Abstract
Factors implicated in the pathophysiology of intestinal inflammation include defects in intestinal epithelial barrier function, abnormal immune responses, and activities of the gut microbiota. Current agents used to treat human Inflammatory Bowels Disease (IBD), chronic inflammation of digestive tract, have serious side effects. In addition, most of these treatments target the damaging factors while not providing pro-healing factors that repair the damaged intestine. Here we provide a method to isolate, purify and characterize a specific population from ginger (ginger-derived nanoparticles: GDNPs 2) with anti-inflammatory activities. GDNPs 2 as a drug vehicle are a novel natural, nontoxic delivery system, which target the inflamed intestinal mucosa, blocks damaging factors while promoting pro-healing factors and could easily be developed for large-scale production aimed at the treatment of IBD.
Keywords: Ginger, Sucrose gradient ultracentrifuge, Nanoparticles, Ginger-derived nanoparticles, GDNPs 2, Natural drug delivery system, Inflammatory Bowels Disease, IBD
Background
Efforts to develop new drug-based therapeutic approaches against Intestinal Bowel Disease (IBD) must overcome several challenges, including issues of delivery, potential off-target effects, safety, toxicity, large-scale production costs, and tissue specificity ( Chen et al., 2017 ). We and other groups have recently demonstrated that artificially synthesized nanoparticles may be used to target low doses of drugs (e.g., siRNAs, proteins and peptides) to specific cell types or tissues ( Xiao et al., 2017 ). However, the nanoparticles synthesized to date have two major limitations: i) each constituent of the synthesized nanoparticles must be examined for potential in vivo toxicity before clinical application; and ii) the production scale is limited. The use of nanoparticles derived from natural sources may overcome these limitations of synthetic nanoparticles. Here, we provide a safe and cost-effective sucrose gradient ultracentrifuge method to isolate, purify and characterize a specific population of nanoparticles from ginger (ginger-derived nanoparticles: GDNPs 2) with anti-inflammatory activities. GDNPs 2 enable encapsulation of various types of therapeutic agents such as DNA, RNA and antibodies. Loading therapeutic agents in ginger-derived nanolipids can be found in a separate publication ( Zhang et al., 2017 ). GDNPs 2 represents a novel natural, nontoxic delivery system that targets the inflamed intestinal mucosa, blocks damaging factors while promoting healing factors and may easily be developed for large-scale production aimed at the treatment of IBD.
Materials and Reagents
Pipette tips 0.1-10 μl, 1-200 μl, 100-1,000 μl (Sorenson Bioscience, catalog numbers: 70600, 70520, 70540)
Pipettes 0.5-10 μl, 10-100 μl and 100-1,000 μl (Eppendorf, catalog number: 13-684-251)
Thin wall, Ultra clear 13.2 ml, 14 x 89 mm ultracentrifuge tubes (Beckman Coulter, catalog number: 344059)
Thick wall, 38 ml, 25 x 89 mm centrifuge tubes polycarbonate (Beckman Coulter, catalog number: 355631)
Thick wall, 70 ml, 38 x 102 mm centrifuge bottles polycarbonate (Beckman Coulter, catalog number: 355655)
Eppendorf Safe-Lock Tubes, 1.5 ml (Eppendorf, catalog number: 022363204)
50 ml conical tubes (Denville Scientific, catalog number: C1062-P)
Formvar®-coated copper grids (Electron Microscopy Sciences, catalog number: FCF300-CU-SC)
Filter paper (VWR, catalog number: 28313-068)
Sterile cell strainer 40 μM (Fisher Scientific, catalog number: 22-363-547)
Capillary cell & plastic cap (Malvern, catalog number: DTS 1070)
Protein quantification assay kit (Bio-Rad, catalog number: 5000002)
Phosphate-buffered Saline (Corning, catalog number: 21-040-CV)
Simulated intestinal fluids (RICCA Chemical, catalog number: 7109-16)
Simulated gastric fluids (RICCA Chemical, catalog number: 7108-16)
Uranyl acetate (Electron Microscopy Sciences, catalog number: 22400-4)
Mica sheet (Electron Microscopy Sciences, catalog number: 71855-15)
Sucrose (Calbiochem, catalog number: 8510) (see Recipes)
Sucrose gradient (8, 30, 45, 60%) (see Recipes)
1% uranyl acetate solution (see Recipes)
Equipment
Juicer (Breville, model: BJE200XL)
Sorvall ST 16R centrifuge (Thermo Fisher Scientific, catalog number: 75004381)
Optima L-90K Ultracentrifuge (Beckman Coulter, catalog number: 969349)
-
Rotors:
J-Lite Series JLA 16.250 16,000 RPM (Beckman Coulter, Serial number O8U 3761)
Type 45 Ti 45,000 RPM (Beckman Coulter, Serial number 99E.2785)
SW 32 Ti 32,000 RPM (Beckman Coulter, Serial number 11U 2963)
Zetasizer (Malvern, model: Nano-ZS90)
Particle size analyzer (Brookhaven Instrument Corps, model: 90PLUS)
Atomic force microscopy instrument (Seiko Instrument Inc., model: SPA400)
Transmission electron microscope (Carl Zeiss, model: LEO 906E)
Milli-Q advantage A10 water purification system (Millipore-sigma, catalog number: C10117)
Semi-micro cuvettes, clear 1.5 ml (Sigma, catalog number: BR759115)
Ultrasonic bath (Branson, catalog number: 3510MTH)
Fluorometric microplate reader (Biotek, model: Synergy 2)
pH meter (Sper Scientific, catalog number: 860031)
-80 °C freezer (So-Low, model: PV85-21)
Procedure
-
Isolation (see Note 1) (Figure 1)
Note: Approximate amounts of GDNPs 2 acquired from 1 kg of ginger is 50-100 ng/ml.
Wash 1 kg of roughly peeled ginger thoroughly with tap water.
Grind sliced ginger with blender to obtain juice.
Centrifuge approximately 550 ml of the ginger juice at 3,000 × g for 20 min at RT.
Centrifuge again at 10,000 × g for 2 h at RT to remove large fibers.
Collect the supernatant and equally distribute (~45 ml) into twelve 70 ml capacity tubes.
Ultracentrifuge the collected supernatant at 4 °C, 150,000 × g for 2 h (Use Type 45 Ti 45,000 RPM). Ultracentrigfuge 6 tubes two times.
Remove supernatant and suspend the pellet in each tube in 5 ml PBS through ultrasonic dispersion. After proper pipetting, all the nanoparticle pellets should be well suspended in PSC solution. If insoluble pellet exists, a cell strainer can be used to remove the insoluble pellet before loading to the gradient centrifugation.
-
Purification
Prepare twelve 38.5 ml capacity tubes filled with 10 ml of each sucrose solution in the order of 8%, 30% and 45% from top to bottom.
Transfer 5 ml of the PBS suspended pellet from each tube to discontinuous sucrose gradient (8%, 30%, 45% [gram/volume]) in the 12 tubes and ultracentrifuge with SW 32 Ti rotor at 4 °C, 150,000 × g for 2 h (see Recipe 1). Ultracentrigfuge 6 tubes two times.
Collect ~2 ml of band 2 and ~0.6 ml of band 1 respectively from 8/30% and 30/45% interfaces by gently dipping pipette tips into the sucrose gradient solution (see Data analysis).
Ultracentrifuge the collected bands at 4 °C, 100,000 × g for 45 min (use Type 45 Ti 30,000 RPM), remove the supernatant.
Wash the pellet with 1 ml PBS to remove the remaining sucrose and then suspend the pellet in 1 ml PBS through ultrasonic dispersion.
-
Measure the concentration of obtained GDNPs (0.25~1.25 mg/ml) using protein quantification assay kit (follow the manufacturer’s manual) and microplate reader at absorbance of 750 nm.
Note: Proteins contained by GDNPs were used to quantitate the GDNPs. The assay is based on the reaction of protein with an alkaline copper tartrate solution and Folin reagent (DC Protein Assay Instruction Manual). Proteins effect a reduction of the Folin reagent by loss of 1, 2, or 3 oxygen atoms, thereby producing one or more of several possible reduced species which have a characteristic blue color with maximum absorbance at 750 nm. Color development is primarily due to the amino acid tyrosine and tryptophan groups.
For short term storage (less than 3 months), GDNPs can be suspended in PBS and stored at -80 °C. Long term stability has not been tested (Only Band 2 for Characterization; Discard Band 1).
-
Characterization (GDNPs 2)
-
Test the nanoparticle for its in vitro stability (see Note 6)
Note: Measure particle size and zeta potential before and after incubation in SGF/IGF for comparison.
Add 1 ml of nanoparticles (1 mg/ml) to 9 ml of simulated gastric fluid (SGF) and simulate intestinal fluid (SIF) in a separate tube.
Mix thoroughly by inverting tubes.
Dilute 0.2 ml of mixture into 1.8 ml PBS.
Determine the particle size and zeta potential before incubation (follow Steps C2 and C3 below).
Incubate the mixture in 37 °C water bath for 30 min.
Determine the particle size and zeta potential after incubation.
-
Measure particle size (Instruction for Steps C1d and C1f, see Data analysis, Figure 2)
Dilute 0.2 ml of mixture into 1.8 ml PBS.
Carefully add all of the diluted mixture to a cuvette without bubbles.
Insert the cuvette to the particle analyzer chamber.
Determine the size of particles.
-
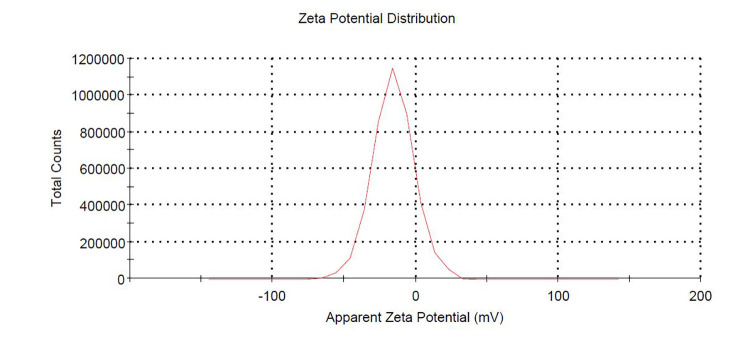
Measure zeta potential (Instruction for Steps C1d and C1f, see Data analysis, Figure 1)
Carefully add 0.8 ml of mixture to capillary cell.
Cover the capillary cell with plastic cap.
Insert the cell to the chamber of zeta potential analyzer.
Determine the numbers for the sample at neutral pH.
-
Acquire transmission electron microscopy (TEM) image (Figure 5)
Deposit a drop of sample onto the surface of a formvar-coated grid.
Add 1% uranyl acetate on top of the sample and wait for 15 s (see Recipe 2).
Carefully absorb the sample and uranyl acetate with tissue without touch the surface.
Dry the sample at room temperature for 5 min.
Scan the sample image with a transmission electron microscope.
-
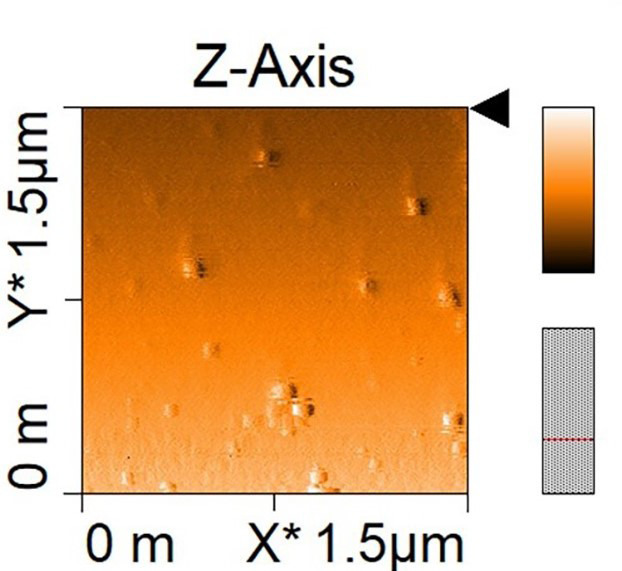
Acquire atomic force microscopy (AFM) image (Figure 6)
Deposit 1 μl of nanoparticle sample to mica sheet.
Dry the sample at room temperature.
Gently rinse the mica sheet with 2.5 μl of distilled water three times.
Dry the sample at room temperature again.
Leave it until the sample become flat.
Scan the sample with area of about 4 x 4 μm and 2-50 nm in height.
-
Figure 1. Ginger juice extraction.
Sliced ginger is squeezed in the juicer to obtain juice for nanoparticle isolation and purification.
Figure 2. Sucrose gradient ultracentrifugation.
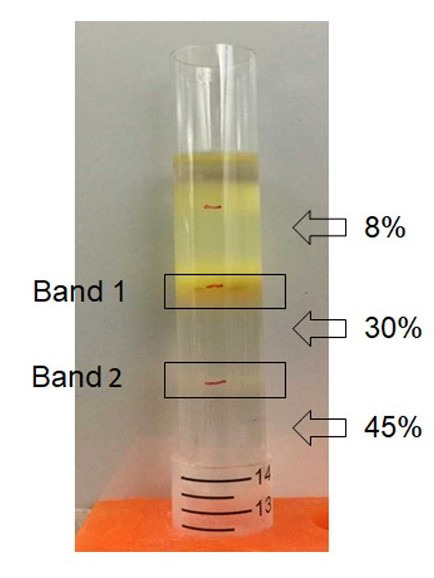
GDNPs were added to 8, 30, 45 % of sucrose gradient and ultracentrifuged for purification.
Figure 5. TEM (Transmission electron microscopy) image of GDNPs 2.
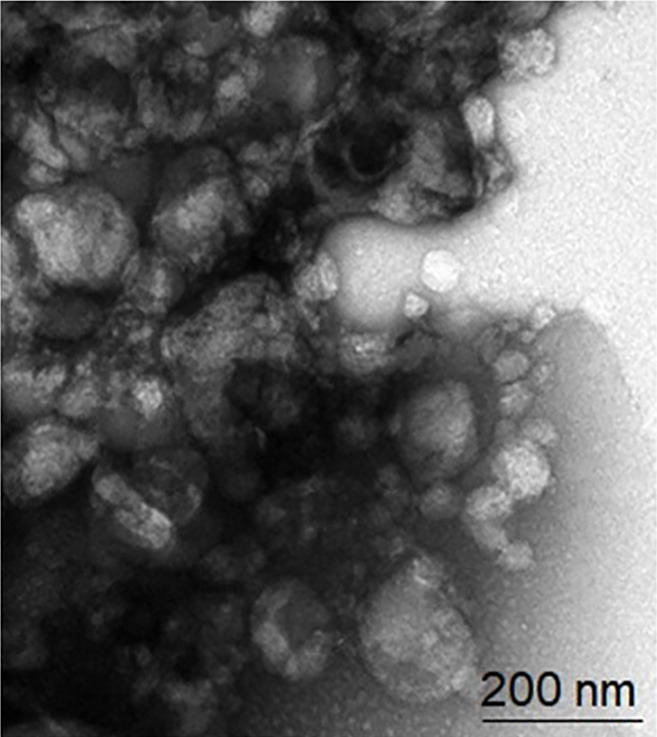
Figure 6. AFM (Atomic force microscopy) image of GDNPs 2.
Data analysis
Sucrose gradient ultracentrifuge methods showed two concentrated bands, 8/30% (band 1) and 30/45% (band 2). Photon correlation spectroscopy (PCS) was used to measure size distribution and zeta potential of the GDNPs. The average nanoparticle size of band 2 was approximately ~115.8 nm in radius (Figure 3). An approximate zeta potential value was ~12 mV at pH 6 for band 2 (Figure 4). Transmission electron microscopy (TEM) and atomic force microscopy (AFM) analysis also confirmed successful isolation and characterization of stable GDNPs (Figures 5 and 6). In vitro stability test for GDNPs 2 confirmed the tolerance of the nanoparticle within harsh environment such as gastric and intestinal tract. Precisely measured particle size and zeta potential of GDNPs 2 ensure the capability to be used as stable drug carrier in human system. Also, TEM and AFM probed ability of GDNPs 2’s electromechanical functionality which is critical for characterization for biological materials.
Figure 3. Measurement of band 2 ginger nanoparticle size.
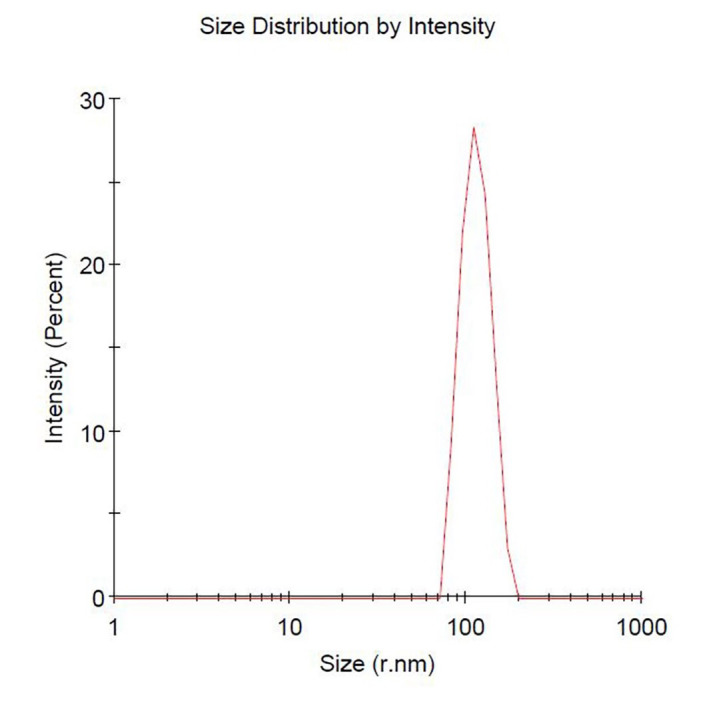
Approximate nanoparticle size was determined to be ~115.8 nm in radius.
Figure 4. Measurement of zeta potential of band 2 ginger nanoparticle.
Approximate zeta potential measured by Malvern zetasizer was ~-12 mV at pH 6.
Notes
Filtrate the sucrose solutions with 0.22 μm membrane filter to eliminate small particles.
Pre-warm water bath to 37 °C.
Be sure to balance the particle size analyzer and zetasizer for at least 15 min before use.
Avoid generating air bubbles in cuvettes for size and zeta potential measurement.
Recipes
-
Sucrose gradient (8, 30, 45%)
Dissolve each 8, 30, 45 g sucrose powder in 70 ml distilled water then make up the volume to 100 ml
Pipette each sucrose solution to make a gradient in the order from the bottom; 45%, 30%, and 8%. For 5 ml raw GDNPs suspension, 10 ml 8%, 10 ml 30%, and 10ml 45% sucrose solution were used. Total 30 ml of sucrose gradient is filled in the 38 ml capacity tubes
-
1% uranyl acetate solution
Dissolve 4 g uranyl acetate powder into 100 ml preheated (50-60 °C) filtered water to prepare 4% uranyl acetate stock solution
Dilute 1 ml 4% uranyl acetate stock solution into 3 ml of filtered water
Acknowledgments
This work was supported by National Institute of Health of Diabetes and Digestive and Kidney (RO1-DK-107739 & RO1-DK-116306 to D.M) and the Department of Veterans Affairs (Merit Award BX002526 to D.M). D. Merlin is a recipient of a Senior Research Career Scientist Award from the Department of Veteran Affairs (BX004476). EV is a recipient of the Career Development Award from the Crohn’s and Colitis Foundation. This protocol was adapted from published work by Zhang et al. (2016).
Competing interests
The authors declare no conflicts of interest within the work.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Chen Q., Xiao B. and Merlin D.(2017). Nanotherapeutics for the treatment of inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 11(6): 495-497. [DOI] [PubMed] [Google Scholar]
- 2. Xiao B., Ma L. and Merlin D.(2017). Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv 14(1): 65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z., Han M. K., Xiao B., Xu C., Srinivasan S. and Merlin D.(2016). Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101: 321-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang M., Wang X., Han M. K., Collins J. F. and Merlin D.(2017). Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine(Lond) 12(16): 1927-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]