ABSTRACT
Background
Dietary fiber reduces body weight and inflammation in clinical trials. It is unclear whether body mass index (BMI) and inflammation might explain the observed association between higher fiber intake and the lower risk of symptomatic knee osteoarthritis (SXKOA).
Objectives
We quantified the extent to which BMI and inflammation influenced the relation between dietary fiber and SXKOA.
Methods
We used longitudinal data from the Osteoarthritis Initiative (OAI) and the Framingham Offspring Osteoarthritis Study. At baseline of each study, men and women (mean age: 61 y) with or at risk of knee osteoarthritis were followed for 48 mo in the OAI. Adults (mean age: 53 y) were followed for 9.5 y in the Framingham study. Dietary fiber intake was estimated using a validated food-frequency questionnaire. Measured weight and height were used to calculate BMI. Serum high-sensitivity C-reactive protein (CRP) was measured in the Framingham study only. Incident SXKOA was defined as new onset of a combination of knee pain and radiographic osteoarthritis. We applied marginal structural models to quantify the mediation through BMI in the OAI and the sequential mediation through BMI and CRP in the Framingham study.
Results
Incident SXKOA occurred in 861 knees among 2876 persons in the OAI and in 143 knees among 971 persons in the Framingham study. In persons whose fiber intake was ≥21 g/d compared with those with intakes <21 g/d, the OR (95% CI) was 0.70 (0.53, 0.91) for the overall association with SXKOA and was 0.93 (0.92, 0.95) for the mediation via BMI (per kg/m2) in the OAI. In the Framingham study, the overall association was 0.57 (0.30, 1.09), the mediation through BMI (via BMI and the influence of BMI on CRP) was 0.94 (0.85, 1.02), and the mediation through CRP (per milligram per liter) was 0.99 (0.84, 1.19).
Conclusion
Our findings suggest that the inverse association of fiber intake and the risk of incident symptomatic knee osteoarthritis is partially mediated by BMI.
Keywords: dietary fiber, knee osteoarthritis, BMI, C-reactive protein, mediation analysis
Introduction
Osteoarthritis, the most common form of arthritis, is one of the 10 leading causes of loss of function and its ranking among diseases as a cause of disability has been increasing with aging and increased rates of global obesity (1). Obesity is a major cause of osteoarthritis in the knee, with a recent meta-analysis suggesting that both overweight and obesity increase the risk of knee osteoarthritis by 2- to 4-fold (2). It is thought that obesity increases the loading across the knee and also augments serum and tissue concentrations of proinflammatory cytokines and adipokines (3), which may themselves accelerate disease development and increase the risk of joint pain (4, 5). C-reactive protein (CRP), a marker of low-grade systemic inflammation, has been related to painful osteoarthritis in a longitudinal study (4), an association further confirmed in a meta-analysis (5). In addition, CRP concentration could be influenced in part by obesity, in which BMI contributes most of the variance in CRP concentrations (6, 7).
Because very few effective treatments without adverse consequences are available in knee osteoarthritis, dietary modification may offer a safe and effective prevention strategy for osteoarthritis (8). Dietary fiber consists of nondigestible or nonabsorbable carbohydrates in the small intestine that are partially or fully fermented in the colon (9). Several clinical trials have shown that dietary fiber reduces body weight (10–12) and lessens systemic inflammation reflected by CRP concentrations (13).
Recently, we reported that a high intake of dietary fiber from all food sources was associated with a lower risk of incident symptomatic knee osteoarthritis (SXKOA) in 2 US prospective cohorts (14). We found that those in the Osteoarthritis Initiative (OAI) whose consumption of dietary fiber placed them in the highest quartile of intake (median intake: 20.6 g/d) had a significantly lower risk of developing SXKOA, with an OR (95% CI) of 0.70 (0.52, 0.94), and that in the Framingham Offspring Osteoarthritis Study (Framingham study), compared with participants in the lowest quartile of fiber intake, those whose consumption placed them in the fourth (highest) quartile (median: 25.5 g/d) had a lower risk, with an OR of 0.39 (95% CI: 0.17, 0.88) (14). These analyses included adjustment for measures of diet quality and other factors associated with healthy lifestyles. Further adjustment for BMI partially weakened the associations (14). We speculated that the protective association of dietary fiber intake against incident knee symptomatic osteoarthritis was mediated, at least in part, through fiber's impact on BMI. As mentioned above, CRP is closely linked with dietary fiber, obesity, and SXKOA, and we hypothesized that CRP was also in the mediation pathway.
