Abstract
Background
In horses and ponies numerous medical conditions are known to be linked with inflammation in different tissues, especially in the liver. Besides affecting other metabolic pathways such as the expression of certain interleukins (IL), inflammation is associated with stress of the endoplasmic reticulum (ER). In particular, ER stress leads to adaptive stress response and can be measured by several markers of inflammatory and stress signalling pathways, like nuclear factor κB (NF-kB).
Objectives
To investigate lipopolysaccharide (LPS)-induced inflammatory reactions and their modulation in horses and ponies by feeding a polyphenol-rich supplement consisting of green tea and curcuma.
Methods
In a cross-over study, 11 animals were allocated to either a placebo or a supplement group and supplemented with 10 g of a blend of green tea and curcuma extract (GCE) or a placebo (calcium carbonate) once daily. After 21 days of supplementation, all animals underwent a LPS challenge to induce moderate systemic inflammation. Blood samples and liver biopsies were taken at standardized time points: 24 hours before and 12 hours after LPS challenge. Inflammatory blood parameters such as serum amyloid A (SAA), haptoglobin and retinol binding protein 4 (RBP4) were measured in serum. Hepatic mRNA levels of selected markers of inflammation such as haptoglobin, tumor necrosis factor α (TNF-α), IL-1β, IL-6, cluster of differentiation 68 (CD68), fibroblast growth factor 21 (FGF-21), NF-κB, activating transcription factor 4 (ATF4) were quantified by RT-qPCR. In addition, liver biopsies were examined histologically for inflammatory alterations.
Results
Blood markers of acute inflammatory response increased after LPS challenge. In the liver, the proinflammatory cytokine IL-1β showed significantly lower mRNA levels after LPS challenge in the supplemented group (P = 0.04) compared to the placebo group. Levels of the hepatic CD68 mRNA increased significantly in the placebo group (P = 0.04). There were no significant differences between supplemented and placebo groups concerning other markers of inflammation and markers of ER stress within the liver. The number of hepatic macrophages were not different after LPS challenge in both feeding groups.
Conclusion
LPS was able to induce inflammation but seemed less suitable to induce ER stress in the horses and ponies. The polyphenol-rich supplement showed some potential to reduce inflammatory responses. Nevertheless, the supplementation did not exert an overall anti-inflammatory effect in horses and ponies.
Keywords: Curcumin, Catechin, Equines, ER-stress, Polyphenols
Introduction
Horses may suffer from medical conditions that result in a low-grade inflammatory state in different tissues, especially in the liver. For example, obesity was recently suggested to induce a low-grade inflammation in horses (Vick et al., 2007). As inflammation may trigger stress of the endoplasmic reticulum (ER), equine obesity might be associated with hepatic ER stress as well (Marycz et al., 2018). ER stress is defined as an imbalance between folded and unfolded or misfolded proteins, which in consequence accumulate in the ER lumen (Zhang & Kaufman, 2008). The accumulation leads to the activation of an adaptive stress response, called the “unfolded protein response” (UPR; Kozutsumi et al., 1988; Hotamisligil, 2010). The UPR is mainly exerted by key mediators, such as nuclear factor κB (NF-κB) and results in an elevated proinflammatory response (Hotamisligil, 2010). Inflammatory cytokines not only trigger the cascade of ER stress but their genes are also known as NF-κB target genes. Therefore, after activation of transcription factor NF-κB the expression of several target genes, such as those for tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 1β (IL-1β), haptoglobin and serum amyloid A (SAA) are induced (Gessner et al., 2013; Chaudhari et al., 2014). Oxidative stress, an imbalance between the excessive formation of oxidants and inadequate antioxidant defenses, activates the NF-κB pathway and is therefore also directly linked with inflammation. Numerous nutraceuticals are available with claims for altering inflammatory responses and reducing ER stress. Recently, the anti-inflammatory and antioxidant properties of selected polyphenol-rich plant metabolites such as tea catechins (Gaur & Agnihotri, 2014) and curcumin, a polyphenol of Curcuma longa (Dulbecco & Savarino, 2013), have been the focus of interest in studies of humans and animal species such as cattle (Winkler et al., 2015) and pigs (Gessner et al., 2013). By interfering with the key regulator NF-κB (Gessner et al., 2013) the production of several inflammatory cytokines such as TNF-α and IL-1β are inhibited (Jurenka, 2009; Gaur & Agnihotri, 2014). As an example, flavonoids derived from grape seeds and grape marc meal had anti-inflammatory effects on TNF-α and SAA production in the duodenal mucosa of pigs (Gessner et al., 2013). Additionally, Winkler et al. (2015) described the potential to increase performance parameters in livestock by feeding a blend of polyphenols derived from green tea and curcuma extract to dairy cows. Data on anti-inflammatory and antioxidative properties of polyphenols are lacking in the equine. Therefore, the aim of the study was to investigate selected markers of inflammation in blood and liver tissue after an inflammatory stimulus with or without supplementation of a green tea and curcuma extract (GCE). We hypothesized that feeding a polyphenol-rich diet has the potential to reduce inflammation and ER stress in horses and ponies.
Materials & Methods
Animals
Five adult Warmblood horses and six adult Shetland ponies owned by the Institute of Animal Nutrition, Nutrition Diseases and Dietetics, University of Leipzig were used in this study. The horses had a mean (±SD) age of 19 ± 5 years and mean (±SD) body weight (BW) of 589 ± 81 kg. Mean (±SD) age of ponies was 9 ± 3 years and mean (±SD) BW was 126 ± 8 kg. The animals were housed in groups on different paddocks with a shelter hut and had free access to water and salt at all times. The project was approved by the Ethics Committee for Animal Rights Protection of the Leipzig District Government (No TVV 34/16), in accordance with German Legislation for Animal Rights and Welfare. All animals were given a thorough physical examination prior to supplementation period to determine that they were healthy.
Supplementation period
All animals were fed meadow grass hay ad libitum before starting the experiment. During adaptation period, all animals were fed meadow grass hay (2 kg/100 kg BW) for 21 days. After adaptation a cross-over study was performed by additionally feeding either 10 g of a blend of green tea (95%) and curcuma (5%) extract (Spicemaster© CP Alpha, Kaesler Nutrition GmbH, Cuxhaven, Germany; total polyphenol content of 20%) or 10 g CaCO3 (Kaesler Nutrition GmbH, Cuxhaven, Germany) as placebo once daily for 21 days. The supplement or the placebo were mixed in 1 kg (horses) or 0.2 kg (ponies) of a commercial feed (Pavo Pferdenahrung GmbH, Vechta Langförden, Germany) and fed to each animal individually. Intake of the supplement or placebo was monitored.
Wash-out period
A 3 month wash-out period was conducted between the change of feeding regime according to the cross-over design. During the wash-out period, animals were fed with meadow grass hay ad libitum and had access to water and salt at all times.
LPS challenge
After 3 weeks of supplementation animals underwent a LPS challenge. An indwelling venous catheter (Braunüle MT, B. Braun Melsungen AG, Melsungen, Germany) was aseptically inserted into the jugular vein. 10 ng/kg BW of LPS (Escherichia coli O55:B5, Sigma-Aldrich Chemie GmbH, Munich, Germany) was mixed in 1,000 mL (horses) or 500 mL (ponies) 0.9% saline (B. Braun Melsungen AG, Melsungen, Germany) and infused over 30 min. Heart rate, respiratory rate, rectal temperature, sweating, appetite, general behaviour and interaction with the examiner were monitored, using a modified pain score protocol proposed by Bussières et al. (2008) before and in 30 min intervals for 3 h after LPS challenge. Each parameter was categorized from 0 (= physiologic) to 3 (= pathologic). Clinical parameters such as colour of oral mucosa, capillary refilling time, digital pulsation and defecation were also monitored in the above-mentioned intervals.
Blood sampling
Blood samples were collected from the jugular vein 24 h before and 12 h after LPS infusion directly into 3 blood collection tubes (Monovette, Sarstedt AG & Co. KG, Nuremberg, Germany) containing K3-EDTA, lithium heparin or a coagulation activator. Lithium heparin and K3-EDTA tubes were centrifuged immediately at 865 g for 10 min. The serum tubes were centrifuged after 30 min of clotting at room temperature under the same conditions. Serum and plasma were frozen in multiple aliquots of 1 mL at −20 °C followed by storage at −80 °C until analysis.
Liver sampling
Liver biopsies were taken 24 h before and 12 h after LPS infusion. The animals were sedated with xylazine i.v. (0.5 mg/kg BW, Proxylaz, bela-pharm GmbH & Co. KG, Vechta, Germany). Biopsies for liver tissue sampling were performed transcutaneously on the right body side in the 12th or 13th intercostal space at the level of the tuber ischiadicum in the horses and approximately 5 cm ventrally to the level of the tuber coxae in the ponies. After clipping, surgical scrubbing, skin disinfection and injection of 2 mL lidocaine hydrochloride s.c and i.m. for local anaesthesia (20 mg/mL, Lidocainhydrochlorid 2%, bela-pharm, Vechta, Germany) a 1 cm vertical skin incision was made in the intercostal space at the cranial side of the rib. Ultrasound-guided biopsy was performed with a fully automated biopsy gun (BIP High Speed Core Cut 2, BIP Biomed. Instrumente & Produkte GmbH, Tuerkenfeld, Germany) using a 12 G x 15 cm biopsy needle. Liver samples were immediately transferred to a sterile tube (CryoPure© Tube, Sarstedt AG & Co. KG, Nuremberg, Germany) and shock frozen in liquid nitrogen (−196 °C). Liver samples were then stored at −80 °C until further analysis. A second liver sample was transferred to a tube containing formalin (10%) and stored at room temperature pending histological examination.
Analyses
SAA and haptoglobin
SAA (Eiken SAA, Eiken Chemical CO, Tokyo, Japan) and haptoglobin (PHASE™ RANGE Haptoglobin, Tridelta Development Limited, Maynoooth, Ireland) were measured by ABX Pentra C400 (HORIBA Europe, Darmstadt, Germany). Quality controls before measurements were performed according to the manufacturer’s instructions.
Retinol binding protein 4
Serum RBP4 was analysed by 12% sodium dodecyl–polyacrylamide gel electrophoresis (SDS-PAGE), using the buffer system of Laemmli (1970). After SDS-PAGE, the proteins were transferred from the gel onto a polyvinylidene difluoride membrane (Merck KGaA, Darmstadt, Germany) for 60 min, and blocked with 5% milk powder in tris-buffered saline with 0.1% Tween 20 (TBST, pH 7.6) for 1 h. The membranes were incubated for 90 min with 1:300 diluted cross-reacting rabbit anti-human RBP4 (DakoCytomation GmbH, Hamburg, Germany). After washing with 0.3% TBST, the membranes were incubated with 1:500 diluted peroxidase-conjugated sheep anti-rabbit IgG (EnVision K4003; DakoCytomation GmbH Germany) for 1 h. The colour reaction was developed using Luminol reaction (Chemiluminescence Blotting Substrate, Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. Band intensity of RBP was read with an imager (Bio-Rad, Munich, Germany) and analysed with the Bio-Rad Discovery software 1.1.
Hepatic mRNA levels
The quantity of mRNA transcripts of inflammatory cytokines in liver tissue was analysed using quantitative real-time PCR. RNA was isolated from liver tissue using a commercial kit (RNeasy® Lipid tissue Mini Kit, QIAGEN GmbH, Hilden, Germany) following the manufacturer’s protocol and RNA quantity was measured by spectrophotometry (NanoVue® Plus, Healthcare Biosciences AB, Munich, Germany). The RNA samples were transcribed into cDNA using a mastermix (random primer, deoxynucleotide triphosphates (dNTPs®), 5x First Strand Buffer, dithiothreitol (DTT)) and SuperScript™ II Reverse Transcriptase (QIAGEN GmbH, Hilden, Germany). All samples were run through standardised protocols using Peltier Thermal Cycler-200 (MJ research, St. Bruno, Canada). Gene sequences encoding the genes of interest TNF-α , IL-6, IL-1β, NF-κB, haptoglobin, CD68, FGF 21 and ATF4 were obtained from the database ensemble (http://www.ensembl.org). The 18S RNA and ribosomal protein L32 (RPL32) were chosen as reference genes. An RNA-probe was used for the 18S RNA. Primer (biomers.net GmbH, Ulm, Germany) validation followed standard procedures. The length varied between 16–24 base pairs, with minimum content of guanine and cytosine bases of 40–60%. When possible, more than 3 terminal t bases and more than 4 repeating structures were avoided. The program Primer3 was used to validate melting temperatures and for preventing possible secondary structures such as hairpins, homodimers and heterodimers. Table 1 gives an overview of the used primer sequences. Standard curves were generated with serial dilutions of pooled cDNA of all samples for quantification of the transcripts. For performing the quantitative real-time PCR the assays were run through a standard program of TaqMan™ (7500 Real Time PCR System, Thermo Fisher Scientific Inc., Schwerte, Germany) with minor modifications. Power SYBR Green PCR Master Mix and TaqMan™ Universal Master Mix II (Thermo Fisher Scientific Inc., Schwerte, Germany) were used. The genes of interest were normalised against the geometric mean of the two reference genes.
Table 1. Primer sequences used to analyse the mRNA levels of genes of interest and reference genes.
| Forward (5’-3’) | Reverse (3’-5’) | |
|---|---|---|
| IL-6 | CCACCTCAAATGGACCACTACTC | TTTTCAGGGCAGAGATTTTGC |
| TNF-α | AAAGGACATCATGAGCACTGAAAG | GGGCCCCCTGCCTTCT |
| CD68 | CTTTGGGCCAAGTTTCTCTTGT | AAGAGGCCGAGGAGGATCAG |
| IL-1β | CGGCAATGAGAATGACCTGT | GCTTCTCCACAGCCACAATG |
| Haptoglobin | AGAAAGCAGCCTGTGGAGAT | AGCCAGACACATAACCCACA |
| NF-kB | GCTTTGTGACAAGGTGCAGA | ACGATCATCTGTGTCTGGCA |
| FGF-21 | GATGATGCCCAGGAGACAGA | AAGTGGAGCGATCCGTACAG |
| ATF4 | TGGTCTCAGACAACAGCAAG | AGCTCATCTGGCATGGTTTC |
| RPL32 | AGCCATCTACTCGGCGTCA | TCCAATGCCTCTGGGTTTC |
Immunohistochemistry
Immunohistochemistry was performed on liver biopsies using an antibody against lysozyme (rabbit pAb, A099, Dako Deutschland GmbH, Hamburg, Germany). Sections were deparaffinized in xylene substitute (Roti-Histol, Carl Roth GmbH & Co KG, Karlsruhe, Germany) and rehydrated using a descending alcohol series. Blocking of endogenous peroxidase activity was carried out by incubation in methanol with 0.5% hydrogen peroxide. Slides were pretreated with 0.05% protease for antigen retrieval. The primary antibody was diluted 1:600 in tris-buffered saline (TBS) and was incubated at 4 °C overnight. As a secondary antibody pig anti rabbit IgG (Vector Laboratories, Burlingame, California) was used together with rabbit PAP (peroxidase-anti-peroxidase; Z113, Dako Deutschland GmbH, Hamburg, Germany) as detection system. Antibody binding was visualized using 3,3′-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO) as chromogen, and sections were counterstained with Papanicolaou solution (Merck KGaA, Darmstadt, Germany). Slides were dehydrated in an ascending alcohol series and mounted with a xylene-based solution. All liver biopsies were examined and semi-quantitatively evaluated dependant on the number of macrophages within each liver biopsy as follows: grade 0 = no macrophages, grade 1 = mild (1–33 macrophages), grade 2 = moderate (34–66 macrophages) and grade 3 = severe (67–100 macrophages). For each sample the number of macrophages was counted in 5 high power fields (HPF, 400x total magnification). The examiner was blind to the treatment group of origin of the samples.
Statistics
The commercial statistical software program STATISTICA© (StatSoft GmbH, Hamburg, Germany) was used for statistical analyses. All data on blood and liver parameters were checked for normal distribution by using Shapiro–Wilks test. As data set was not normally distributed Wilcoxon test for non-parametric data was used for analyses. Level of significance was set at P < 0.05. Data are shown as median and 25./75. percentiles. Immunohistochemical results were assessed and are presented descriptively.
Results
There were no feed refusals of the compound feed mixed either with GCE or placebo for horses and ponies. In addition, there were no recorded hay refusals in either group during the supplementation period.
LPS challenge
All animals showed behavioural signs of discomfort (e.g., yawning, pawing and anorexia) up to 180 min after LPS infusion (data not shown). Rectal temperature increased significantly (P < 0.0001) from mean (±SD) basal temperature of 37.3 ± 0.31 °C up to mean (±SD) maximum temperature of 38.5 ± 0.59 °C after LPS challenge.
Blood parameters
SAA and haptoglobin
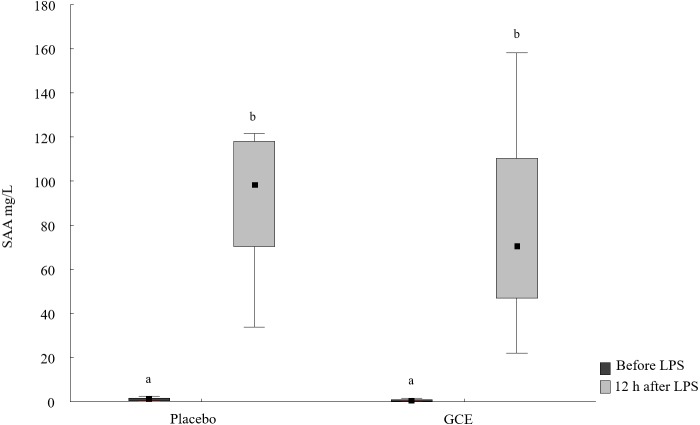
SAA concentrations increased 12 h after LPS challenge compared to basal SAA levels for both the GCE group (P = 0.003) and placebo group (P = 0.008, see Fig. 1 and Table 2). The increase in SAA was not significantly different between GCE and placebo supplementation groups. Twelve hours after LPS infusion, haptoglobin increased compared to baseline samples in the GCE supplemented animals (P = 0.005; see Table 2) but not in the placebo group. However, serum haptoglobin levels were not significantly different between feeding groups at both time points.
Figure 1. SAA concentrations before and 12 hours after lipopolysaccharide (LPS) challenge in horses and ponies fed placebo or green tea and curcuma extract (GCE).
Data are shown as median (squares), 25./75. percentiles (boxes), minimum and maximum (whiskers). Different superscript letters indicate significant differences (P < 0.05).
Table 2. Markers of inflammation in serum before and 12 hours after lipopolysaccharide (LPS) challenge in horses and ponies fed placebo or green tea and curcuma extract (GCE).
Values are presented as median (25./75. percentiles). Different superscript letters indicate significant differences (P < 0.05) within a row.
| Parameter | Placebo | GCE | ||
|---|---|---|---|---|
| Before LPS | 12 h after LPS | Before LPS | 12 h after LPS | |
| SAA [µg/mL] | 1.2a (0.3/1.9) | 98.4b (70.2/118) | 0.6a (0.1/1.2) | 70.7b (46.8/111) |
| Haptoglobin [mg/mL] | 1.5ab (1.1/1.8) | 1.6ab (1.2/2.5) | 1.3a (1.2/1.7) | 1.7b (1.3/1.8) |
| RBP4 [µg/mL] | 4.7a (3.5/5.9) | 4.2a (2.7/5.6) | 4.2a (3/6.1) | 4.1a (2.5/5.6) |
Serum RBP4 concentrations
Serum RBP4 concentrations did not show significant changes, either by LPS stimulus or between feeding group (see Table 2).
Hepatic mRNA levels
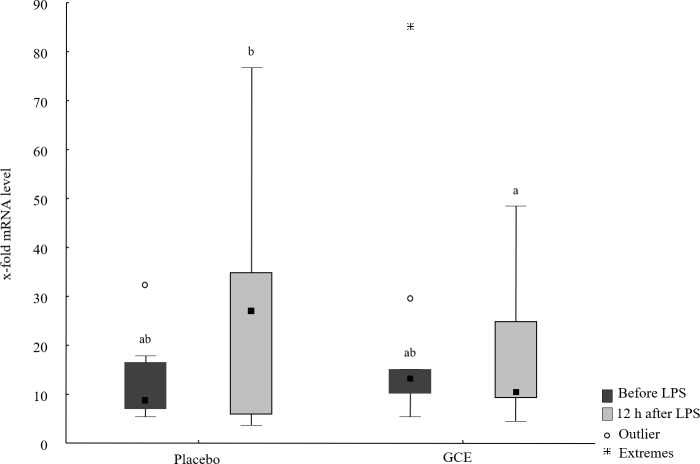
Baseline mRNA levels of all hepatic parameters were not different between the GCE group compared to placebo group. mRNA levels of TNF-α, IL-6, NF-κB and ATF4 did not show significant changes induced by LPS or by supplementation. In the placebo group, mRNA levels of haptoglobin were higher (P = 0.03) after LPS challenge compared to baseline. In GCE group, LPS challenge did not significantly change mRNA levels of haptoglobin. However, mRNA levels of haptoglobin were not significantly different between feeding groups at either time point. Levels of CD68 mRNA were higher (P = 0.04) after LPS challenge compared to baseline in the placebo group but not in the GCE group. Comparing both feeding groups 12 h after LPS revealed no significant differences in mRNA levels of CD68. After LPS challenge mRNA levels of IL-1β tended to be higher in placebo group but differences were not statistically significant. Levels of IL-1β mRNA in GCE group were not altered significantly by LPS challenge. Twelve hours after LPS, GCE group had significantly lower mRNA levels in IL-1β than did the placebo group (P = 0.04, see Fig. 2). Levels of FGF21 mRNA did not show significant changes induced by LPS challenge in both feeding groups although levels in GCE group showed a trend towards reduction after LPS challenge (P = 0.05, see Table 3). Levels of FGF21 mRNA were not significantly different between feeding groups after LPS.
Figure 2. Liver mRNA levels of IL-1β before and 12 hours after lipopolysaccharide (LPS) challenge in horses and ponies fed placebo or green tea and curcuma extract (GCE).
Data are shown as median (squares), 25./75. percentiles (boxes), minimum and maximum (whiskers). Different superscript letters indicate significant differences (P < 0.05).
Table 3. Hepatic mRNA levels of markers of inflammation and stress of the endoplasmic reticulum (ER) before and 12 hours after lipopolysaccharide (LPS) challenge in horses and ponies fed placebo or green tea and curcuma extract (GCE).
Values are presented as median (25./75. percentiles). Different superscript letters indicate significant differences (P < 0.05) within a row.
| Parameter | Placebo | GCE | ||
|---|---|---|---|---|
| Before LPS | 12 h after LPS | Before LPS | 12 h after LPS | |
| TNF-α | 7.66a (2.98/10.3) | 4.37a (3.94/9.68) | 4.32a (3.12/6.2) | 7.88a (3.86/12) |
| Haptoglobin | 1.03a (0.87/1.33) | 1.35b (1.24/1.49) | 1.18ab (0.64/1.7) | 1.51ab (1.15/1.7) |
| NF-κB | 2.21a (1.18/3.76) | 2.12a (1.63/3.32) | 2.04a (1.59/2.87) | 3.70a (2.22/4.63) |
| ATF4 | 1.87a (0.96/3.24) | 1.38a (1.06/1.58) | 1.72a (1.23/2.77) | 1.19a (0.51/1.87) |
| CD68 | 4.58a (2.8/9.45) | 8.63b (6.02/11.8) | 5.49ab (3.65/6.65) | 11.58b (6.96/16) |
| FGF21 | 1.46ab (0.12/5.18) | 0.15b (0.05/0.59) | 1.65a (0.14/6.36) | 0.08ab (0.02/1.6) |
| IL-6 | 7.71a (3.64/74.9) | 4.29a (2.05/8.22) | 1.81a (1.7/3.78) | 3.55a (2.35/9.08) |
| IL-1β | 8.63ab (6.91/16.6) | 26.93b (5.79/34.9) | 13.19ab (10.13/15.1) | 10.53a (9.14/24.9) |
Immunohistochemistry
Macrophages were found in all liver samples (see Table 4). In the placebo group the amount of grade 1 samples was lower after LPS challenge (10%) compared to before LPS (30%). In contrast, the number of grade 2 and 3 samples was higher after LPS challenge (grade 2: 50 to 60%; grade 3: 20 to 30%). Contrary to placebo, the GCE group had a higher number of grade 1 samples from 60 to 70% after LPS challenge, whereas the number of grade 2 samples was lower (30 to 20%). The number of samples staged as grade 3 in GCE group remained unchanged after LPS challenge. Comparing feeding groups after LPS stimulus, most liver biopsies of the placebo group (60%) had a moderate number of hepatic macrophages (grade 2) whereas in the GCE group most liver biopsies (70%) had a low number of hepatic macrophages (grade 1, see Table 4).
Table 4. Hepatic number of macrophages before and 12 hours after lipopolysaccharide (LPS) challenge horses and ponies fed placebo or green tea and curcuma extract (GCE).
Values are presented as absolute numbers and percentages of the subpopulation. Grade 0 = no macrophages, grade 1 = 1-33 macrophages, grade 2 = 34-66 macrophages, grade 3 = 67-100 macrophages.
| Grade | Placebo | GCE | ||
|---|---|---|---|---|
| Before LPS | 12 h after LPS | Before LPS | 12 h after LPS | |
| 0 | 0/10 | 0/10 | 0/10 | 0/10 |
| 1 | 3/10 (30%) | 1/10 (10%) | 6/10 (60%) | 7/10 (70%) |
| 2 | 5/10 (50%) | 6/10 (60%) | 3/10 (30%) | 2/10 (20%) |
| 3 | 2/10 (20%) | 3/10 (30%) | 1/10 (10%) | 1/10 (10%) |
Discussion
In the present study, we hypothesized that feeding a polyphenol-rich diet for 21 days to equines would mitigate the inflammatory response in blood and liver tissue to an inflammatory stimulus. For this purpose, we used a feed additive consisting of green tea and curcuma extract with a total polyphenol content of 20%. As reviewed by Gaur & Agnihotri (2014), green tea provides one of the best sources for polyphenols. Products based on green tea contain high amounts of catechins as the active polyphenols. Catechins such as epigallocatechin gallates belong to the group of flavonoids (Lipiński et al., 2017). The polyphenol curcumin is one of three groups of curcuminoids and the main component of turmeric, a spice which is derived from the plant Curcuma longa (Jurenka, 2009).
In the literature, there are equivocal results concerning inflammatory parameters response to the effect of polyphenols on different tissues and animal species and more particularly between in vitro and in vivo studies. Tea catechins and curcumin demonstrated anti-inflammatory activity in vitro in human epithelial cells (Jobin et al., 1999; Wheeler et al., 2004; Biswas et al., 2005). However in vivo, the generally poor bioavailability of oral polyphenols is a challenge in the field of nutraceuticals since Nakagawa, Okuda & Miyazawa (1997) found only 0.2–2.0% of epigallogatechin-3-gallate to be available in human plasma after oral administration. Levels of tea catechins in blood and liver tissue have also been studied previously by Kim et al. (2000) in rats and mice after repeated oral doses of green tea. The authors demonstrated that green tea catechins could be detected in several tissues in these species. Nevertheless, liver tissue seems to not be significantly affected by tea catechins, as the hepatic levels were low compared to other tissues such as intestines or bladder (Kim et al., 2000). To the best of our knowledge, there are very few studies on the bioavailability of polyphenols in equines. Wein & Wolffram (2013) found quercetin, a flavonoid, to be abundant in equine plasma which is only comparable to some of the polyphenols we used in our study. Still, no data on the availability of polyphenols derived from green tea and curcuma in equine plasma is available. A limitation of the current study was that plasma and tissue levels of polyphenols were not examined in our animals but bioavailability of polyphenols was presumed to be low due to the minor dietary effects by the GCE post LPS challenge.
Nevertheless, the selected dosage of polyphenols in the present study was in the upper range of what was found in the literature. Winkler et al. (2015) fed a product consisting of green tea and curcuma at an estimated dose of 0.04–0.1 g polyphenols per 100 kg BW to dairy cows. In the present study horses were fed 0.3 g per 100 kg BW of polyphenols daily, and ponies were given 1.6 g per 100 kg BW. However, most effects in livestock were found in performance parameters such as a higher milk yield in dairy cows (Winkler et al., 2015) or an improved gain to feed ratio in pigs (Gessner et al., 2013). Performance parameters were not in the focus in the present study as the horses and ponies were kept under maintenance conditions and were adults and non-reproductive.
Regarding the daily doses of GCE in our animals, the ponies were given approximately 5 times higher doses per 100 kg BW compared to the horses. However, there was no significant difference in hepatic IL-1β mRNA levels comparing ponies and horses which underlines the assumed low bioavailability of the used polyphenols.
According to recent studies in livestock, a polyphenol-rich diet might be beneficial for animals such as cattle and pigs whose demands of high growth and milk production performance can trigger chronic inflammatory conditions (Gessner et al., 2013; Winkler et al., 2015). Since it was postulated by Winkler et al. (2015) that cattle are usually kept under conditions of highly elevated metabolic stress and chronic inflammation we used a LPS challenge as a well-established inflammatory model to induce a low grade inflammation in the equine animals (Oliveira-Filho et al., 2011; Tadros & Frank, 2012; Vinther et al., 2015). In consequence, we were able to evaluate the discussed anti-inflammatory effects of GCE supplementation under standardized inflammatory conditions. In the present study, inflammation induced by LPS challenge was verified by several inflammatory parameters in blood and liver tissue such as increased SAA and serum haptoglobin concentrations and increased mRNA levels of CD68 and haptoglobin in liver tissue. According to Jacobsen et al. (2006), in healthy horses SAA concentrations were found to be ≤ 2.3 mg/L by using turbidimetry as the measurement principle. Therefore, all of our animals were within or close to the postulated range with all values of SAA being ≤ 2.6 mg/L prior to LPS challenge. Additionally, all animals were in the reference range of 2–10 g/L for haptoglobin according to a review by Crisman, Scarratt & Zimmerman (2008) which included different analytical methods, such as turbidimetry. The suitability of SAA and haptoglobin as inflammatory markers in horses was demonstrated by Hultén et al. (2002) where both parameters significantly increased due to an experimentally-induced non-infectious arthritis. Therefore, the SAA response to LPS induced inflammatory conditions by increasing to more than 100-fold higher concentrations compared to baseline was expected. Contrary to our hypothesis, feeding the GCE did not have an anti-inflammatory effect on the inflammatory blood parameters since concentrations did not differ from placebo group. However, it remains open if a longer lasting sampling procedure, e.g., 24 h after LPS might have an impact on the outcome of SAA and especially on haptoglobin as both parameters seem to reach their peak values later than 12 h after an inflammatory stimulus. Although levels of SAA start to increase early at 6–12 h after inflammation, peak values can be expected after 36–48 h (Hultén et al., 2002; Jacobsen & Andersen, 2007). Serum haptoglobin increased significantly 24 h after inflammation with peak values at 48–96 h (Hultén et al., 2002; Vinther et al., 2016).
As reviewed by Gessner, Ringseis & Eder (2017), oxidative stress is directly linked with inflammation in farm animals. RBP4, which is mainly produced in the liver, seems to play a role in both conditions. Besides its carrier function as specific plasma retinol transporter (Zabetian-Targhi et al., 2015), RBP4 was suggested to have a role in the induction of oxidative stress and vascular inflammation in vitro in human endothelial cells through activation of NF-κB (Farjo et al., 2012). Since serum levels of RBP4 and mRNA levels of NF-κB in the liver in the current study were not altered by the inflammatory stimulus, the comparably low dose of LPS seemed less suitable to induce oxidative stress in our horses and ponies. Other parameters that are related to oxidative stress were not examined.
Macrophage invasion from blood into affected tissues during inflammation plays a crucial role in the progression of inflammation (Imamura et al., 2005). Liver macrophages, called Kupffer cells (KC), are promotors of the inflammatory cascade in hepatic tissue. We observed changes in numbers of hepatic macrophages but with a maximum variation of only 20% in course of treatment with LPS in both feeding groups. Since the results on hepatic macrophages should only be used as numerical references, the effect of GCE feeding and LPS challenge remains open. However, due to the changes we found in the liver samples, this might be an interesting aspect of further research. Furthermore, the invasion of macrophages is, among other things, mediated by release of cytokines such as TNF-α, IL-1β and IL-6 (Tacke, 2012) which mostly failed to be induced in our horses and ponies by the LPS challenge. Besides immunohistochemistry of KCs, Baldus et al. (1998) proposed analysis of CD68 to be a sensitive method to specify the amount of KCs as an increased number of hepatic CD68+ macrophages were found in conditions of moderate and severe inflammation such as hepatitis B. Cai et al. (2005) identified the role of CD68 as a marker for the activation of resident KCs. As mRNA levels of CD68 increased after LPS challenge in the placebo group, but not in the GCE group, GCE seemed to have an inhibiting effect on the activation of KCs and might therefore impair the subsequent inflammatory processes in the liver. However, a significant difference between the two feeding groups concerning macrophage activation could not be demonstrated. It can be concluded that our low-dose-LPS challenge might not have caused a significant invasion of hepatic macrophages according to the few numerical changes in liver samples. Due to elevated mRNA levels of CD68 an increased activation of resident KCs can still be assumed.
We also investigated mRNA levels of different inflammatory markers to assess protective effects of GCE on inflammatory signalling pathways in the liver. Haptoglobin is mainly produced in liver tissue and was induced under conditions of acute inflammation in rat liver cells (Wang et al., 2001). Our findings confirmed that haptoglobin was induced by LPS in liver tissue of horses and ponies, since it showed a significant increase after LPS challenge in the placebo group. However, a significant increase was not observed in the GCE group. In accordance with our hypothesis, we found an anti-inflammatory effect by GCE on IL-1β, a proinflammatory cytokine, as hepatic mRNA levels after LPS challenge were significantly lower in the GCE group compared to the placebo group. These findings agree with in vitro studies on mouse fibroblasts, where polyphenols were able to reduce expression of IL-1β through inhibition of the NF-κB pathway (Gonzales & Orlando, 2008). In a cell culture study curcumin reduced the production of proinflammatory cytokines when treating isolated lymphocytes collected from old horses with polyphenols (Siard, McMurry & Adams, 2016). In addition, tea catechins reduced activation of NF-κB in LPS-treated mouse-derived peritoneal macrophages in vitro (Lin & Lin, 1997). Gessner et al. (2013) used a polyphenol-rich diet consisting of grape seed and grape marc meal extract and found NF-kB and other target genes, like TNF-α and SAA to be suppressed in the duodenal mucosa, but not in the liver in pigs. Similarly, in the present study, NF-κB and its target genes, such as TNF-α and IL-6, were unaltered at all time points. Due to animal welfare concerns liver tissue was only obtained once after LPS challenge. Therefore, the second sampling point was set at 12 h after LPS but might have not been able to detect a presumably early reaction of the examined cytokines.
