Abstract
Evolution of eukaryotes from simple cells to complex multicellular organisms remains a mystery. Our postulate is that cytoskeletal stiffening is a necessary condition for evolution of complex multicellular organisms from early simple eukaryotes. Recent findings show that embryonic stem cells are as soft as primitive eukaryotes-amoebae and that differentiated tissue cells can be two orders of magnitude stiffer than embryonic stem cells. Soft embryonic stem cells become stiff as they differentiate into tissue cells of the complex multicellular organisms to match their microenvironment stiffness. We perhaps see in differentiation of embryonic stem cells (derived from inner cell mass cells) the echo of those early evolutionary events. Early soft unicellular organisms might have evolved to stiffen their cytoskeleton to protect their structural integrity from external mechanical stresses while being able to maintain form, to change shape, and to move.
Keywords: Cytoskeleton, Force, Bacteria, Amoebae, Eukaryotes
How eukaryotes have originated is at the center of debate in evolutionary biology. Various models on the evolution of eukaryotes have been proposed [1–6]. Recent molecular phylogeny evidence appears to support the three-domain model of bacteria, archaea, and eucarya [1] and seems to deviate from the models of evolution from prokaryotes to eukaryotes [2,3,5,6]. However, these models are not well-suited to address late stages of evolution, i.e., how complex forms of multicellular organisms have evolved from simple eukaryotes. Life could have stayed simple eukaryotes like their single-celled ancestors or like amoebae. Contributions from genome evolution [7] and cell-cell adhesion molecule cadherins [8] have been proposed as some of the necessary conditions for the evolution of the complex multicellular animal organisms. The question then is: what are other conditions that must be met for the complex multicellular animals to evolve?
We propose a cell stiffness postulate: for a complex multicellular animal to evolve, early soft unicellular organisms must evolve to stiffen their cytoskeleton to protect their structural integrity from being irreversibly damaged by external mechanical stresses while being able to maintain form, to change shape, and to move.
To provide support for this postulate, let us first examine stiffness values of various cell types. It is known that, due to the presence of a rigid cell wall of peptidoglycan, a bacterium has a cell stiffness of ~1000 kPa [9]. Archaea whose membrane consists of stiff protein surface layers (S-layers) possibly also have a stiffness of ~1000 kPa [10]. Although the cell stiffness of the very first eukaryotes is not known, simple primitive eukaryotes such as an amoeba has a stiffness of ~0.1 kPa [11] that is at least 4 orders of magnitude lower than that of bacteria or that of archaea. The stiffness of the plasma membrane likely contributes to less than 1% of the total cell stiffness [12] and hence the majority of the cell stiffness originates from that of the cytoskeleton. Do all modern-day cell types in a complex multicellular land animal have a similar stiffness as an amoeba? The answer appears to be no.
Now let us examine the stiffness of various cell types in an animal. An embryonic stem cell has a stiffness of ~0.5 kPa [13]. Neural cells generally have a stiffness of ~0.1–0.5 kPa [14]. A differentiated cell has a stiffness of ~5 kPa [13], similar to that of a mesenchymal stem cell [15]. A typical tissue cell (e.g., a smooth muscle cell, an endothelial cell, or a fibroblast) has a stiffness of ~1–5 kPa [16]. A skeletal muscle cell has a stiffness of ~12 kPa [17]. The stiffest cells in a modern-day animal appear to be the skeletal muscle cells that generate extremely high stresses themselves. It may not be a coincidence that these muscle cells have a very stiff cytoskeleton to withstand its own stresses, as the muscle cells need to generate high stresses to perform various essential functions of the organism. Similarly, besides closing a wound, one of fibroblasts’ main tasks is to secrete matrix proteins to produce extracellular polymers and to pull on the matrix polymers to generate specific patterns and forms [18]. Therefore, it is not surprising that stiffness of the fibroblast is very high. In a similar vein, a smooth muscle cell needs to generate high stresses to regulate the caliber of a lumen, whether it is a blood vessel, a gastrointestinal lumen, or an airway in the lung. Therefore, smooth muscle cell stiffness would also be very high. It is probably true that most types of tissue cells that can remodel its own microenvironment matrix protein polymers have stiffness of similar magnitudes. It is interesting that the differentiated cells that generally have much lower stiffness are those that are either an amoeboid-like cell (e.g., a neutrophil) or a neural cell of the central nervous system. It is important to note that the process of cytoskeletal stiffening does not have to be irreversible during differentiation. For example, a stiff mesenchymal stem cell becomes softer when it differentiates into an adipocyte that typically has a stiffness of ~0.6–0.9 kPa [19], possibly after depositing a soft extracellular matrix (ECM).
Since archaea and bacteria are both rigid cells, the question raises whether the very first ancestor cells of all eukaryotes are soft or rigid cells. It is not clear at this time. Nevertheless, one thing appeared to be happening after penetration of its membrane by an aerobic aproteobacterium or after phagocytosis of the α-proteobacterium: a newly formed cell was a soft cell that did not have the rigid wall as the archaea and the bacteria did. This primitive eukaryote was probably as soft as today’s amoebae.
How did then the primitive soft eukaryotes evolve to be stiffer to give rise to modern day different eukaryotes of various stiffnesses? One way to stiffen these soft cells was to cluster transmembrane cell-matrix adhesion molecule integrins [20,21] and to cluster cell-cell adhesion molecule cadherins [22]. Another way was to express crosslinking/bundling proteins to stiffen the cytoskeleton [23,24]. Moreover, myosin-II (both a crosslinking protein and a force generator) might have evolved [25] to form actomyosin bundles to generate large forces for power generation and for prestress-dependent cell shape stability [14,15,26–28]. The stiffening responses of the primitive eukaryotes were also likely to be triggered when they were pulled by nearby cells via cell-cell adhesion molecule cadherins [22] and when they pulled on the stiff ECM via cell-matrix adhesion molecule integrins [14]. The nuclear lamina is a fibrillar network that lies underneath the nuclear envelope in eukaryotes. The nuclear lamina consists of lamins (e.g., lamin A/C and lamin B) that are a stiff polymer structure [29] to provide protection to the genome from being damaged by mechanical stress [30]. It is increasingly evident that the nucleus can be viewed as a mechanosensor [31] in which lamins are an important component responsible for differentiation [32] and for regulation of transcription factors [33]. It is recently revealed that the nuclear envelope and lamina are physically tethered to the cytoskeleton via the linker of neucleoskeleton and cytoskeleton (LINC) complex to propagate force signals [34] for the chromatin to sense forces to activate genes [35]. Most differentiated eukaryotes have a nucleus that is several folds stiffer than its cytoskeleton [29,36]. Therefore, as cells begin to differentiate during embryonic development [37,38], their cytoskeleton and nucleus should become stiffened to accommodate the need to withstand high mechanical stresses in their microenvironment. However, it is important that the cytoskeleton and the nucleus should not have stiffened too much. Because if all modern day eukaryotes were still as stiff as those bacteria or archaea, we might not have a modern-day complex animal whose cells are able to readily change shapes during development and under physiological conditions. Although the 1000 kPa stiffness of the bacteria is beneficial for the bacteria to survive in the presence of large external mechanical stresses or osmotic challenges, it is just too rigid for shape change. It is so stiff that they cannot change their shape easily to perform spreading, to migrate through porous extracellular matrices, and/or to contract to change cell shape since it is energetically too costly. This is probably why the modern-day stiffest animal cell (the skeletal muscle cell) has a modulus of only ~10 kPa and not 1000 kPa. In contrast, simple cells like amoeba are too soft (0.1 kPa) to sustain any large mechanical stresses to form a complex multicellular organism, although they do form a colony of cells under certain conditions. It is well known that these cells have a very soft and dynamic cytoskeleton [11]. Therefore, although the dynamic nature of the cytoskeleton alone cannot explain the evolution of the complex multicellular organisms, it facilitates cell shape change. The first principle for survival is probably to protect the integrity of the cell (i.e., without structural failure in the presence of changes in mechanical stresses or osmotic pressures) while still being able to perform necessary physiological functions. Neurons appear to be specialized cells whose primary function is to conduct electrical/chemical signaling. That is probably why they are soft (~0.1–0.5 kPa) and reside deep inside other tissues in the animal body to be protected against mechanical insults.
Cell shape is determined by the organization of the cytoskeleton whose stiffness, in turn, is determined by the clustering of adhesion molecules like integrins and cadherins, crosslinking and bundling of the cytoskeletal filament systems, and the prestress generated by myosin-II motor proteins. The final shape of the cell is the result of the balances of those external and internal forces, the overall stiffness of the cell, the cell-matrix and/or cell-cell adhesion, the stiffness of the ECM, and the shape and organization of the cytoskeleton. It is known that a normal cell cannot survive if its plasma membrane is detached from the underneath cytoskeleton for long. Therefore, cell shape per se, while being an easily measurable parameter, is not a mechanistic factor in a complex multicellular organism.
