ABSTRACT
The mechanistic target of rapamycin (mTOR) signaling pathway coordinates environmental and intracellular cues to control eukaryotic cell growth. As a pivot point between anabolic and catabolic processes, mTOR complex 1 (mTORC1) signaling has established roles in regulating metabolism, translation and autophagy. Hyperactivity of the mTOR pathway is associated with numerous human diseases, including diabetes, cancer and epilepsy. Pharmacological inhibition of the mTOR pathway can extend lifespan in a variety of model organisms. Given its broad control of essential cellular processes and clear relevance to human health, there is extensive interest in elucidating how upstream inputs regulate mTORC1 activation. In this Cell Science at a Glance article and accompanying poster, we summarize our understanding of how extracellular and intracellular signals feed into the mTOR pathway, how the lysosome acts as an mTOR signaling hub, and how downstream signaling controls autophagy and lysosome biogenesis.
KEY WORDS: Cell growth, Lysosome, mTORC1, Autophagy, Signaling, Nutrients, Amino acids, Glucose
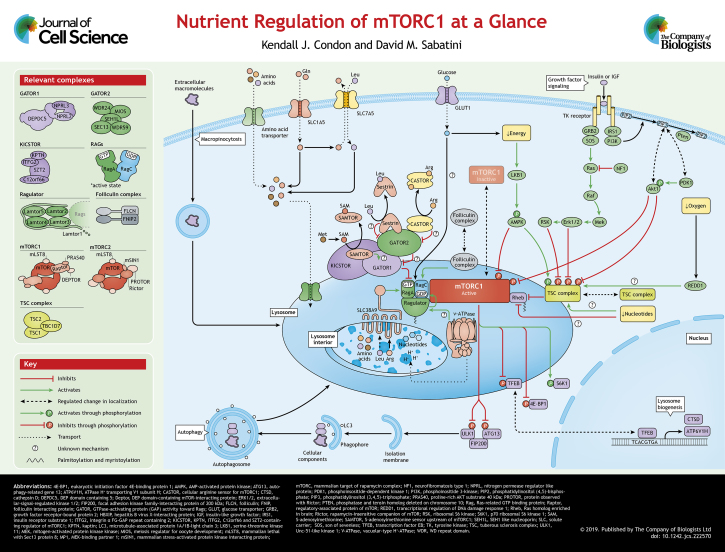
Summary: A summary of how extracellular and intracellular signals feed into the mTOR pathway, how the lysosome acts as an mTOR signaling hub, and how downstream signaling controls autophagy and lysosome biogenesis.
Introduction
As a major regulator of eukaryotic cell growth, the mechanistic (formerly known as ‘mammalian’) target of rapamycin (mTOR) protein kinase responds to intra- and extra-cellular signals. To facilitate growth, mTOR coordinates the synthesis and recycling of essential biomolecules, such as lipids, proteins and nucleotides. While mTOR is tightly controlled under physiological conditions, the loss of negative regulation can lead to the unrestrained cell growth found in cancers, benign tumors or in epilepsy (Guertin and Sabatini, 2007; Tee et al., 2002; Yuskaitis et al., 2018). Conversely, a decrease in mTOR activation is associated with longevity (Bjedov et al., 2010; Wu et al., 2013). Such a broad role in cell growth necessitates a specialized regulation of mTOR kinase activity.
The serine/threonine protein kinase mTOR is a conserved member of the phosphoinositide 3-kinase (PI3K)-related kinase family and is the key component of two distinct complexes termed mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Both complexes share mTOR, the catalytic subunit, mammalian lethal with Sec13 protein 8 (mLST8), a protein that might stabilize mTOR, and DEP-domain-containing mTOR-interacting protein (Deptor), a negative regulator of mTOR (Kim et al., 2003; Peterson et al., 2009). mTORC1 is distinguished from mTORC2 by the presence of the regulatory-associated protein of mTOR (Raptor, also known as RPTOR), a protein responsible for mTORC1 localization and substrate recruitment, and the proline-rich AKT substrate of 40 kDa (PRAS40, also known as AKT1S1), an insulin-responsive mTORC1 inhibitor (Aylett et al., 2016; Hara et al., 2002; Kim and Sabatini, 2004; Sancak et al., 2007). mTORC2 contains mSIN1, Protor and the rapamycin-insensitive companion of mTOR (Rictor), a protein that may have analogous function to Raptor (Frias et al., 2006; Pearce et al., 2007; Sarbassov et al., 2004) (see poster). These distinct complexes perform separate functions in the cell: mTORC1 primarily controls cell growth, while mTORC2 participates in the control of cell survival and proliferation (Saxton and Sabatini, 2017). Here, we focus on the regulation of mTORC1 in response to nutrient availability in the cytosol and lysosome.
As a major depot for recycled biomolecules, the lysosome is an important point of regulation for mTORC1 signaling. With roughly 60 acid hydrolases, the lysosome degrades macromolecules so that their constituent building blocks can be reused within the cell. These macromolecules arrive at the lysosome through two major pathways. The first route, autophagy (reviewed by Bento et al., 2016), is a cellular process in which damaged and unnecessary organelles or proteins are targeted for destruction and constituent biomolecules are recycled. Through a step-wise process, a double-membraned autophagosome is formed and encapsulates intracellular cargo. The autophagosome then fuses with the late endosome or lysosome to generate an autolysosome, where the contents are degraded (Luzio et al., 2007; Xu and Ren, 2015). The second path, endocytosis, involves engulfment of extracellular material into a vesicle, which then enters into the endosomal maturation process and terminates with lysosomal degradation (Doherty and McMahon, 2009).
