Abstract
Introduction:
Despite advances in treatment, head and neck squamous cell carcinoma (HNSCC) survival rates remain stagnant. Current treatment is associated with significant toxicities and includes chemotherapy, radiation, surgery and few targeted treatments. Targeted treatments, epidermal growth factor receptor (EGFR)-targeted agent, cetuximab, and immune checkpoint inhibitors, pembrolizumab and nivolumab, show improved toxicity profiles and modestly improved survival in select patients. An urgent need remains to identify novel targeted treatments for single-agent or combined therapy use.
Areas covered:
Multitargeted kinase inhibitors are small molecule inhibitors with limited toxicity. This review will focus on early-stage investigations of multitargeted tyrosine kinase inhibitors (m-TKIs) (those that target at least two tyrosine kinases) for HNSCC. Preclinical and early trials investigating m-TKIs for various disease settings of HNSCC will be evaluated for efficacy, identification of significant biomarkers and potential for combination therapy.
Expert opinion:
Few single agent m-TKIs have demonstrated efficacy in unselected HNSCC populations. The most promising clinical results have been obtained when m-TKIs are tested in combination with other therapies, including immunotherapy, or in mutation-defined subgroups of patients. The future success of m-TKIs will rely on identification, in preclinical models and clinical trials, of predictive biomarkers of response and mechanisms of innate and acquired resistance.
Keywords: head and neck cancer, immunotherapy, multitargeted kinase inhibitors, squamous cell carcinoma, tyrosine-kinase inhibitors
1. Background
Head and neck squamous cell carcinoma (HNSCC) encompasses a broad array of neoplasms originating from the oral cavity, pharynx and larynx. The majority, > 90%, of HNSCC originate in the squamous epithelium of the upper aerodigestive tract [1]. Head and neck squamous cell carcinoma is the sixth most common cancer by incidence and accounts for 5.0% of worldwide cancers. Further, the incidence of this cancer is increasing in developed countries with 63,000 new cases reported annually in the US [2].
HNSCC is associated with various environmental and lifestyle risk factors such as tobacco and alcohol use, as well as more recently, human papilloma virus (HPV)-linked oropharyngeal cancers [3]. Most patients with HNSCC present with locally advanced, stage III or IV disease. Standard treatment for early stage HNSCC is radiation or surgery. Therapy for advanced staged disease generally includes a combination of surgery, radiation and chemotherapy [4]. Considerable treatment-related morbidities are associated with this multi-modal approach, and 5-year survival rates are approximately 60% [5].
There has been a major effort in the past few decades to develop more targeted therapies for HNSCC treatment, with a total of three agents being approved since 2006 (the EGFR monoclonal antibody cetuximab, and the PD-1 immune checkpoint inhibitors pembrolizumab and nivolumab). Activation of numerous signaling pathways has been implicated in HNSCC cell survival, proliferation, angiogenesis, and/or inflammation, and various molecules in these pathways have emerged as potential drug targets. Molecular targeting agents currently under clinical investigation in head and neck cancer include novel EGFR monoclonal antibodies, HER3 (human EGFR receptor 3) monoclonal antibodies, serine/threonine-specific protein kinase inhibitors, cyclin-dependent kinase inhibitors and tyrosine kinase inhibitors (TKIs) [6].
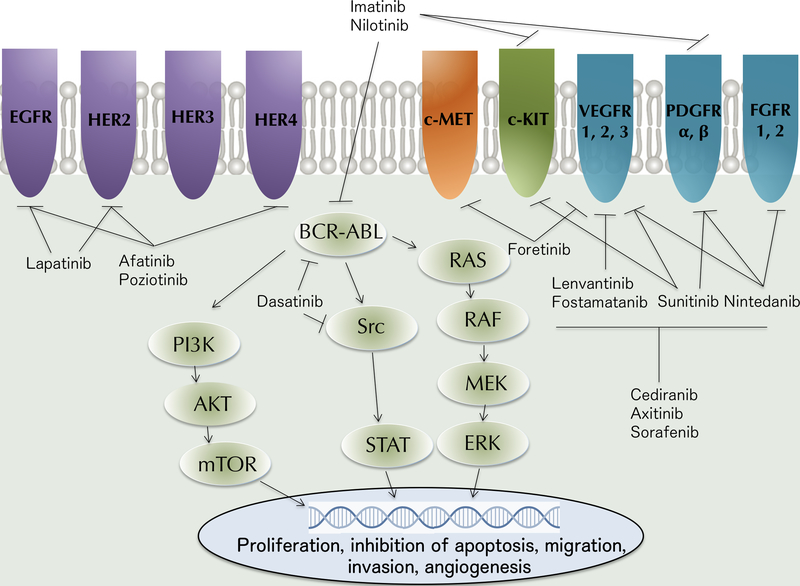
TKIs refer to small molecule drugs that target a variety of tyrosine kinase receptors, such as EGFR, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), vascular endothelial growth factor receptor (VEGFR), c-KIT, RET and c-MET, whose downstream pathways are collectively implicated in tumorigenesis [7]. Additional targets include non-receptor tyrosine kinases such as BCR-ABL and SRC, as well as serine-threonine kinases such as RAF/MEK (Figure 1). Identification of diverse mutational profiles in HNSCCs as well as intrinsic and acquired resistance to current treatments has led to an increased interest in investigation of TKIs that target more than one tyrosine kinase, known as multi-target tyrosine kinase inhibitors (m-TKIs). For the purpose of this review, tyrosine kinase inhibitors with significant pharmacokinetic activity against at least two tyrosine kinases, currently in early stages of development were included. Drugs targeting other cellular kinases or drugs in development for solid tumors other than HNSCC were excluded.
Figure 1: Emerging investigated multi-targeted kinase inhibitors in head and neck squamous cell carcinoma.
Multi-target kinase inhibitors (m-TKIs) currently being tested in clinical trials target various molecular pathways. m-TKIs compete with ATP for binding to receptor tyrosine kinases to inhibit downstream signaling. This pictorial representation of common tumorigenic molecular pathways shows downstream signaling of epidermal growth factor receptor (EGFR) and HER family receptors, which activates the RAS/RAF/MEK/ERK pathways and PI3K/AKT/mTOR pathways, supporting tumor cell proliferation and survival. EGFR/HER kinase inhibitors include lapatinib, afatinib and poziotinib. Briefly, VEGFR, PDGFR, FGFR, MET, KIT and BCR-ABL activation also leads to downstream activation of a variety of molecular pathways including RAS/RAF/MEK/ERK, STAT and PI3K/AKT pathways resulting in proliferation, survival and, in some cases, angiogenesis. These pathways are inhibited in various combinations by several m-TKIs.
AKT: v-AKT Murine thymoma viral oncogene; BCR-ABL: Breakpoint cluster region protein-Abelson murine leukemia viral oncogene homolog 1; ERK: Extracellular signal-regulated kinase; FGFR: Fibroblast growth factor receptor; HER: Human EGFR; KIT: Also called c-KIT tyrosine kinase; c-MET: Also called c-MET tyrosine kinase or HGF receptor; MEK: MAPK/ERK kinase; mTOR: Mammalian target of rapamycin; PDGFR: Platelet derived growth factor receptor; PI3K: Phosphatidylinositol-3-kinase; RAF: Rapidly accelerated fibrosarcoma kinase; RAS: Rat sarcoma protein; c-SRC: Sarcoma-family kinase; STAT: Signal transducer and activator of transcription; VEGFR: Vascular endothelial growth factor receptor.
This review will focus on current developments in clinical investigation of multi-targeted kinase inhibitors for the treatment of head and neck cancer, with a focus on preclinical and clinical phase I and II trials that have been completed or are currently active/recruiting.
2. Existing treatments
2.1. Conventional treatment
Agents currently approved by the US Food and Drug Administration (FDA) for the treatment of head and neck cancer include conventional chemotherapy drugs including cisplatin, methotrexate, 5-flurouracil [5-FU], bleomycin and docetaxel, as well as a small number of targeted molecular therapies. Current standard of care for patients with locally advanced (LA) HNSCC is concomitant platinum-based chemoradiotherapy (CRT) or surgery followed by adjuvant radiation or chemoradiation. For patients with recurrent and/or metastatic (R/M) HNSCC, platinum-based chemotherapy plus 5-FU has a response rate (RR) of 30–40% and median survival of 6–9 months [8]. However, these cytotoxic chemotherapy drugs are non-specific and are accompanied by treatment-associated toxicity. Patients with platinum-resistant disease have few options and very poor prognosis with second-line therapies [9].