One open question that could be asked is the following: is it the cytoskeleton stiffening or the changed mechanical microenvironment that drives organ evolution? Of all the organs and tissues in an organism, only the cells are the “live” components that can actively “sense” changes in their microenvironments, including mechanical microenvironments. The ECM that is the main component of the mechanical microenvironments that can be remodeled by the living cells and by the ambient environment such as radiation [39]. Part of the cytoskeletal stiffening of the cell is through the generation of active tension in the cytoskeleton that is utilized by the cells to mechanically remodel their microenvironment [18]. In addition, for the cells to secrete enzymes to remodel it enzymatically. Therefore, it is possible that both the cytoskeletal stiffening and the changed mechanical microenvironment contributes to organ evolution but the living cells dictate organ evolution by actively sensing changes in their microenvironments. It is reported recently that cell stiffness increases with intracellular crowding as a result of water efflux induced cell volume reduction [40], although the experiments are generally performed in short time scales (e.g., tens of seconds to minutes via osmotic challenges). Consistent with this notion is the fact that an animal egg that is very soft (the cytoplasm of an unfertilized frog egg has a stiffness of ~0.01 kPa [41]) and is generally much bigger in volume than a stiff differentiated cell. It remains to be determined in the future, however, how much intracellular crowding has to increase to account for the cell volume reduction and the cell stiffness elevation to impact on multicellular evolution.
It has been proposed that the principles of tension-dependent integrity (tensegrity) of the cytoskeleton might have guided the evolution of the first cells on the earth [42]. A different hypothesis is that fluidization of the cytoskeleton during invasion might manifest early evolutionary adaptations of the eukaryotic cell to material properties of a soft inert microenvironment [43]. It is possible that the capacity to maintaining the cell shape stability in the presence of mechanical challenges and the dynamic malleability of the cytoskeleton both are necessary for animal cells to evolve. However, for a complex multicellular land animal to evolve and to perform challenging mechanical tasks (e.g., movement, running, jumping, climbing, etc.), it also needs to develop stiff tissue cells such as skeletal muscle cells. These cells are generally as stiff as their substrates. The evolution of stiff ECM polymers of cartilage and of rigid bones provided the animal with additional mechanical strength and stiffness to sustain impacts of the gravitational forces and/or external mechanical insults by predators. In addition to changes in mechanical environments, changes in chemical environments such as estrogen deficiency induce osteoporosis in the rigid bone and result in lower stiffness and higher plastic deformation in the bone than the control bones [44], influencing the overall capacity of the organism to sustain large compressive stresses. It is interesting that differentiation of stem cell like cells leading to cell stiffening has been demonstrated in tumor. Melanoma tumor-repopulating cells, which express high levels of self-renewal gene Sox2, are extremely soft (cell stiffness is ~0.15 kPa, 4-fold lower than their differentiated counterpart cells) [45]. These cells are highly tumorigenic and metastatic, enabling them to change cell shape easily to extravasate and to invade the 3D ECM in vivo in a zebrafish model [46]. The cell stiffness postulate is testable: if one finds that a complex land animal that can generate huge mechanical stresses and can sustain great external stresses but is still composed of only very soft cells (~0.1 kPa) in the absence of the stiff ECM and rigid bones, then this postulate is disproven.