In this study, we conducted mediation analyses to quantify to what extent the reduction in SXKOA from dietary fiber could be explained by the association of dietary fiber with BMI and CRP. Identifying the causal pathway for the association of fiber intake with knee osteoarthritis may inform a prevention strategy for this common disabling disease. Here, we used a new method that allowed us to consider mediation by both BMI and CRP but also whether change in BMI influenced CRP in the association of fiber intake with risk of SXKOA.
Methods
Study population
The OAI is a multicenter prospective cohort of 4796 US men (41.5%) and women aged 45–79 y, with or at risk of knee osteoarthritis, recruited between 2004 and 2006 to investigate risk factors for osteoarthritis. Knee pain was assessed, and weight-bearing radiographs of both knees were obtained at baseline and annually until 48 mo in the current analysis (15). The Framingham Offspring Study was initiated in 1971 persons comprising adult children of the original Framingham study and spouses of these offspring participants (16). The Framingham offspring subjects were invited to participate in the osteoarthritis study if either or both parent members of the original cohort had received radiographs for the evaluation of osteoarthritis (17). There were 1268 eligible persons (men: 43.9%; mean age: 53.2 y) enrolled in the study in 1993–1994 (offspring exam 5), at which time weight-bearing radiographs of both knees at full extension were obtained along with a survey about knee pain. Participants were re-examined using the same assessments 9.5 y later at exam 7 (2002–2005) as an ancillary study of the Framingham Heart Study (18). In both studies, participants who had rheumatoid arthritis or other forms of inflammatory arthritis were excluded (15, 18). Institutional review board approval was obtained from all OAI study sites for the OAI and from Boston University for the Framingham Osteoarthritis Study.
Assessment of dietary fiber
Dietary fiber intake was assessed at baseline and has been described previously (14). Briefly, in the OAI, dietary fiber intake was estimated using the Block Brief 2000 semiquantitative FFQ (19) and has been shown to have a moderate to high correlation for dietary fiber (r = 0.50–0.66) in the validation studies (20). In the Framingham study, habitual dietary intake was recorded at exam 5 (1993–1994) as the baseline of the present study and 4 y later at exam 6 (1995–1998) via the validated Harvard semiquantitative FFQ of 126 food items (21). The correlation coefficient for dietary fiber was 0.68 in the validation studies (21–23). The nutrient values for the Block FFQ were estimated primarily on the basis of the nutrient-composition values, which were developed in the NHANES II (19); the Harvard FFQ relied primarily on the USDA food-composition database supplemented by other published data (21).
We used 21 g fiber intake/d as the cutoff to define high and low fiber intake in this study. This cutoff is based on our findings in the OAI and the Framingham study (14). That is, in the OAI, those whose consumption fell into the highest quartile of dietary total fiber (median intake: 20.6 g/d) and those in the Framingham study who consumed at the upper third (19.1 g/d) or upper fourth (25.5 g/d) quartile had significantly lower risks of developing incident SXKOA (14). In addition, 21 g fiber/d is the Adequate Intake in the DRIs for women aged ≥50 y (24). To have sufficient power to detect significant results in both men and women in the cohorts, we used 21 g dietary fiber/d as a cutoff to conduct mediation analysis.
Assessments of BMI and high-sensitivity CRP
OAI
Body weight and height were measured without shoes at baseline and at each annual examination. Weight was measured with a scale in lightweight clothing without heavy jewelry or wallets. Standing height was measured with a wall-mounted stadiometer with a movable headboard (15).
Framingham study
Body weight and height were measured at baseline and every 4 y without shoes. Weight was measured in lightweight clothing, and height was measured using a mounted stadiometer. At the beginning of the seventh examination cycle (1998–2001), blood samples were collected and serum high-sensitivity CRP (milligrams per liter), serving as a marker for systemic inflammation, was measured using a Dade Behring BN100 nephelometer (25, 26). The intra- and interassay CVs were 3.2% and 5.3%, respectively (25).
Other covariates
In both studies, demographic characteristics, history of knee surgery (including knee replacement), medication use, tobacco and alcohol use, and physical activity were collected at baseline. Only participants with valid fiber intake (no missing values) and calorie intake (≥500 and <4200 kcal/d for men and <4000 kcal/d for women) were included in the analyses. To estimate the level of physical activity, we used the Physical Activity in the Elderly Scale (PASE) score in the OAI and the Physical Activity Index (PAI) in the Framingham study. The PASE score was computed by the products of empirically derived activity weights in 3 domains (household, occupational, and leisure) times the activity frequencies (hours per week) (27). The PAI was used to capture subjects’ usual activities in a typical 24-h day; the total score summed the products of activity frequency (hours per day) multiplied by a weight based on oxygen consumption (liters per minute) required for that level of activity (28).