2.2. Approved targeting treatments in head and neck cancer
There are few FDA approved molecular targeting agents available to treat HNSCC. They include only cetuximab, an epidermal growth factor receptor (EGFR) inhibitor [10], and, more recently, nivolumab and pembrolizumab, inhibitors of PD-1 [11,12]. Cetuximab, a monoclonal antibody, was approved by the FDA in 2006 and was the first newly approved drug for use in HNSCC in decades. Cetuximab is approved in combination with radiation for LA disease, in combination with platinum-based chemotherapy and 5-FU for first-line treatment of R/M HNSCC and as a monotherapy for R/M disease after failed platinum-based chemotherapy [10, 13,14]. Nivolumab, a monoclonal antibody that inhibits the interaction of the immune checkpoint receptor PD-1 with its ligands PD-L1 and PD-L2, has been approved as a single-agent in recurrent HNSCC following failure of platinum-based chemotherapy [11]. Similarly, pembrolizumab, a monoclonal antibody with the same target as nivolumab, has been approved as a monotherapy in R/M HNSCC following failure of platinum-based chemotherapy [12, 15].
2.3. Tyrosine kinase inhibitors
Tyrosine kinases are a subset of protein kinases. Receptor tyrosine kinases contain an extracellular ligand-binding domain and cytoplasmic tyrosine kinase, signal-generating region. Upon binding of growth factors and other ligands, receptor tyrosine kinases undergo receptor dimerization, leading to transfer a phosphate group from ATP to an intracellular protein and downstream signaling [16]. Tyrosine kinase inhibitors are small, orally available molecules which are able to pass through the cell membrane and compete with the ATP binding site of various onocogenic tyrosine kinases [17]. Many tyrosine kinase inhibitors have affinity for more than one tyrosine kinase binding site [18–20].
Tyrosine kinase inhibitors have become one of the most important classes of targeting agents in cancer treatment. Over the past 3 decades, the FDA has approved a total of 42 kinase inhibitors for use in the treatment of various malignancies [21]. Unlike conventional therapies which do not discriminate between rapidly dividing cells and cancer cells, TKIs have a higher selectivity against tumor cells, thereby minimizing toxicities. Most TKIs are active against multiple targets at clinical doses; however, a small subset is specific for a single target such as erlotinib and gefitinib, which solely inhibit EGFR [22].
Given the critical biological functions that kinases perform in the cell and the frequency of kinase mutations in cancers, TKIs have become an intensively investigated group of drugs. In numerous cancers TKIs have been shown to stabilize tumor progression, have minimal side effects compared to cytotoxic CRT and exhibit synergistic effects in vitro when combined with radiation and chemotherapy [23, 24]. Furthermore, TKIs are low molecular weight compounds that can be given orally and are well absorbed across the gastrointestinal tract [25].
TKIs have been approved by the FDA for the treatment of various hematologic and lymphoid malignancies such as ALL, AML, CLL, CML, mantle cell lymphoma, marginal zone lymphoma and polycythemia vera. They have also been approved for treatment of various solid malignancies such as breast, differentiated hepatocellular, thyroid, pancreatic and colorectal cancer, NSCLC, melanoma, renal cell carcinoma and soft tissue sarcomas. Many of these FDA approved TKIs are being studied in HNSCC (Table 1). To date, no TKIs, single or multi-targeted, have been approved for use in the treatment of head and neck cancer; however, there are a number of currently active phase I and II clinical trials testing m-TKIs for HNSCC in various treatment settings (Table 2).
Table 1:
Competitive environment table of the major tyrosine kinase inhibitors currently under development for HNSCC treatment.
| Drug | Target(s) | Developmental stage in HNSCC | FDA approved treatment | |
|---|---|---|---|---|
| EGFR targeting | Erlotinib* | EGFR (HER 1) | Phase III | EGFR-mutated NSCLC |
| Gefitinib* | EGFR (HER 1) | Phase III | EGFR-mutated NSCLC | |
| Lapatinib | EGFR and HER2 | Phase III | HER2-positve breast cancer | |
| Afatinib | EGFR, HER2 and HER4 | Phase III | EGFR-mutated NSCLC | |
| Poziotinib | EGFR, HER2 and HER4 | Phase II | NSCLC w/ S768 mutations | |
| Neratinib | EGFR, HER2 and HER4 | Preclinical | HER2-positive breast cancer | |
| Vandetanib | EGFR, VEGFR, and RET | Phase II | Medullary thyroid cancer | |
| VEGFR targeting | Sunitinib | VEGFR 1–3, PDGFR ⍺/β, FLT-3, c-KIT, RET and BRAF | Phase II | RCC |
| Sorafenib | VEGFR 1–3, PDGFRβ, FLT-3, c-KIT, RET and BRAF | Phase II | HCC, RCC, thyroid cancer | |
| Cediranib | VEGFR 1–3, PDGFR ⍺/β and c-KIT | Phase II | None | |
| Pazopanib | VEGFR 1–3, PDGFR ⍺/β and c-KIT | Phase II | RCC | |
| Nintedanib | VEGFR1–3, PDGFR and FGFR1–2 | Phase II | Idiopathic pulmonary fibrosis, NSCLC | |
| Axitinib | VEGFR 1–3, PDGFRβ and c-KIT | Phase II | RCC | |
| Lenvatinib | VEGFR 1–3, FGFR 1–4, PDGFR ⍺, c-KIT and RET | Phase I | HCC, thyroid cancer | |
| Motesanib | VEGFR 1–3 and c-KIT | Preclinical | None | |
| Fostamatinib | VEGFR, SYK, FLT3, aurora A kinase and RET | Phase II | Chronic immune thrombocytopenia | |
| Foretinib | VEGFR2 and c-MET | Phase II | None | |
| Bcr-Abl targeting | Imatinib | BCR-ABL, DDR, c-KIT, PDGFR and CSF-1R | Phase II | CML, Ph+ ALL, GIST and MDS |
| Dasatinib | BCR-ABL and SRC family | Phase II | CML and Ph+ ALL | |
| Nilotinib | BCR-ABL, PDGFRα and c-KIT | Phase I | Ph+ CML | |
| Ponatinib | BCR-ABL, VEGFR, PDGFR | Phase II | Ph+ ALL, CML |
: denotes single-target tyrosine kinase inhibitor; BCR-ABL: Breakpoint cluster region protein-Abelson murine leukemia viral oncogene homolog 1, BRAF: B-rapidly accelerated fibrosarcoma kinase, CML: Chronic myeloid leukemia, CSF-1R: Colony stimulating factor 1 receptor, DDR: Discoidin domain receptor, EGFR: Epidermal growth factor receptor, FGFR: Fibroblast growth factor receptor, FLT3: FMS-like tyrosine kinase 3, GIST: Gastrointestinal stromal tumors, HCC: Hepatocellular carcinoma, HER: Human epidermal growth factor receptor, KIT: Also called c-KIT tyrosine kinase, MDS: Myelodysplatic syndrome, NSCLC: Non-small cell lung cancer, PDGFR: Platelet derived growth factor receptor, Ph+ ALL: Philadelphia chromosome-positive acute lymphoblastic leukemia, Ph+ CML: Philadelphia chromosome-positive chronic myelogenous leukemia, RCC: Renal cell carcinoma, RET: Rearranged during transfection, SRC: Sarcoma-family kinase, SYK: Spleen tyrosine kinase, VEGFR: Vascular endothelial growth factor receptor
Table 2:
Multi-targeted tyrosine kinase inhibitor active phase I/II clinical trials for HNSCC treatment.