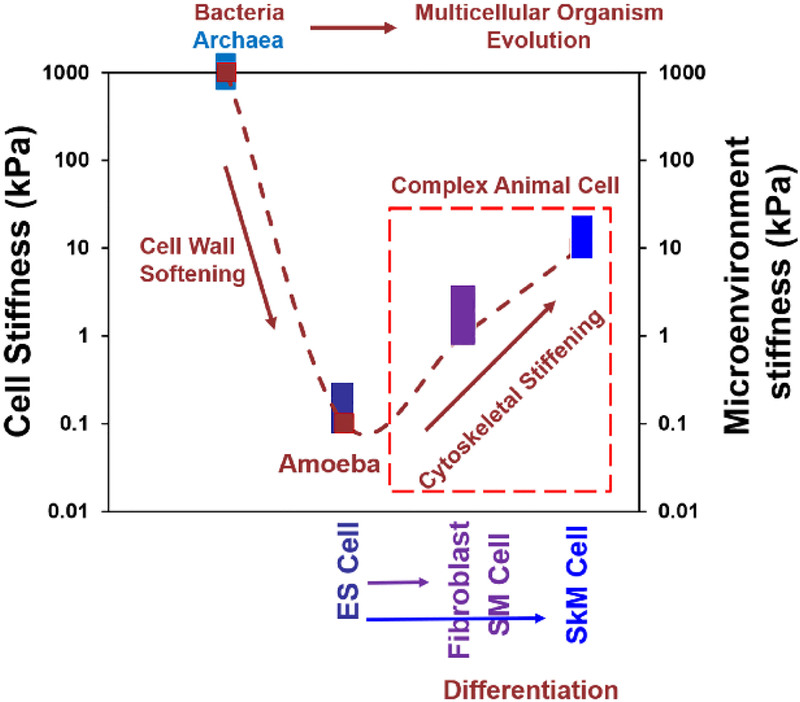
It has been reported that matching cell stiffness with that of its substrate is critical in forming striation in skeletal muscle cells [17] and in optimizing cardiomyocyte beating [47]. However, stiffness matching may not be limited to skeletal muscle cells and cardiomyocytes. The adaptability of the cell stiffness to the stiffness of the ECM could be a critical feature for a multicellular life to evolve. Embryonic cells and fibroblasts adapt and change their stiffness to match their mechanical microenvironments [48–53]. For a complex multicellular land animal to evolve, it must adapt its different tissue cells’ stiffness to their local micromechanical environment. If the earliest unicellular eukaryotes had a cell stiffness of ~0.1 kPa, their stiffness might match that of nutrient-rich uncompacted soft ocean sediments deposited ~2 billion years ago. These cells would be favored to evolve because they would be better able to perform mechanical functions optimally when material properties of the cell match those of very soft paste-like microenvironments. Evidence suggests that archaea are the ancestor of eukaryotic cells [54–56]. High stiffness of archaea (~1000 kPa) that is similar to that of bacteria endows their ability to cope with the challenges of changing environmental mechanical stresses and osmotic pressures. It appears that soft embryonic stem cells become stiffer as they differentiate into tissue cells of the complex multicellular organisms to match their microenvironment stiffness. We perhaps see in the differentiation of embryonic stem cells (derived from inner cell mass cells of the blastocyst) the echo of those early evolutionary events of complex multicellular animals (Fig. 1).
Fig. 1.
Cytoskeletal stiffening during differentiation might echo evolution events from simple eukaryotes to complex animal cells. When an embryonic stem (ES) cell (derived from inner cell mass cells of the blastocyst) differentiates into germ layer cells and then into fibroblasts, smooth muscle (SM) cells, or skeletal muscle (SKM) cells, the cell stiffness increases by ~2 orders of magnitude: from ~0.1–0.5 kPa to 12 kPa [13,16,17]. Stiffness of an amoeba is ~0.1 kPa [11], which is ~4 orders of magnitude lower than that of a bacterium [9] or that of an archaeon [10]. The microenvironmental stiffness of ES cells is 0.1–1 kPa [37,48]; that of vascular smooth muscle cells is ~20 kPa [49]; that of skeletal muscle cells is 20–50 kPa [17,47]; that of fibroblasts is 1–10 kPa [32,51–53]; that of amoebae is probably ~0.1 kPa; those of bacteria and archaea are assumed to vary from low (0.01 kPa) to high (thousands of kPa). We hypothesize that differentiation of embryonic stem cells (days to weeks) echoes those early evolutionary events (millions of years). We propose a model that cytoskeletal stiffening is necessary for evolution from early simple eukaryotes to complex multicellular land animal cells. This working model for evolution and survival of multicellular land animals in mechanically stressed environments complements the models of genome evolution [7] and cadherin evolution [8].
The evolution of the genome and of the cadherins has been proposed as some of the necessary conditions for a multicellular animal to evolve [7,8]. In this report, we propose, at the functional level, a necessary condition of cytoskeletal stiffening for the evolution of the complex land animals. The postulate of the cytoskeletal stiffening evolution complements that of the genome evolution and of the cadherin evolution. If all the cells in a multicellular land animal organism were still as soft as those primitive organisms like amoebae, there probably would be no emergence of complex multicellular animals that are able to exhibit various forms and patterns, and to generate and sustain large mechanical stresses. The evolution of the cytoskeleton based on the strategy of crosslinking/bundling and tension-dependent prestress stiffening might be a key determinant whether a present-day animal can evolve. These are likely achieved by the selective pressures of evolution. If these were not the case, we might have seen a planet that was still filled with rigid-walled bacteria, archaea, and soft simple organisms like amoebae.
Acknowledgments
The authors thank former and current lab members for their experimental findings that have contributed to the formation of the current postulate. This work was supported by the National Institutes of Health (Grant GM072744) and funds from Huazhong University of Science and Technology. Ning Wang acknowledges the support from the Hoeft Endowed Professorship in Engineering at University of Illinois at Urbana-Champaign.
References
- 1.Pace NR: Mapping the Tree of Life: Progress and Prospects. Microbiol. Mol. Biol. Rev 73, 565–576 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavalier-Smith T: Cell evolution and Earth history: stasis and revolution. Philos Trans R Soc Lond B Biol Sci. 361, 969–1006 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalier-Smith T: Predation and eukaryote cell origins: A coevolutionary perspective. Int. J. Biochem. Cell. Biol 41, 307–322 (2009) [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen B, Fletcher IR, Brocks JJ, et al. : Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101–1104 (2008) [DOI] [PubMed] [Google Scholar]
- 5.Cox CJ, Foster PG, Hirt RP, et al. : The archaebacterial origin of eukaryotes. Proc. Natl. Acad. Sci. U.S.A 105, 20356–20361 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidov Y, Jurkevitch E: Predation between prokaryotes and the origin of eukaryotes. Bioessays 31, 748–757 (2009) [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Trillo I, Burger G, Holland PWH, et al. : The origins of multicellularity: a multi taxon genome initiative. Trends Genet. 23, 113–118 (2007) [DOI] [PubMed] [Google Scholar]
- 8.Abedin M, King N: The premetazoan ancestry of cadherins. Science. 319, 946–948(2008) [DOI] [PubMed] [Google Scholar]
- 9.Francius G, Domenech O, Mingeot-Leclercq MP, et al. : Direct Observation of Staphylococcus aureus Cell Wall Digestion by Lysostaphin. J. Bacteriol 190, 7904–7909(2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt H: Mechanism of osmoprotection by archaeal S-layers: A theoretical study. J. Struct. Biol 160, 190–199 (2007) [DOI] [PubMed] [Google Scholar]
- 11.Reichl EM, Ren YX, Morphew MK, et al. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr. Biol 18, 471–480 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waugh R, Evans EA: Thermoelasticity of Red Blood-Cell Membrane. Biophys. J 26, 115–131 (1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury F, Na S, Li D, et al. : Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater 9, 82–88 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Discher DE, Janmey P, Wang YL: Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005) [DOI] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, et al. : Matrix elasticity directs stem cell lineage specification. Cell. 126, 677–689 (2006) [DOI] [PubMed] [Google Scholar]
- 16.Janmey PA, McCulloch CA: Cell mechanics: Integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng 9, 1–34 (2007) [DOI] [PubMed] [Google Scholar]
- 17.Engler AJ, Griffin MA, Sen S, et al. : Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol 166, 877–887 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meshel AS, Wei Q: Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat. Cell Biol 7,157–164 (2005) [DOI] [PubMed] [Google Scholar]
- 19.Darling EM, Topel M, Zauscher S, et al. : Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech 41, 454–464 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Butler JP, Ingber DE: Mechanotransduction across the Cell-Surface and through the Cytoskeleton. Science, 260, 1124–1127 (1993) [DOI] [PubMed] [Google Scholar]
- 21.Roca-Cusachs P, Gauthier NC, del Rio A, et al. : Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. U.S.A 106, 16245–16250 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.le Duc Q, Shi Q, Blonk I, et al. :. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol 189, 1107–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Na S, Chowdhury F, Tay B, et al. : Plectin contributes to mechanical properties of living cells. Am. J. Physiol-Cell. Physiol 96, C868–C877 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasza KE, Nakamura F, Hu S, et al. : Filamin A Is Essential for Active Cell Stiffening but not Passive Stiffening under External Force. Biophys. J 96, 4326–4335(2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson RF, Langford GM: Myosin superfamily evolutionary history. Anat Rec 268, 276–289 (2002) [DOI] [PubMed] [Google Scholar]
- 26.Wang N, Naruse K, Stamenovic D, et al. : Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. U.S.A 98, 7765–7770 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N, Tolic-Norrelykke IM, Chen JX, et al. : Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol-Cell. Physiol 282 C606–C616 (2002) [DOI] [PubMed] [Google Scholar]
- 28.Gardel ML, Nakamura F, Hartwig JH, Crocker JC, et al. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc. Natl. Acad. Sci. U.S.A 103, 1762–1767 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajerowski JD, Dahl KN, Zhong FL, et al. : Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. U.S.A 104, 15619–15624 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith L, Cho S, Discher DE: Mechanosensing of matrix by stem cells: From matrix heterogeneity, contractility, and the nucleus in pore-migration to cardiogenesis and muscle stem cells in vivo. Semin. Cell Dev. Biol 71, 84–98 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby TJ, Lammerding J: Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol 20, 373–381 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swift J, Ivanovska IL, Buxboim A, et al. : Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 341, 2140104 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho CY, Jaalouk DE, Vartiainen MK, et al. : Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 497, 507–511(2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang N, Tytell JD, Ingber DE: Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell. Biol 10, 75–82(2009) [DOI] [PubMed] [Google Scholar]
- 35.Tajik A, Zhang YJ, Wei FX, et al. : Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater 15, 1287–1296 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniotis AJ, Chen CS, Ingber DE: Demonstration of mechanical connections between integrins cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U.S.A 94, 849–854 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majkut S, Idema T, Swift J, et al. : Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol 2, 2434–9 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poh YC, Chen JW, Hong Y, et al. : Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun 5, 4000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JP,Borde BH, Bordeleau F, et al. : Clinical doses of radiation reduce collagen matrix stiffness. APL Bioengineering 2, 031901 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo M, Pegoraro AF, Mao A, et al. : Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci U S A. 114, E8618–E8627 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentine MT, Perlman ZE, Mitchison TJ:Weitz DA. Mechanical properties of Xenopus egg cytoplasmic extracts. Biophys J. 88, 680–9 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingber DE: Mechanical control of tissue growth: Function follows form. Proc. Natl. Acad. Sci. U.S.A 102,11571–11572 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan R, Park CY, Lin YC, et al. : Reinforcement versus Fluidization in Cytoskeletal Mechanoresponsiveness. PLoS One. 4, e5486 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Niu G, Dong NX, et al. : Osteoporosis affects both post-yield microdamage accumulation and plasticity degradation in vertebra of ovariectomized rats. Acta Mechanica Sinica 33, 267–273 (2017) [Google Scholar]
- 45.Tan Y, Tajik A, Chen J, et al. : Matrix softness regulates plasticity of tumour repopulating cells via H3K9 demethylation and Sox2expression. Nat Commun. 5, 4619(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Zhou W, Jia Q, et al. : Efficient extravasation of tumor-repopulating cells depends on cell deformability. Sci. Rep 6, 19304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engler AJ, Carag-Krieger C, Johnson CP, et al. : Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci 121, 3794–3802 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolahi KS, Donjacour A, Liu X, et al. : Effect of substrate stiffness on early mouse embryo development. PLoS One 7, e41717 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sehgel NL, Vatner SF, Meininger GA: “Smooth Muscle Cell Stiffness Syndrome”—Revisiting the Structural Basis of Arterial Stiffness. Front. Physiol 6, 335(2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng T, Yue M, Aslam MN, et al. : Neuronal Protein 3.1 deficiency leads to reduced cutaneous scar collagen deposition and tensile strength due to impaired transforming growth factor-β! to -β3 translation. Am. J. Pathol 187, 292–303. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solon J, Levental I, Sengupta K, et al. : Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. Biophys. J 93, 4453 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marquez JP, Genin GM, Zahalak GI, et al. : The relationship between cell and tissue strain in three-dimensional bio-artificial tissues. Biophys. J 88, 778–89. (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marquez JP, Elson EL, Genin GM: Whole cell mechanics of contractile fibroblasts: relations between effective cellular and extracellular matrix moduli. Philos. Trans. A. Math. Phys. Eng. Sci 368, 635–54. (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woese CR, Kandler O, Wheelis ML: Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. U.S.A 87, 4576–4579 (1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woese CR: The universal ancestor. Proc. Natl. Acad. Sci. U.S.A 95, 6854 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eme L, Spang A, Lombard J, et al. : Archaea and the origin of eukaryotes. Nat. Rev. Microbiol 15, 711–723 (2017) [DOI] [PubMed] [Google Scholar]