVPV/PeN appears earlier than other known dimorphic cell phenotypes, such as TH and KISS (Figure 1; Poling & Kauffman, 2013; Waters & Simerly, 2009), and might potentially influence the development of the mouse AVPV/PeN. CRF signaling through CRFR1 has been implicated in normal brain developmental processes. Specifically, CRF/CRFR1 signaling has been shown to facilitate dendrite and synapse formation in the developing olfactory bulb (Garcia et al., 2014). Notably, AVPV/PeN CRFR1 cell number also appears to decrease between P0 and P60 in both sexes, a decline we have previously reported (Rosinger, Jacobskind, Park, Justice, & Zuloaga, 2017). This decrease in CRFR1 cell number might reflect ongoing apoptosis in the AVPV during the postnatal period.
We next sought to determine the role of circulating gonadal steroid hormones on the maintenance of the AVPV/PeN CRFR1-GFP-ir nucleus in adulthood. We gonadectomized P60 female and male mice, and following a 6-week recovery period, found no alterations in CRFR1GFP cells in animals of either sex (Figure 7). The lack of effect following gonadectomy suggests that this nucleus is organized during the perinatal period, and not sensitive to circulating androgens and estrogens in adulthood. These results contrast those reported for other sexually dimorphic cell phenotypes in the AVPV such as TH and KISS. Ovariectomy increases TH-ir in rats and decreases KISS-ir in mice (Brock & Bakker, 2013; Simerly, 1989). Estrus phase was not accounted for in the present study, therefore it is possible that a difference could be observed in proestrus (when estrogens are elevated) compared to ovariectomized females. However, a similar comparison of freely cycling and ovariectomized mice (6 weeks) revealed a decrease in kisspeptin within the same region (Brock & Bakker, 2013). Furthermore, our findings reveal near exact CRFR1-GFP cell numbers between OVX and intact mice, indicating that circulating estrogens likely have little effect on this cell phenotype. It remains possible that other hormones such as those elevated during the maternal period (e.g. prolactin, glucocorticoids) might alter CRFR1 levels, however, further investigation is required. Regardless, in males, the organizational influence of perinatal testosterone exposure is sufficient enough to maintain an absence of expression in adulthood, even after removal of the primary source of androgens.
4.3. Potential role of AVPV/PeN CRFR1 in sexually dimorphic stress regulation
Considering the well-established sex differences in behavioral and hormonal responses to stress, we sought to investigate the involvement of CRFR1-GFP-ir in the AVPV/PeN during a single acute psychogenic stressor. CRFR1-GFP containing cells showed extensive activation illustrated through high levels of pCREB co-expression following a single 30-min restraint. Additionally, the few CRFR1-GPF cells present in males were not activated following restraint. This finding indicates a discrete population of cells that is engaged in the female, but not the male, brain and may therefore represent a unique site of regulation for the female stress response. The sustained pCREB response (2 hours after stress onset) appears to reflect a prolonged stimulation of pCREB which is induced by phosphorylation of calcium/calmodulin dependent protein kinase II (CaMKII). The temporal induction of hypothalamic pCREB following restraint mirrors that of phosphorylated CaMKII indicating that a sustained increase in CAMKII might drive the sustained pCREB response (Kwon et al., 2006). The resulting CREB activation might therefore regulate expression of various CREB-associated genes. It is important to note that increases in pCREB within CRFR1 cells doesn’t necessarily indicate that pCREB elevations are due to activation of CRFR1. Future studies utilizing CRFR1 antagonists are warranted to determine whether pCREB induction is indeed due to CRF actions at CRFR1. CRFR1 receptor antagonists have been shown to reduce immediate early gene responses following restraint in the paraventricular nucleus (Imaki et al., 1995), however whether similar effects occur for AVPV/PeN pCREB is currently unknown.
The potential CRF inputs that might contact/activate AVPV/PeN CRFR1 cells remain unknown. Anatomical studies in the rat indicate several preoptic, hypothalamic, amygdala regions project to the AVPV. These include the medial preoptic area, paraventricular hypothalamus, bed nucleus of the stria terminalis, central amygdala, and medial amygdala (Canteras et al., 1992; 1995; Hutton et al, 1998; Simerly & Swanson, 1988). Each of these regions contain CRF-expressing cells and represent potential sites for CRF regulation of AVPV/PeN R1 cells.
We further demonstrate that the majority of CRFR1-GFP cells co-localize GR, indicating a site at which circulating glucocorticoids can influence this cell group in female rodents. Another CRFR1 cell group in the paraventricular hypothalamus has been shown to be upregulated by circulating glucocorticoids and glucocorticoid actions on CRFR1 cells during chronic stress have been proposed as a mechanism of hormonal and potentially behavioral adaptation to chronic stress (Ramot et al., 2017). Whether AVPV/PeN CRFR1 cells are similarly regulated by glucocorticoids and their involvement in adaptation to chronic stress is currently unknown.
Gonadal hormones and actions at their cognate receptors strongly influence HPA axis tone and anxiety/depression-like behaviors. Androgens including testosterone or metabolite 5α-dihydrotestoterone, effectively reduce the adrenocorticotropic hormone and CORT responses to stress (Handa et al., 1994; Lund, Hinds, & Handa, 2006) while the opposite is true of estrogens, which enhance glucocorticoid responses and impair glucocorticoid-mediated negative feedback (Burgess and Handa, 1992; Handa & Weiser, 2014; Goel, Workman, Lee, Innala, & Viau, 2014; Viau, 2002; Weiser & Handa, 2009). ERβ and AR binding induce anxiolytic and HPA axis suppressing effects (Lund, Hinds, & Handa, 2006; Zuloaga, Morris, Jordan, & Breedlove, 2008). On the contrary ERα binding generally produces anxiogenic effects and impairs HPA axis negative feedback, shown through studies utilizing ERα-specific agonists (e.g., propylpyrazoletriol; Rossi et al., 2010; Weiser & Handa, 2009). However, what remains largely unknown are the specific brain regions and cell phenotypes upon which gonadal hormones can induce effects on stress-related functions.
Given the known role of CRFR1 in regulation of behavioral and hormonal stress responses, the presence of ERα in CRFR1 cells represents an intriguing potential site for estrogen regulation of these functions. Furthermore, the virtual absence of ERα/CRFR1 cells in the male AVPV/PeN indicates this is a key site for female-specific regulation of stress functions via ERα. Although the AVPV/PeN has not traditionally been considered a central region for regulation of stress responsivity, it projects to other hypothalamic and limbic regions involved in the regulation of behavioral and hormonal stress functions including the paraventricular hypothalamus, arcuate nucleus, and periaqueductal gray (Gu & Simerly, 1997). Future studies are warranted to determine the specific connectivity of AVPV/PeN CRFR1-GFP-ir cells with other hypothalamic and limbic structures. It is also possible that these cells play a lesser role in mediating stress effects on other neural systems and are primarily a stressor-responsive circuit. Potential functions noted above may include CRF regulation of the luteinizing hormone surge, ovulation, and maternal behaviors which are functions known to be controlled by AVPV cells (Herbison, 2008; Scott et al., 2015). Additionally, neurons in the AVPV have also been shown to regulate feeding and drinking behaviors and may therefore serve as a site through which stress induced CRF might mediate these functions (Bealer & Johnson, 1980; Johnson & Buggy, 1978).