| Drug | Target(s) | Setting | Developmental stage in HNSCC |
Status | Clinical trial ID |
|
|---|---|---|---|---|---|---|
| EGFR Targeting | Lapatinib | EGFR and HER2 | Capecitabine | Phase II | Active, not recruiting | NCT01044433 |
| Radiation and cisplatin | Phase II | Active, not recruiting | NCT01711658 | |||
| Carboplatin and paclitaxel | Phase II | Active, not recruiting | NCT01612351 | |||
| Afatinib | EGFR, HER2 and HER4 | Radiation or chemotherapy and radiation | Phase I | Recruiting | NCT01783587 | |
| Monotherapy* | Phase II | Recruiting | NCT03088059 | |||
| Cetuximab | Phase II | Recruiting | NCT02979977 | |||
| Poziotinib | EGFR, HER2 and HER4 | Monotherapy* | Phase II | Recruiting | NCT03292250 | |
| Vandetanib | EGFR, VEGFR, and RET | Monotherapy | Phase II | Active, not recruiting | NCT01414426 | |
| VEGFR Targeting | Sorafenib | VEGFR 1–3, PDGFRβ, FLT-3, c-KIT, RET and BRAF | Carboplatin and paclitaxel | Phase II | Active, not recruiting | NCT00494182 |
| Nintedanib | VEGFR1–3, PDGFR and FGFR1–2 | Monotherapy* | Phase II | Recruiting | NCT03292250 | |
| Lenvatinib | VEGFR 1–3, FGFR 1–4, PDGFRα, c-KIT and RET | Cetuximab | Phase I/Ib | Recruiting | NCT03524326 | |
| Pembrolizumab | Phase I/Ib | Active, not recruiting | NCT03006887 | |||
| Axitinib | VEGFR1–3, PDGFRβ and c-KIT | Monotherapy | Phase II** | Recruiting | NCT02762513 | |
| Bcr-Abl targeting | Nilotinib | BCR-ABL, PDGFRα and c-KIT | Cetuximab | Phase I | Recruiting | NCT01871311 |
: Patient criteria for biomarkers included in study;
: Expansion Trial
BCR-ABL: Breakpoint cluster region protein-Abelson murine leukemia viral oncogene homolog 1, BRAF: B-rapidly accelerated fibrosarcoma kinase, EGFR: Epidermal growth factor receptor, FGFR: Fibroblast growth factor receptor, FLT3: FMS-like tyrosine kinase 3, HER: Human epidermal growth factor receptor, KIT: Also called c-KIT tyrosine kinase, PDGFR: Platelet derived growth factor receptor, RET: Rearranged during transfection, VEGFR: Vascular endothelial growth factor receptor
3. Scientific rationale
3.1. Current challenges in therapy
Despite advances in multimodal HNSCC treatments, including targeted therapies, survival rates have remained unchanged. One of the major challenges in HNSCC treatment and a key contributor to the stagnation of survival rates is drug resistance. Intrinsic resistance occurs when cancer cells are inherently insensitive to a treatment, while acquired resistance occurs when treated cancer cells become insensitive following treatment. While platinum-based chemotherapy represents first-line treatment for HNSCC, chemoresistance greatly limits the effectiveness of these and other therapies [26]. With respect to cetuximab therapy, EGFR is upregulated in early stages of HNSCC pathogenesis, and approximately 90% of HNSCC tumors have widespread EGFR expression [27, 28]. However, despite ubiquitous EGFR expression, the objective response (OR) to cetuximab treatment in R/M HNSCC patients with platinum-resistant disease is only 13% [13]. The effective treatment of only a subset of patients with cetuximab has largely been attributed to widespread resistance mechanisms and the paucity of predictive biomarkers to guide therapy. In addition, an interim analysis of a phase III trial investigating side effects and long-term quality of life in HPV+ oropharyngeal cancer patients found that cetuximab and radiation was associated with decreased overall and progression-free survival versus cisplatin and radiation [29].
3.2. Potential role for m-TKIs in HNSCC treatment
As the understanding of the genetic and proteomic landscape of HNSCC increases, there has been an accompanying expansion in the identification of potential new drug targets. Of particular interest are molecules involved in intrinsic and acquired resistance, especially in the context of cetuximab treatment [30]. Various groups have shown that upregulation and activation of multiple receptor tyrosine kinases (RTK) results in resistance to cetuximab in HNSCC [28–33]. Increased activation of multiple tyrosine kinases enables tumor cells to proliferate in the face of selective pressure from a targeted drug treatment such as cetuximab. Examples of RTKs that have been implicated in resistance pathways include HER2, HER3, VEGF and MET. Targeting the activity of one or more of these RTKs leads to improved anti-tumor effects of cetuximab-containing regimens [34–37]. Because of their broad activity profiles and action against various key tyrosine kinases, m-TKIs have the potential to overcome these resistance pathways and enhance the effects of current treatments.
4. Competitive environment
4.1. Single-target TKIs
There are a several ongoing clinical trials investigating TKI clinical safety and efficacy in HNSCC. Single-target TKIs, such as gefitinib and erlotinib, have not been shown to be clinically effective in the treatment of unselected HNSCC patients. Gefitinib, an EGFR TKI, is approved for use in non-small cell lung cancer (NSCLC) [38]; however, a phase III trial comparing gefitinib versus intravenous (IV) methotrexate showed no significant improvement in overall survival (NCT00206219) [39]. A second phase III trial compared gefitinib with placebo in previously treated advanced oesophageal cancer and also reported no difference in median survival (NCT01243398) [40]. Erlotinib, another single-target EGFR TKI was approved for use in NSCLC and pancreatic cancer. A phase II trial showed that erlotinib in combination with cisplatin and radiotherapy did not result in improved response rate and progression-free survival (PFS) in HNSCC [41], though no further clinical trials have been conducted. To date, EGFR tyrosine kinase inhibition has generally been associated with low response rates compared to standard of care, primarily due to the lack of predictive biomarkers to identify HNSCC patients who are most likely to respond.
4.2. EGFR targeting agents
Agents with activity against multiple EGFR receptors are the most extensively studied group of m-TKIs in HNSCC. Both lapatinib, a reversible EGFR (HER1) and HER2 inhibitor, and afatinib, an irreversible pan-HER inhibitor, have undergone phase III testing in HNSCC. Poziotinib, another irreversible pan-HER inhibitor, is approved for the treatment of non-small cell lung cancer with S768 mutations, and has also reached phase II clinical testing.
Lapatinib has been extensively studied in HNSCC. In 2010, lapatinib was approved for first-line combination treatment of metastatic, HER2-positive breast cancer and continues to be tested in HNSCC clinical trials. Lapatinib has been tested as a substitute for cetuximab since evidence suggests that HNSCC overexpression of HER2 may lead to greater activity of lapatinib versus cetuximab; however, in a phase II trial testing lapatinib for HNSCC, only two patients were HER+ and neither of these patients responded to treatment. [42]. A phase III trial combining lapatinib with chemoradiation in patients with high-risk features after surgical treatment of stage III/IV HNSCC showed no benefit and demonstrated additional toxicity compared to placebo (NCT00424255) [43]. A recent phase II trial tested lapatinib and capecitabine, an oral pro-drug of 5-FU, in R/M HNSCC and met its primary objective of survival comparable to the combination of cisplatin, 5-FU and cetuximab while maintaining a tolerable toxicity profile (NCT01044433) [42]. A second phase II trial conducted by the same group tested induction therapy with lapatinib in combination with carboplatin and paclitaxel prior to transoral surgery, followed by risk-adapted adjuvant therapy. This combination therapy yielded high response rates and excellent long-term outcomes with no patients recurring or dying on study follow-up, and 29 of 39 surgical patients avoiding post-operative radiation [44]. An ongoing randomized, placebo-controlled phase II trial of 142 patients comparing radiation therapy with cisplatin versus radiation therapy with cisplatin and lapatinib in non-HPV LA HNSCC may provide more insights into the use of lapatinib concurrently with radiation (NCT01711658).
Afatinib, an ErbB family inhibitor, is also being actively studied as a potential therapy in HNSCC. Afatinib has demonstrated clinical activity in EGFR-mutated lung cancer, and preclinical data suggests afatinib is more effective than the TKIs lapatinib, erlotinib, and neratinib in HN5 xenografts [45]. There are five currently active phase I/II trials and one active phase III trial testing afatinib in various treatment combinations for HNSCC. Clinical trials involving afatinib have focused on the identification of predictive biomarkers in both treatment and analysis stages (Table 3). A phase II study testing afatinib in the neoadjuvant setting showed only partial response according to the Response Evaluation Criteria in Solid Tumors (RECIST)v1.1; however, this study identified the Cluster3-hypoxia gene signature and TP53 status as potential predictive biomarkers of ErbB family inhibitors [46]. In addition, a phase III trial completed in 2016 identified subgroups of patients who may achieve increased benefit from afatinib based on prespecified tumor biomarkers (p16-negative, EGFR-amplified, HER3-low, PTEN-high). Of 326 sub-group selected patients, median progression free survival was increased in afatinib over methotrexate in patients with p16-negative [2.7 versus 1.6 months; HR 0.70 (95% CI 0.50–0.97)], EGFR-amplified [2.8 versus 1.5 months; HR 0.53 (0.33–0.85)], HER3-low [2.8 versus 1.8 months; HR 0.57 (0.37–0.88)], and PTEN-high [1.6 versus 1.4 months; HR 0.55 (0.29–1.05)] tumors [47]. Based on these findings, an ongoing biomarker-based study (UPSTREAM: NCT03088059) is testing the effects of afatinib versus the standard of care in R/M HNSCC patients who are p16-negative and either cetuximab naïve or who have high PTEN or a HER2 mutation/amplification (NCT03088059). Afatinib has demonstrated comparable efficacy to cetuximab, improved outcomes compared with IV methotrexate in HNSCC refractory to first-line platinum-based therapy, and improved preoperative response according to RECIST1.1 versus placebo [48]. Other ongoing phase I/II trials include testing afatinib versus placebo in untreated, non-metastatic HNSCC (NCT01824823), afatinib in combination with cetuximab for R/M HNSCC previously treated with platinum-based regimen or immune checkpoint inhibitor, with exploratory biomarker analysis of pre- and post-treatment tumor biopsies (NCT02979977), and afatinib with radiation versus chemotherapy and radiation in high risk HNSCC (NCT01783587). Another currently active phase II trial will test various targeted therapies, including afatinib, sunitinib and dasatinib, for patients with advanced, refractory solid tumors, including HNSCC (NCT02465060). Patients with EGFR or HER2 activating mutations will be included in the afatinib treatment group. A phase I/II study testing afatinib in combination with carboplatin and paclitaxel induction chemotherapy was terminated due to low accrual and high toxicity (NCT01732640).