Assessment of SXKOA (study outcome)
In both the OAI and Framingham cohorts, a positive response to the question of “During the past 30 days, have you had pain, aching, or stiffness in your right/left knee on most days?” was defined as knee pain. All persons obtained a fixed flexion posterior-anterior radiograph, which was read centrally for Kellgren and Lawrence grade. Incident SXKOA was defined as new onset of a combination of knee pain and radiographic osteoarthritis (i.e., Kellgren and Lawrence grade ≥2) in the same knee during the follow-up.
Statistical analysis
In both cohorts, we used marginal structural models under the counterfactual framework to partition the total effect of the exposure of interest into the indirect effect from the remaining direct effect (29). Because CRP measurement was not available in the OAI, BMI was assessed as the sole mediator. In the Framingham study, both BMI and CRP were assessed for their potential mediation in the association between dietary fiber and incident SXKOA with the use of the method developed by Steen et al. (30). We postulated that BMI would affect CRP in the association between dietary fiber and the risk of incident SXKOA. Under the counterfactual framework (29), the total effect (overall association) of dietary fiber on the risk of incident SXKOA was decomposed into the natural direct effect (i.e., the association of dietary fiber and the risk of SXKOA not through a given mediator) and the natural indirect effect (i.e., the association of fiber intake and the risk of SXKOA through a giver mediator) (29). The directed acyclic graph for each cohort is depicted in Figure 1.
FIGURE 1.
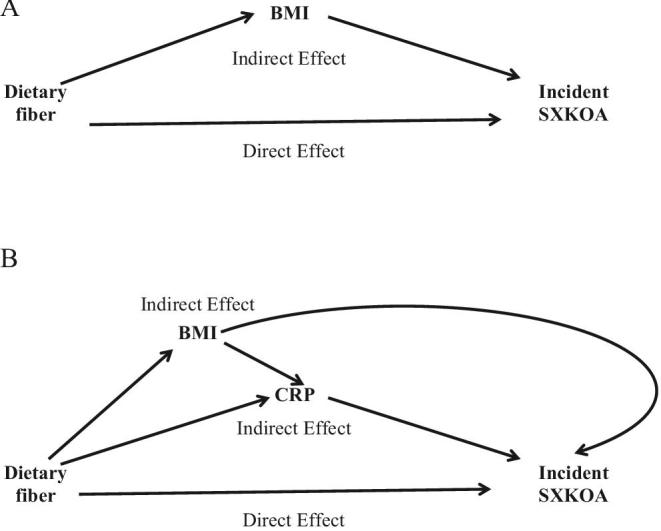
Directed acyclic graphs for the decompositions of association between dietary fiber and risk of incident SXKOA mediated by BMI (Osteoarthritis Initiative; n = 2876) (A) or by BMI and CRP (Framingham Osteoarthritis Study; n = 971) (B). CRP, C-reactive protein; SXKOA, symptomatic knee osteoarthritis.
To examine to what extent the association between dietary fiber intake and the risk of incident SXKOA was mediated through BMI and CRP, respectively, we first evaluated whether BMI or CRP met the criteria as a potential mediator (31). Specifically, we assessed 1) whether dietary fiber intake was associated with BMI or CRP and 2) whether there was an association of BMI or CRP with the risk of incident SXKOA. To do so, we first performed linear regression analyses to assess the relation of dietary fiber (dichotomized as ≥21 compared with <21 g/d) with BMI or with CRP, and then we carried out logistic regression analyses between BMI or CRP and the risk of incident SXKOA.
We used generalized estimating equations to account for the correlation between 2 knees of each individual in the regression models (32). Both BMI (kg/m2) and CRP (milligrams per liter) were considered as continuous variables in the following models. In our regression models, we controlled for age (years), sex (men or women), race (white or nonwhite in the OAI), total energy intake (kilocalories per day), education (below college or college or above), tobacco use (never, former, or current smokers), physical activity (PASE in the OAI or PAI in the Framingham study; continuous), and intake of other dietary components that have been suggested to be related to knee osteoarthritis (33–36), including vitamin C (milligrams per day), vitamin K (micrograms per day), polyunsaturated fat (grams per day), and saturated fat (grams per day) in the OAI. In the Framingham study, we adjusted for diet quality to account for dietary confounders using the Dietary Guidelines Adherence Index (DGAI-2010) applied to the baseline FFQ (37). There was no evidence that suggested differences of results between men and women in the association of fiber intake with incident SXKOA. All of the statistical analyses were conducted using SAS version 9.3 (SAS Institute), except for the decomposition of the sequential mediator effects, for which the CIs and inference were based on the 1000 bootstrap samples using the R software (30).
Results
Of 4796 participants in the OAI at baseline, 540 (11.2%) subjects were excluded from the analyses due to loss to follow-up at the 48-mo visit, extreme caloric intake or missing fiber intake (n = 205), or knee replacement at baseline (n = 56). Of the remaining participants (n = 3995), we further excluded 1020 persons who had prevalent SXKOA and 99 persons with missing SXKOA status, leaving 2876 persons (5752 knees) with 869 incident SXKOA knees for inclusion in the analyses. Among the 1268 participants in the Framingham study, we excluded 131 persons (11.9%) due to loss of follow-up and 166 persons due to missing data on dietary fiber intake; thus, 971 persons (1941 knees) including 131 incident SXKOA knees were included in the analyses (see flowchart in Supplemental Figure 1).
The baseline characteristics for each cohort are shown in Table 1 by the intake levels of dietary fiber (≥21 compared with <21 g/d). In both studies, participants who consumed ≥21 g/d had lower mean BMIs, were less likely to smoke tobacco, and were more physically active than those who consumed <21g dietary fiber/d. They also had higher total energy intakes and higher intakes of PUFAs, vitamin C, and vitamin K. In the Framingham study, those who had a higher fiber intake consumed a healthier diet as reflected by the DGAI-2010 and had lower concentrations of serum CRP.
TABLE 1.
Baseline preincident symptomatic knee osteoarthritis characteristics of participants in the OAI and the Framingham Offspring Osteoarthritis Study1
| Dietary fiber | ||||||
|---|---|---|---|---|---|---|
| OAI (n = 2876) | Framingham study (n = 971) | |||||
| <21 g/d | ≥21 g/d | <21 g/d | ≥21 g/d | |||
| (n = 2363) | (n = 513) | P | (n = 649) | (n = 322) | P | |
| Age at baseline, y | 61.0 ± 9.1 | 61.5 ± 9.3 | 0.06 | 53.2 ± 7.8 | 55.3 ± 8.3 | <0.001 |
| Male sex, n (%) | 953 (40.3) | 252 (49.1) | <0.001 | 292 (45.0) | 150 (46.4) | 0.56 |
| Race (white), n (%) | 2027 (85.8) | 432 (84.2) | 0.20 | 649 (100) | 322 (100) | — |
| BMI, kg/m2 | ||||||
| At baseline | 28.1 ± 4.7 | 27.8 ± 4.6 | 0.02 | 27.2 ± 4.6 | 26.6 ± 4.6 | <0.001 |
| At median follow-up | 28.1 ± 4.7 | 27.8 ± 4.9 | 0.02 | 27.7 ± 4.8 | 27.2 ± 4.7 | <0.001 |
| Tobacco use, n (%) | <0.001 | <0.001 | ||||
| Never | 1873 (79.3) | 387 (75.4) | 541 (83.4) | 299 (92.9) | ||
| Former smoker | 403 (17.1) | 113 (22.0) | 8 (1.2) | 2 (0.6) | ||
| Current smoker | 87 (3.7) | 13 (2.5) | 100 (15.4) | 42 (6.5) | ||
| Education, n (%) | 0.13 | <0.001 | ||||
| Less than college | 832 (35.2) | 168 (32.8) | 452 (69.6) | 200 (62.1) | ||
| College or above | 1531 (64.8) | 345 (67.2) | 197 (30.4) | 122 (37.9) | ||
| Physical activity2 (PASE for OAI; PAI for Framingham) | 151 (100–211) | 164 (108–231) | <0.001 | 35 (32, 38) | 35 (33, 38) | 0.048 |
| Prevalence of radiographic osteoarthritis, n (%) | 732 (31.0) | 160 (31.2) | 0.89 | 46 (7.1) | 22 (6.7) | 0.74 |
| Pain, aching or stiffness, n (%) | 485 (20.5) | 101 (19.7) | 0.55 | 102 (15.7) | 50 (15.4) | 0.004 |
| Total energy, kcal/d | 1274 ± 447 | 1930 ± 566 | <0.001 | 1633 ± 470 | 2309 ± 588 | <0.001 |
| Total dietary fiber, g/d | 13 ± 4.2 | 27 ± 5.9 | <0.001 | 14 ± 4.9 | 28 ± 6.3 | <0.001 |
| Dietary quality score (DGAI 2010)3 | — | — | — | 55 ± 9 | 64 ± 5 | <0.001 |
| Saturated fats, g/d | 17 ± 8.3 | 23 ± 11 | <0.001 | 20 ± 8.0 | 26 ± 12.1 | <0.001 |
| PUFAs, g/d | 10 ± 4.7 | 15 ± 6.2 | <0.001 | 12 ± 4.7 | 17 ± 6.3 | <0.001 |
| Vitamin C, mg/d | 100 ± 53 | 165 ± 69 | <0.001 | 200 ± 220 | 294 ± 275 | <0.001 |
| Vitamin K, µg/d | 146 ± 106 | 337 ± 237 | <0.001 | 128 ± 64 | 231 ± 125 | <0.001 |
| Serum high-sensitivity CRP,2 mg/L | — | — | — | 2.0 (0.9–4.9) | 1.9 (0.9–3.9) | 0.02 |
1Values are means ± SDs unless otherwise indicated. CRP, C-reactive protein; DGAI-2010, Dietary Guidelines Adherence Index 2010; OAI, Osteoarthritis Initiative; PAI, Physical Activity Index; PASE, Physical Activity Scale for the Elderly.
2Values are medians (IQRs).
3Dietary Guidelines Adherence Index 2010 (37), excluding fiber.
The associations of dietary fiber with either BMI (kg/m2) at different time points or CRP (log-transformed) were significant (P values ranged from <0.001 to 0.02). The relation between BMI at different time points and incident SXKOA was highly significant (P < 0.001). Similarly, CRP was related to incident SXKOA (P < 0.002). These results suggested that BMI and CRP were potential mediators.
The total effect, the natural direct effect, and the natural indirect effect of dietary fiber on the risk of incident SXKOA mediated by BMI or CRP are shown in Table 2. All effect estimates were significant in the OAI in which BMI was the single mediator. In the OAI, BMI significantly mediated the association of dietary fiber with SXKOA, comparing those who consumed ≥21 g dietary fiber/d with those who consumed <21 g/d (OR: 0.93; 95% CI: 0.92,0.95). In the Framingham study, the analysis of the sequential mediation roles of BMI and CRP suggested a similar effect size for BMI as that in the OAI; however, the bootstrap CI for the estimated effect size of the natural indirect effect slightly overlapped the null for BMI (OR: 0.94; 95% CI: 0.85, 1.02). The mediation by CRP in the association between fiber intake and SXKOA after accounting for BMI was null (OR: 0.99, 95% CI: 0.84,1.19). For both studies, the natural direct effect was significant.
TABLE 2.
Natural direct and natural indirect effects of dietary fiber on incident symptomatic knee osteoarthritis for single-mediator mediation with BMI at baseline or CRP as a mediator in the OAI and the Framingham Offspring Osteoarthritis Study1
| Dietary fiber | ||
|---|---|---|
| <21 g/d | ≥21 g/d | |
| OAI | ||
| n | 2363 | 513 |
| Median (IQR) fiber intake, g/d | 12.5 (9.3–15.6) | 25.2 (22.7–29.0) |
| OA knees, n/total knees (%) | 727/4726 (15.4) | 142/1026 (13.8) |
| Total effect2 | 1.00 (ref) | 0.70 (0.53,0.91)3 |
| Direct effect | 1.00 (ref) | 0.76 (0.58, 0.99) |
| Indirect effect via BMI | 1.00 (ref) | 0.93 (0.92,0.95) |
| Framingham study | ||
| n | 649 | 322 |
| Fiber intake median (IQR), g/d | 14.8 (11.9–17.5) | 25.6 (23.1–29.9) |
| OA knees, n/total knees (%) | 92/1297 (7.1) | 39/644 (6.0) |
| Total effect4 | 1.00 (ref) | 0.57 (0.30, 1.09) |
| Direct effect | 1.00 (ref) | 0.55 (0.25, 0.98) |
| Indirect effect via BMI directly and BMI→CRP | 1.00 (ref) | 0.94 (0.85, 1.02) |
| Indirect effect via CRP only | 1.00 (ref) | 0.99 (0.84,1.19) |
1CRP, C-reactive protein; OA, osteoarthritic; OAI, Osteoarthritis Initiative; PAI, Physical Activity Index; PASE, Physical Activity Scale for the Elderly; ref, reference.
2Model adjusted for age (years), sex (men or women), race (white or nonwhite), total energy intake (kilocalories), education (less than college or college or higher), tobacco use (never, former, or current smoker), physical activity (PASE, continuous), intake of other dietary factors including polyunsaturated fat (grams per day), vitamin C (milligrams per day), vitamin D (International units per day), vitamin E (milligrams α tocopherol per day), vitamin K (micrograms per day), dairy products (servings per day), and saturated fat (grams per day).
3OR; 95% CI in parentheses (all such values).
4Model adjusted for age (years), sex (men or women), total energy intake (kilocalories), education (less than college or college or higher), current cigarette smoking status (never or current), physical activity level (PAI, continuous), and diet quality using the modified dietary guidelines 2010 score (37) (excluding dietary fiber).
Discussion
Our results showed that the association of dietary fiber with a lower risk of incident SXKOA was, at least partially, mediated through BMI in 2 longitudinal studies with distinct study designs and populations. In addition, our findings suggest that BMI affected serum CRP in mediating the association of dietary fiber with incident SXKOA. To our knowledge, this study is the first to provide causal insights into how dietary fiber may lower the risk of a disease outcome by examining multiple sequential mediators—here, in the case of knee osteoarthritis.
The effect of dietary fiber on reduced body weight has been shown in several clinical trials (10, 11, 38). The significant positive relation of BMI with a higher risk of knee osteoarthritis is well established from study populations, regardless of regions or ethnic backgrounds (2). In the meta-analysis conducted by Zheng and Chen (2), both overweight and obesity were significantly associated with a higher risk of knee osteoarthritis by 2- and 4-fold, respectively; a BMI increment of 5 was related to a significant 35% higher risk (2). In our earlier report, controlling for BMI attenuated the risk estimates for the longitudinal association of dietary fiber with incident SXKOA in both the OAI and Framingham study (14). In the present study, results from the mediation analyses further confirmed that BMI either at baseline (see Table 2) or the midpoint of the study course (data not shown) mediated, at least in part, the association of dietary fiber with incident SXKOA. Hence, our results showed that dietary fiber may result in a lower body weight, because those who consumed more dietary fiber were likely to have a lower BMI over time and this, in turn, reduced their risk of developing SXKOA.
In the Framingham study, which used marginal structural models to assess sequential mediators as described in Steen et al. (30), we found that the mediation via BMI, which included the pathway through BMI itself and that through the impact of BMI on serum CRP, had a similar effect size to the mediation via BMI only in the OAI. Furthermore, the sole meditation by CRP under the influence of BMI produced a null effect. These results suggest that BMI appears to be the primary mediator in the causal pathway for the association between dietary fiber and the risk of incident SXKOA. In the previously published meta-analysis, there were no significant differences in the concentrations of serum CRP detected between persons with and without osteoarthritis (5), after adjustment for BMI, indicating that body weight status plays an influential role in systemic inflammation. Our findings were consistent with this observation because we found a marginal natural indirect effect of CRP (OR: 0.98; 95% CI: 0.96, 1.0) when we modeled CRP as a single mediator (i.e., BMI was neither adjusted as a confounder nor modeled as a mediator) in the marginal structural model. Because obesity promotes excessive white adipose tissue, which is a key regulator of systemic inflammation (39), and because systemic inflammation may further affect synovitis (3) and increase knee pain (5, 40, 41), it is possible that dietary fiber lowers BMI, which reduces both mechanical loading and systemic inflammation. Together, our analyses suggested that BMI preceded and affected serum CRP concentration in the causal pathways between dietary fiber and the risk of incident SXKOA.
Moreover, we noticed that the natural direct effect was significant in both cohorts. This suggests that other mechanisms in addition to BMI and CRP could play a role in the beneficial association between dietary fiber and the risk of SXKOA. For instance, dietary fiber may affect the gut microbiome (42) and hormones (43) to promote other health benefits to reduce SXKOA. In addition, dietary fiber may decrease absorption of macronutrients and promote satiety, both of which can affect caloric intake and weight status (44). Other healthful effects of dietary fiber include lowering blood pressure (45) and cholesterol (46) and improving glycemic control (47) and cytokines and chemokines, all of which are related to osteoarthritis (48–50). A reduction in these metabolic risk factors and inflammatory markers may further contribute to the protective role of dietary fiber in SXKOA (51).
There are several strengths of this study. We used data from 2 well-executed prospective cohorts to assess mediation in an attempt to explain part of the causal relation between dietary fiber and SXKOA. We had measured body weight and height in both studies, which would reduce potential biases from self-reported measures of these anthropometric variables. Second, the consistent findings of the mediation via BMI from 2 different cohorts increase generalizability of the results, because each cohort has distinct demographic and baseline characteristics. Nonetheless, our study has limitations. First, self-reported dietary intake via semiquantitative FFQs is prone to measurement error and potentially results in nondifferential misclassification of dietary fiber; however, such bias is likely to lead to an underestimate or attenuation of the observed associations. The selection criteria for participants in the OAI might introduce collider bias by conditioning on BMI; nevertheless, such bias is likely to underestimate the true association between dietary fiber and SXKOA (14). Self-reported physical activity may not sufficiently reflect participants’ physical activity in real life. The residual confounding from this variable as other (self-reported) measures in observational studies is unavoidable. However, the relation between physical activity and knee osteoarthritis remains unclear, even in randomized controlled trials (52, 53). Another limitation is that, in the Framingham study, we used general estimating equation models to estimate the total effect of fiber intake on the risk of SXKOA, and then we used a different approach [i.e., the sequential mediation models proposed by Steen et al. (30)] to estimate the natural direct effect and the natural indirect effect. Because this sequential mediation model does not provide effect estimates for the total effect, we believe that the use of 2 different models may explain a slightly lower effect estimate for the total effect than the natural direct effect in the Framingham study. Finally, conducting mediation analyses under the counterfactual framework for causal inference relies on several assumptions, including no (substantial) unmeasured confounding or model mis-specifications. These assumptions are difficult to verify.
In conclusion, our data in 2 longitudinal studies suggest that a high fiber intake may lower the risk of SXKOA, at least in part, through optimizing BMI, whereas serum CRP is possibly affected by BMI in the association between dietary fiber and SXKOA. Alternative pathways such as the role of microbiome should be explored in future studies.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—ZD, DTF, and YZ: conception and design of study; JN, ZD, DTF, and SRJ: acquisition of data; ZD and SRJ: statistical analysis; ZD: drafting the manuscript and primary responsibility for final content; ZD, PFJ, SL, SRJ, DTF, and YZ: data interpretation and revising the manuscript critically for important intellectual content; and all authors: made a substantial contribution to the development of the manuscript and read and approved the final manuscript.
Notes
Author disclosures: ZD, SRJ, JN, DTF, PFJ, SL, and YZ, no conflicts of interest. The funders (NIH and USDA) had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- CRP
C-reactive protein
- OAI
Osteoarthritis Initiative
- PAI
Physical Activity Index
- PASE
Physical Activity in the Elderly Scale
- SXKOA
symptomatic knee osteoarthritis.
References
- 1. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open 2015;5(12):e007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015;54(4):588–600. [DOI] [PubMed] [Google Scholar]
- 4. Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis 2013;72(4):535–40. [DOI] [PubMed] [Google Scholar]
- 5. Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, Ding C. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74(4):703–10. [DOI] [PubMed] [Google Scholar]
- 6. Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, Brook GJ, Levy Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord 2004;28(5):674–9. [DOI] [PubMed] [Google Scholar]
- 7. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282(22):2131–5. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26(3):355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Association of Cereal Chemists The definition of dietary fiber. Cereal Food World 2001;112–26. [Google Scholar]
- 10. Jull AB, Ni Mhurchu C, Bennett DA, Dunshea-Mooij CA, Rodgers A. Chitosan for overweight or obesity. Cochrane Database Syst Rev 2008;3:CD003892. [DOI] [PubMed] [Google Scholar]
- 11. Ma Y Olendzki BC Wang J Persuitte GM Li W Fang H Merriam PA Wedick NM Ockene IS Culver AL et al.. Single-component versus multicomponent dietary goals for the metabolic syndrome: a randomized trial. Ann Intern Med 2015;162(4):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grube B, Chong PW, Lau KZ, Orzechowski HD. A natural fiber complex reduces body weight in the overweight and obese: a double-blind, randomized, placebo-controlled study. Obesity (Silver Spring) 2013;21(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiao J, Xu JY, Zhang W, Han S, Qin LQ. Effect of dietary fiber on circulating C-reactive protein in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Food Sci Nutr 2015;66(1):114–9. [DOI] [PubMed] [Google Scholar]
- 14. Dai Z NJ, Zhang Y, Jacques P, Li S, Felson D.T. Dietary intake of fibre and risk of knee osteoarthritis in two US prospective cohorts. Ann Rheum Dis 2017;76(8):1411–1419. [DOI] [PubMed] [Google Scholar]
- 15. Nevitt M, Felson DT, Lester G. The Osteoarthritis Initiative—protocol for the cohort study 2005;74 Available from: https://oai.epi-ucsf.org/datarelease/docs/studydesignprotocol.pdf (accessed on August 8, 2018).
- 16. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4(4):518–25. [DOI] [PubMed] [Google Scholar]
- 17. Felson DT, Couropmitree NN, Chaisson CE, Hannan MT, Zhang Y, McAlindon TE, LaValley M, Levy D, Myers RH. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham study. Arthritis Rheum 1998;41(6):1064–71. [DOI] [PubMed] [Google Scholar]
- 18. Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, Hunter DJ, Amin S, Rogers G, Booth SL. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum 2007;56(1):129–36. [DOI] [PubMed] [Google Scholar]
- 19. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 20. Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology 1990;1(1):58–64. [DOI] [PubMed] [Google Scholar]
- 21. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135(10):1114–26; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 22. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 23. Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127(1):188–99. [DOI] [PubMed] [Google Scholar]
- 24. Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate. fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 25. Dhingra R, Gona P, Nam BH, D'Agostino RB, Sr., Wilson PW, Benjamin EJ, O'Donnell CJ. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am J Med 2007;120(12):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vlad SC, Neogi T, Aliabadi P, Fontes JD, Felson DT. No association between markers of inflammation and osteoarthritis of the hands and knees. J Rheumatol 2011;38(8):1665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46(2):153–62. [DOI] [PubMed] [Google Scholar]
- 28. Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham Study. Arch Intern Med 1979;139(8):857–61. [PubMed] [Google Scholar]
- 29. Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol 2012;176(3):190–5. [DOI] [PubMed] [Google Scholar]
- 30. Steen J, Loeys T, Moerkerke B, Vansteelandt S. Flexible mediation analysis with multiple mediators. Am J Epidemiol 2017;186(2):184–93. [DOI] [PubMed] [Google Scholar]
- 31. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Zhang B, Wise B, Niu J, Zhu Y. Statistical approaches to evaluating the effect of risk factors on the pain of knee osteoarthritis in longitudinal studies. Curr Opin Rheumatol 2009;21(5):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr 2011;14(4):709–15. [DOI] [PubMed] [Google Scholar]
- 34. McAlindon TE, Jacques P, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Levy D, Felson DT. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum 1996;39(4):648–56. [DOI] [PubMed] [Google Scholar]
- 35. Neogi T, Booth SL, Zhang YQ, Jacques PF, Terkeltaub R, Aliabadi P, Felson DT. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum 2006;54(4):1255–61. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Davies-Tuck ML, Wluka AE, Forbes A, English DR, Giles GG, O'Sullivan R, Cicuttini FM. Dietary fatty acid intake affects the risk of developing bone marrow lesions in healthy middle-aged adults without clinical knee osteoarthritis: a prospective cohort study. Arthritis Res Ther 2009;11(3):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sauder KA Proctor DN Chow M Troy LM Wang N Vita JA Vasan RS Mitchell GF Jacques PF Hamburg NM et al.. Endothelial function, arterial stiffness and adherence to the 2010 Dietary Guidelines for Americans: a cross-sectional analysis. Br J Nutr 2015;113(11):1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 2012;142(4):710–6. [DOI] [PubMed] [Google Scholar]
- 39. Odegaard JI, Ganeshan K, Chawla A. Adipose tissue macrophages: amicus adipem? Cell Metab 2013;18(6):767–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, Basdevant A, Clement K, Bardin T, Chevalier X. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis 2011;70(1):139–44. [DOI] [PubMed] [Google Scholar]
- 41. Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther 2011;13(6):R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 2013;4(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papathanasopoulos A, Camilleri M. Dietary fiber supplements: effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 2010;138(1):65–72, e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev 2001;59(5):129–39. [DOI] [PubMed] [Google Scholar]
- 45. Hollaender PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2015;102(3):556–72. [DOI] [PubMed] [Google Scholar]
- 46. Evans CE, Greenwood DC, Threapleton DE, Cleghorn CL, Nykjaer C, Woodhead CE, Gale CP, Burley VJ. Effects of dietary fibre type on blood pressure: a systematic review and meta-analysis of randomized controlled trials of healthy individuals. J Hypertens 2015;33(5):897–911. [DOI] [PubMed] [Google Scholar]
- 47. Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev 2013;71(12):790–801. [DOI] [PubMed] [Google Scholar]
- 48. Valcheva R, Hotte N, Gillevet P, Sikaroodi M, Thiessen A, Madsen KL. Soluble dextrin fibers alter the intestinal microbiota and reduce proinflammatory cytokine secretion in male IL-10-deficient mice. J Nutr 2015;145(9):2060–6. [DOI] [PubMed] [Google Scholar]
- 49. Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann Clin Biochem 2011;48(Part 3):233–7. [DOI] [PubMed] [Google Scholar]
- 50. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7(1):33–42. [DOI] [PubMed] [Google Scholar]
- 51. Abourazzak F, Talbi S, Lazrak F, Azzouzi H, Aradoini N, Keita S, Errasfa M, Harzy T. Does metabolic syndrome or its individual components affect pain and function in knee osteoarthr itis women? Curr Rheumatol Rev 2015;11(1):8–14. [PubMed] [Google Scholar]
- 52. Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2015;1:CD004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cooper C, Coggon D. Physical activity and knee osteoarthritis. Lancet 1999;353(9171):2177–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