4.4. Summary
Overall, we report a novel sexually dimorphic expression of CRFR1-GFP in the female AVPV/PeN. Sexual differentiation of this nucleus occurs via testosterone conversion to estrogen, likely via actions at ERα. On the contrary, this nucleus is unaffected by removal of circulating gonadal hormones via gonadectomy in adulthood. These cells are also activated by psychological stress and co-express GR indicating the potential for circulating glucocorticoids to alter their function. The function of this sexually dimorphic CRFR1 population is currently unknown, although co-localization patterns with ERα indicate a potential role in the estrogen regulation of stress functions. Lower levels of co-localization with KISS and TH also suggest these cells as potential sites for stress/CRF regulation of reproductive and maternal functions.
Acknowledgments
Kasia Szafranska and Shannon Park are acknowledged for their technical assistance. Support was provided by University at Albany research initiation funds.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology 2003; 144(6):2580–2587. [DOI] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. Journal of Neuroscience 2002; 22(1):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2 deficient mice: sexually dichotomous responses. Journal of Neuroscience 2003; 23(12): 5295–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Research 2016; 1641(Pt B):177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer SL, Johnson AK. Preoptic-hypothalamic periventricular lesions after food-associated drinking and circadian rhythms. Journal of Comparative Physiology and Psychology 1980; 94(3):547–55. [DOI] [PubMed] [Google Scholar]
- Bingham B, Wang NX, Innala L, Viau V. Postnatal aromatase blockade increases c-fos mRNA responses to acute restraint stress in adult male rats. Endocrinology 2012; 153(4):16031608. [DOI] [PubMed] [Google Scholar]
- Borrow AP, Handa RJ. Estrogen Receptors Modulation of Anxiety-Like Behavior. Vitamins and Hormones 2017; 103:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology 2013; 154(8):2739–2749. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology 1992; 131(3):1261–1269. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Estrogen-induced alterations in the regulation of mineralocorticoid and glucocorticoid receptor messenger RNA expression in the female rat anterior pituitary gland and brain. Molecular and Cellular Neuroscience 1993; 4(2):191–198. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. Journal of Comparative Neurology 1992; 324(2):143–79. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology 1992; 360(2):213–45. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology 2009; 5(7):374–381. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Research 1999; 835:1–9. [DOI] [PubMed] [Google Scholar]
- Damjanovic SS, Antic JA, Ilic BB, Cokic BB, Ivovic M, Ognjanovic SI, … Paunovic I. Glucocorticoid receptor and molecular chaperones in the pathogenesis of adrenal incidentalomas: potential role of reduced sensitivity to glucocorticoids. Molecular Medicine 2013; 18:1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva BS, Rovaris DL, Schuch JB, Mota NR, Cupertino RB, Aroche AP, … Bau CHD. Effects of corticotropin-releasing hormone receptor one SNPs on major depressive disorder are influenced by sex and smoking status. Journal of Affective Disorders 2016; 205:282288. [DOI] [PubMed] [Google Scholar]
- Dimitrov E, Usdin TB. Tuberoinfundibular peptide of 39 residues modulates the mouse hypothalamic-pituitary-adrenal axis via paraventricular glutamatergic neurons. The Journal of Comparative Neurology 2010; 518(21):4375–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ (2004), Deletion of bax eliminates sex differences in the mouse forebrain. Proceedings of the National Academy of Sciences 2004; 101(37):13666–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology 2004; 29:475–485. [DOI] [PubMed] [Google Scholar]
- Garcia I, Paramjit K, Burak T, Ortiz-Guzman J, Huang L, Herman AM, Chaboub L, … Arenkiel BR (2014), Local corticotropin releasing hormone (CRH) signals to its receptor CRHR1 during postnatal development of the mouse olfactory bulb. Brain Structure and Function 2014; 221:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carmona JA, Baroja-Mazo A, Milanes MV, Laorden ML. Sex differences between CRF1 receptor deficient mice following Naloxone-precipitated morphine withdrawal in a conditioned place aversion paradigm: implication of HPA axis. PLoS ONE 2015; 10(4):e0121125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). Journal of Clinical Psychiatry 2015; 76:155–162. [DOI] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Comprehensive Physiology 2014; 4(3):1121–1155. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. The Journal of Comparative Neurology 1997; 384:142–164. [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology and Behavior 1994; 55:117–124. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Frontiers in Neuroendocrinology 2014; 35:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Annals of the New York Academy of Science 1995; 771:92–104. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Research Reviews 2008; 57(2):277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA. Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. Journal of Neuroscience 2016; 36(14):4093–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton LA, Gu G, Simerly RB. Development of a sexually dimorphic projection from the bed nuclei of the stria terminalis to the anteroventral periventricular nucleus in the rat. Journal of Neuroscience 1998; 18(8):3003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Wang XQ, Demura H. Intracerebroventricular administration of corticotropin-releasing factor antagonist attenuates c-fos mRNA expression in the paraventricular nucleus after stress. Neuroendocrinology 1995; 61(4):445–52. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Buggy J. Periventricular preoptic-hypothalamus is vital for thirst and normal water economy. Am J Physiol 1978; 234(3):R122–9. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, … Shah NM. The androgen receptor governs the execution, but not programming, or male sexual and territorial behaviors. Neuron 2010; 66(2):260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. The Journal of Comparative Neurology 2008; 511(4):479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya M, Tsuda MC, Sagoshi S, Nagata K, Morimoto C, Tha Thu CK. Regional difference in sex steroid action on formation of morphological sex differences in the anteroventral periventricular nucleus and principle nucleus of the bed nucleus of the stria terminalis. PLoS ONE 2014; 9(11):e112616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas CA, Lu YL, Richardson HN. Adolescent drinking targets corticotropin-releasing factor (CRF) peptide labeled cells in the central amygdala of male and female rats. Neuroscience 2013; 249:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DA, Varnum MM, Krentzel AA, Krug S, Forger NG. Differential control of sex differences in estrogen receptor α in the bed nucleus of the stria terminalis and anteroventral periventricular nucleus. Neuroendocrinology 2013; 154(10):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, …Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry 1987; 51(1):8–19. [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Knox AM, Lin YS, Milligan SR, Lightmant SL, O’Byrne KT. Corticotrophin-releasing factor alters the timing of puberty in the female rat. Journal of Neuroendocrinology 2010; 22(2):102–109. [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, … O’Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. Journal of Neuroendocrinology 2009; 21:1:20–29. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Korets R, & Silver R Expression of the circadian clock gene Period 1 in neuroendocrine cells: An investigation using mice with a Per1:GFP transgene. European Journal of Neuroscience 2003; 17(2):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience 2006; 142(4):1281–92. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. Journal of Neuroscience 2006; 26:1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology 2005; 146:797–807. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. At the frontier of epigenetics of brain sex differences. Frontiers in Behavioral Neuroscience 2015; 9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Wilson MA. A sexually dimorphic population of CRF neurons in the medial preoptic area. Neuroendocrinology 1994; 5:653–656. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. The Journal of Comparative Neurology 2004; 473(2):270–91. [DOI] [PubMed] [Google Scholar]
- Milosevic A, Liebmann T, Knudsen M, Schintu N, Svenningsson P, Greengard P. Cell- and region-specific expression of depression-related protein p11 (S100a10) in the brain. The Journal of Comparative Neurology 2017; 525(4):955–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM Sexual differentiation of the vertebrate nervous system. Nature Neuroscience 2004; 7(10):1034–9. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S Sex differences in unipolar depression: evidence and theory. Psychological Bulletin 1987; 101(2):259–82. [PubMed] [Google Scholar]
- Omoto Y, Imamov O, Warner M, Gustafsson JA. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. Proceedings of the National Academy of Sciences 2005; 102:1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Thompson MK, Handa AZ, Handa RJ. Distribution and chemical composition of estrogen receptor β neurons in the paraventricular nucleus of the female and male mouse hypothalamus. The Journal of Comparative Neurology 2017;525(17):3666–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang H. Atlas of the developing mouse brain at E17.5, P0, and P6, vol. 1 New York: Elsevier; 2007. [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology 1993; 133(6):3058–61. [DOI] [PubMed] [Google Scholar]