Table 3:
m-TKI completed or active phase I/II clinical trials with genomic biomarker driven patient subgroups for treatment and/or analysis.
| Trial Name | Clinical Trial ID |
Stage | Drug | Treatment setting |
Genomic subgroups |
Results |
|---|---|---|---|---|---|---|
| Trials testing EGFR targeting agents | ||||||
| Neoadjuvant Afatinib Window Study in HNSCC | NCT01538381 | II | Afatinib | Neoadjuvant, previously untreated | No pre-treatment groups | Partial response according to RECISTv1.1, FDG-PET hypoxic gene signature and TP53 status potential activity biomarkers |
| Biomarkers of Activity and Efficacy of BIBW2992 in U/N (PREDICTOR) | NCT01415674 | II | Afatinib | Neoadjuvant, previously untreated | No pre-treatment groups | Improved response according to RECIST1.1 and PERCIST compared to no treatment (no access to biomarker data) |
| Biomarker-based Study in R/M SCCHN (UPSTREAM) | NCT03088059 | II | Afatinib | R/M after platinum therapy versus standard of care | EGFR amplification, PTEN-high, HER2 mutation or amplification | Active: pending |
| Dual Inhibition of EGFR: Afatinib and Cetuximab for Advanced HNSCC | NCT02979977 | II | Afatinib | R/M after platinum therapy or immune checkpoint inhibitor | No pre-treatment groups | Active: pending (exploratory biomarker analysis) |
| LUX-Head & Neck 1 | NCT01345682 | III | Afatinib | R/M after platinum therapy vs methotrexate | p16-negative, EGFR-amplified, HER3-low, PTEN-high | Increased PFS compared to methotrexate in all sub-groups |
| Trials testing BCR-ABL targeting agents | ||||||
| Dasatinib for R/M HNSCC | NCT00507767 | II | Dasatinib | Platinum-refractory R/M HNSCC | No pre-treatment groups | No significant activity reported, cytokine biomarker analysis showed increased MIF in rapidly progressing patients |
| Imatinib Mesylate in Patients With Salivary Gland Cancer | NCT00045669 | II | Imatinib | Unresectable or metastatic salivary gland cancer | salivary gland cancers expressing c-KIT | Terminated: overexpression of WT c-KIT was not sufficient for clinical benefit |
| Trials testing various targeting agents | ||||||
| Targeted Therapy Directed by Genetic Testing in Patients With Advanced Refractory Solid Tumors (MATCH) | NCT02465060 | II | Afatinib | Advanced refractory cancers | EGFR or HER2 activating mutation | Active: pending |
| Dasatinib | DDR2, S768R, I638F, or L239R mutation | Active: pending | ||||
| Sunitinib | c-KIT mutation | Active: pending | ||||
| Translational Biomarker Driven UMbrella Project for Head and Neck (TRIUMPH) | NCT03292250 | II | Nintedanib | R/M after platinum therapy | PDGFR, VEGFR or FGFR mutation | Active: pending |
| Poziotinib | HER mutation | Active: pending | ||||
| Comparison of Biomarker Modulation by Inhibition of EGFR and/or SRC Family | NCT00779389 | I | Dasatinib/Erlotinib | Operable tumors, neoadjuvant treatment versus placebo | No pre-treatment groups | pMAPK baseline expression associated with erlotinib sensitivity (P=0.099), pSTAT3 baseline expression associated with dasatinib resistance (P=0.02), pSTAT3, pSRC, pMET & IL-6 not associated with response |
EGFR: Epidermal growth factor receptor, FDG-PET: Fluorodeoxyglucose-positron emission tomography, FGFR: Fibroblast growth factor receptor, HER: Human EGFR, IL-6: Interleukin 6, KIT: Also called c-KIT tyrosine kinase, R/M: Recurrent/metastatic, MIF: Macrophage migration inhibitory factor, PDGFR: Platelet derived growth factor receptor, PERCIST: Positron Emission Tomography Response evaluation criteria in solid tumors, PFS: Progression free survival, pMET: phosphorylated MET tyrosine kinase, pSRC: Sarcoma-family kinase, pSTAT: phosphorylated Signal transducer and activator of transcription, PTEN: Phosphatase and tensin homolog, RECIST: Response evaluation criteria in solid tumors, SCCHN: squamous cell carcinoma of head and neck, TP53: Tumor protein 53, U/N: Untreated/Non-metastatic, VEGFR: Vascular endothelial growth factor receptor.
4.3. Potential radiosensitizers
Radioresistance in HNSCC cells occurs via a variety of mechanisms, including upregulation of MAPK, PI3K and VEGF, a strong regulator of angiogenesis. Sunitinib, sorafenib and vandetanib are orally available m-TKIs, which are potentially effective in combination with radiotherapy.
Sorafenib is an inhibitor of VEGFR and PDGFR as well as intracellular serine/threonine kinases (Raf-1, B-Raf) [49]. Sorafenib was approved by the FDA in 2017 for use in hepatocellular carcinoma (HCC) after it demonstrated single-agent efficacy in patients with advanced HCC versus placebo [50]. Sorafenib is also a first-line treatment for metastatic renal cell carcinoma and was approved for treatment of radioiodine-resistant metastatic differentiated thyroid cancer (DTC) in 2014. Preclinical data suggests that sorafenib treatment prior to irradiation of HNSCC cell lines increases radiosensitivity by blocking the repair of DNA double-strand breaks and decreasing clonogenic survival [51,52]. An early phase II clinical trial showed tolerability but poor response (less than 20% confirmed RR) of single-agent sorafenib administered to chemotherapy naïve, advanced and metastatic HNSCC patients [53]. An attempt to combine sorafenib with radiation led to a dose escalation trial of neoadjuvant sorafenib and concurrent sorafenib, cisplatin and radiation (NCT00627835). However, this trial was withdrawn after the site decided to not open the study. Another Phase II trial combining sorafenib and cetuximab treatment showed only modest response and no clinical benefit of sorafenib plus cetuximab versus single-agent cetuximab in R/M HNSCC [54]. Despite the low efficacy of sorafenib as a single agent or in combination with cetuximab, a recent phase II clinical trial is testing sorafenib in combination with various chemotherapeutic agents. An active phase II trial is combining sorafenib with carboplatin, a platinum-based agent, and paclitaxel, a taxane, in patients with R/M HNSCC (NCT00494182), with results pending.
Sunitinib.
While no completed study has tested sorafenib with radiotherapy, trials have tested sunitinib (SU11248), a similar m-TKI, in combination with radiotherapy and as a single agent in HNSCC treatment. Sunitinib is a VEGFR, PDGFR, FLT3 and c-KIT inhibitor [55], which, similar to sorafenib, has garnered interest in HNSCC treatment for its anti-angiogenic effects. Sunitinib was shown to attenuate radioresistance of two different HNSCC cell lines in vitro, presumably through blockade of downstream ERK activation [52]. There is significant preclinical evidence that sunitinib induces transient vascular normalization in solid tumors, improving tumor oxygenation, which is known to increase radiotherapy sensitivity [56,57]. An early phase IB trial combining sunitinib with external beam radiation therapy (EBRT) for head and neck cancer, pelvic cancer, nervous system neoplasms and thoracic neoplasms yielded acceptable toxicities and adverse events (NCT00437372). However, a follow-up phase II clinical trial completed in 2010 found sunitinib to show low single-agent activity in R/M HNSCC, necessitating early closure of the study (NCT00387335) [58]. A similar study was terminated due to frequent grade 3–4 toxicities with single-agent sunitinib treatment of R/M HNSCC (NCT00408252). Preclinical data suggests that combining sunitinib and cetuximab treatment in an orthotopic head and neck cancer model leads to an additive decrease in tumor growth, while the addition of radiation completely abolished tumor growth [59]. As a result, a phase I trial was conducted treating LA/R HNSCC patients with a combination of sunitinib, cetuximab and radiation therapy (NCT00906360). This trial was terminated suggesting that the use of sunitinib in combination with radiotherapy is likely limited by toxicities.
Vandetanib.
Another potential radiosensitizer is vandetanib, an EGFR, VEGFR and RET inhibitor FDA approved for medullary thyroid cancer. In preclinical testing, vandetanib with cisplatin radiosensitized human HNSCC cells in vitro and in vivo. A combination of vandetanib, cisplatin and radiation was superior to other treatments in antitumoral effects, prolonged survival and decreased lymph node metastases in orthotopic, nude mice model xenografts [60]. Another in vitro, human tumor xenograft experiment showed that a clinically relevant dose of vandetanib leads to enhanced antitumor effects of radiation by inhibition of both EGFR and VEGFR signaling [61]. A phase I study determined tolerability (only 5 of 30 patients were discontinued due to adverse events) of vandetanib in combination with radiotherapy with or without cisplatin in patients with previously untreated, locally advanced HNSCC [62]. However, no further phase II testing has been attempted. Phase II trials testing vandetanib in other contexts have not shown enough clinical activity to warrant further investigation. A trial testing vandetanib with docetaxel for R/M HNSCC showed only marginal improvement of PFS (NCT00459043) [63], while another trial testing vandetanib with conventional chemotherapy was terminated due to withdrawal of the drug supply (NCT00720083).
