Abstract
Background
Antiretroviral treatment (ART) management is challenging for individuals in resource-limited settings presenting for third-line ART because of complex resistance patterns, partly due to limited access to viral load (VL) monitoring.
Methods
A5288 was a phase IV, third-line ART strategy study conducted at 19 urban sites in 10 countries in HIV-1 infected adults that enrolled participants with confirmed plasma HIV-1 RNA (VL) ≥1000 copies per mL after >24 weeks of protease inhibitor (PI)-based second-line ART. The primary objective was to use antiretrovirals (ARV: raltegravir, etravirine and ritonavir-boosted darunavir) and diagnostic monitoring technologies, including VL, genotyping and adherence support to achieve VL suppression in ≥65% of participants. ART history plus real-time drug resistance genotypes were used to assign participants to one of four cohorts: Cohort A (no lopinavir resistance) stayed on second-line ART; Cohorts B (B1: best available nucleoside reverse transcriptase inhibitors [NRTIs] + ritonavir-boosted darunavir + raltegravir, B2: ritonavir-boosted darunavir + raltegravir + etravirine), C (ritonavir-boosted darunavir + raltegravir + tenofovir/emtricitabine or tenofovir+lamivudine) and D (best available NRTIs + ritonavir-boosted darunavir + raltegravir) were defined by increasing levels of resistance and received appropriate regimens including new ARVs. A randomized comparison among participants in Cohort B was performed (cohorts B1, B2 and B3). This trial was registered with ClinicalTrials.gov, .
Findings
From February 2013 to December 2015, 545 participants were enrolled, with 287 (53%) assigned to Cohort A, 74 to B1 (13.5%), 72 to B2 (13 %), 8 to B3(1.5 %). 70 (13%) to C, and 34 (6%) to D. Overall, 64% (95% CI 60, 68%) of participants achieved VL≤ 200 copies per mL at week 48, with proportions varying from 44% to 88%, 88%,100%, 90% and 74% in Cohorts A, B1,B2, B3, C and D. Cohort A, remained on their second-line PI, and had the most participants with Grade ≥3 adverse events (51%).
Interpretation
Third-line ART regimens assigned by algorithm and containing new drugs were highly effective in participants with lopinavir resistance. By contrast, of participants without lopinavir resistance who were assigned to continue their second-line ART less than 50% achieved viral suppression. This subgroup requires additional interventions. Targeted real-time genotyping to select third-line ART can appropriately allocate more costly ARVs to those with greater levels of HIV drug resistance.
Funding National Institutes of Health
Introduction
The World Health Organization recommended in 2013 that low and middle-income countries (LMIC) develop policies for third-line antiretroviral therapy (ART) and developing effective policies requires data.1 In 2015, more than 500,000 people in LMIC) received second-line ART but fewer than 1% were estimated to be receiving third-line regimens.2 However, with an increase in access to viral load testing,3 which enables accurate and timeline detection of virological failure, it is likely that the numbers of persons living with HIV who fail second-line ART will increase, creating more need for third-line ART.3 Although antiretroviral regimen recommendations and use in LMIC have recently changed to include better tolerated regimens, it is likely that common reasons for virological failure will continue to be due to multi-class resistance and suboptimal adherence.4 Identification of the most appropriate third-line regimens is a high priority to sustain both individual and public health benefits of ART.
Individuals who have first-and second-line ART failure are a particular challenge in LMIC, as they likely have been exposed to medications from the three most widely used classes of antiretrovirals (ARVs) including nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs). There are a wide range of resistance patterns associated with 2nd line failure in LMIC although a substantial fraction of persons failing second-line ART may also have wild-type virus.5–8
Randomized controlled trials and observational cohorts support use of ritonavir-boosted darunavir, etravirine, dolutegravir and raltegravir containing regimens in treatment-experienced adults.9–12 Previous studies have shown that NRTIs can be safely omitted if a new optimized regimen contains multiple fully or partially active ARVs.13 Potential benefits of this NRTI-sparing strategy include reduced pill burden and, potentially, decrease in long-term NRTI-associated toxicity. However, most studies evaluating these strategies have been conducted in middle- or high-income settings and little available data about second-line regimen failure in LMICs and outcomes with third-line regimens exist.14,15
There is a need to better characterize individuals experiencing ART failure on second-line ART in LMIC and define the best options for third-line ART. To address this important knowledge gap, the AIDS Clinical Trials Group (ACTG) protocol A5288 was designed as a prospective, multi-center study in LMIC to evaluate use of newer antiretroviral drugs and contemporary management tools, including population-based sequencing to select appropriate ARVs, plasma viral load (VL) monitoring and interventions to improve adherence among individuals presenting with second-line virologic failure. To determine the benefit of continuing NRTI use with NRTI resistance, a subset of participants with PI resistance was randomized to regimens that did or did not include NRTIs. Our study hypothesis was that use of newer drugs and contemporary management tools would achieve virologic suppression in at least 65% of participants.
Methods:
Study design and participants
A5288 was an open-label phase IV, prospective interventional strategy study at 19 urban sites in 10 countries in Africa (Kenya, Malawi, South Africa, Uganda, and Zimbabwe), Latin America (Brazil, Haiti and Peru), and Asia (India and Thailand). We recruited, HIV-1-infected adults (≥18 years) with confirmed plasma VL values of ≥1000 copies per mL (two consecutive measurements at least 1 week apart) after at least 24 weeks of a second-line PI-based regimen. Participants needed to have experience with drugs in three classes (i.e., NRTIs, NNRTIs, and PIs). Exclusion criteria included pregnancy, active tuberculosis or rifampin exposure within 2 weeks prior to entry and prior exposure to ritonavir-boosted darunavir or etravirine. The protocol with the complete inclusion and exclusion criteria is available at the Appendix page 19. Participants had visits every 3 months. Each site had the approval of their ethics committees. Written informed consent was obtained from each participant.
Procedures
After the initial VL test, we confirmed VL ≥1000 copies per mL with an additional test using the Abbott HIV-1 RealTime assay (Abbott Laboratories), per manufacturer’s instructions. The DAIDS Virology Quality Assurance (VQA) Program at Rush University (Chicago, IL) certified all laboratories. Upon confirmation of the VL ≥1000 copies per mL, we performed real-time population-based HIV drug resistance testing at ACTG regional laboratories at FIOCRUZ, Brazil; YRG Care, India and BARC-SA and Lancet Laboratories, South Africa, using laboratory developed assays that were VQA certified. Sequencing was performed with an ABI Prism 3130-Avant Genetic Analyzer (Applied Biosystems, USA) in Brazil and India and an ABI Prism 3500 Genetic Analyzer (Applied Biosystems, USA) in South Africa. Samples unable to be amplified by the laboratory developed assay were amplified at the same laboratory using the FDA-approved ViroSeq HIV-1 Genotyping System (v.2.0), per manufacturer’s instructions (Celera Diagnostics, Alameda, CA).
We identified known HIV drug resistance mutations in the viral population sequences and calculated resistance scores using the Stanford Drug resistance database (version 6.2). This was the available version at the time of study enrollment, and we consistently used it across all resistance reports for participant management and analysis. We used these scores to determine HIV drug resistance for all NRTIs, NNRTIs and PIs currently used in LMIC. For etravirine and darunavir, we used the scores from Janssen, the makers of these ARVs, as they were the best predictor of response to etravirine and darunavir observed during clinical development of both drugs.16
We classified the levels of HIV drug resistance into five distinct categories: susceptible, potential low-level resistance, low-level resistance, intermediate resistance and high-level resistance. For statistical analysis, we defined resistance as being in the intermediate or high-level resistance categories. For etravirine and darunavir, we used only susceptible and resistant classifications. For etravirine, a score below 2.5 was classified as susceptible.17,18 For darunavir, we classified the sample as resistant if there were ≥3 darunavir associated mutations. At screening, enrollment, and on a quarterly basis, participants underwent the following safety testing: hematology, serum chemistries, creatinine to permit calculation of estimated creatinine clearance, liver function tests, glucose, cholesterol and triglycerides, CD4/CD8, HIV RNA, urinalysis and for women, pregnancy testing.
Cohort assignment
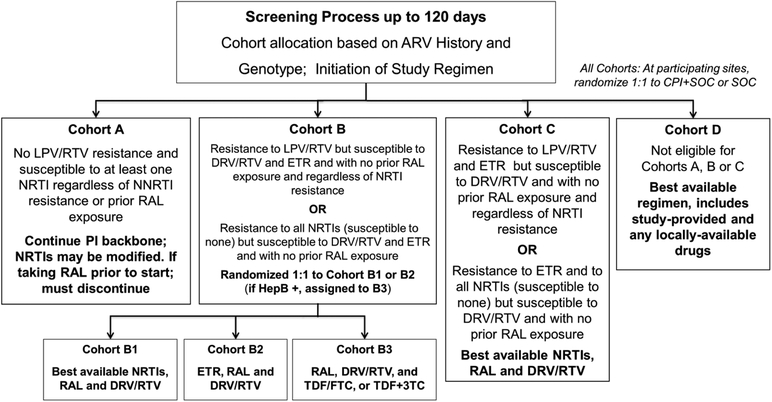
We used real-time HIV drug resistance results, treatment history and any historical resistance results to assign participants to one of four treatment cohorts (A, B, C, or D) (Figure 1). Target study sample size was 500 participants with no restriction on number per cohort. Following receipt of each potential participant’s resistance report, the site investigator made an initial cohort and regimen recommendation for each potential participant. For Cohort A, switch of NRTIs to study-provided NRTIs was allowed but not required; for Cohort B and C at least two NRTIs were proposed. As individuals enrolled in Cohort D had more complex resistance patterns, they could use any of the study and/or locally provided ARVs to build the most appropriate ART regimen
Figure 1: Cohort Definitions, Assignment and Antiretroviral Regimens.
Figure showing criterion by which participants were assigned to the respective cohorts and the regimen for each.
Figure 1 definitions: ARV = antiretroviral; CPI = cell-phone intervention; SOC = standard of care; NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; HepB = hepatitis B; 3TC = lamivudine; DRV = darunavir; ETR = etravirine; FTC = emtricitabine; LPV = lopinavir; RAL = raltegravir; RTV = ritonavir; TDF = tenofovir
To explore whether either etravirine or best available NRTIs (both in combination with ritonavir-boosted darunavir and raltegravir) resulted in better outcomes, we did a randomized comparison among participants in Cohort B without detectable hepatitis B surface antigen (Cohorts B1 and B2). Participants with detectable hepatitis B surface antigen were assigned to Cohort B3. We performed the randomization through a web-based computer system after confirmation of eligibility. The randomization was block stratified by the randomized adherence support intervention (see below), with dynamic site balancing to avoid large imbalances within each site.
The A5288 Clinical Management Committee (CMC) reviewed the site’s recommendations, and either agreed or suggested an alternative cohort and/or regimen. After CMC approval of cohort and regimen, we enrolled participants and followed them until the last enrollee reached 48 weeks.
Oral study drug doses were: raltegravir 400 mg twice a day, darunavir 600 mg with ritonavir 100 mg twice a day, etravirine 200 mg twice a day, tenofovir disoproxil fumerate 300 mg daily and emtricitabine 200 mg daily (provided as Truvada®).
We conducted a randomized adherence support strategy study among participants in all cohorts at 17 of the 19 sites to assess the effect of a cell phone-based adherence support intervention plus standard of care adherence support versus standard of care adherence support. The findings are reported separately.19
In the event of a confirmed virologic failure (VF: two successive HIV-1 RNA levels ≥1000 copies per mL at or after 24 weeks of follow-up), we performed another genotype resistance test and assigned participants to a cohort/ARV regimen using a similar approach to that at study entry (including the possibility of staying on the same regimen).
Outcomes
The primary outcome measure was virologic suppression at week 48, defined as VL ≤200 copies per mL. We considered participants who died or were lost to follow-up before week 48 as not having VL ≤200 copies per mL at week 48. We considered participants missing a week 48 VL measurement as not having VL ≤200 copies per mL unless the immediately preceding and succeeding measurements were both ≤200 copies per mL. Pre-specified secondary outcomes included time to confirmed virologic failure (VF), defined as time from enrollment to the first VL ≥1000 copies per mL at or after 24 weeks, which was confirmed by the next measurement. Other secondary endpoints included new resistance-associated mutations, CD4+T-cell counts, death, AIDS-defining events, non-AIDS defining events, grade 3 or 4 adverse events (AEs) (defined by the Division of AIDS Table for Grading AEs, Version 1.0).
Statistical analysis
We hypothesized that the study population could achieve a ≥ 65% rate of suppression of HIV-1 RNA to ≤ 200 copies per mL at 48 weeks. This was based on the study team’s assessment based on information from sites and from the literature of what proportion of participants might be in each cohort assuming no effect of the cell phone intervention over SOC adherence support (20%, 25%, 25%, 5%, 15% and 10% in Cohorts A, B1, B2, B3, C and D, respectively), and what proportion might achieve this level of suppression in each cohort (50%, 70%, 90%, 70%, 70% and 50%, respectively). We chose the sample size of 500 to provide high precision in estimating the proportion suppressed in the overall study population, specifically for the 95% confidence interval to have width no more than plus/minus 4.4%. The study was not specifically powered to address the randomized comparison in Cohort B. There were no pre-specified sample sizes for the cohorts and the number of participants in each cohort reflected the resistance profile of individuals failing second line ART who were enrolled.
The primary study objective involved estimation of the percentage of participants with HIV-1 RNA ≤200 copies per mL at week 48 with an associated 95% confidence interval calculated using the normal approximation to the binomial distribution. As assignment to cohort was determined by genotypic resistance profile and treatment history, outcome results for each cohort are descriptive with 95% confidence intervals. We defined the identification of new resistance-associated mutations at the time of virologic failure as the development of a mutation, not present at screening, identified and scored by the Stanford HIVDB 6.2 algorithm. We did not count changes in mixture mutations as new mutations. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, North Carolina). This trial was registered with ClinicalTrials.gov, .
Role of the funding source
The U.S. National Institutes of Health funded the study and had an oversight role in development and monitoring of the study; one author (CG) was an employee of this sponsor, a member of the study team, and involved in the conduct, analyses, and conclusions drawn from the study. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
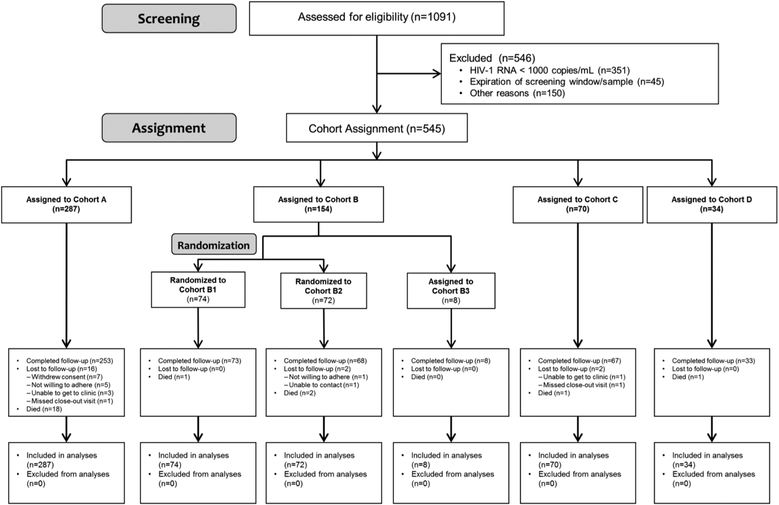
Between January 10th 2013 and September 10th 2015, we performed 1,091 screenings; 545 participants were enrolled from February 22nd 2013 to December 21st 2015 with the last participant completing follow-up in February 2017 (Figures 1 & 2). The most common reason for screening failure was confirmatory VL< 1000 copies per mL (64%). The median time between the collection of the screening sample for centralized resistance testing and enrollment was 8.7 weeks (range 2.1–14.7 weeks). The CMC agreed with the initial site recommendation for cohort assignment and antiretroviral regimen for 84% of the participants. When the CMC disagreed with the site selection, there was a discussion about the issue, and the CMC explained their interpretation of the available data (treatment history, relevant clinical laboratory data, genotype result and interpretation, and requirements to be assigned to a specific cohort/subcohort). The CMC asked that the disputed request be modified so the cohort and regimen were compliant with CMC’s interpretation.
Figure 2: Trial Profile.
The progress of all participants from screening through assignment/randomization and analysis are displayed. Key outcomes are identified within each block. Populations for analyses are also summarized.
Overall, we assigned 287 participants (53%) to Cohort A, 154 (28%) to B, 70 (13%) to C, and 34 (6%) to D. In Cohort B, 8 participants (1% of 545) had active hepatitis B infection and were assigned to Cohort B3. We randomized the remaining 146 participants assigned to Cohort B to either Cohort B1 (74 participants [14% of all 545 participants] or Cohort B2 (72 participants [13%]). In Cohort A, 176 participants (61%) continued a ritonavir-boosted lopinavir-based regimen and 109 (38%) received a ritonavir-boosted atazanavir-based regimen. In Cohort D, we selected a diverse range of regimens: all participants’ regimens except one included ritonavir-boosted darunavir (21 [62%] of the 34 participants), etravirine (19 [56%]) and/or raltegravir (21 [62%]). We listed all ARV regimens used in Appendix pages 2–4.
Table 1 displays baseline characteristics. Participants were relatively young adults, and nearly half were female. Of note, the majority in Cohort A were female, but less than half were in all other cohorts. Study participants had relatively advanced immunosuppression. Median time of ART exposure was 7.9 years (IQR 5.9–10.2) and time since NNRTI-based regimen discontinuation was 3.3 years (IQR 1.9–5.0). Sixty-eight percent of participants were taking tenofovir as part of the second-line ART regimen ongoing at screening. A small percentage (12%) used tenofovir with their initial ART regimen. All participants, except three (who had taken fosamprenavir), had prior exposure to lopinavir (n=276; 51%), atazanavir (n=148; 27%) or both (n=118; 22%).
Table 1:
Baseline characteristics of the study population by Cohort
| A | B | C | D | Total | ||
|---|---|---|---|---|---|---|
| (n=287) | (n=154) | (n=70) | (n=34) | (n=545) | ||
| Age (years) | Median (IQR) | 40 (33, 46) | 42 (36, 48) | 42 (35, 46) | 42(38, 48) | 41 (35, 47) |
| Sex (n, %) | Female | 160 (56%) | 61 (40%) | 23 (33%) | 14(41%) | 258 (47%) |
| Male | 127 (44%) | 93 (60%) | 47 (67%) | 20 (59%) | 287 (53%) | |
| Ethnicity (n, %) | Hispanic or latino | 38 (13%) | 16 (10%) | 1 (1%) | 8 (24%) | 63 (12%) |
| Not hispanic or latino | 241 (84%) | 129 (84%) | 60 (86%) | 24 (71%) | 454 (83%) | |
| Unknown or not reported | 8 (3%) | 9 (6%) | 9 (13%) | 2 (6%) | 28 (5%) | |
| Region (n, %) | Africa | 148 (52%) | 85 (55%) | 32 (46%) | 18 (53%) | 283 (52%) |
| Asia | 62 (22%) | 41 (27%) | 35 (50%) | 8 (24%) | 146 (27%) | |
| South America | 43 (15%) | 19 (12%) | 1 (1%) | 8 (24%) | 71 (13%) | |
| Caribbean | 34 (12%) | 9 (6%) | 2 (3%) | 0 (0%) | 45 (8%) | |
| CD4 cell count (cells/mm3) | Median (IQR) | 171 (72, 288) | 191 (68, 315) | 161 (71, 289) | 189 (62, 361) | 175 (71, 308) |
| n (%) < 50 cells/mm3 | 50 (17%) | 29 (19%) | 9 (13%) | 7 (21%) | 95 (17%) | |
| HIV-1 RNA (log10 copies/mL) | Median (IQR) | 4.3 (3.3, 4.9) | 4.6 (3.7, 5.3) | 4.6 (3.7, 5.4) | 4.2 (3.7, 5.1) | 4.4 (3.5, 5.2) |
| n (%) ≥ 100,000 copies/mL | 68 (24%) | 60 (39%) | 27 (39%) | 12 (35%) | 167 (31%) | |
| HIV-1 subtype | C | 138 (48%) | 68 (44%) | 51 (73%) | 12 (35%) | 269 (49%) |
| B | 74 (26%) | 27 (18%) | 3 (4%) | 8 (24%) | 112 (21%) | |
| Al | 49 (17%) | 33 (21%) | 8 (11%) | 7 (21%) | 97 (18%) | |
| D | 12 (4%) | 18 (12%) | 3 (4%) | 2 (6%) | 35 (6%) | |
| CRF01_AE | 8 (3%) | 5 (3%) | 3 (4%) | 3 (9%) | 19 (3%) | |
| Other | 6 (2%) | 3 (2%) | 2 (3%) | 2 (6%) | 13 (2%) | |
| Hepatitis B surface antigen (n, %) | Positive | 14 (5%) | 8 (5%) | 3 (4%) | 4 (12%) | 29 (5%) |
| Body Mass Index (kg/m2) (n, %) | Underweight (< 18) | 51 (18%) | 20 (13%) | 13 (19%) | 6 (18%) | 90 (16%) |
| Normal (18 – <25) | 162 (57%) | 93 (60%) | 44 (63%) | 16 (47%) | 315 (58%) | |
| Overweight (25 – <30) | 47 (16%) | 29 (19%) | 8 (11%) | 9 (26%) | 93 (17%) | |
| Obese (30+) | 27 (9%) | 12 (8%) | 5 (7%) | 3 (9%) | 47 (9%) | |
| Years on antiretroviral therapy | Median (IQR) | 7.9 (5.5, 10.0) | 8.0 (6.0, 10.9) | 8.1 (6.2, 9.9) | 7.8 (5.6, 10.1) | 7.9 (5.9, 10.2) |
| Years from last NNRTI to entry | Median (IQR) | 3.2 (1.8, 5.0) | 3.5 (2.1, 6.1) | 3.0 (1.8, 4.3) | 3.8 (2.4, 4.7) | 3.3 (1.9, 5.0) |
| NRTI Exposure (n, %) | Tenofovir (TDF) | 232 (81%) | 132 (86%) | 66 (94%) | 28 (82%) | 458 (84%) |
| Zidovudine (ZDV) | 229 (80%) | 122 (79%) | 52 (74%) | 26 (76%) | 429 (79%) | |
| Lamivudine (3TC) | 287 (100%) | 153 (99%) | 70 (100%) | 34 (100%) | 544 (100%) | |
| Stavudine (D4T) | 152 (53%) | 93 (60%) | 48 (69%) | 17 (50%) | 310 (57%) | |
| TDF use in initial 1st line regimen | n (%) | 38 (13%) | 17 (11%) | 5 (7%) | 3 (9%) | 63 (12%) |
| TDF use during NNRTI use | n (%) | 81 (28%) | 45 (29%) | 19 (27%) | 7 (21%) | 152 (28%) |
| TDF use in 2nd line regimen ongoing at screening | n (%) | 174 (61%) | 108 (70%) | 59 (84%) | 27 (79%) | 368 (68%) |
| TDF use in initial 1st line regimen and in 2nd line regimen ongoing at screening | n (%) | 14 (5%) | 6 (4%) | 0 (0%) | 3 (9%) | 23 (4%) |
IQR is defined as the inter-quartile range and displays the 1st and 3rd quartiles, respectively. Baseline was defined as the last evaluation on or before the date of study entry. Summaries of ARV drug exposure were derived using data collected during the screening period.
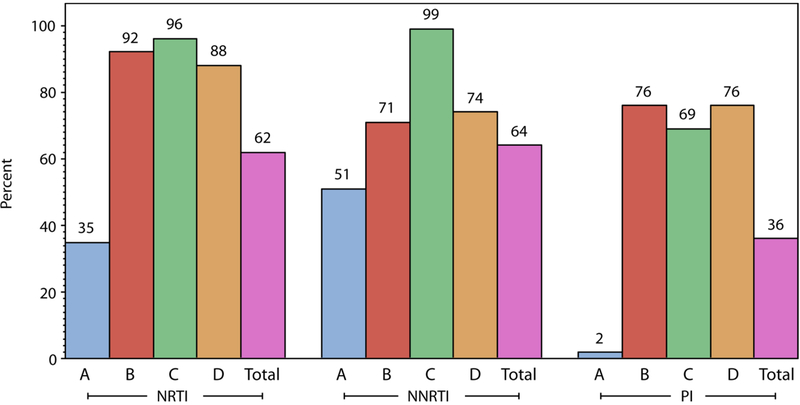
At screening, 78% (424 of the 545 participants) had at least one mutation indicating resistance to an NRTI (62%), NNRTI (64%) or PI (36%) (Table 2 and Figure 3). Resistance to 0, 1, 2 and all 3 drug classes was present in 22%, 20%, 30% and 27% respectively (Table 1). The most frequent NRTI mutations were M184V/I (57% of participants) followed by thymidine analogue mutations (TAMs) at codons 215 (27%), 67 (23%), 70 (19%), 219 (20%), 41 (21%); K65R occurred in only 3% of participants. The most frequent NNRTI mutations were at codons 103 (34%), 190 (19%) and 181 (15%); 78% of participants were susceptible to etravirine. We detected PI resistance in 36%, with 75% susceptible to lopinavir, 70% susceptible to atazanavir and 97% susceptible to darunavir. The most frequent major PI mutations were at codons 46 (22%), 71 (23%), 82 (21%) and 54 (20%). Of note, in Cohort A, 41% of participants had no resistance to a NRTI, NNRTI or PI and 99% were susceptible to lopinavir (Table 2).
Table 2:
Baseline Resistance by Cohort
| A | B | C | D | Total | ||
|---|---|---|---|---|---|---|
| (n=287) | (n=154) | (n=70) | (n=34) | (n=545) | ||
| Resistance Profile affecting Cohort Assignment | ||||||
| DRV/RTV | Susceptible | 287 (100%) | 154 (100%) | 70 (100%) | 17 (50%) | 528 (97%) |
| ETR | Susceptible | 247 (86%) | 154 (100%) | 2 (3%) | 23 (68%) | 426 (78%) |
| ATV/RTV | Susceptible | 283 (99%) | 58 (38%) | 31 (44%) | 10 (29%) | 382 (70%) |
| LPV/RTV | Susceptible | 285 (99%) | 72 (47%) | 41 (59%) | 11 (32%) | 409 (75%) |
| LPV/RTV and NRTI resistance | Resistance to LPV/RTV; not resistant to all NRTIs | 1 (0%) | 59 (38%) | 20 (29%) | 19 (56%) | 99 (18%) |
| Resistance to LPV/RTV; resistance to all NRTIs | 1 (0%) | 23 (15%) | 9 (13%) | 4 (12%) | 37 (7%) | |
| Susceptible to LPV/RTV; not resistant to all NRTIs | 284 (99%) | 49 (32%) | 22 (31%) | 8 (24%) | 363 (67%) | |
| Susceptible to LPV/RTV; resistance to all NRTIs | 1 (0%) | 23 (15%) | 19 (27%) | 3 (9%) | 46 (8%) | |
| Drug Class Resistance | ||||||
| NRTI, NNRTI or PI | Resistance | 170 (59%) | 153 (99%) | 69 (99%) | 32 (94%) | 424 (78%) |
| NRTI | Resistance | 101 (35%) | 142 (92%) | 67 (96%) | 30 (88%) | 340 (62%) |
| NNRTI | Resistance | 147 (51%) | 109 (71%) | 69 (99%) | 25 (74%) | 350 (64%) |
| PI | Resistance | 5 (2%) | 117 (76%) | 48 (69%) | 26 (76%) | 196 (36%) |
| Number of classes with resistance | 0 | 117 (41%) | 1 (1%) | 1 (1%) | 2 (6%) | 121 (22%) |
| 1 | 90 (31%) | 16 (10%) | 2 (3%) | 2 (6%) | 110 (20%) | |
| 2 | 77 (27%) | 59 (38%) | 19 (27%) | 11 (32%) | 166 (30%) | |
| 3 | 3 (1%) | 78 (51%) | 48 (69%) | 19 (56%) | 148 (27%) | |
| Selected Resistance Mutations | ||||||
| NRTI mutation | K65 | 5 (2%) | 4 (3%) | 7 (10%) | 2 (6%) | 18 (3%) |
| M184 | 87 (30%) | 131 (85%) | 64 (91%) | 28 (82%) | 310 (57%) | |
| Thymidine Analog mutation (TAM) | M41 | 5 (2%) | 60 (39%) | 32 (46%) | 15 (44%) | 112 (21%) |
| D67 | 6 (2%) | 75 (49%) | 33 (47%) | 14 (41%) | 128 (23%) | |
| K70 | 7 (2%) | 59 (38%) | 30 (43%) | 8 (24%) | 104 (19%) | |
| L210 | 2 (1%) | 18 (12%) | 9 (13%) | 2 (6%) | 31 (6%) | |
| T215 | 10 (3%) | 76 (49%) | 40 (57%) | 20 (59%) | 146 (27%) | |
| K219 | 8 (3%) | 60 (39%) | 30 (43%) | 11 (32%) | 109 (20%) | |
| NNRTI mutation | ||||||
| K103 | 90 (31%) | 63 (41%) | 20 (29%) | 10 (29%) | 183 (34%) | |
| G190 | 37 (13%) | 24 (16%) | 32 (46%) | 10 (29%) | 103 (19%) | |
| Y181 | 32(11%) | 0 (0%) | 45 (64%) | 6 (18%) | 83 (15%) | |
| PI mutation | ||||||
| M46 | 2 (1%) | 74 (48%) | 26 (37%) | 16 (47%) | 118 (22%) | |
| A71 | 19 (7%) | 60 (39%) | 32 (46%) | 13 (38%) | 124 (23%) | |
| V82 | 2 (1%) | 74 (48%) | 26 (37%) | 10 (29%) | 112 (21%) | |
| I54 | 2 (1%) | 71 (46%) | 20 (29%) | 18 (53%) | 111 (20%) |
HIV drug resistance mutations were identified and resistance scores were calculated using the Stanford Drug resistance database (version 6.2). These scores were used to determine HIV drug resistance for all NRTIs, NNRTIs and PIs currently used in LMIC except for etravirine and darunavir. The levels of HIV drug resistance were classified into five distinct categories: susceptible, potential low-level resistance, low-level resistance, intermediate resistance, and high-level resistance. For statistical analysis, resistance was defined as being in the intermediate or high-level categories. For etravirine and darunavir, susceptible and resistant classifications were used. For etravirine a score below 2.5 was classified as susceptible, and darunavir was classified as resistant if there were ≥3 darunavir associated mutations. The number of antiretroviral classes with resistance were restricted to NRTI, NNRTI and PI. Drugs included in the interpretation of nucleoside reverse transcriptase inhibitor (NRTI) class resistance were lamivudine (3TC), emtricitabine (FTC), abacavir (ABC), zidovudine (ZDV), stavudine (D4T), didanosine (DDI) and tenofovir (TDF); drugs included for non-nucleoside reverse transcriptase inhibitor class resistance were efavirenz (EFV), nevirapine (NVP) and etravirine (ETR); and drugs included for protease inhibitor (PI) class resistance were atazanavir (ATV), darunavir (DRV), indinavir (IDV), lopinavir (LPV), nelfinavir (NFV), saquinavir (SQV), tipranavir (TPV) and fosamprenavir (FPV). Individual drugs chosen to be displayed in the table were those most associated with cohort assignment.
Figure 3: Baseline Resistance by Cohort and Drug Class.
Showing the percentage of participants in each cohort with resistance to at least one drug in the class. Drug resistance interpretation for the study used the Stanford HIVDB 6.2 algorithm, but with rules for etravirine (ETR) and darunavir (DRV) resistance modified for this study. Drugs included in the interpretation of nucleoside reverse transcriptase inhibitor (NRTI) class resistance were lamivudine (3TC), emtricitabine (FTC), abacavir (ABC), zidovudine (ZDV), stavudine (D4T), didanosine (DDI) and tenofovir (TDF); drugs included for non-nucleoside reverse transcriptase inhibitor class resistance were efavirenz (EFV), nevirapine (NVP) and etravirine (ETR); and drugs included for protease inhibitor (PI) class resistance were atazanavir (ATV), darunavir (DRV), indinavir (IDV), lopinavir (LPV), nelfinavir (NFV), saquinavir (SQV), tipranavir (TPV) and fosamprenavir (FPV).
Of the 545 participants, 502 (92%) completed the protocol-specified follow-up (F/U), 23 (4%) died during follow-up (18 of them in Cohort A) and only 20 participants (4%) were lost to follow-up (Figure 2). Median duration of F/U across cohorts was 72 weeks. Eighty-three participants (15%) permanently discontinued the initial CMC-approved regimen. Twenty-three participants (4% of the 545 participants) discontinued due to VF, 19 (3%) due to death, 14 (3%) due to adverse events, 10 (2%) due to non-compliance, 9 (2%) due to loss to F/U, and the remaining 8 (1%) due to other reasons. Overall, the estimated percentage permanently discontinuing the initial CMC-approved regimen was 8.6% at 48 weeks (95% confidence interval: 6.5 to 11.2%), and was higher for Cohort A compared to other cohorts: 13.2% for Cohort A, and between 0% and 4.3% for Cohorts B1, B2, B3, C and D.
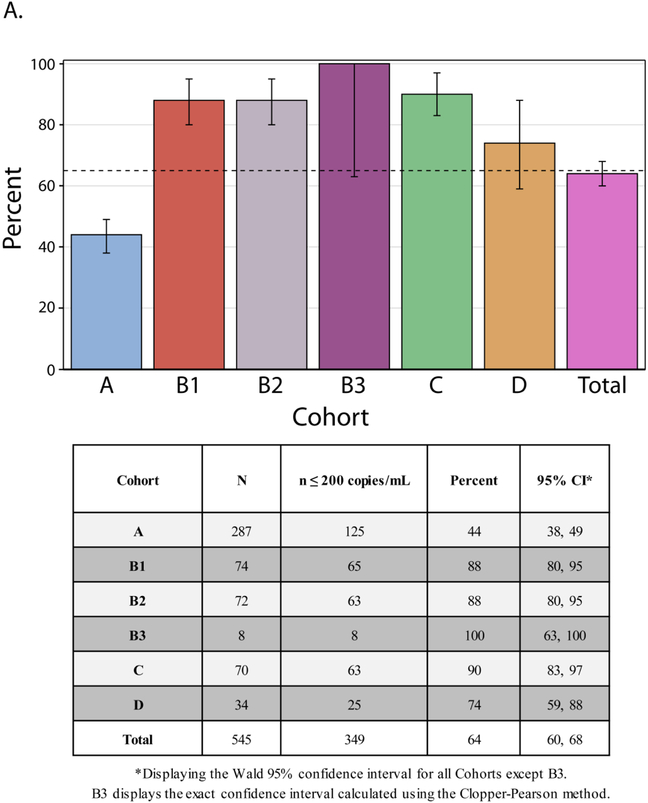
Of the 545 participants, 349 (64%) (95% CI 60, 68%) achieved VL≤ 200 copies per mL at week 48 (Figure 4, panel A). Viral suppression was substantially lower in Cohort A (44%) than other Cohorts (74% to 100%). We observed no difference in the primary virologic outcome between the randomized Cohorts B1 and B2 (88% for both). There was also no statistically significant difference by adherence support intervention so the results herein are not stratified by intervention arm.19
FIGURE 4: PANEL ‘A’ – HIV-1 RNA ≤ 200 copies/mL at week 48 with accompanying table:
Showing the percentage of participants in each cohort with HIV-1 RNA ≤ 200 copies/mL at week 48, irrespective of the antiretroviral regimen being taken at the time. The black dashed line at 65% represents the pre-defined suppression rate evaluated on the study. The week 48 measurement was the measurement closest to exactly 48 weeks (i.e. 7×48=336 days) after the date of study entry within a window of 295 to 378 days after study entry, inclusive. Participants who died or were lost to follow-up before week 48 are considered as not having HIV-1 RNA ≤200 copies/mL at week 48. Participants with a missing HIV-1 RNA measurement at week 48 were considered as not having HIV-1 RNA ≤200 copies/mL at week 48 unless the immediately preceding and immediately succeeding HIV-1 RNA measurements were both ≤200 copies/mL.
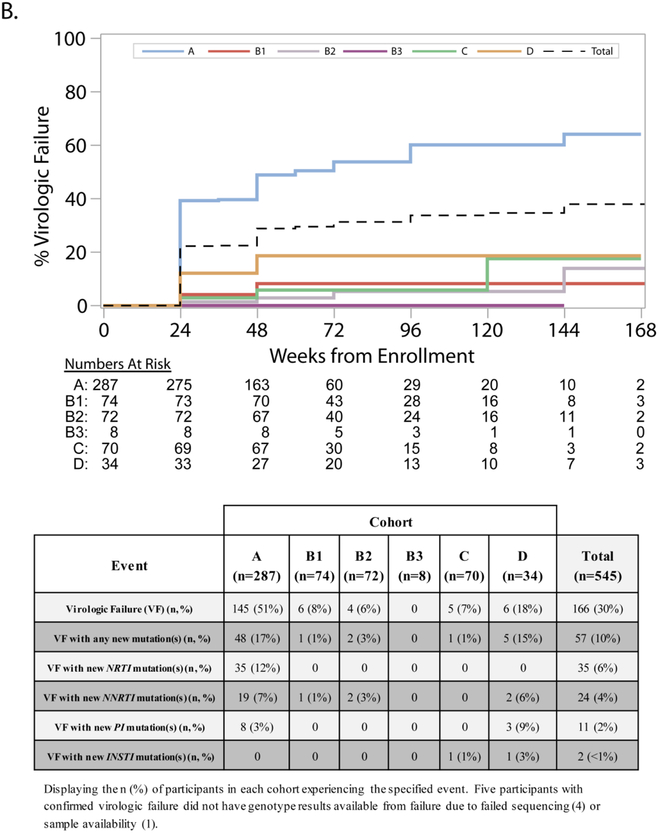
Confirmed VF at or after week 24 occurred in 30% of participants in the overall study population, with a significantly higher rate among participants in Cohort A (Figure 4 panel B). Across all cohorts, 10% of participants experienced VF with new drug resistance mutations: 17% in Cohort A, 15% in Cohort D (15%) and 3% or lower in other Cohorts. We observed new NRTI mutations in 6% of participants at VF, mostly among those in Cohort A, and new NNRTI and PI mutations in 4% and 2% of participants, respectively.
FIGURE 4-. PANEL ‘B’– Cumulative incidence of virologic failure and accompanying table displaying development of resistance associated mutations.
Shown are the cumulative percentage of participants who experienced confirmed virologic failure over time. At-risk numbers for each cohort and time point are displayed. For participants who did not experience confirmed virologic failure (including those who died), censoring was at the last HIV-1 RNA measurement on or before the end of follow up. Virologic failure was defined as two consecutive HIV-1 RNA values greater than or equal to 1000 copies/mL at or after 24 weeks on study. Evaluations for virologic failure included in the analysis occurred on or after 22 weeks (specifically, on or after 154 days from study entry) to allow for the scheduled visit window for the week 24 visit. The identification of new resistance-associated mutations was defined as the development of a mutation, not present at screening, identified and scored by the Stanford HIVDB 6.2 algorithm. Changes in mixture mutations were not counted as new mutations. Participants indicated as having developed new mutations may have lost mutations present at screening.
At week 48, the mean change in CD4 from baseline was 65 cells per μL (48, 82) in Cohort A, 86 cells per μL (−53, 225) in Cohort B3, 135 cells per μL (56, 214) in Cohort D, and 157(119, 195), 158(117, 200) and 160 cells per μL (126, 193) in Cohorts B1, B2 and C, respectively.
Overall, 236 participants (43%) experienced Grade 3 or higher adverse events during follow-up, ranging from 24% in Cohort C to 51% in Cohort A (Table 3). Thirty-one participants (6%) experienced new AIDS-defining diagnoses including 11 with pulmonary tuberculosis. Hypertension was the most frequent new non-AIDS diagnosis (n=13, 2%).
Table 3:
Grade 3 or higher adverse events*, deaths, AIDS-defining events, non-AIDS-defining events, hospitalizations, and pregnancies
| Cohort | |||||||
|---|---|---|---|---|---|---|---|
| A | B1 | B2 | B3 | C | D | Total | |
| (n=287) | (n=74) | (n=72) | (n=8) | (n=70) | (n=34) | (n=545) | |
| Adverse Events | |||||||
| Participants with Adverse Events (Gr ≥3) | 147 (51%) | 26 (35%) | 28 (39%) | 3 (38%) | 17 (24%) | 15 (44%) | 236 (43%) |
| Participants with Clinical Adverse Events (Gr ≥3)# | 77 (27%) | 14 (19%) | 15 (21%) | 1 (13%) | 10 (14%) | 8 (24%) | 125 (23%) |
| Infections | 46 (16%) | 4 (5%) | 6 (8%) | 1 (13%) | 4 (6%) | 2 (6%) | 63 (12%) |
| General | 20 (7%) | 2 (3%) | 3 (4%) | 1 (13%) | 2 (3%) | 2 (6%) | 30 (6%) |
| Weight loss | 16 (6%) | 1 (1%) | 2 (3%) | 0 (0%) | 1 (1%) | 1 (3%) | 21 (4%) |
| Fever | 6 (2%) | 0 | 0 | 1 (13%) | 1 (1%) | 2 (6%) | 10 (2%) |
| Gastrointestinal | 18 (6%) | 4 (5%) | 1 (1%) | 1 (13%) | 0 | 0 | 24 (4%) |
| Diarrhea | 7 (2%) | 2 (3%) | 0 | 0 | 0 | 0 | 9 (2%) |
| Metabolic | 13 (5%) | 1 (1%) | 2 (3%) | 0 | 0 | 3 (9%) | 19 (3%) |
| Neurological | 11 (4%) | 1 (1%) | 2 (3%) | 1 (13%) | 0 | 1 (3%) | 16 (3%) |
| Hematologic | 9 (3%) | 2 (3%) | 1 (1%) | 0 | 0 | 1 (3%) | 13 (2%) |
| Anemia | 7 (2%) | 0 | 1 (1%) | 0 | 0 | 1 (3%) | 9 (2%) |
| Musculoskeletal | 5 (2%) | 3 (4%) | 1 (1%) | 1 (13%) | 1 (1%) | 0 | 11 (2%) |
| Pulmonary | 8 (3%) | 0 | 1 (1%) | 0 | 0 | 0 | 9 (2%) |
| Vascular | 5 (2%) | 2 (3%) | 2 (3%) | 0 | 1 (1%) | 1 (3%) | 11 (2%) |
| Participants with Laboratory Adverse Events (Gr ≥3)# | 102 (36%) | 15 (20%) | 20 (28%) | 3 (38%) | 12 (17%) | 11 (32%) | 163 (30%) |
| Chemistry | |||||||
| High bilirubin | 49 (17%) | 0 | 1 (1%) | 0 | 2 (3%) | 1 (3%) | 53 (10%) |
| Low phosphorus | 10 (3%) | 2 (3%) | 1 (1%) | 0 | 1 (1%) | 3 (9%) | 17 (3%) |
| High aspartate aminotransferase | 7 (2%) | 1 (1%) | 1 (1%) | 1 (13%) | 3 (4%) | 1 (3%) | 14 (3%) |
| High glucose | 7 (2%) | 2 (3%) | 1 (1%) | 0 | 1 (1%) | 1 (3%) | 12 (2%) |
| Low sodium | 7 (2%) | 0 | 1 (1%) | 1 (13%) | 2 (3%) | 1 (3%) | 12 (2%) |
| Hematology | |||||||
| Low neutrophil count | 12 (4%) | 3 (4%) | 4 (6%) | 0 | 3 (4%) | 2 (6%) | 24 (4%) |
| Low hemoglobin | 13 (5%) | 2 (3%) | 3 (4%) | 0 | 0 | 1 (3%) | 19 (3%) |
| Low platelet count | 8 (3%) | 1 (1%) | 0 | 0 | 0 | 0 | 9 (2%) |
| Metabolic | |||||||
| High low density lipoprotein cholesterol | 7 (2%) | 4 (5%) | 7 (10%) | 0 (0%) | 2 (3%) | 2 (6%) | 22 (4%) |
| High total cholesterol | 5 (2%) | 4 (5%) | 5 (7%) | 0 | 1 (1%) | 1 (3%) | 16 (3%) |
| High triglycerides | 3 (1%) | 2 (3%) | 2 (3%) | 0 | 1 (1%) | 1 (3%) | 9 (2%) |
| Clinical Events | |||||||
| Deaths | 18 (6%) | 1 (1%) | 2 (3%) | 0 | 1 (1%) | 1 (3%) | 23 (4%) |
| AIDS-defining illnesses | 21 (7%) | 2 (3%) | 3 (4%) | 0 | 2 (3%) | 3 (9%) | 31 (6%) |
| Pulmonary tuberculosis# | 9 (3%) | 1 (1%) | 1 (1%) | 0 | 0 | 0 | 11 (2%) |
| Non-AIDS defining events | 11 (4%) | 7 (9%) | 4 (6%) | 0 | 5 (7%) | 7 (21%) | 34 (6%) |
| Hypertension# | 7 (2%) | 2 (3%) | 0 | 0 | 1 (1%) | 3 (9%) | 13 (2%) |
| Hospitalizations | 48 (17%) | 8 (11%) | 9 (13%) | 1 (13%) | 6 (9%) | 4 (12%) | 76 (14%) |
| Pregnancies | 8 (3%) | 1 (1%) | 2 (3%) | 0 | 3 (4%) | 0 | 14 (3%) |
Events do not add to the total of the category since participants can have multiple events across categories.
Displaying categories with at least 2% of the total enrollees.
Discussion
A5288 was a large, multi-country clinical trial in LMIC for individuals experiencing virologic failure on second-line ART. We enrolled a study population from diverse cultural, socioeconomic and geographic settings who had a wide range of viral resistance patterns. Our strategy resulted in a 64% viral suppression rate at 48 weeks of follow-up, largely reflecting a greater than anticipated proportion of failure to suppress among participants who stayed on a second-line regimen.
Our multi-site prospective study in adults of a third-line therapy algorithm based upon genotyping adds substantially to the available information about management of second-line failure in LMIC. Previous reports about this population have generally been single center, observational reports with relatively small patient numbers or reports of drug resistance patterns without outcome data.14,15,20 A report from a Zimbabwe national program for individuals with virological failure of second-line PI-based treatment (assessed adherent by pill counts) reported that 14% of the 86 who underwent genotyping had wild-type HIV.20 Among 39 patients from that program who had no PI mutations and continued second-line ART with adherence support, only a minority (8, or 20%) achieved virologic suppression by 24 weeks; whereas similar to our study, the 36 individuals with PI resistance treated with new drugs had a substantially higher rate of virological suppression (29 [81%] <50 copies per mL). A retrospective observational study of 152 patients from the South African private health care sector with PI mutations who were treated with a variety of new drugs reported that 83% achieved viral load of <400 copies per mL after a median of 2.5 years.14,15 A public sector third-line ART program from South Africa reported outcomes in 82% of the 144 individuals (all with PI resistance) started on darunavir-containing regimens; 79% achieved <400 copies per mL after 6 months.14 Interestingly, this program was organized with a virtual committee that reviewed data, very similar to the CMC in our study. Our report of prospective outcome data for over 500 individuals from across the globe presenting with second-line failure whose ART was selected on the basis of genotyping and drug history validates and extends the smaller, single center reports.
Less than half of those enrolled in Cohort A, who had the least resistance at screening, and who therefore remained on a second-line ART regimen, attained virologic suppression at 48 weeks. Several observational studies in LMIC (as noted above) and the Thilao clinical trial in West Africa20–22 reported that a substantial proportion of individuals can re-suppress after second-line failure with continuation of the same regimen. Few cohort A participants developed new resistance mutations at failure underscoring the probable contribution of non-adherence. Early identification of those failing a second-line regimen, without significant resistance, who are not able to re-suppress, is essential to avoiding disease progression and ongoing HIV transmission. An interplay of individual, interpersonal, socio-cultural and health system factors, as well as structural issues, can affect treatment adherence. For example, ARV shortages and stock-outs have been associated with increased unstructured treatment interruption and mortality.23 Our study eliminated these structural barriers and offered significant support; nonetheless, we observed a high rate of treatment failure with a potentially efficacious regimen. Further work is needed to improve outcomes in this group.
Women represented the majority of participants enrolled in Cohort A, as opposed to the cohorts with more resistance at enrollment. Compared to men, a higher risk of treatment discontinuation due to intolerability has been observed among women with ritonavir-boosted atazanavir and ritonavir-boosted lopinavir based ART.24 Higher rates of second-line ART discontinuation may help to explain the higher proportion of women with preserved susceptibility to boosted PIs.
On a global scale, tenofovir/lamivudine/dolutegravir (TLD) is being recommended as first-line and second-line ART, and as a switch for those individuals using an NNRTI or PI-based regimen, regardless of virologic suppression. Our finding that a significant majority of enrollees in the current study had known mutations that confer resistance to NRTIs with M184V almost universally prevalent and a high prevalence of TAMs raises concern regarding a strategy of switching to TLD from a second-line PI-based regimen, as dolutegravir might end up unintendedly being the only active drug of TLD regimens, resulting in virologic failure and dolutegravir resistance.25 Our finding that the prevalence of K65R at study entry was minuscule and lower than reported for first-line ART failure26 may be related to agonistic molecular interactions between reverse transcriptase inhibitor resistance mutations27 or effective suppression of K65R by PI-containing regimens.28
Simpler and better-tolerated second-line regimens are a major unmet need. In a recent study of dolutegravir and two NRTIs compared with ritonavir-boosted lopinavir and two NRTIs as second-line treatments, dolutegravir was superior to ritonavir-boosted lopinavir.29 How the new WHO guidelines recommending dolutegravir-based regimens as first and second-line therapy will impact the incidence of second-line failures in the future is unknown.
The proportion of participants susceptible to ritonavir-boosted darunavir at study entry was reassuring and in line with our expectations given limited ritonavir-boosted darunavir availability in the countries participating in this study during the timeframe in which it was undertaken. We found that regimens containing ritonavir-boosted darunavir and raltegravir with or without etravirine were highly effective and safe for participants with ritonavir-boosted lopinavir resistance, corroborating the evidence that targeted real-time drug resistance testing can appropriately allocate more costly ARVs to those with greater resistance.
We have proposed an algorithmic approach in A5288, which could serve as a compromise between individualized regimens and having only one or two third-line regimens available. As treatment access has expanded, so has the number of persons who may be appropriate for third-line ART. Some middle-income countries are making such therapy available and our results may be relevant to them. WHO has introduced the concept of differentiated care and it is possible that third-line ART should be made more widely accessible, even in low-income settings. We demonstrated the feasibility of using real-time drug resistance and viral load testing in countries around the world, including locations where these tests have not been routinely used. Although simpler resistance tests are in development that do not rely on sequencing of PCR-amplified regions of the virus, the multitude of potential mutations arising after two prior ART regimens and the broadening targets of ART (protease, reverse transcriptase, and integrase) will complicate the interpretation and implementation of such technologies.
A major strength of this study is its diverse population, having participants from multiple countries and continents. The number of countries and sites conducting the study suggests wide clinical relevance of third-line ART. Furthermore, we used local definitions of ART failure, allowing large numbers of individuals to be screened for potential participation. Another strength is our requirement for confirmed virologic failure which limited enrollment to individuals who had not been successful with their second-line treatment; 66% of potential enrollees were no longer eligible because of having improved viral suppression between their initial and confirmatory viral load tests.
This approach is similar to that adopted in some of the sites reporting management of second-line failures, where adherence support is provided before they are assessed as failing second-line ART and those without PI resistance do not get treated with new drugs.14,15
Nevertheless, some limitations should also be acknowledged. First, we had a delay, albeit short, between our collection of blood for resistance testing and switch to the study-assigned regimen – where there could have been accumulation of resistance during this interval that impacted study outcomes. The timing between the baseline resistance test and study entry appeared to be an important aspect in the cost-effectiveness of our strategy.30 Second, the study involved more frequent VL testing, clinic visits and staff contact than what usually occurs in clinical practice, thus possibly limiting generalizability of the results. Third, we cannot predict whether outcomes in Cohort A would have been better if they had also started novel antiretrovirals.
In conclusion, targeted real-time HIV-1 drug resistance testing was a useful treatment management tool for ensuring appropriate allocation of expensive third-line drug options. In these individuals, the combination of darunavir and raltegravir with either etravirine or recycled NRTIs was highly effective and safe for individuals in diverse LMIC who were failing second-line therapy with resistance to lopinavir. For individuals on failing second-line ART without resistance to lopinavir and limited resistance to NRTIs, better tolerated second-line regimens, as well as adherence interventions, will be needed to improve treatment outcomes. While the impact on the global rollout of dolutegravir-containing regimens on rates of virological failure is unknown, the importance of non-adherence that this study demonstrates makes it likely that virological failure will still occur and 3rd line ART will still be needed.
Supplementary Material
Acknowledgements
This study was supported by the National Institute of Allergy and Infectious Diseases (award numbers UM1 AI068636, UM1 AI068634 [ACTG Statistical and Data Management Center], UM1 AI069423, and UM1 AI069481). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases. AbbVie, Gilead Sciences, Janssen Pharmaceuticals, and Merck & Company provided the study drugs. We thank Dimagi, the study participants, the other members of the A5288 team, the staff members at the virology and genotyping laboratories for their work in testing for the primary endpoint and the genotyping, and the staff at the sites and grants supporting their work, including grants. We thank Dimagi, which consulted individually with the sites to tailor the set-up and maintenance of the automated system, addressing issues such as how to prevent power interruptions from disrupting service.
Footnotes
Declaration of interests
ACC reports grants from NIH during the conduct of the study; personal fees from Merck & Co., grants from Bristol-Myers-Squibb, outside the submitted work.
JWM reports grants from NIAID/NIH, during the conduct of the study; personal fees from University of Pittsburgh, grants from Gilead Sciences, grants from Janssen Pharmaceuticals, personal fees from Bristol-Myers Squibb, personal fees from Gilead Sciences, personal fees from Janssen Pharmaceuticals, personal fees from Merck, personal fees from Xi’an Yufan Biotechnologies, other from Cocrystal Pharma, Inc., outside the submitted work; In addition, Dr. Mellors has a patent Patent #: 8,815,829 pending.
CLW reports personal fees from IPM (International Partnership for Microbicides), personal fees from Right-to-Care, personal fees from MSD-MERCK, outside the submitted work.
JR reports grants from NIH during the conduct of the study.
MDH reports grants from NIH during the conduct of the study.
RG reports grants from NIH during the conduct of the study; personal fees from Pfizer, outside the submitted work.
RTS reports grants from National Institute of Allergy and Infectious Diseases, during the conduct of the study; grants from Gilead Sciences, grants and personal fees from Monogram Biosciences, grants from Pfizer, personal fees from CytoDyn, personal fees from VIR, outside the submitted work.
VM reports grants from NIH, during the conduct of the study.
LM reports grants from The National Institute of Allergy and Infectious Diseases, National Institutes of Health, during the conduct of the study; grants from Janssen Pharmaceutica, grants from Merck Sharp & Dohme Corp, grants from ViiV Healthcare, grants from Johnson and Johnson, grants from Pfizer Pharmaceuticals, non-financial support from Kowa Pharmaceuticals America, non-financial support from Sanofi-Aventis, grants from Bristol Myers Squibb, outside the submitted work.
All other authors declare no competing interests.
References
- 1.World Health Organization (WHO). Consolidated ARV guidelines, June 2013. https://www.who.int/hiv/pub/guidelines/arv2013/art/thirdlineart/en/index2.html. Accessed: 21-Jan-2019.
- 2.World Health Organization (WHO). Antiretroviral medicines in low-and-middle-income countries: forecasts of global and regional demand for 2014–2018: technical report, 2015. https://www.who.int/hiv/pub/amds/arv-forecast2014-2018/en/. Accessed: 21-Jan-2019.
- 3.Gupta A, Juneja S, Vitoria M, et al. Projected Uptake of New Antiretroviral (ARV) Medicines in Adults in Low-and Middle-Income Countries: A Forecast Analysis 2015–2025. PLoS One 2016; 11(10): e0164619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cambiano V, Bertagnolio S, Jordan MR, et al. Predicted levels of HIV drug resistance: potential impact of expanding diagnosis, retention, and eligibility criteria for antiretroviral therapy initiation. AIDS 2014; 28 Suppl 1: S15–23. [DOI] [PubMed] [Google Scholar]
- 5.Cohen K, Stewart A, Kengne AP, et al. A clinical prediction rule for protease inhibitor resistance in patients failing second-line antiretroviral therapy. J Acquir Immune Defic Syndr 2019;80(3):325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzaule SC, Hamers RL, Mukui I, et al. Emergence of untreatable, multidrug-resistant HIV-1 in patients failing second-line therapy in Kenya. AIDS 2017; 31(10): 1495–8. [DOI] [PubMed] [Google Scholar]
- 7.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Protease Inhibitor Resistance Is Uncommon in HIV-1 Subtype C Infected Patients on Failing Second-Line Lopinavir/r-Containing Antiretroviral Therapy in South Africa. AIDS Res Treat 2011; 2011: 769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawadogo S, Shiningavamwe A, Roscoe C, et al. Human Immunodeficiency Virus-1 Drug Resistance Patterns Among Adult Patients Failing Second-Line Protease Inhibitor-Containing Regimens in Namibia, 2010–2015. Open Forum Infect Dis 2018; 5(2): ofy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382(9893): 700–8. [DOI] [PubMed] [Google Scholar]
- 10.Arathoon E, Bhorat A, Silaghi R, et al. Week 48 results of a Phase IV trial of etravirine with antiretrovirals other than darunavir/ritonavir in HIV-1-infected treatment-experienced adults. J Int AIDS Soc 2014; 17(4 Suppl 3): 19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210(3): 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vingerhoets J, Calvez V, Flandre P, et al. Efficacy of etravirine combined with darunavir or other ritonavir-boosted protease inhibitors in HIV-1-infected patients: an observational study using pooled European cohort data. HIV Med 2015; 16(5): 297–306. [DOI] [PubMed] [Google Scholar]
- 13.Tashima KT, Smeaton LM, Fichtenbaum CJ, et al. HIV Salvage Therapy Does Not Require Nucleoside Reverse Transcriptase Inhibitors: A Randomized, Controlled Trial. Ann Intern Med 2015; 163(12): 908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorhouse M, Maartens G, Venter WDF, et al. Third-Line Antiretroviral Therapy Program in the South African Public Sector: Cohort Description and Virological Outcomes. J Acquir Immune Defic Syndr 2019; 80(1): 73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meintjes G, Dunn L, Coetsee M, et al. Third-line antiretroviral therapy in Africa: effectiveness in a Southern African retrospective cohort study. AIDS Res Ther 2015; 12: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Meyer S, Vangeneugden T, van Baelen B, et al. Resistance profile of darunavir: combined 24-week results from the POWER trials. AIDS Res Hum Retroviruses 2008; 24(3): 379–88. [DOI] [PubMed] [Google Scholar]
- 17.Vingerhoets J, Azijn H, Fransen E, et al. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J Virol 2005; 79(20): 12773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vingerhoets J, Tambuyzer L, Azijn H, et al. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled Phase III clinical studies. AIDS 2010; 24(4): 503–14. [DOI] [PubMed] [Google Scholar]
- 19.Gross R, Ritz J, Hughes MD, Salata R, Mugyeni P, Hogg E, Wieclaw L, Godfrey C, Wallis CL, Mellors JW, Mudhune VO, Badal-Faesen S, Grinsztejn B, Collier ACC. Two-way Cellphone Intervention Compared with Standard of Care Adherence Support after Second Line Antiretroviral Therapy Failure: A Multinational Randomized Clinical Trial. The Lancet Digital Health (2019. in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chimbetete C, Katzenstein D, Shamu T, et al. HIV-1 Drug Resistance and Third-Line Therapy Outcomes in Patients Failing Second-Line Therapy in Zimbabwe. Open Forum Infect Dis 2018; 5(2): ofy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Health 2016; 21(9): 1131–7. [DOI] [PubMed] [Google Scholar]
- 22.Moh R, Benalycherif A, Gabillard D, et al. 48-weeks efficacy of a third-line based on darunavir plus raltegravir regimen in HIV-infected adults who failed second-line protease inhibitor-based regimen in sub-Saharan Africa, ANRS 12269 THILAO study 9th IAS Conference on HIV Science; 2017; Paris, France; 2017. [Google Scholar]
- 23.Meloni ST, Chaplin B, Idoko J, et al. Drug resistance patterns following pharmacy stock shortage in Nigerian Antiretroviral Treatment Program. AIDS Res Ther 2017; 14(1): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squires KE, Johnson M, Yang R, et al. Comparative gender analysis of the efficacy and safety of atazanavir/ritonavir and lopinavir/ritonavir at 96 weeks in the CASTLE study. J Antimicrob Chemother 2011; 66(2): 363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuritzkes DR. Resistance to dolutegravir--a chink in the armor? J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TenoRes Study G Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16(5): 565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh UM, Bacheler L, Koontz D, Mellors JW. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J Virol 2006; 80(10): 4971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherrer AU, Boni J, Yerly S, et al. Long-lasting protection of activity of nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) by boosted PI containing regimens. PLoS One 2012; 7(11): e50307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19(3): 253–64. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzana SB, Hughes MD, Grinsztejn B, et al. Genotype assays and third-line ART in resource-limited settings: a simulation and cost-effectiveness analysis of a planned clinical trial. AIDS 2012; 26(9): 1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.