- Poling MC, Kauffman AS. Organizational and activational effects of sex steroids on kisspeptin neuron development. Frontiers in Neuroendocrinology 2013; 34:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra. The Journal of Comparative Neurology 2007; 503(1):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Field PM. A sexual difference in the preoptic area of the rat forebrain. Journal of Physiology 1971; 218. [PubMed] [Google Scholar]
- Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, Chen A. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nature Neuroscience 2017; 20(3):385–388. [DOI] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, Park SG, Justice NJ, Zuloaga DG. Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: a novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience 2017; 361:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DV, Dai Y, Thomas P, Carrasco GA, DonCarlos LL, Muma NA, Li Q. Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinology 2010; 35:1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Meijer OC, de Kloet ER. Specificity of glucocorticoid receptor primary antibodies for analysis of receptor localization patterns in cultured cells and rat hippocampus. Brain Research 2010; 1331:1–11. [DOI] [PubMed] [Google Scholar]
- Schussler P, Kluge M, Gamringer W, Wetter TC, Yassouridis A, Uhr M, … Steiger A. Corticotropin-releasing hormone induces depression-like changes of sleep electroencephalogram in healthy women. Psychoneuroendocrinology 2016; 74:302–307. [DOI] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 2015; 525(7570):519–522. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology 2005; 146(4):1973–82. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. Journal of Physiology 2005; 563(Pt 1):265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Neuroendocrinology 2010; 151:5807–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Research Molecular Brain Research 1989; 6(4):297–310. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. Journal of Comparative Neurology 1988; 270(2):209–42. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, … Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998; 20(6):1093–1102. [DOI] [PubMed] [Google Scholar]
- Subbannayya T, Balakrishnan L, Sudarshan G, Advani J, Kumar S, Mahmood R, Keshava Prasad TS. An integrated map of corticotropin-releasing hormone signaling pathway. Journal of Cell Communication and Signaling 2013; 7:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Schulkin J, Meerveld BGV. Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain. Neuropshychopharmacology 2014; 39:2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, … Schmidt MV. Prefrontal Cortex Corticotropin-Releasing Factor Receptor 1 Conveys Acute StressInduced Executive Dysfunction. Biological Psychiatry 2016; 80(10):743–753. [DOI] [PubMed] [Google Scholar]
- Viau V Functional cross-talk between the hypothalamic-pituitary-gonadal and –adrenal axes. J Neuroendocrinology 2002; 14:506–513. [DOI] [PubMed] [Google Scholar]
- Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. Journal of Neuroscience 2009; 29(31):9714–9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RP, Rivalan M, Bangasser DA, Deussing JM, Ising M, Wood SK, … Summers CH. Evidence for the role of corticotropin-releasing factor in major depressive disorder. Neuroscience and Biobehavioral Reviews 2015; 58:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wealthington JM, Hamki A, Cooke BM. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. The Journal of Comparative Neurology 2014; 522:1284–1298. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Price KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 1996; 137(12):5747–5750. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 2009; 159(2):883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, … Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. Journal of Psychopharmacology and Experimental Therapeutics 2007; 323(3):846–854. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Jordan CL, Breedlove SM. Sexual Differentiation of the Brain New Encyclopedia of Neuroscience; Squire L, editor. 2010. [Google Scholar]
- Zuloaga DG, Jordan CL, Breedlove SM. The organizational role of testicular hormones and the androgen receptor in anxiety-related behaviors and sensorimotor gating in rats. Endocrinology 2011; 152(4):1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Hormones and behavior 2008; 54:758–767. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor β expression in the mouse forebrain: age and sex differences. The Journal of Comparative Neurology 2014; 522(2):358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]