4.4. Potent anti-angiogenesis agents
Other anti-angiogenic TKIs, which target VEGFR but have not been shown in preclinical models to be radiosensitizers include lenvatinib, nintedanib and axitinib. Cumulative evidence suggests that concomitant targeting of VEGFR and the EGFR signaling pathway has the potential to circumvent acquired resistance to EGFR inhibitors such as cetuximab [64,65].
Lenvatinib.
A pan-VEGFR inhibitor, lenvatinib, which is approved for treatment of hepatocellular carcinoma, is being tested in two different clinical trials for HNSCC. Based on the potential role of VEGFR signaling in EGFR resistance, a phase I study with 16 participants is being conducted to determine the maximum tolerated dose of lenvatinib when combined with cetuximab (NCT03524326). In addition, cumulative evidence suggests that angiogenesis and immunosuppression occur simultaneously in various solid tumors [66]. Preclinical, in vivo studies in pancreatic, breast and brain cancer mouse models have shown that VEGFR blockade leads to PD-L1 over-expression and anti-PD-L1 therapy sustains response to VEGFR blockade [67]. Lenvatinib is the only VEGFR targeted m-TKI being tested in combination with an immune targeting drug. KEYNOTE-146, is multicenter, open-label basket phase I/Ib trial evaluating lenvatinib (20 mg/d) in combination with PD-1 inhibitor pembrolizumab (200 mg every 3 weeks) in patients with select solid tumors including RCC, endometrial carcinoma, NSCLC, urothelial cancer, melanoma and HNSCC (NCT02501096). Based on preliminary results, the FDA has approved Breakthrough Therapy designation for lenvatinib with pembrolizumab for advanced/metastatic endometrial carcinoma and advanced RCC. For HNSCC, preliminary results showed an objective RR of 40.9% (9 of 22 patients) and a median PFS of 8.2 months [68]. This is an improvement from a response rate of 15–18% for pembrolizumab monotherapy and a response rate of less than 10% for lenvatinib monotherapy [14].
Nintedanib, a VEGFR, FGFR and PDGFR inhibitor, is being studied in patients with salivary gland tumors, which account for <5% of HNSCCs [69]. Nintedanib has been explored as a potent anti-angiogenic drug and was approved for NSCLC as well as a rare lung condition, idiopathic pulmonary fibrosis (Table 1). Preclinical data showed that in various human solid tumor xenografts, nintedanib (50–100 mg/kg) has anti-tumor efficacy, delays or eliminates tumor growth, and reduces vessel density and vessel integrity after 5 days of exposure [70]. An open-label, multicenter phase II trial was conducted in South Korea with 20 R/M salivary gland cancer patients. Nintedanib was found to have an acceptable toxicity profile, and while it did not yield a partial response, it achieved a disease-control rate of 75% and 6-month PFS of 60%, warranting further investigation (NCT02558387) [71]. An ongoing phase II trial, the Translational biomarker Driven Umbrella Project for Head and Neck (TRIUMPH), is being conducted by the same group (NCT03292250). This umbrella trial is centrally screening HNSCC patients following platinum-based chemotherapy and assigning patients to a molecularly defined sub-trial with matched target agents. Following next-generation sequencing (NGS), patients are assigned to an EGFR/HER2 inhibitor – poziotinib, FGFR inhibitor – nintedanib, PI3K inhibitor – BYL719, cell cycle (CDK4/6) inhibitor – abemaciclib, or anti PD1/PD-L1 – durvalumab +/ tremelimumab group. A total of 259 patients are enrolled, with an estimated study completion date of 2020.
Axitinib.
Axitinib is a VEGFR1–3, PDGFRβ and c-KIT inhibitor which has been approved for the treatment of renal cell carcinoma. It is a highly selective and potent inhibitor of VEGFR’s, especially when compared to other anti-angiogenic m-TKIs such as sunitinib and sorafenib which have a broader spectrum of targets [72]. A phase II trial in patients with heavily pre-treated R/M HNSCC showed that single-agent axitinib is well-tolerated with no severe bleeding events, although only 19 patients achieved full planned dose since 73% of patients discontinued due to disease progression [73]. The overall response rate was 6.7% (two partial responses) with a median PFS of 3.7 months and median overall survival of 10.2 months. Analysis of cytokines in treated patients revealed that a persistent increase in IL-8 was seen in all patients with no response to axitinib, suggesting a possible immune-mediated resistance. Given the favorable median survival versus conventional chemotherapy, an expansion trial of this study is currently ongoing (NCT02762513).
4.5. BCR-ABL targeting agents
The m-TKIs imatinib, dasatinib, nilotinib and ponatinib target the BCR-ABL (breakpoint cluster region-Abelson murine leukemia viral oncogene homolog 1) protein, along with other tyrosine kinases. The t(9;22) translocation, known as the Philadelphia chromosome, encodes an unregulated tyrosine kinase, the BCR-ABL oncogene, that promotes cell proliferation and apoptosis avoidance through downstream activation of the RAS/MAPK, PI3K/AKT and JAK/STAT. This BCR-ABL translocation is observed in 95% of patients with chronic myelogenous leukemia (CML) [74]. Imatinib, dasatinib, nilotinib and ponatinib have been approved for CML and other leukemias and are currently being investigated for HNSCC (Table I).
Imatinib.
While the clinical efficacy of imatinib for CML depends on its activity against BCR-ABL, its primary target in some HNSCCs is c-KIT, a commonly over-expressed RTK in specific head and neck neoplasms such as adenoid cystic carcinoma (ACC) [75]. Evidence of c-KIT overexpression in human ACC tumor specimens led to a phase II clinical trial testing single-agent imatinib for c-KIT-expressing ACC tumors (Table 3). Of 15 assessed patients, no objective responses were observed, resulting in termination of the study, and no additional evaluation was warranted (NCT00045669) [76]. Imatinib’s demonstrated inefficacy for ACC is consistent with more recent literature suggesting that the frequency of c-KIT mutations in ACC is significantly lower than previously reported [77].
Nilotinib, an improved first-line therapy for CML, has similar targets as imatinib including BCR-ABL, PDGFR and c-KIT. However, nilotinib demonstrates a 10 to 30-fold increased potency against BCR-ABL tyrosine kinase activity compared to imatinib [78,79]. In preclinical, in vitro studies, nilotinib has been shown to reduce EGFR expression in HPV-negative HNSCC cells [80]. Therefore, an ongoing phase I trial with 22 currently enrolled patients is testing a 28-day cycle of nilotinib with cetuximab for R/M HNSCC (NCT01871311). In addition, a recent preclinical study testing the effect of nilotinib on SRC and c-KIT expression in HPV-positive and HPV-negative HNSCC in vitro found that nilotinib significantly reduced c-KIT expression, while increasing SRC expression in HPV-positive cells. By contrast, nilotinib treatment did not lead to significant changes in SRC expression in HPV-negative cells [81]. These results suggest a potential for nilotinib to be used for HPV-positive tumors, with the caveat of SRC overexpression.
Dasatinib.
Dasatinib is a BCR-ABL and SRC-family tyrosine kinase inhibitor. SRC-family kinases play a central role in oncogenic signaling pathways, making SRCs a potential drug target regardless of SRC mutation status. One member of the SRC-family of kinases, c-SRC, mediates EGFR signals and can act as an upstream EGFR activator, promoting tumor survival and growth [82]. A recent review suggested that based on current understanding of these pathways, there is significant evidence for combining EGFR and SRC inhibition [83]. A phase II clinical trial combining anti-EGFR drug, cetuximab, and dasatinib for R/M HNSCC was terminated due to the principal investigator leaving the institution (NCT01488318). A phase I trial testing dasatinib, cetuximab and radiation with or without cisplatin was also terminated due to low accrual (NCT00882583). Given that combined inhibition of EGFR and SRC is synergistic in HNSCC cell lines [84] and that SRC mediates erlotinib resistance in HNSCC [85], one group conducted a phase I placebo-controlled window trial testing erlotinib, dasatinib, both agents combined or placebo in patients with operable, stage II-Va HNSCC (NCT00779389). Patients were treated for 7–21 days preoperatively, and tumor specimens were evaluated for expression of EGFR and SRC pathway proteins before and after treatment. While preclinical models suggested synergy effects of EGFR and SRC inhibition, a decrease in tumor size by erlotinib was not enhanced by addition of dasatinib, and erlotinib with or without dasatinib yielded a significantly greater decrease in tumor size versus dasatinib or placebo. In addition, while neither erlotinib nor dasatinib altered post-treatment expression of the selected biomarkers (pSTAT1, 3, pAKT, pSRC, pMAPK, pEGFR, pMET, pFAK, cMet, EGFR, vimentin, E-cadherin, HER2 and HER3), there was an association of baseline signaling biomarkers to sensitivity/resistance. Namely, baseline pMAPK expression was associated with erlotinib sensitivity (P=0.099), and baseline pSTAT3 expression was associated with dasatinib resistance (P=0.02), leading to identification of potential biomarkers for future studies [86]. These results suggest that pre-treatment selection of patients with low baseline pSTAT3 expression may increase the effect of dasatinib.
Meanwhile, a preclinical in vivo study showed that addition of dasatinib to combined cetuximab and radiation therapy unexpectedly leads to increased tumor growth in FaDu and A431 xenografts, likely resulting from increased DNA synthesis and angiogenesis associated with RAS, AKT and ERK1/2 activation and SRC inhibition [87]. While c-SRC is implicated in EGFR signaling, this preclinical data suggests potential clinical futility of a combined treatment regimen. In addition, a phase II trial testing single-agent dasatinib for R/M HNSCC failed to demonstrate significant anti-tumor activity, despite c-SRC inhibition (NCT00507767) [88]. Upon biomarker analysis, a significant increase in Macrophage Migration Inhibitory Factor (MIF) was detected in rapidly progressing patients [88]. An in vitro study showing significant upregulation of STAT3 activation and downstream signaling following sustained c-SRC inhibition in various HNSCC cell lines might explain dasatinib’s low anti-tumor activity and suggest a potential role for dasatinib combined with STAT3 or Janus-activated kinase inhibitors in HNSCC treatment [89]. In addition, preclinical data in other cancers such as NSCLC suggest a potential for combined targeted therapy of dasatinib with mTOR inhibitors [90,91].
Ponatinib.
Ponatinib is a BCR-ABL inhibitor that also targets VEGFR and FGFR and has been approved for treatment of chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia. Despite termination of a phase III clinical trial of ponatinib for CML due to a high occurrence of arterial thrombotic events [92], ponatinib is indicated for CML patients with treatment refractory to dasatinib and nilotinib, those who have a T315I mutation, or those for whom imatinib is not indicated (NCT01207440). A copy number analysis of 144 head and neck cancer tissue samples and 20 cell lines revealed frequent amplification of FGF19, though amplification of FGFR1 was found in only one cell line. Inhibition of the FGF pathway with ponatinib holds potential clinical utility in overcoming EGFR resistance. In addition, it was shown that ponatinib was effective as a single agent in HNSCC cell lines and showed synergistic effect when combined with gefitinib [93]. Despite encouraging preclinical data, a phase II clinical trial testing ponatinib for HNSCC and NSCLC was terminated due to an adverse event, pancreatitis, occurring in one of two enrolled patients with NSCLC (NCT01761747). Another trial conducted in advanced medullary thyroid cancer was also terminated due to drug toxicities, with one patient developing gastric hemorrhage and one unexplained death (NCT01838642). Both terminated trials indicate that ponatinib carries more significant toxicity compared to similar m-TKIs, and there are no currently active clinical trials evaluating ponatinib in HNSCC.
5. Conclusions
Existing CT agents approved for HSNCC are non-specific and have considerable toxicity. Targeted agents, such as monoclonal antibodies cetuximab, nivolumab, and pembrolizumab are effective, however only in a subset of patients and can lose efficacy due to acquired resistance. Tyrosine kinase inhibitors have emerged as an important subclass of targeted cancer drugs for various solid and hematologic tumors. Their ability to target multiple kinases at a time allows for inhibition of various tumorigenic signaling pathways concomitantly. m-TKIs are increasingly being investigated for treatment of head and neck neoplasms. Across the board, single-agent use of m-TKIs has not yielded clinically relevant anti-tumor activity, with several trials being terminated in early phases of development. EGFR targeting m-TKIs, lapatinib and afatinib, have demonstrated potential as a less toxic substitute to cetuximab, particularly in pre-operative settings and in select patient groups, respectively. Radio-sensitizing drug, sunitinib, did not show clinical efficacy and demonstrated significant toxicities when combined with radiotherapy, though efficacy of sorafenib with radiotherapy remains unexplored. Sorafenib showed low single agent efficacy and no added benefit when combined with cetuximab, though combination studies of sorafenib with chemotherapeutic agents are currently active. Another radio-sensitizing drug, vandetanib, showed promising preclinical efficacy and tolerability in a Phase I trial when combined with radiotherapy and cisplatin. However, no follow-up Phase II trial has been conducted and other combinations resulted in no improvement of efficacy. Other angiogenesis inhibitors such as lenvatinib have warranted continued development, particularly in combination with immune targeting drugs, while axitinib and nintedanib have shown promise in selected patients based on tumor mutational profiles. Finally, Bcr-Abl targeting drugs imatinib, nilotinib, dasatinib and ponatinib have demonstrated higher toxicity than other m-TKIs and are less efficacious. However preclinical data showing efficacy of dasatinib and other BCR-ABL targeting agents with STAT3/JAK and mTOR inhibitors in select patient populations with solid tumors, such as NSCLC patients with EGFR mutations, suggests potential for future testing of these drugs as combination therapies in select HNSCC patient populations.
6. Expert Opinion
Despite similar histologic appearances, studies to date have demonstrated that individual HNSCC tumors harbor distinct characteristics that may guide treatment selection. For example, an increased understanding of the biology of HNSCC associated with human papillomavirus (HPV) has revealed that, in general, HPV-associated HNSCC has a better prognosis than HNSCC that are HPV-negative. More recently, we have learned that HPV-positive HNSCC is not responsive to the FDA-approved EGFR monoclonal antibody cetuximab [94, 95]. The fact that it took nearly 12 years to determine that HPV represented a negative predictive biomarker for cetuximab therapy underscores the need to systematically, prospectively characterize tumors and iteratively assess clinical responses in the context of tumor biology.
Identification of predictive biomarkers (both positive and negative) should guide the use of targeted therapies in HNSCC, including m-TKIs. Tumors that are dependent on these kinases for survival and growth are expected to be most responsive to m-TKIs. For example, a tumor with a gain of function mutation in an oncogenic kinase that is a target of a m-TKI, would be expected to be more responsive than a tumor that harbors two wild-type, unamplified alleles for the kinase. Elucidation of the genetic landscape of HNSCC over the past 7 years has revealed alterations that activate kinases (such as amplification or mutation) in both HPV-positive and HPV-negative tumors [96,97]. It is plausible that such kinase activations may represent predictive biomarkers for m-TKI therapy in this cancer. The most informative clinical trial should incorporate tumor profiling (such as next generation sequencing) so that the biology of each patient’s tumor, enrolled in the trial, can be assessed in the context of treatment responses. In addition, the most successful use of m-TKIs is likely to be based on strong evidence of antitumor activity in relevant preclinical models including well-characterized HNSCC cell lines, patient-derived xenografts and immunocompetent HNSCC models. Most agents or combination of agents are administered to patients without robust preclinical evidence of efficacy in specific genetic contexts. The 2016 FDA-approval of pembrolizumab and nivolumab and the observation that many of the pathways inhibited by m-TKIs are also upregulated in tumor immunosuppression, underscores the promise of testing m-TKIs in combination with immune checkpoint inhibitors [11,15]. There is also is a growing understanding of the effect of m-TKIs on other oncogenic pathways implicated in tumor immune microenvironment such as the IL-6/JAK/STAT pathway, for which there are targeted agents in clinical development. The potential for combined efficacy of a broad class of immunotherapies (in addition to the two FDA-approved agents to date) and m-TKIs represents a significant opportunity for future investigation.
There are numerous challenges to effectively incorporate m-TKIs into HNSCC treatment including the heterogeneity of human tumors, incomplete understanding of mechanisms of innate or acquired treatment resistance, and the limited and varied use of tumor profiling in the management of HNSCC. The genomic heterogeneity of HNSCC has become evident through preclinical and clinical studies, as well as through the comprehensive genomic characterization of HNSCC by The Cancer Genome Atlas (TCGA). Increasing understanding of the heterogeneity of HNSCC has led investigators to conduct a number of clinical trials using m-TKIs with the goal of blocking multiple important tumorigenic signaling pathways or reversing pathways of resistance to conventional or other targeted therapies. However, the majority of these studies test m-TKIs as single agents or in combination with cetuximab in unselected patients and have not demonstrated significant clinical efficacy. The most successful of these trials, such as those testing afatinib [47], and the most promising such as those testing nintedanib (NCT03292250), have included a systematic identification of potential biomarkers for response to treatment and then tested the drug in groups with the corresponding tumor mutational profile following tumor biopsy and next generation sequencing.
Future success of these trials will depend on relying on robust preclinical data to define predictive biomarkers and guide treatment as well as application of these data to trial design. Even when targeted sequencing is obtained, cumulative evidence to date suggests that the sequencing results may vary depending on both the region of the tumor sampled and the sequencing platforms employed. Elucidation of treatment resistance is likely to depend on more detailed studies in relevant preclinical models, which can inform the design of clinical trials. In addition, tumor biopsies are not routinely required after enrollment into clinical trials so that when a patient develops resistance to the regiment tested, we often do not have the opportunity to analyze the tumor and determine plausible resistance mechanisms. Ideally, patients being treated with m-TKIs on a clinical protocol can be enlisted to undergo tumor biopsies at clinically relevant time points (such as in the setting of tumor progression) to capture treatment resistance mechanisms. Overall, the successful use of m-TKIs will depend on developing a clear understanding of which HNSCC patients are likely to benefit from therapy based on tumor characterization such as targeted sequencing followed by discussion at molecular tumor boards. Combinations of m-TKIs and immune checkpoint inhibitors will require testing in immunocompetent preclinical models and/or prospective analysis of patients enrolled in clinical trials.
Article Highlights:
Due to incomplete understanding of treatment resistance and the heterogeneity of HNSCC tumors, current targeted therapies, such as the EGFR targeting monoclonal antibody, cetuximab, have not significantly improved treatment outcomes of HNSCC.
Multi-targeted kinase inhibitors have been approved for use in various hematologic and other solid tumors, either as monotherapy or in combination with other therapies.
Various multi-targeted tyrosine kinase inhibitors are in the early stages of development for HNSCC and include EGFR targeting agents, potential radiosensitizers which target PDGFR and VEGFR, BCR-ABL targeting agents, and potent anti-angiogenesis agents.
While single agent m-TKIs have not been shown to improve clinical outcomes in HNSCC to date, m-TKIs with the most promising clinical results are being tested in combination with current FDA-approved therapies and/or in mutation-defined patient subgroups.
Future work in this field requires the identification of predictive biomarkers to increase clinical responses to m-TKIs alone or in combination with standard of care including immune modulating agents.
Acknowledgments
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Bibliography.
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Vigneswaran N, Williams MD. Epidemiological Trends in Head and Neck Cancer and Aids in Diagnosis. Oral and maxillofacial surgery clinics of North America. 2014;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A cancer journal for clinicians. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denaro N, Russi E,G, Adamo V, Merlano M,C, State-of-the-Art and Emerging Treatment Options in the Management of Head and Neck Cancer: News from 2013. Oncology 2014;86:212–229. [DOI] [PubMed] [Google Scholar]
- 5.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–396. [DOI] [PubMed] [Google Scholar]
- 6.Kozakiewicz P, Grzybowska-Szatkowska L. Application of molecular targeted therapies in the treatment of head and neck squamous cell carcinoma. Oncology letters. 2018;15(5):7497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. [DOI] [PubMed] [Google Scholar]
- 8.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol 2004;22(9):1743–52. [DOI] [PubMed] [Google Scholar]
- 9.Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol 2012;13:35–46. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578.* A landmark randomized Phase III trial comparing cetuximab with radiotherapy versus radiotherapy alone, looking at survival benefit.
- 11.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheth S, Weiss J. Pembrolizumab and its use in the treatment of recurrent or metastatic head and neck cancer. Future Oncology. 2018;14(16):1547–1558. [DOI] [PubMed] [Google Scholar]
- 13.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2007;25(16):2171–2177.**Influential study showing that the response rate to cetuximab in the single-agent phase is only 13%, a key driver behind the urgent need for development of novel targeted therapies.
- 14.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. [DOI] [PubMed] [Google Scholar]
- 15.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. The Lancet Oncology. 2016;17(7):956–965. [DOI] [PubMed] [Google Scholar]
- 16.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315(3):971–979. [DOI] [PubMed] [Google Scholar]
- 18.Anastassiadis T, Deacon SW, Devarajan K. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol 2011;29(11):1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MI Hunt JP, Herrgard S. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29(11):1046–51. [DOI] [PubMed] [Google Scholar]
- 20.Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig P, et al. The target landscape of clinical kinase drugs. Science. 2017;358(6367):eaan4368. doi: 10.1126/science.aan4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Food & Drug Administration. New drugs at FDA: CDER's new molecular entities and new therapeutic biological products. FDA; https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm592464.htm (2018). [Google Scholar]
- 22.Ferguson FM, Gray NS. Kinase inhibitors: The road ahead. Nature Reviews Drug Discovery. 2018;17(5):353–377. [DOI] [PubMed] [Google Scholar]
- 23.Steeghs N, Nortier JW, Gefllderblom H. Small molecule tyrosine kinase inhibitors in the treatment of solid tumors: An update of recent developments. Annals of surgical oncology. 2007;14(2):942–953. [DOI] [PubMed] [Google Scholar]
- 24.Chun PY, Feng FY, Scheurer AM, Davis MA, Lawrence TS, Nyati MK. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66(2):981–988. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors—impact on future treatment strategies. Nature reviews Clinical oncology. 2010;7(9):493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taneja C, Allen H, Koness RJ, Radie-Keane K, Wanebo HJ. Changing patterns of failure of head and neck cancer. Archives of Otolaryngology–Head & Neck Surgery. 2002;128(3):324–327. [DOI] [PubMed] [Google Scholar]
- 27.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53(15):3579–3584. [PubMed] [Google Scholar]
- 28.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncology. 2009;45(4–5):324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onita B, Lester DRT, Iman AA, Jergin C, Shawn I. Comparison of high-dose cisplatin-based chemoradiotherapy and cetuximab-based bioradiotherapy for p16-positive oropharyngeal squamous cell carcinoma in the context of revised HPV-based staging. Reports of Practical Oncology & Radiotherapy. 2018;23(5):451–457.*Cetuximab decreases overall and progression-free survival versus cisplatin and radiation in HPV-positive patients.
- 30.Brand TM, Iida M, Wheeler DL. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer biology & therapy. 2011;11(9):777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madoz-Gúrpide J, Zazo S, Chamizo C, Casado V, Caramés C, Gavín E, Cristóbal I, García-Foncillas J, Rojo F. Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer. J Transl Med. 2015;13(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang N, Wang D, Hu Z, Shin HJ, Qian G, Rahman MA, Zhang H, Amin AR, Nannapaneni S, Wang X, et al. Combination of anti‐HER3 antibody MM‐121/SAR256212 and cetuximab inhibits tumor growth in preclinical models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2014;13:1826–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27(28):3944–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen L, Benlimame N, Nie Z, et al. Differential regulation of tumor angiogenesis by distinct ErbB homo-and heterodimers. Mol Biol Cell. 2002;13(11):4029–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A molecularly annotated platform of patient-derived xenografts ('xenopatients') identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer discov. 2011;1(6):508–523. [DOI] [PubMed] [Google Scholar]
- 36.Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer discov. 2013;3(6):658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu S, Fu W, Xu W, Yang Y, Cruz M, Berezov SD, Jorissen D, Takeda H, Zhu W. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res. 2015;75(1):159–170. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch FR, Sequist LV, Gore I, et al. Long-term safety and survival with gefitinib in select patients with advanced non–small cell lung cancer: Results from the US IRESSA clinical access program (ICAP). Cancer. 2018;124(11):2407–2414. [DOI] [PubMed] [Google Scholar]
- 39.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(11):1864–1871. [DOI] [PubMed] [Google Scholar]
- 40.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. The Lancet Oncology. 2014;15(8):894–904. [DOI] [PubMed] [Google Scholar]
- 41.Martins RG, Parvathaneni U, Bauman JE, Sharma AK, Raez LE, Papgikos MA, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: A randomized phase II trial. J Clin Oncol. 2013;31(11):1415–1421.* Adding erlotinib to CRT did not increase response rate or progression-free survival.
- 42.Weiss JM, Bagley S, Hwang W, et al. Capecitabine and lapatinib for the first-line treatment of metastatic/recurrent head and neck squamous cell carcinoma. Cancer. 2016;122(15):2350–2355. [DOI] [PubMed] [Google Scholar]
- 43.Harrington K, Temam S, Mehanna H, et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: A phase III, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2015;33(35):4202–4209. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JM, Grilley-Olson JE, Deal AM, Zevallos JP, Chera BS, Paul J, et al. Phase 2 trial of neoadjuvant chemotherapy and transoral endoscopic surgery with risk-adapted adjuvant therapy for squamous cell carcinoma of the head and neck. Cancer. 2018;124(14):2986–2992.** Neo-adjuvant lapatinib and chemotherapy yields high response rates and excellent long-term outcomes
- 45.Young NR, Soneru C, Liu J, et al. Afatinib efficacy against squamous cell carcinoma of the head and neck cell lines in vitro and in vivo. Targeted oncology. 2015;10(4):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machiels J, Bossi P, Menis J, et al. Activity and safety of afatinib in a window preoperative EORTC study in patients with squamous cell carcinoma of the head and neck (SCCHN). Annals of Oncology. 2018;29(4):985–991. [DOI] [PubMed] [Google Scholar]
- 47.Cohen EE, Licitra LF, Burtness B, et al. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Annals of Oncology. 2017;28(10):2526–2532.** Phase III clinical trial showing that sub-group selection leads to increased median progression free survival for afatinib over IV methotrexate
- 48.Specenier P, Vermorken J. Afatinib in squamous cell carcinoma of the head and neck. Expert Opin Pharmacother. 2016;17(9):1295–1301. [DOI] [PubMed] [Google Scholar]
- 49.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. [DOI] [PubMed] [Google Scholar]
- 50.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 51.Laban S, Steinmeister L, Gleibner L, et al. Sorafenib sensitizes head and neck squamous cell carcinoma cells to ionizing radiation. Radiotherapy and Oncology. 2013;109(2):286–292. [DOI] [PubMed] [Google Scholar]
- 52.Affolter A, Samosny G, Heimes A, et al. Multikinase inhibitors sorafenib and sunitinib as radiosensitizers in head and neck cancer cell lines. Head Neck. 2017;39(4):623–632. [DOI] [PubMed] [Google Scholar]
- 53.Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 2010;28(20):3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert J, Schell MJ, Zhao X, Murphy B, Tanvetyanon T, Leon ME, Neil D, Haigentz M, Saba M, Nieva J, Bishop J, Sidransky D, Ravi R, Bedi A, Chung CH. A randomized phase II efficacy and correlative studies of cetuximab with or without sorafenib in recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2015;51(4):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. [DOI] [PubMed] [Google Scholar]
- 56.Hillman GG, Singh-Gupta V, Al-Bashir AK, et al. Monitoring sunitinib-induced vascular effects to optimize radiotherapy combined with soy isoflavones in murine xenograft tumor. Translational oncology. 2011;4(2):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto S, Batra S, Saito K, Yasui H, Choudhuri R, Gadisetti C, Subramanian S, Devasahayam N, Munasinghe JP, Mitchell JB, et al. Anti-angiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011:71(20):6350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choong NW, Kozloff M, Taber D, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs. 2010;28(5):677–683. [DOI] [PubMed] [Google Scholar]
- 59.Bozec A, Sudaka A, Toussan N, Fischel J, Etienne-Grimaldi M, Milano G. Combination of sunitinib, cetuximab and irradiation in an orthotopic head and neck cancer model. Annals of Oncology. 2009;20(10):1703–1707. [DOI] [PubMed] [Google Scholar]
- 60.Sano D, Matsumoto F, Valdecanas D, Zhao M, Molkentine DP, Takahashi Y, et al. Vandetanib restores head and neck squamous cell carcinoma cells' sensitivity to cisplatin and radiation in vivo and in vitro. Clinical Cancer Research. 2011:17(7):1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gustafson DL, Frederick B, Merz AL, Raben D. Dose scheduling of the dual VEGFR and EGFR tyrosine kinase inhibitor vandetanib (ZD6474, Zactima®) in combination with radiotherapy in EGFR-positive and EGFR-null human head and neck tumor xenografts. Cancer Chemother Pharmacol. 2008;61(2):179–188. [DOI] [PubMed] [Google Scholar]
- 62.Papadimitrakopoulou VA, Frank SJ, Cohen EW, Hirsch FR, Myers JN, Heymach JV, et al. Phase I study of vandetanib with radiation therapy with or without cisplatin in locally advanced head and neck squamous cell carcinoma. Head Neck. 2016;38(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Limaye S, Riley S, Zhao S, O’Neill A, Posner M, Adkins D, Jaffa Z, Clark J, and Haddad R. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol. 2013;49(8):835–841. [DOI] [PubMed] [Google Scholar]
- 64.Van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. International Journal of Cancer. 2005;117(6):883–888. [DOI] [PubMed] [Google Scholar]
- 65.Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: A role for altered tumor angiogenesis. Cancer Res. 2001;61(13):5090–5101. [PubMed] [Google Scholar]
- 66.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nature Reviews Immunology. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 67.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Rmili CW, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D, De Palma M. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Science translational medicine. 2017;9(385):eaak9670. [DOI] [PubMed] [Google Scholar]
- 68.Taylor MH, Rasco DW, Brose MS, et al. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2018;36(15):6016. [Google Scholar]
- 69.Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973–2007 surveillance, epidemiology, and end results data. Cancer. 2012;118(18):4444–4451. [DOI] [PubMed] [Google Scholar]
- 70.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–4782. [DOI] [PubMed] [Google Scholar]
- 71.Kim Y, Lee SJ, Lee JY, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: A multicenter phase 2 study (korean cancer study group HN14–01). Cancer. 2017;123(11):1958–1964. [DOI] [PubMed] [Google Scholar]
- 72.Patson B, Cohen RB, Olszanski AJ. Pharmacokinetic evaluation of axitinib. Expert Opin Drug Metab Toxicol. 2012;8(2):259–270. [DOI] [PubMed] [Google Scholar]
- 73.Swiecicki PL, Zhao L, Belile E, et al. A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Invest New Drugs. 2015;33(6):1248–1256. [DOI] [PubMed] [Google Scholar]
- 74.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164–172. [DOI] [PubMed] [Google Scholar]
- 75.Mino M, Pilch BZ, Faquin WC. Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma. Modern Pathology. 2003;16(12):1224–1231. [DOI] [PubMed] [Google Scholar]
- 76.Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: A princess margaret hospital phase II consortium study. J Clin Oncol. 2005;23(3):585–590. [DOI] [PubMed] [Google Scholar]
- 77.Moskaluk CA, Frierson HF Jr, El-Naggar AK, Futreal PA. C-kit gene mutations in adenoid cystic carcinoma are rare. Modern Pathology. 2010;23(6):905–906. [DOI] [PubMed] [Google Scholar]
- 78.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant bcr-abl. Cancer Cell. 2005;7(2):129–141. [DOI] [PubMed] [Google Scholar]
- 79.Saglio G, Kim D, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. [DOI] [PubMed] [Google Scholar]
- 80.Kramer B, Hock C, Birk R, Sauter A, Stuck BA, Hörmann K, Schultz JD, Aderhold C. Targeted therapies in HPV-positive and-negative HNSCC–alteration of EGFR and VEGFR-2 expression in vitro. Anticancer Res. 2016;36(6):2799–2807. [PubMed] [Google Scholar]
- 81.Kramer B, Kneissle M, Birk R, Rotter N, Aderhold C. Tyrosine kinase inhibition in HPV-related squamous cell carcinoma reveals beneficial expression of cKIT and src. Anticancer Res. 2018;38(5):2723–2731. [DOI] [PubMed] [Google Scholar]
- 82.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular src and epidermal growth factor receptor. Proceedings of the National Academy of Sciences. 1999;96(4):1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol. 2008;35(3):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278(34):31574–83. [DOI] [PubMed] [Google Scholar]
- 85.Stabile LP, He G, Lui VWY, Thomas SM, Henry C, Gubish CT, et al. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clinical Cancer Research. 2013;19(2):380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bauman JE, Duvvuri U, Gooding WE, Rath TJ, Gross ND, Song J, et al. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI insight. 2017;2(6):e90449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baro M, De Llobet LI, Figueras A, Skvortsova I, Mesia R, Balart J. Dasatinib worsens the effect of cetuximab in combination with fractionated radiotherapy in FaDu-and A431-derived xenografted tumours. Br J Cancer. 2014;111(7):1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brooks HD, Glisson BS, Bekele BN, Ginsberg LE, El-Naggar A, Culotta KS, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011;117(10):2112–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained src inhibition results in STAT3 activation and cancer cell survival via altered JAK-STAT3 binding. Cancer Res. 2009;69(5):1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen B, Xu X, Luo J, Wang H, Zhou S. Rapamycin enhances the anti-cancer effect of dasatinib by suppressing src/pi3k/mtor pathway in nsclc cells. PLoS One. 2015;10(6):e0129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haltia UM, Andersson N, Yadav B, Farkkila A, Kulesskiy E, Kankainen M, Tang J, Butzow R, Riska A, Leminen A, Heikinheimo M, Kallioniemi O, Unkila-Kallio L, Wennerberg K, Aittokallio T, Anttonen M. Systematic drug sensitivity testing reveals synergistic growth inhibition by dasatinib or mtor inhibitors with paclitaxel in ovarian granulosa cell tumor cells. Gynecol Oncol 2017;144(3):621–630. [DOI] [PubMed] [Google Scholar]
- 92.Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. The Lancet Oncology. 2016;17(5):612–621. [DOI] [PubMed] [Google Scholar]
- 93.Rieke DT, Zuo Z, Endhardt K, Keck M, Khattri A, Mahmutogulu D, Leung K, Dinali M El, Braegelmann J, Seiwert TY. Fibroblast growth factors in head and neck cancer: Genetic alterations and therapeutic targeting with ponatinib. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, Illinois Washington,DC Philadelphia: AACR; 2012. p. 7. [Google Scholar]
- 94.Cohen EE, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. The Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 95.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Sturgis EM, Burtness B, Ridge JA, Ringash J, Galvin J, Yao M, Koyfman SA, Blakaj DM, Razaq MA, Colevas AD, Beitler JJ, Jones CU, Dunlap NE, Seaward SA, Spencer S, Galloway TJ, Phan J, Dignam JJ, Le QT. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. The Lancet. 2019;393(10166):40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]