Beside the above discussed parameters of inflammation, upregulation of FGF21 has been postulated as a sensitive marker of ER stress as FGF21 levels increased in rat hepatocytes in vitro and in vivo due to experimentally induced ER stress (Schaap et al., 2013). Under conditions of ER stress FGF21 is induced via an ATF4 dependent pathway where ATF4 acts as a transcriptional effector in the UPR cascade (Schaap et al., 2013; Kim et al., 2015; Wan et al., 2018). Liver FGF21 mRNA levels were reduced in dairy cows in the peri-parturient phase and therefore under elevated metabolic stress by feeding an extract consisting of green tea and curcuma similar to the additive used in the present study (Winkler et al., 2015). Nevertheless, to the best of the author’s knowledge, the properties of FGF21 and ATF4 in conditions of ER stress have not been previously reported in equine animals. In contrast to Winkler et al. (2015), hepatic levels of FGF21 and ATF4 in our study were not significantly altered by feeding GCE. Although LPS was shown to induce ER stress in vitro in mice (Endo et al., 2006), the LPS challenge did not induce FGF21 and ATF4 in our animals and was therefore apparently insufficient to induce significant hepatic ER stress, presumably due to a low dosage. As a result, the evaluation of the impact of GCE supplementation on ER stress was limited.
Conclusion
In conclusion, our findings indicate that feeding a supplement containing green tea and curcuma extract has some potential to mediate inflammatory reactions in horses and ponies. However, an overall anti-inflammatory effect by the polyphenols was not apparent. Inflammation in our animals was indicated by several inflammatory parameters in blood and liver tissue but the parameters that are linked to ER stress remained unchanged throughout the study. This suggests that the inflammatory model was not suitable to induce ER stress in our animals, leaving the effects of flavonoids and curcuminoids on hepatic ER stress uncertain in this species. Further studies are needed for a better understanding of the bioavailability and tissue distribution of polyphenols from green tea and curcuma extract in horses and ponies.
Supplemental Information
Values are presented as median (25./75. percentiles).
Acknowledgments
The authors are grateful to S. Berthold, D. Kern, S. Klemann, M.Wacker and J. Tietke for providing technical support.
Funding Statement
This work was supported by Kaesler Nutrition GmbH. Support was also received from Leipzig University within the program of Open Access Publishing. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Janine Starzonek, Email: janine.starzonek@vetmed.uni-leipzig.de.
Ingrid Vervuert, Email: ingrid.vervuert@vetmed.uni-leipzig.de.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Janine Starzonek conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Katja Roscher conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Matthias Blüher, Manuela Hirz and Jens Raila analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Dominique Blaue and Carola Schedlbauer conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Ingrid Vervuert conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, fund raising.
Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The project was approved by the Ethics Committee for Animal Rights Protection of the Leipzig District Government (No TVV 34/16), in accordance with German Legislation for Animal Rights and Welfare.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.
References
- Baldus et al. (1998).Baldus SE, Zirbes TK, Weidner IC, Flucke U, Dittmar E, Thiele J, Dienes HP. Comparative quantitative analysis of macrophage populations defined by CD68 and carbohydrate antigens in normal and pathologically altered human liver tissue. Analytical Cellular Pathology. 1998;16(3):141–150. doi: 10.1155/1998/192975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas et al. (2005).Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxidants & Redox Signaling. 2005;7(1–2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- Bussières et al. (2008).Bussières G, Jacques C, Lainay O, Beauchamp G, Leblond A, Cadoré J-L, Desmaizières L-M, Cuvelliez SG, Troncy E. Development of a composite orthopaedic pain scale in horses. Research in Veterinary Science. 2008;85(2):294–306. doi: 10.1016/j.rvsc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cai et al. (2005).Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nature Medicine. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari et al. (2014).Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Frontiers in Cellular Neuroscience. 2014;8:1–15. doi: 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisman, Scarratt & Zimmerman (2008).Crisman MV, Scarratt WK, Zimmerman KL. Blood proteins and inflammation in the horse. Veterinary Clinics of North America: Equine Practice. 2008;24(2):285–297. doi: 10.1016/j.cveq.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Dulbecco & Savarino (2013).Dulbecco P, Savarino V. Therapeutic potential of curcumin in digestive diseases. World Journal of Gastroenterology. 2013;19(48):9256–9270. doi: 10.3748/wjg.v19.i48.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo et al. (2006).Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of Caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. The Journal of Immunology. 2006;176(10):6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- Farjo et al. (2012).Farjo KM, Farjo RA, Halsey S, Moiseyev G, Ma J-X. Retinol-Binding Protein 4 induces inflammation in human endothelial cells by an NADPH oxidase- and Nuclear Factor Kappa B-dependent and Retinol-independent mechanism. Molecular and Cellular Biology. 2012;32(24):5103–5115. doi: 10.1128/MCB.00820-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur & Agnihotri (2014).Gaur S, Agnihotri R. Green tea: a novel functional food for the oral health of older adults. Geriatrics & Gerontology International. 2014;14(2):238–250. doi: 10.1111/ggi.12194. [DOI] [PubMed] [Google Scholar]
- Gessner et al. (2013).Gessner DK, Fiesel A, Most E, Dinges J, Wen G, Ringseis R, Eder K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Veterinaria Scandinavica. 2013;55(1):1–10. doi: 10.1186/1751-0147-55-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner, Ringseis & Eder (2017).Gessner DK, Ringseis R, