Abstract
Ovarian cancers are the most common cause of gynecological death, and the five-year survival rate for women diagnosed with epithelial ovarian carcinoma (EOC) remains extremely low at only 47%. Recent studies have highlighted the importance of the anti-tumor immune response in determining EOC clinical outcomes, and much research is currently being undertaken in an effort to reverse tumor immune evasion. One mechanism known to promote tumor immune evasion in multiple cancer types is tryptophan catabolism. Here we review the potential role of two rate-limiting enzymes that evolved separately to catabolize tryptophan, indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase 2 (TDO2), that may be active in ovarian cancers and result in the production of immune suppressive catabolites. Research to date has focused on IDO inhibitors, currently in clinical trials, but these therapies fail to inhibit TDO2. However, our mining of publically available data from clinical specimens suggest that TDO2 may also need to be targeted in ovarian cancer.
Keywords: epithelial ovarian carcinoma, tryptophan, TDO2, IDO1
Introduction
Ovarian cancers are the most common cause of death from gynecological disease in the United States. In 2019, it is estimated there will be 22,530 new cases of ovarian cancer and 13,980 deaths. High-grade serous ovarian carcinoma (HGSC), a subtype of epithelial ovarian carcinoma (EOC), accounts for around 70% of all ovarian adenocarcinomas and are most prevalent in postmenopausal women [1]. Women typically present with non-specific symptomatology such as abdominal distension, early satiety, and rarely with associated lymphadenopathy [1]. Thus, the vast majority of HGSCs present in either stage III with dissemination throughout the peritoneal cavity or stage IV with extensive lymph node involvement [1]. Thus, the five-year survival rate for patients with advanced stage disease (III/IV) is remarkably low at 30%, with high rates of recurrent disease [1, 2].
Unlike most carcinomas, in EOC the main method of metastasis is through non-hematogenous spread. EOCs metastasize throughout the peritoneal cavity by direct seeding, in which cancer cells shed off the primary tumor into the peritoneal cavity [3]. Historically, EOC was thought to arise from the ovarian surface epithelium, but it is now accepted that it arises from the fallopian tube epithelium [1, 4]. Like other carcinomas, EOC can undergo an epithelial to mesenchymal transition (EMT) [5] that facilitates survival in anchorage-independent conditions [6, 7]. EOC cells shed from the fallopian tube and survive anchorage-independent conditions to attach to the ovary and multiple metastatic sites within the peritoneal cavity, making operative resection difficult and minimally effective [7–9]. Normal epithelial cells undergo apoptosis in response to being detached from the basement membrane and this specific type of cell death is termed ”anoikis” [10]. The ability of carcinoma cells to survive in suspension is defined as anoikis resistance [6, 7], and this contributes significantly to the metastatic potential of EOC [11]. Current treatment protocols involve initial debulking and staging surgery followed by systemic and/or intraperitoneal chemotherapy, typically with a combination of platinum- and taxane-based agents [6–9]. Unfortunately, EOC often recurs within 2 years of initial diagnosis as chemo-resistant disease, which contributes to a poor overall survival rate [12].
The exact mechanisms that facilitate direct seeding, survival during dissemination and peritoneal spread of EOC remain unclear and there is significant interest in understanding this process and developing agents to prevent or target metastatic EOC. The use and development of immunotherapy to promote anti-tumor immunity is expanding. In EOC, an increase in tumor-infiltrating T cells conveys a delayed time to recurrence [13], suggesting that immune stimulation through checkpoint inhibition should reduce EOC tumor burden. However, response rates to immune checkpoint inhibition (i.e., programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1)) in HGSC patients is only about 15% [14, 15], indicating a need to investigate additional mechanisms of EOC immune evasion. Suppression of cytotoxic T cells, significantly contributes to ovarian cancer progression [16, 17]. Immunosuppressive pathways such as tryptophan catabolism may contribute mechanistically to this process and play a critical role in determining clinical outcomes [18, 19]. This review presents rationale for investigating tryptophan catabolism in the context of EOC.
Tryptophan catabolism
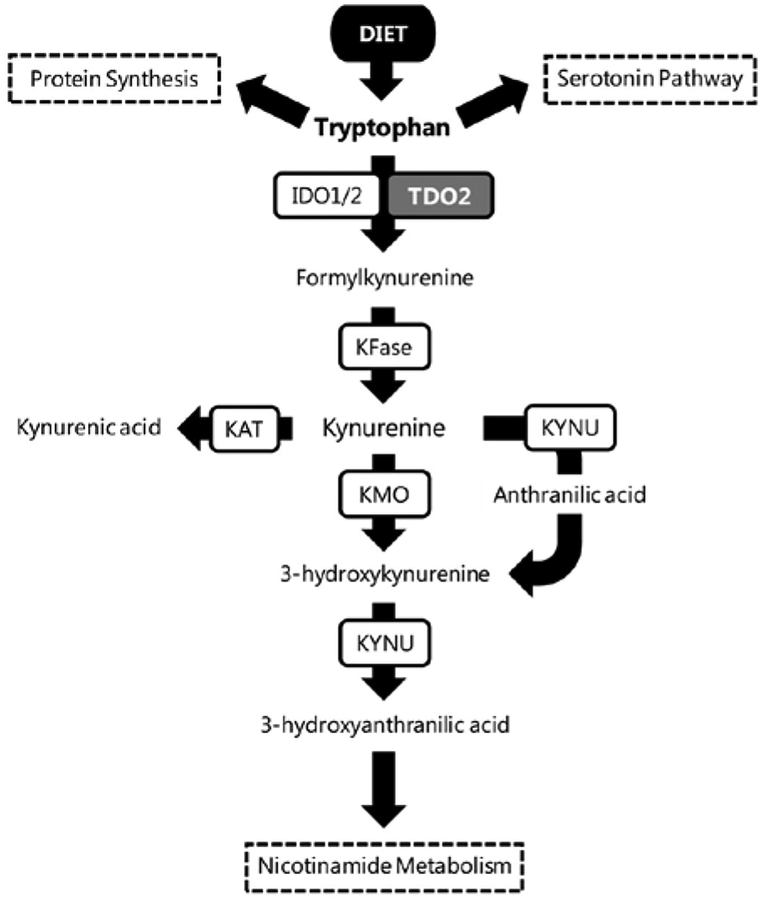
Tryptophan is an essential amino acid that is ingested through the diet. In the cell, tryptophan can be used in several different ways, such as protein synthesis, production of serotonin, production of kynurenine or ultimately through the kynurenine pathway to generate nicotinamide adenine dinucleotide (NAD+; Figure 1) [20]. There are three rate-limiting enzymes that can catabolize tryptophan into kynurenine: indoleamine 2,3-dioxygenase 1 (IDO1), indoleamine 2,3-dioxygenase 2 (IDO2), and tryptophan 2,3-dioxygenase 2 (TDO2) [20, 21].
Figure 1.
The tryptophan catabolism pathway. The essential amino acid tryptophan is catabolized through three pathways: protein synthesis, the serotonin pathway and nicotinamide metabolism. IDO, indoleamine 2,3-dioxygenase; TDO, tryptophan 2,3-dioxygenase; KFase (also known as AFMID), arylformamidase; KAT (AADAT), kynurenine aminotransferase; KYNU, kynureninase; KMO (K3H), kynurenine 3-hydroxylase.
IDO1 and IDO2 are highly expressed in the placenta, lung, and intestine. Under normal conditions minimal tryptophan is catabolized by these enzymes; however, they become highly active during immunological responses [21]. TDO2 is expressed in hepatocytes and is responsible for the majority of tryptophan catabolism under normal conditions [21]. The expression and function of TDO2 is regulated by a number of factors, including inflammatory stimuli [22], glucocorticoids [22, 23], heme-molecules [24], and via feedback inhibition by NADPH (nicotinamide adenine dinucleotide phosphate) [25]. Increased levels of tryptophan catabolism via the kynurenine pathway have been found in many human malignancies including, but not limited to, leukemia, glioma, lymphoma, breast cancer, hepatobiliary cancer and melanoma [26–30]. Additionally, the L-type amino acid transporter 1 (LAT1/SLC7A5), which transports large neutral amino acids, such as tryptophan, into cells, is overexpressed in many cancers including breast and ovarian clear cell carcinoma [31] and is associated with poor prognosis and chemo-resistance [32, 33].
Immune regulation by tryptophan catabolism
The immune system has multiple mechanisms for self-regulation, one of which is tolerance, which serves to dampen its response to infection and inflammatory insult in order to prevent over activation and the destruction of self tissues. This is also important during pregnancy to protect the fetus from the maternal immune system and tryptophan catabolism has been extensively studied in this context [34]. The kynurenine pathway has been shown to be an important player in this process of immune modulation [35]. Under normal conditions, T cells secrete IFNγ (interferon-gamma) which leads to upregulation of IDO1 in antigen-presenting cells (APCs). APCs are then able to catabolize local tryptophan, depleting it from the microenvironment to starve pathogens of tryptophan. In response to certain pathogens, such as Chlamydia pneumoniae and Toxoplasma gondii, the relative lack of tryptophan also leads to slowed CD4 T cell replication [36, 37]. Upregulation of IDO1 and TDO2 and increased levels of kynurenine metabolites have also been shown to suppress CD4 T cell proliferation and induce T cell death. This mechanism of T cell regulation has been proposed as a contributing factor to immune suppression in several disease states, including cancer [20, 21].
Metabolites of tryptophan catabolism act by both autocrine and paracrine mechanisms to promote tumorigenesis and tumor evasion from immune detection. Kynurenine acts in an autocrine fashion through the aryl hydrocarbon receptor (AhR) to provide anti-apoptotic signals that enhance survival under anchorage-independent conditions by upregulation of key genes that promote cell growth and survival [30, 38]. Through paracrine action, kynurenine can also suppress anti-tumor cytotoxic T cell function [30, 38]. Cultured human plasmacytoid dendritic cells (PDCs) exposed to inflammatory stimuli can induce class switching from CD4+ T cells to CD4+FOXP3+CD25+ regulatory T cells (Tregs) via upregulation of IDO [39], which then suppress the activity of cytotoxic T cells. The functionality of Tregs can be decreased by the IDO inhibitor (1-methyl-D-tryptophan), but is restored by the addition of kynurenine, suggesting that IDO is a critical factor in T cell regulation [39]. Furthermore, when T cells are exposed to IDO in vitro, CD4 and CD8 T cell proliferation is inhibited both by depletion of tryptophan and through kynurenine metabolite-mediated cell death [40, 41].
Role of IDO and TDO in cancer
Tryptophan catabolism and the kynurenine pathway have been highly studied in the field of oncology. Upregulation and increased activity of IDO1/2 or TDO2 have been found in a variety of cancers and are thought to have an important role in disease progression. Tumors that express IDO1/2 or TDO2 can suppress and evade the anti-tumor immune response [30, 40, 42]. Tumor cells that express IDO have fewer tumor-infiltrating lymphocytes (TILs), indicating that tryptophan catabolism can aid tumors in immune evasion [43].
The kynurenine pathway plays a role in progression of many tumors types, such as human meningioma, where IDO is highly expressed and the tryptophan metabolites 5-hydroxy indole acetic acid (5-HIAA) and kynurenine are increased [44]. In triple negative breast cancer (TNBC), TDO2 protein levels increased rapidly during conditions of anchorage-independent survival and are also increased by inflammatory activators of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [38]. TDO2 is a direct target of the microRNA-200c (miR-200c) in more indolent breast cancer subtypes, but continues to be expressed in TNBC due to their loss of miR-200c [45].
An important measure of tryptophan metabolisms that has been indicated in prognostication of different cancers is the kynurenine-to-tryptophan ratio (KTR), which when elevated indicates that tryptophan is being metabolized through the kynurenine pathway by IDO1/2 or TDO2. For example, clear cell renal carcinoma patients with a higher KTR have lower survival rates indicating that tryptophan catabolism is an important prognostic indicator [46]. Likewise, increased KTR was also found to be associated with increased squamous cell lung cancer risk [47]. Our study in presurgical plasma samples from patients with breast cancer versus healthy patients did find that tryptophan was significantly lower in the plasma of breast cancer patients with estrogen receptor alpha-negative tumors than normal donors; however contrary to expected results, kynurenine was also lower in the cancer patient plasma compared to cancer-free controls [31]. Although we predicted, based on prior studies from other types of cancer including breast [28], that tryptophan would be lower and kynurenine higher in breast cancer patients, our study had a low percentage of patients with advanced stage disease, where KTR might be expected to be higher due to increased disease burden. Additionally, we had a relatively young patient cohort than is typical for most breast cancer studies. Kynurenine production by tumor cells may not be enough to result in increased plasma levels of circulating kynurenine and the kidneys are efficient in catabolizing kynurenine [31].
IDO and TDO in ovarian cancer
IDO1/2 have been studied in EOC and it has been postulated that they could serve as effective therapeutic targets for this disease [17, 19]. In advanced EOC, the presence of intratumoral TILs correlates with improved clinical outcomes [13]. In fact, 56% of surgically resected EOCs sampled have high IDO expression that correlates with decreased levels of CD8+ T cells [19]. Interestingly, increased levels of IDO are further positively correlated with impaired survival in patients with serous type ovarian cancer [18]. Gene expression profiles of HGSC cells show that IDO positively correlates with chemo-resistance, reduced survival, and poor prognosis [48]. Indeed, IDO upregulation promoted peritoneal metastasis of ovarian cancer by creating an immunosuppressive environment within the peritoneal cavity [49].
Several clinical studies involving IDO inhibition in ovarian cancer are ongoing (Table 1). One trial is investigating genetic polymorphisms within IDO and how they affect the outcome of EOC patients [50]. Other studies are investigating presurgical treatment with IDO1 inhibition using Epacadostat (an IDO1-specific inhibitor) in combination with other agents in stage III-IV EOC to determine if this treatment stops the growth of tumor cells. In particular, one study, , is testing Epacadostat to treat patients with platinum-resistant ovarian, fallopian tube, or peritoneal cancers, while another, , is investigating the effectiveness of the combination of Epacadostate with anti-PD-1 immunotherapy.
Table 1.
Clinical research on IDO-targeted therapies in ovarian cancer.
| Trial name | NCT # | Study synopsis |
|---|---|---|
| How our immune system can help fight cancer | Tregs are immunosuppressive and promote cancer progression by inhibiting the anti-tumor immune response. In most T cell populations tryptophan depletion leads to decreased activity and viability, but Tregs are less susceptible to tryptophan depletion than other T cell populations. We hypothesize that genetic polymorphisms within IDO alter enzymatic activity within Treg populations and affect patient outcomes. This study will examine IDO polymorphisms in EOC tumors and ascites. | |
| Epacadostat before surgery in treating patients with newly diagnosed stage III-IV epithelial ovarian, fallopian tube, or primary peritoneal cancer | Epacadostat is an IDO1 inhibitor that may reduce tumor cell growth. This study will examine how neoadjuvant treatment with epacadostat affects disease progression (and adverse reactions) in newly diagnosed stage III-IV epithelial ovarian, fallopian tube or primary peritoneal cancers. | |
| DEC-205/NY-ESO-1 fusion protein CDX-1401, poly ICLC, and IDO1 inhibitor INCB024360 in treating patients with ovarian, fallopian tube, or primary peritoneal cancer in remission | Antigens, such as the cancer-specific antigen NY-ESO-1 protein, are found on many cancer cells and assist the immune system in targeting and destroying cancer cells. Some tumors, however, express IDO which promotes tumor growth and progression by suppressing the immune system. This study will determine the side effects, best dose and initial effectiveness of combining the IDO inhibitor INCB024360 with a cancer vaccine (DEC-205/NY-ESO-1 fusion protein CDX-1401) and an immune stimulant (poly ICLC). The goal is to generate a stronger, longer lasting anti-tumor immune response in patients with ovarian, fallopian tube and primary peritoneal cancers in remission. | |
| Study of DPX-Survivac vaccine therapy and epacadostat in patients with recurrent ovarian cancer | This study will determine the safety and immunomodulatory effects of combining the IDO1 inhibitor epacadostat with the immunotherapeutic vaccine DPX-Survivac and cyclophosphamide chemotherapy in patients with recurrent ovarian, fallopian tube or peritoneal cancers. | |
| A phase 2 study of the IDO inhibitor INCB024360 versus tamoxifen for subjects with biochemical-recurrent-only EOC, PPC or FTC following complete remission with first-line chemotherapy | This randomized study will examine the effectiveness of the IDO inhibitor INCB024360 compared to tamoxifen in biochemical recurrent ovarian cancer patients following complete remission with first-line chemotherapy. | |
| Intraperitoneal natural killer cells and INCB024360 for recurrent ovarian, fallopian tube, and primary peritoneal cancer | This study will determine the maximum tolerated dose of the IDO inhibitor INCB024360 when administered as part of a larger regimen of haploidentical donor NK cells and IL-2 following a non-myeloablative cyclophosphamide/fludarabine chemotherapy regimen in recurrent ovarian, fallopian tube and primary peritoneal cancers. | |
| Safety and efficacy of CRS-207 with epacadostat in platinum-resistant ovarian, fallopian or peritoneal cancer (SEASCAPE) | This study will determine the safety and potential efficacy of the investigational cancer drugs CRS-207 (an immune stimulant), epacadostat (an IDO1 inhibitor) and pembrolizumab (anti-PD-1 immunotherapy) in patients with platinum-resistant ovarian, fallopian tube or peritoneal cancers. | |
| Pembrolizumab and epacadostat treating participants with recurrent, persistent or progressive ovarian clear cell carcinoma | This phase II trial will determine the effectiveness of the combination of pembrolizumab and epocadostat in treating patients with ovarian clear cell carcinoma. |
EOC, epithelial ovarian carcinoma; FTC, fallopian tube cancer; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; NK, natural killer; PD-1, programmed cell death protein-1; PPC, primary peritoneal cancer; Tregs, regulatory CD4 T cells.
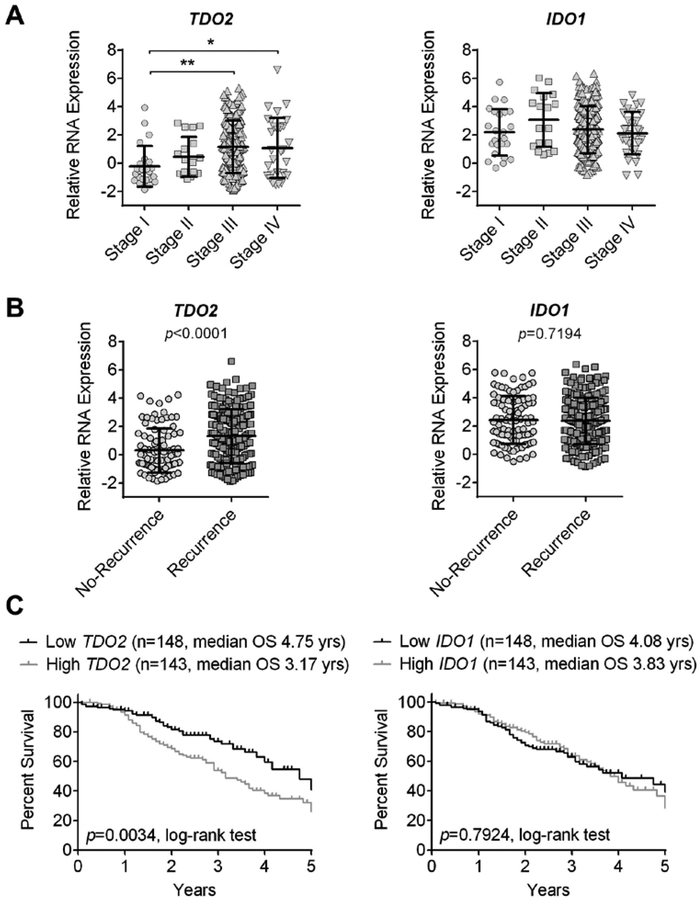
TDO2 has been less well-studied in ovarian cancer. A study by Hsu et al. in 2012 looking at IκB kinases (IKKs) and their role in ovarian cancer invasion and metastasis showed that TDO2 is expressed in ovarian cancer cell lines. This study examined how IKK inhibitors affect gene expression and found that in addition to expected changes in genes involved in cellular motility and inflammation,TDO2 and another enzyme in the kynurenine pathway, kynureninase (KYNU), appear to play a role in IKK signaling [51], though the authors did not further investigate the role of trypophan catabolism in this process [51]. Gene expression similarities between EOC and TNBC [52] prompted us to examine whether TDO2 or IDO were more well correlated with tumor progression and outcome in EOC, since in breast cancer we found that TDO2 was correlated with clinical parameters, while IDO1 was not [31]. In the Tothill ovarian cohort (n = 293, [53]) we examined correlations between TDO2 and IDO1 and ovarian cancer outcomes. We found that while TDO2 was significantly associated with disease stage, recurrence and survival in ovarian cancer, IDO1 was not (Figure 2). These data indicate that TDO2 may be an important potential target in ovarian cancer and provides a rationale for further investigation into testing drugs that target TDO2 or dual inhibition of both TDO2 and IDO in EOC.
Figure 2.
TDO2 and IDO1 levels in ovarian cancer patients from the Tothill cohort (n = 293). RNA expression was compared between (A) disease stage, (B) recurrence status, and (C) the five-year survival rate of patients with high versus low levels of expression based on the median. Comparisons were statistically analyzed using one-way ANOVA for disease stage (TDO2 p = 0.003, IDO1 p = 0.262), unpaired t-test for recurrence, and log-rank for survival; mean ± standard deviation, * p < 0.05, ** p < 0.01.
Conclusion
Ovarian cancers are the most common cause of gynecological death. Unfortunately, EOC treatment is also extremely difficult since chemo-resistance often develops rapidly. The tryptophan catabolism pathway is well known to play a role in the aggressive nature of cancer. Higher levels of IDO1 and IDO2 and decreased TILs have been reported in more aggressive late-stage EOC compared to early stage EOC. TDO2, on the other hand, has not been examined in EOC. Gene expression data from clinical specimens suggests that TDO2 correlates with disease progression and may be a relevant target in the treatment of EOC. Thus, further exploration into the role of TDO2 in serous ovarian carcinoma is warranted.
ACKNOWLEDGMENTS
The authors thank Drs. Michael Gordon and Thomas Rogers for their assistance with the analysis of publically available ovarian cancer datasets.
ABBREVIATIONS:
- 5-HIAA
5-hydroxy indole acetic acid
- AhR
aryl hydrocarbon receptor
- APCs
antigen presenting cells
- EMT
epithelial to mesenchymal transition
- EOC
epithelial ovarian carcinoma
- HGSC
high grade serous ovarian carcinoma
- KYNU
kynureninase
- IHC
immunohistochemistry
- IDO
indoleamine 2,3-dioxygenase
- IKKs
IκB kinases
- KTR
kynurenine-to-tryptophan ratio
- NAD
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells)
- PD-1
programmed death protein 1
- PDCs
plasmacytoid dendritic cells
- PD-L1
programmed death-ligand 1
- TILs
tumor infiltrating lymphocytes
- TDO2
tryptophan 2,3-dioxygenase 2
- TNBC
triple-negative breast cancer
- Tregs
regulatory T cells
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
REFERENCES
- 1.Clement PB MD; Young Robert H. MD, FRCPath. (2014) Atlas of Gynecologic Surgical Pathology, Third edn., Elsevier Inc. [Google Scholar]
- 2.Howlader NNAM; Krapcho M; Miller D; Bishop K; Kosary CL; Yu M; Ruhl J; Tatalovich Z; Mariotto A; Lewis DR; Chen HS; Feuer EJ; Cronin KA 2014. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- 3.Erickson BK, Conner MG and Landen CN Jr. 2013. The role of the fallopian tube in the origin of ovarian cancer. Am. J. Obstet. Gynecol, 209, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nucci MR MD; Oliva Esther MD; Goldblum John R MD. (2009) Gynecologic Pathology: A Volume in the Series: Foundations in Diagnostic Pathology, First edn., Elsevier Inc. [Google Scholar]
- 5.Thiery JP 2003. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol, 15, 740. [DOI] [PubMed] [Google Scholar]
- 6.Simpson CD, Anyiwe K and Schimmer AD 2008. Anoikis resistance and tumor metastasis. Cancer Lett, 272, 177. [DOI] [PubMed] [Google Scholar]
- 7.Paoli P, Giannoni E and Chiarugi P 2013. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta, 1833, 3481. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA 2006. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med, 354, 34. [DOI] [PubMed] [Google Scholar]
- 9.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK and Sonke GS 2018. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med, 378, 230. [DOI] [PubMed] [Google Scholar]
- 10.Frisch SM and Francis H 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol, 124, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK and Richer JK 2009. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol. Cancer Ther, 8, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose S, Tanabe H, Nagayoshi Y, Hirata Y, Narui C, Ochiai K, Isonishi S, Takano H and Okamoto A 2018. Retrospective analysis of sites of recurrence in stage I epithelial ovarian cancer. J. Gynecol. Oncol, 29, e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC and Coukos G 2003. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med, 348, 203. [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A and Wigginton JM 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med, 366, 2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T and Konishi I 2015. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J. Clin. Oncol, 33, 4015. [DOI] [PubMed] [Google Scholar]
- 16.Reinartz S, Finkernagel F, Adhikary T, Rohnalter V, Schumann T, Schober Y, Nockher WA, Nist A, Stiewe T, Jansen JM, Wagner U, Muller-Brusselbach S and Muller R 2016. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol, 17, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang WT, Adams SF, Tahirovic E, Hagemann IS and Coukos G 2012. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol, 124, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takao M, Okamoto A, Nikaido T, Urashima M, Takakura S, Saito M, Saito M, Okamoto S, Takikawa O, Sasaki H, Yasuda M, Ochiai K and Tanaka T 2007. Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncol. Rep, 17, 1333. [PubMed] [Google Scholar]
- 19.Inaba T, Ino K, Kajiyama H, Yamamoto E, Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O and Kikkawa F 2009. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol. Oncol, 115, 185. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y and Guillemin GJ 2009. Kynurenine pathway metabolites in humans: disease and healthy States. Int. J. Tryptophan Res, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takikawa O 2005. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun, 338, 12. [DOI] [PubMed] [Google Scholar]
- 22.Brooks AK, Lawson MA, Smith RA, Janda TM, Kelley KW and McCusker RH 2016. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. J. Neuroinflammation, 13, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inayoshi Y, Kaneoka H, Machida Y, Terajima M, Dohda T, Miyake K and Iijima S 2005. Repression of GR-mediated expression of the tryptophan oxygenase gene by the SWI/SNF complex during liver development. J. Biochem, 138, 457. [DOI] [PubMed] [Google Scholar]
- 24.Ren S and Correia MA 2000. Heme: A regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch. Biochem. Biophys, 377, 195. [DOI] [PubMed] [Google Scholar]
- 25.Badawy AA 2002. Tryptophan metabolism in alcoholism. Nutr. Res. Rev, 15, 123. [DOI] [PubMed] [Google Scholar]
- 26.Weinlich G, Murr C, Richardsen L, Winkler C and Fuchs D 2007. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology, 214, 8. [DOI] [PubMed] [Google Scholar]
- 27.Masaki A, Ishida T, Maeda Y, Suzuki S, Ito A, Takino H, Ogura H, Totani H, Yoshida T, Kinoshita S, Narita T, Ri M, Kusumoto S, Inagaki A, Komatsu H, Niimi A, Ueda R, Utsunomiya A, Inagaki H and Iida S 2015. Prognostic Significance of Tryptophan Catabolism in Adult T-cell Leukemia/Lymphoma. Clin. Cancer Res, 21, 2830. [DOI] [PubMed] [Google Scholar]
- 28.Lyon DE, Walter JM, Starkweather AR, Schubert CM and McCain NL 2011. Tryptophan degradation in women with breast cancer: A pilot study. BMC Res. Notes, 4, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ and Chabot JA 2014. A biological basis for depression in pancreatic cancer. HPB (Oxford), 16, 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W and Platten M 2011. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478, 197. [DOI] [PubMed] [Google Scholar]
- 31.Greene LI, Bruno TC, Christenson JL, D’Alessandro A, Culp-Hill R, Torkko K, Borges VF, Slansky JE and Richer JK 2018. A Role for Tryptophan-2,3-dioxygenase in CD8 T-cell Suppression and Evidence of Tryptophan Catabolism in Breast Cancer Patient Plasma. Mol. Cancer Res, 17, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaira K, Nakamura K, Hirakawa T, Imai H, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Tsukamoto N, Oyama T, Asao T and Minegishi T 2015. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am. J. Transl. Res, 7, 1161. [PMC free article] [PubMed] [Google Scholar]
- 33.Furuya M, Horiguchi J, Nakajima H, Kanai Y and Oyama T 2012. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci, 103, 382. [DOI] [PubMed] [Google Scholar]
- 34.Badawy AA 2015. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep, 35, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F and Puccetti P 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature, 511, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfefferkorn ER 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. U. S. A, 81, 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta SL, Carlin JM, Pyati P, Dai W, Pfefferkorn ER and Murphy MJ Jr. 1994. Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect. Immun, 62, 2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D’Alessandro A, Hansen KC and Richer JK 2015. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res, 75, 4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Liang XQ, Peterson AJ, Munn DH and Blazar BR 2008. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol, 181, 5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U and Ferrara GB 2002. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med, 196, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A and Mellor AL 1999. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med, 189, 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC and Scherle PA 2010. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood, 115, 3520. [DOI] [PubMed] [Google Scholar]
- 43.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ 2003. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Med, 9, 1269. [DOI] [PubMed] [Google Scholar]
- 44.Talari NK, Panigrahi M, Madigubba S, Challa S and Phanithi PB 2016. Altered tryptophan metabolism in human meningioma. J. Neurooncol, 130, 69. [DOI] [PubMed] [Google Scholar]
- 45.Rogers TJ, Christenson JL, Greene LI, O’Neill KI, Williams MM, Gordon MA, Nemkov T, D’Alessandro A, Degala GD, Shin J, Tan AC, Cittelly DM, Lambert JR and Richer JK 2018. Reversal of triple-negative breast cancer EMT by miR-200c decreases tryptophan catabolism and a program of immunosuppression. Mol. Cancer Res, 17, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucarelli G, Rutigliano M, Ferro M, Giglio A, Intini A, Triggiano F, Palazzo S, Gigante M, Castellano G, Ranieri E, Buonerba C, Terracciano D, Sanguedolce F, Napoli A, Maiorano E, Morelli F, Ditonno P and Battaglia M 2017. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol. Oncol, 35, 461 e15. [DOI] [PubMed] [Google Scholar]
- 47.Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, Gunter MJ, Seckl MJ, Travis RC, Wareham N, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, Bueno-de-Mesquita HB, Boeing H, Wientzek A, Kuehn T, Kaaks R, Tumino R, Agnoli C, Palli D, Naccarati A, Aicua EA, Sanchez MJ, Quiros JR, Chirlaque MD, Agudo A, Johansson M, Grankvist K, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G, Weiderpass E, Riboli E, Brennan PJ, Vineis P and Johansson M 2014. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol. Biomarkers Prev, 23, 461. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T and Urashima M 2005. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin. Cancer Res, 11, 6030. [DOI] [PubMed] [Google Scholar]
- 49.Tanizaki Y, Kobayashi A, Toujima S, Shiro M, Mizoguchi M, Mabuchi Y, Yagi S, Minami S, Takikawa O and Ino K 2014. Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of ovarian cancer by inducing an immunosuppressive environment. Cancer Sci, 105, 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.How Our Immune System Can Help Fight Cancer. (2018). Retrieved from http://clinicaltrials.gov.
- 51.Hsu S, Kim M, Hernandez L, Grajales V, Noonan A, Anver M, Davidson B and Annunziata CM 2012. IKK-epsilon coordinates invasion and metastasis of ovarian cancer. Cancer Res, 72, 5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.network, C. G. A. 2012. Comprehensive molecular portraits of human breast tumours. Nature, 490, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, Traficante N, Fereday S, Hung JA, Chiew YE, Haviv I, Gertig D, DeFazio A and Bowtell DD 2008. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res, 14, 5198. [DOI] [PubMed] [Google Scholar]