Extensive crosstalk between mTORC1 and the lysosome grants the cell exquisite sensitivity to changes in the environment. In this ‘Cell Science at a Glance’ and the accompanying poster, we will discuss a lysosome-centric model of mTORC1 activation, the machinery required, and the relationship between mTORC1 and the lysosome.
A coincidence detector on the lysosomal surface
At the lysosomal surface, an mTORC1 recruitment complex and an mTORC1 activator colocalize so that mTORC1 can only be activated when both growth factors and nutrients are present, thus acting as an ‘AND’ gate. Growth factors and cellular stress signals feed into one side of the AND gate and culminate in the activation of the small GTPase Rheb, which resides on the lysosomal membrane and directly stimulates mTOR kinase activity when it is bound to GTP (Inoki et al., 2003a; Long et al., 2005). These signals regulate the nucleotide state of Rheb (GDP- versus GTP-bound), thereby licensing mTORC1-dependent phosphorylation of its targets. Nutrients, such as amino acids and glucose, regulate the other side of the AND gate through the heterodimeric Rag GTPases. In order to interact with Rheb, mTORC1 must be recruited to the lysosomal membrane, a translocation that requires the Rags. Composed of RagA or RagB and RagC or RagD, the Rags are only competent to recruit mTORC1 when RagA or RagB is bound to GTP and RagC or RagD is bound to GDP (Efeyan et al., 2013; Sancak et al., 2008, 2010). Coordination of these signals via the coincidence detector ensures that mTORC1 kinase activity is only turned on when all the requisites for cell growth are fulfilled.
Inputs into mTORC1
Growth factors, nucleotides, energy and oxygen
The tuberous sclerosis complex (TSC) is a major mediator of growth factor and cellular stress signaling to mTORC1. TSC is a complex composed of TSC1, TSC2 and TBC1D7, which acts as a GTPase-activating protein (GAP) for Rheb and therefore serves as a negative regulator of mTORC1 signaling (Dibble et al., 2012; Li et al., 2004). Several pathways converge on TSC to regulate mTORC1 in response to growth factors (see poster). Upon insulin stimulation, insulin and/or insulin-like growth factor-1 (IGF-1) stimulate PI3K signaling, which recruits and activates AKT1 (hereafter AKT). Active AKT then phosphorylates TSC2, leading to its dissociation from the lysosome and its substrate Rheb (Inoki et al., 2002; Manning et al., 2002; Menon et al., 2014; Potter et al., 2002). Furthermore, both the WNT and Ras signaling pathways affect mTORC1 signaling through TSC phosphorylation (Inoki et al., 2006; Mendoza et al., 2011; Roux et al., 2004). Independently of TSC, PRAS40, a component of mTORC1, relays growth factor signals to mTORC1, and in the absence of active AKT, PRAS40 negatively regulates mTORC1. Once AKT is activated by the presence of growth factors, it directly phosphorylates PRAS40 and causes its dissociation from the complex, thereby relieving inhibition (Sancak et al., 2007; Vander Haar et al., 2007).
A depletion of cellular nucleotide pools inhibits mTORC1 through two distinct mechanisms. Although both mechanisms are responsive to lowered purine levels, they differ with respect to the molecule sensed and the machinery required for sensing. Acute sensing occurs in response to lowered adenylate levels and is mediated by TSC (Hoxhaj et al., 2017). In contrast, prolonged deprivation of guanylate inhibits mTORC1 by inducing Rheb degradation (Valvezan et al., 2017). However, the molecular underpinnings of these mechanisms are largely unknown.
Under energetic stress, 5′ AMP-activated protein kinase (AMPK) signaling inactivates mTORC1 (Inoki et al., 2003b). When AMP levels rise, AMPK is activated and inhibits mTORC1 through activating phosphorylation of TSC and inhibiting phosphorylation of Raptor (Gwinn et al., 2008; Shaw et al., 2004) (see poster). As a fundamental energetic substrate, glucose is important for maintaining energetic homeostasis. Glucose transport across the plasma membrane is mediated by glucose transporters, such as Glut1. Interestingly, inhibition of this transporter selectively inhibits mTORC1 signaling (Kang et al., 2019). Although glucose starvation can induce energetic stress, it has been reported that glucose sensing may occur through the Rags (Efeyan et al., 2013). Much like energetic stress, hypoxic conditions also oppose cell growth. Conditions of low oxygen inhibit mTORC1 through both the activation of AMPK, as discussed above, and the transcriptional activation of the negative regulator DNA damage response 1 (REDD1), which is thought to inhibit mTORC1 via TSC activation (Brugarolas et al., 2004) (see poster).
Amino acids
Amino acids regulate mTORC1 activation through the Rag GTPases, which direct the translocation of mTORC1 from an ill-defined, but likely cytosolic location, to the lysosome via a nutrient-sensitive interaction with Raptor (Sancak et al., 2008, 2010). Recruitment of mTORC1 to the lysosomal surface depends on the Rag nucleotide-loading state, which is tightly regulated by numerous mechanisms, including interactions within the Rag heterodimers themselves (Sancak et al., 2010). In order to precisely control mTORC1 activation, RagA or RagB, and RagC or RagD communicate through inter-subunit interactions that generate a series of stable and transient states (Shen et al., 2017). This regulation also may act as a backstop for aberrant mTORC1 activation.
In addition to this inter-subunit regulation, several complexes act upstream of the Rag heterodimer to control its nucleotide-loading state (see poster). Ragulator, a pentameric complex containing Lamtor1–Lamtor5, affects the nucleotide loading of RagC or RagD through a noncanonical guanine nucleotide exchange factor (GEF) mechanism, in which GTP release is accelerated (Bar-Peled et al., 2012; Shen and Sabatini, 2018). Moreover, Ragulator localizes the Rags to the lysosome via palmitoylation and myristoylation at the N-terminus of Lamtor1 (Nada et al., 2009; Sancak et al., 2010; Wunderlich et al., 2001). Structurally, Lamtor1, the subunit responsible for anchoring Ragulator to the lysosomal surface, wraps around the Lamtor2–Lamtor3 and Lamtor4–Lamtor5 heterodimers. Here, Lamtor1, Lamtor2 and Lamtor3 act as a platform for the Rags (de Araujo et al., 2017; Mu et al., 2017; Su et al., 2017; Yonehara et al., 2017; Zhang et al., 2017).
Another complex acting on the Rags is ‘GAP activity towards the Rags’ (GATOR1), which consists of three components: Nprl2, Nprl3 and DEPDC5. GATOR1 functions as a GAP toward RagA and RagB; accordingly, it is a negative regulator of the pathway (Bar-Peled et al., 2013). Extensive biochemical and structural evidence suggests that GATOR1 interacts with the Rags through two distinct binding modes, termed the inhibitory and GAP modes. The inhibitory mode, wherein GATOR1 is not competent to act as a GAP toward RagA or RagB, is characterized by Rag binding to DEPDC5, while the GAP mode consists of Rag binding to Nprl2 and/or Nprl3 (Shen et al., 2018). These data, and the identification of a critical arginine residue on Nprl2, indicate that the GAP function lies within Nprl2 (Shen et al., 2019). This model proposes that remodeling of the GATOR1–Rag interaction is required to regulate the Rag nucleotide state. However, additional structural studies are required to understand how this conversion occurs at the surface of the lysosome.
In order to interact with the Rags, GATOR1 must be localized to the lysosome. This localization requires KPTN-, ITFG2-, C12orf66- and SZT2-containing regulator of TOR (KICSTOR), a complex that acts as a scaffold for GATOR1 (see poster). Deletion of any KICSTOR component leads to the mislocalization of GATOR1 and renders cells insensitive to amino acid starvation (Peng et al., 2017; Wolfson et al., 2017). It remains unclear whether KICSTOR plays additional roles in the regulation of GATOR1 activity. At the lysosome, GATOR1 also interacts with the GATOR2 complex, which is composed of MIOS, WDR59, WDR24, Seh1L and SEC13. While its function remains elusive, it is a positive regulator of the pathway and acts either upstream of or in parallel to GATOR1 (Bar-Peled et al., 2013).
Two additional complexes regulate the Rags (see poster). The first, the lysosomal v-ATPase, contributes to mTORC1 signaling through an unknown mechanism that involves an interaction with Ragulator (Bar-Peled et al., 2012; Zoncu et al., 2011). Functionally, this protein complex sets up a pH gradient that acidifies the lysosome and thus facilitates its degradative capacity (Cotter et al., 2015). Similarly, disruption of lysosomal trafficking hinders mTORC1 activation (Bridges et al., 2012; Kvainickas et al., 2019). While it is not fully understood how the v-ATPase affects mTORC1 signaling, small-molecule regulation of the v-ATPase increases lysosome acidification and inhibits mTORC1 (Chung et al., 2019). The second, the folliculin complex (FLCN with FNIP1 or FNIP2), has GAP activity for RagC and/or RagD. Amino acids regulate the localization of the Folliculin complex to the lysosome, though it is not known how this recruitment occurs (Petit et al., 2013; Tsun et al., 2013). FLCN may also promote mTORC1-mediated phosphorylation of the transcription factors TFEB and TFE3 (Wada et al., 2016).
Amino acid sensors
The amino acid sensors each directly bind an amino acid or amino acid-derived molecule and relay that signal to mTORC1 through the Rag GTPases (see poster). Specific amino acids appear to be more important for mTORC1 signaling than others, though the reasons for this are largely speculative. For example, leucine starvation has a potent inhibitory effect on mTORC1 and the leucine sensor Sestrin2 mediates this signal (Anthony et al., 2000; Wolfson et al., 2016). Mechanistically, upon leucine starvation, monomeric Sestrin2 binds to and antagonizes GATOR2, which leads to mTORC1 inhibition. When leucine is present, Sestrin2 directly binds leucine, which results in its dissociation from GATOR2 (Saxton et al., 2016b). Sestrin2 has two homologs, Sestrin1 and Sestrin3. Of these, Sestrin1 is thought to act in a similar manner to Sestrin2, while Sestrin3 binds to GATOR2, but does not bind leucine at high affinity (Wolfson et al., 2016). As Sestrin3 is an endogenous inhibitor of the mTORC1 pathway, it would be interesting to investigate whether Sestrin3 expression could impact the range of leucine levels detected.
Similar to what is seen for Sestrin2, the cytosolic arginine sensor, CASTOR1, directly binds to cytosolic arginine and inhibits mTORC1 through its interaction with GATOR2 and subsequent opposition of GATOR2 activity (Chantranupong et al., 2016). The mode of interaction differs from the Sestrins in that CASTOR1 acts as a homodimer and binds two arginine molecules at the interface of the aspartate kinase chorismate mutase, TyrA (ACT) domains (Saxton et al., 2016a). Likewise, the CASTOR1 homolog CASTOR2 can form a heterodimer with CASTOR1 and bind GATOR2, but its interaction with GATOR2 is insensitive to arginine. Interestingly, CASTOR1 is not the only arginine sensor to communicate with mTORC1. SLC38A9 is an essential, arginine-gated lysosomal amino acid transporter that allows mTORC1 to sense both cytosolic and lysosomal levels of arginine (Jung et al., 2015; Wang et al., 2015; Wyant et al., 2017). SLC38A9 directly affects the RagA or RagB nucleotide state through an arginine-regulated GEF mechanism. Additionally, SLC38A9 directly binds arginine within its first transmembrane helix (TM1) (Lei et al., 2018). Upon arginine binding, the N-terminus of SLC38A9 promotes GTP-bound RagA and/or RagB, which pushes the Rags toward an active state (Shen and Sabatini, 2018).
The identification of the S-adenosylmethionine (SAM) sensor SAMTOR indicates that mTORC1 is not only responsive to amino acids themselves, but also to their metabolic products. SAMTOR binds directly to SAM, which is generated from methionine and serves as an essential cellular metabolite in one-carbon metabolism (Gu et al., 2017). This sensor differs from the other cytosolic amino acid sensors Sestrin2 and CASTOR1 in that it does not appear to interact with GATOR2. Although the molecular details are not fully understood, SAMTOR acts through a physical interaction with KICSTOR and GATOR1 to inhibit mTORC1 in the absence of SAM.
mTORC1 sensing at the lysosome and regulation of autophagy
mTORC1 responds to amino acids in the cytosol and those generated by protein degradation in the lysosome. When amino acids are present in the environment, amino acid transporters at the plasma membrane, such as SLC1A5 and SLC7A5, supply cytosolic amino acids that activate mTORC1 signaling. Conversely, when cytosolic amino acids are absent, mTORC1 signaling is inhibited and autophagy is initiated to restore lysosomal amino acid pools through protein degradation. To prevent a futile cycle between mTORC1-stimulated mass accumulation and autophagic degradation, mTORC1 acutely controls autophagic flux through the direct phosphorylation of two proteins required for the initiation of autophagosome formation: Unc-51 like autophagy activating kinase (ULK) 1 and ULK2, and ATG13 (Hosokawa et al., 2009; Kim et al., 2011) (see poster).
Over the course of long-term starvation, lysosomal protein degradation restores amino acid levels in the lysosome. Interestingly, starvation-induced autophagy can be targeted; emerging evidence suggests that ribosomes, which constitute a large fraction of the protein and nucleotides in the cell, may serve as a crucial reservoir of resources and may be selectively degraded (Kraft et al., 2008). This process is referred to as ribophagy and is regulated by mTORC1 through the autophagy receptor NUFIP1–ZNHIT3 (Wyant et al., 2018). Ribosomal degradation not only contributes to the restoration of amino acid levels, but also that of the nucleotide pool, which is essential for cell survival under prolonged nutrient starvation (Guo et al., 2016). Release of these amino acids from the lysosome relies on the arginine-gated transport activity of SLC38A9. Consequently, SLC38A9 is required for the cell to use protein as a nutrient (Wyant et al., 2017). Furthermore, the importance of SLC38A9 in lysosomal amino acid efflux is striking in pancreatic cancer cell lines that rely on uptake and ingestion of extracellular proteins through macropinocytosis in order to obtain nutrients (Commisso et al., 2013; Davidson et al., 2017).
Once amino acids are released into the cytosol, they can reactivate mTORC1 and can be used in growth-promoting processes that are regulated by mTORC1. Reactivation under long-term starvation conditions is autophagy dependent and critical for cell survival. mTORC1 activation at this stage may allow the cell to translate proteins that are transcribed at the onset of starvation. A further consequence of long-term starvation is the consumption of lysosomes through the generation of autolysosomes. In this case, mTORC1 reactivation is required for the reformation of lysosomes through the autophagic lysosome reformation (ALR) pathway, wherein proto-lysosomal tubules are extruded from autolysosomes and the resulting vesicles mature into a restored pool of lysosomes (Yu et al., 2010).
mTORC1 also controls the localization of several downstream effectors through phosphorylation (see poster). The mTORC1 substrates TFE3, MITF and TFEB, members of the of the microphthalmia family of basic helix-loop-helix–leucine-zipper (bHLH-Zip) transcription factors (MiT family), are transcription factors that control lysosome biogenesis genes, together referred to as the coordinated lysosomal expression and regulation (CLEAR) gene network (Palmieri et al., 2011; Sardiello et al., 2009; Settembre et al., 2011). This downstream transcriptional program allows the cell to metabolically adapt to environmental stresses by controlling autophagic flux, lysosome number and lysosome function (Nnah et al., 2019; Raben and Puertollano, 2016; Settembre et al., 2012). Under nutrient-replete conditions, TFEB is confined to the cytosol by phosphorylation and is only able to enter the nucleus when mTORC1 is inhibited. For mTORC1 to phosphorylate TFE3 and/or TFEB, these transcription factors must be recruited to the lysosomal surface through an interaction with the active Rags (Martina et al., 2012). Furthermore, FLCN may regulate the localization of TFE3 and TFEB in an mTORC1-independent manner (El-Houjeiri et al., 2019). Understanding this regulation is an interesting research focus, as it may reconcile the observations that FLCN acts both as a tumor suppressor and a positive regulator of mTORC1 activation (Baba et al., 2006; Tsun et al., 2013).
Conclusions
To be an effective cell growth ‘decision maker’, mTORC1 must survey cellular contents before initiating processes that build mass. Elucidating the machinery that enables cytosolic sensing sheds light on how specific amino acids dynamically regulate mTORC1 activation. Ultimately, these signals feed into mTORC1 activation through a machinery localized to and embedded in the lysosomal membrane, making the lysosome a hub for mTORC1 signaling. This sets up a truly elegant system, in which mTORC1 can surveil the contents of both the cytosol and lysosome at once. Furthermore, feedback between mTORC1 and the lysosome coordinates autophagy to enable cell survival under nutrient-depleted conditions. It is clear that the convergence of mTORC1 signaling and the lysosome benefits the cell in many ways.
Acknowledgements
We thank Grace Liu, Charles Adelmann and Max Valenstein for critical reading of the manuscript. We apologize to those authors whose primary work we did not directly reference due to space restrictions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work was supported by grants from the National Institutes of Health (NIH) to D.M.S. (R01 CA103866, R01 CA129105, and R37 AI047389), from the Lustgarten Foundation, and fellowship support from the National Science Foundation (NSF) to K.J.C. (2016197106). D.M.S. is an investigator of the Howard Hughes Medical Institute and an American Cancer Society Research Professor. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.222570.supplemental.
References
- Anthony J. C., Yoshizawa F., Anthony T. G., Vary T. C., Jefferson L. S. and Kimball S. R. (2000). Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 130, 2413-2419. 10.1093/jn/130.10.2413 [DOI] [PubMed] [Google Scholar]
- Aylett C. H., Sauer E., Imseng S., Boehringer D., Hall M. N., Ban N. and Maier T. (2016). Architecture of human mTOR complex 1. Science 351, 48-52. 10.1126/science.aaa3870 [DOI] [PubMed] [Google Scholar]
- Baba M., Hong S. B., Sharma N., Warren M. B., Nickerson M. L., Iwamatsu A., Esposito D., Gillette W. K., Hopkins R. F. III, Hartley J. L. et al. (2006). Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl. Acad. Sci. USA 103, 15552-15557. 10.1073/pnas.0603781103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L., Schweitzer L. D., Zoncu R. and Sabatini D. M. (2012). Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196-1208. 10.1016/j.cell.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L., Chantranupong L., Cherniack A. D., Chen W. W., Ottina K. A., Grabiner B. C., Spear E. D., Carter S. L., Meyerson M. and Sabatini D. M. (2013). A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100-1106. 10.1126/science.1232044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento C. F., Renna M., Ghislat G., Puri C., Ashkenazi A., Vicinanza M., Menzies F. M. and Rubinsztein D. C. (2016). Mammalian autophagy: how does it work? Annu. Rev. Biochem. 85, 685-713. 10.1146/annurev-biochem-060815-014556 [DOI] [PubMed] [Google Scholar]
- Bjedov I., Toivonen J. M., Kerr F., Slack C., Jacobson J., Foley A. and Partridge L. (2010). Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35-46. 10.1016/j.cmet.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D., Fisher K., Zolov S. N., Xiong T., Inoki K., Weisman L. S. and Saltiel A. R. (2012). Rab5 proteins regulate activation and localization of target of rapamycin complex 1. J. Biol. Chem. 287, 20913-20921. 10.1074/jbc.M111.334060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J., Lei K., Hurley R. L., Manning B. D., Reiling J. H., Hafen E., Witters L. A., Ellisen L. W. and Kaelin W. G. Jr. (2004). Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893-2904. 10.1101/gad.1256804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantranupong L., Scaria S. M., Saxton R. A., Gygi M. P., Shen K., Wyant G. A., Wang T., Harper J. W., Gygi S. P. and Sabatini D. M. (2016). The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153-164. 10.1016/j.cell.2016.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. Y., Shin H. R., Berdan C. A., Ford B., Ward C. C., Olzmann J. A., Zoncu R. and Nomura D. K. (2019). Covalent targeting of the vacuolar H(+)-ATPase activates autophagy via mTORC1 inhibition. Nat. Chem. Biol. 15, 776-785. 10.1038/s41589-019-0308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C., Davidson S. M., Soydaner-Azeloglu R. G., Parker S. J., Kamphorst J. J., Hackett S., Grabocka E., Nofal M., Drebin J. A., Thompson C. B. et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633-637. 10.1038/nature12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter K., Stransky L., McGuire C. and Forgac M. (2015). Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem. Sci. 40, 611-622. 10.1016/j.tibs.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S. M., Jonas O., Keibler M. A., Hou H. W., Luengo A., Mayers J. R., Wyckoff J., Del Rosario A. M., Whitman M., Chin C. R. et al. (2017). Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235-241. 10.1038/nm.4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo M. E. G., Naschberger A., Furnrohr B. G., Stasyk T., Dunzendorfer-Matt T., Lechner S., Welti S., Kremser L., Shivalingaiah G., Offterdinger M. et al. (2017). Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science 358, 377-381. 10.1126/science.aao1583 [DOI] [PubMed] [Google Scholar]
- Dibble C. C., Elis W., Menon S., Qin W., Klekota J., Asara J. M., Finan P. M., Kwiatkowski D. J., Murphy L. O. and Manning B. D. (2012). TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol. Cell 47, 535-546. 10.1016/j.molcel.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G. J. and McMahon H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857-902. 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- Efeyan A., Zoncu R., Chang S., Gumper I., Snitkin H., Wolfson R. L., Kirak O., Sabatini D. D. and Sabatini D. M. (2013). Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493, 679-683. 10.1038/nature11745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Houjeiri L., Possik E., Vijayaraghavan T., Paquette M., Martina J. A., Kazan J. M., Ma E. H., Jones R., Blanchette P., Puertollano R. et al. (2019). The transcription factors TFEB and TFE3 link the FLCN-AMPK signaling axis to innate immune response and pathogen resistance. Cell Rep. 26, 3613-3628.e3616. 10.1016/j.celrep.2019.02.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias M. A., Thoreen C. C., Jaffe J. D., Schroder W., Sculley T., Carr S. A. and Sabatini D. M. (2006). mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16, 1865-1870. 10.1016/j.cub.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Gu X., Orozco J. M., Saxton R. A., Condon K. J., Liu G. Y., Krawczyk P. A., Scaria S. M., Harper J. W., Gygi S. P. and Sabatini D. M. (2017). SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 358, 813-818. 10.1126/science.aao3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin D. A. and Sabatini D. M. (2007). Defining the role of mTOR in cancer. Cancer Cell 12, 9-22. 10.1016/j.ccr.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Guo J. Y., Teng X., Laddha S. V., Ma S., Van Nostrand S. C., Yang Y., Khor S., Chan C. S., Rabinowitz J. D. and White E. (2016). Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 30, 1704-1717. 10.1101/gad.283416.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E. and Shaw R. J. (2008). AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214-226. 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J. and Yonezawa K. (2002). Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177-189. 10.1016/S0092-8674(02)00833-4 [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N. et al. (2009). Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981-1991. 10.1091/mbc.e08-12-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxhaj G., Hughes-Hallett J., Timson R. C., Ilagan E., Yuan M., Asara J. M., Ben-Sahra I. and Manning B. D. (2017). The mTORC1 signaling network senses changes in cellular purine nucleotide levels. Cell Rep. 21, 1331-1346. 10.1016/j.celrep.2017.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Li Y., Zhu T., Wu J. and Guan K.-L. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648-657. 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- Inoki K., Li Y., Xu T. and Guan K. L. (2003a). Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829-1834. 10.1101/gad.1110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Zhu T. and Guan K. L. (2003b). TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577-590. 10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]
- Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K. et al. (2006). TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955-968. 10.1016/j.cell.2006.06.055 [DOI] [PubMed] [Google Scholar]
- Jung J., Genau H. M. and Behrends C. (2015). Amino acid-dependent mtorc1 regulation by the lysosomal membrane protein SLC38A9. Mol. Cell. Biol. 35, 2479-2494. 10.1128/MCB.00125-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. A., O'Neill D. J., Machl A. W., Lumpkin C. J., Galda S. N., Sengupta S., Mahoney S. J., Howell J. J., Molz L., Hahm S. et al. (2019). Discovery of small-molecule selective mTORC1 inhibitors via direct inhibition of glucose transporters. Cell Chem Biol. 26, 1203-1213.e13. 10.1016/j.chembiol.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Kim D.-H. and Sabatini D. M. (2004). Raptor and mTOR: subunits of a nutrient-sensitive complex. Curr. Top. Microbiol. Immunol. 279, 259-270. 10.1007/978-3-642-18930-2_15 [DOI] [PubMed] [Google Scholar]
- Kim D.-H., Sarbassov D. D., Ali S. M., Latek R. R., Guntur K. V., Erdjument-Bromage H., Tempst P. and Sabatini D. M. (2003). GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11, 895-904. 10.1016/S1097-2765(03)00114-X [DOI] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B. and Guan K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132-141. 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C., Deplazes A., Sohrmann M. and Peter M. (2008). Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat. Cell Biol. 10, 602-610. 10.1038/ncb1723 [DOI] [PubMed] [Google Scholar]
- Kvainickas A., Nägele H., Qi W., Dokládal L., Jimenez-Orgaz A., Stehl L., Gangurde D., Zhao Q., Hu Z., Dengjel J. et al. (2019). Retromer and TBC1D5 maintain late endosomal RAB7 domains to enable amino acid–induced mTORC1 signaling. J. Cell Biol. 218, 3019 10.1083/jcb.201812110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H. T., Ma J., Sanchez Martinez S. and Gonen T. (2018). Crystal structure of arginine-bound lysosomal transporter SLC38A9 in the cytosol-open state. Nat. Struct. Mol. Biol. 25, 522-527. 10.1038/s41594-018-0072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Corradetti M. N., Inoki K. and Guan K. L. (2004). TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 29, 32-38. 10.1016/j.tibs.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Long X., Lin Y., Ortiz-Vega S., Yonezawa K. and Avruch J. (2005). Rheb binds and regulates the mTOR kinase. Curr. Biol. 15, 702-713. 10.1016/j.cub.2005.02.053 [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Pryor P. R. and Bright N. A. (2007). Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622 10.1038/nrm2217 [DOI] [PubMed] [Google Scholar]
- Manning B. D., Tee A. R., Logsdon M. N., Blenis J. and Cantley L. C. (2002). Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-Kinase/Akt pathway. Mol. Cell 10, 151-162. 10.1016/S1097-2765(02)00568-3 [DOI] [PubMed] [Google Scholar]
- Martina J. A., Chen Y., Gucek M. and Puertollano R. (2012). MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903-914. 10.4161/auto.19653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M. C., Er E. E. and Blenis J. (2011). The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 36, 320-328. 10.1016/j.tibs.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S., Dibble C. C., Talbott G., Hoxhaj G., Valvezan A. J., Takahashi H., Cantley L. C. and Manning B. D. (2014). Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 156, 771-785. 10.1016/j.cell.2013.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z., Wang L., Deng W., Wang J. and Wu G. (2017). Structural insight into the Ragulator complex which anchors mTORC1 to the lysosomal membrane. Cell Discovery 3, 17049 10.1038/celldisc.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Hondo A., Kasai A., Koike M., Saito K., Uchiyama Y. and Okada M. (2009). The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK–ERK pathway to late endosomes. EMBO J. 28, 477-489. 10.1038/emboj.2008.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnah I. C., Wang B., Saqcena C., Weber G. F., Bonder E. M., Bagley D., De Cegli R., Napolitano G., Medina D. L., Ballabio A. et al. (2019). TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy 15, 151-164. 10.1080/15548627.2018.1511504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri M., Impey S., Kang H., di Ronza A., Pelz C., Sardiello M. and Ballabio A. (2011). Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852-3866. 10.1093/hmg/ddr306 [DOI] [PubMed] [Google Scholar]
- Pearce L. R., Huang X., Boudeau J., Pawlowski R., Wullschleger S., Deak M., Ibrahim A. F., Gourlay R., Magnuson M. A. and Alessi D. R. (2007). Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 405, 513-522. 10.1042/BJ20070540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Yin N. and Li M. O. (2017). SZT2 dictates GATOR control of mTORC1 signalling. Nature 543, 433-437. 10.1038/nature21378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T. R., Laplante M., Thoreen C. C., Sancak Y., Kang S. A., Kuehl W. M., Gray N. S. and Sabatini D. M. (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137, 873-886. 10.1016/j.cell.2009.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. S., Roczniak-Ferguson A. and Ferguson S. M. (2013). Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J. Cell Biol. 202, 1107-1122. 10.1083/jcb.201307084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. J., Pedraza L. G. and Xu T. (2002). Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4, 658-665. 10.1038/ncb840 [DOI] [PubMed] [Google Scholar]
- Raben N. and Puertollano R. (2016). TFEB and TFE3: linking lysosomes to cellular adaptation to stress. Annu. Rev. Cell Dev. Biol. 32, 255-278. 10.1146/annurev-cellbio-111315-125407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P. P., Ballif B. A., Anjum R., Gygi S. P. and Blenis J. (2004). Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA 101, 13489-13494. 10.1073/pnas.0405659101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A. and Sabatini D. M. (2007). PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 25, 903-915. 10.1016/j.molcel.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L. and Sabatini D. M. (2008). The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496-1501. 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y., Bar-Peled L., Zoncu R., Markhard A. L., Nada S. and Sabatini D. M. (2010). Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290-303. 10.1016/j.cell.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P. and Sabatini D. M. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296-1302. 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., Medina D. L., Valenza M., Gennarino V. A., Di Malta C., Donaudy F., Embrione V., Polishchuk R. S. et al. (2009). A gene network regulating lysosomal biogenesis and function. Science 325, 473 10.1126/science.1174447 [DOI] [PubMed] [Google Scholar]
- Saxton R. A. and Sabatini D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell 169, 361-371. 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Saxton R. A., Chantranupong L., Knockenhauer K. E., Schwartz T. U. and Sabatini D. M. (2016a). Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature 536, 229-233. 10.1038/nature19079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R. A., Knockenhauer K. E., Wolfson R. L., Chantranupong L., Pacold M. E., Wang T., Schwartz T. U. and Sabatini D. M. (2016b). Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351, 53-58. 10.1126/science.aad2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Di Malta C., Polito V. A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P. et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332, 1429-1433. 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Zoncu R., Medina D. L., Vetrini F., Erdin S., Erdin S., Huynh T., Ferron M., Karsenty G., Vellard M. C. et al. (2012). A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095-1108. 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Bardeesy N., Manning B. D., Lopez L., Kosmatka M., DePinho R. A. and Cantley L. C. (2004). The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91-99. 10.1016/j.ccr.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Shen K. and Sabatini D. M. (2018). Ragulator and SLC38A9 activate the Rag GTPases through noncanonical GEF mechanisms. Proc. Natl. Acad. Sci. USA 115, 9545-9550. 10.1073/pnas.1811727115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Choe A. and Sabatini D. M. (2017). Intersubunit crosstalk in the Rag GTPase heterodimer enables mTORC1 to respond rapidly to amino acid availability. Mol. Cell 68, 821 10.1016/j.molcel.2017.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Huang R. K., Brignole E. J., Condon K. J., Valenstein M. L., Chantranupong L., Bomaliyamu A., Choe A., Hong C., Yu Z. et al. (2018). Architecture of the human GATOR1 and GATOR1-Rag GTPases complexes. Nature 556, 64-69. 10.1038/nature26158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Valenstein M. L., Gu X. and Sabatini D. M. (2019). Arg-78 of Nprl2 catalyzes GATOR1-stimulated GTP hydrolysis by the Rag GTPases. J. Biol. Chem. 294, 2970-2975. 10.1074/jbc.AC119.007382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M.-Y., Morris K. L., Kim D. J., Fu Y., Lawrence R., Stjepanovic G., Zoncu R. and Hurley J. H. (2017). Hybrid structure of the RagA/C-ragulator mTORC1 activation complex. Mol. Cell 68, 835-846.e833. 10.1016/j.molcel.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee A. R., Fingar D. C., Manning B. D., Kwiatkowski D. J., Cantley L. C. and Blenis J. (2002). Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. USA 99, 13571-13576. 10.1073/pnas.202476899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsun Z. Y., Bar-Peled L., Chantranupong L., Zoncu R., Wang T., Kim C., Spooner E. and Sabatini D. M. (2013). The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol. Cell 52, 495-505. 10.1016/j.molcel.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvezan A. J., Turner M., Belaid A., Lam H. C., Miller S. K., McNamara M. C., Baglini C., Housden B. E., Perrimon N., Kwiatkowski D. J. et al. (2017). mTORC1 couples nucleotide synthesis to nucleotide demand resulting in a targetable metabolic vulnerability. Cancer Cell 32, 624-638.e625. 10.1016/j.ccell.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Haar E., Lee S.-I., Bandhakavi S., Griffin T. J. and Kim D.-H. (2007). Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316-323. 10.1038/ncb1547 [DOI] [PubMed] [Google Scholar]
- Wada S., Neinast M., Jang C., Ibrahim Y. H., Lee G., Babu A., Li J., Hoshino A., Rowe G. C., Rhee J. et al. (2016). The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 30, 2551-2564. 10.1101/gad.287953.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Tsun Z. Y., Wolfson R. L., Shen K., Wyant G. A., Plovanich M. E., Yuan E. D., Jones T. D., Chantranupong L., Comb W. et al. (2015). Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188-194. 10.1126/science.1257132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson R. L., Chantranupong L., Saxton R. A., Shen K., Scaria S. M., Cantor J. R. and Sabatini D. M. (2016). Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43-48. 10.1126/science.aab2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson R. L., Chantranupong L., Wyant G. A., Gu X., Orozco J. M., Shen K., Condon K. J., Petri S., Kedir J., Scaria S. M. et al. (2017). KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1. Nature 543, 438-442. 10.1038/nature21423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Liu J., Chen E. B., Wang J. J., Cao L., Narayan N., Fergusson M. M., Rovira I. I., Allen M., Springer D. A. et al. (2013). Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 4, 913-920. 10.1016/j.celrep.2013.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich W., Fialka I., Teis D., Alpi A., Pfeifer A., Parton R. G., Lottspeich F. and Huber L. A. (2001). A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J. Cell Biol. 152, 765-776. 10.1083/jcb.152.4.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyant G. A., Abu-Remaileh M., Wolfson R. L., Chen W. W., Freinkman E., Danai L. V., Vander Heiden M. G. and Sabatini D. M. (2017). mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 171, 642-654.e12. 10.1016/j.cell.2017.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyant G. A., Abu-Remaileh M., Frenkel E. M., Laqtom N. N., Dharamdasani V., Lewis C. A., Chan S. H., Heinze I., Ori A. and Sabatini D. M. (2018). NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360, 751-758. 10.1126/science.aar2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. and Ren D. (2015). Lysosomal physiology. Annu. Rev. Physiol. 77, 57-80. 10.1146/annurev-physiol-021014-071649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara R., Nada S., Nakai T., Nakai M., Kitamura A., Ogawa A., Nakatsumi H., Nakayama K. I., Li S., Standley D. M. et al. (2017). Structural basis for the assembly of the Ragulator-Rag GTPase complex. Nat. Commun. 8, 1625 10.1038/s41467-017-01762-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., McPhee C. K., Zheng L., Mardones G. A., Rong Y., Peng J., Mi N., Zhao Y., Liu Z., Wan F. et al. (2010). Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465, 942-946. 10.1038/nature09076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuskaitis C. J., Jones B. M., Wolfson R. L., Super C. E., Dhamne S. C., Rotenberg A., Sabatini D. M., Sahin M. and Poduri A. (2018). A mouse model of DEPDC5-related epilepsy: Neuronal loss of Depdc5 causes dysplastic and ectopic neurons, increased mTOR signaling, and seizure susceptibility. Neurobiol. Dis. 111, 91-101. 10.1016/j.nbd.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wang R., Wang Z., Wang X., Wang F. and Ding J. (2017). Structural basis for Ragulator functioning as a scaffold in membrane-anchoring of Rag GTPases and mTORC1. Nat. Commun. 8, 1394 10.1038/s41467-017-01567-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y. and Sabatini D. M. (2011). mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678-683. 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]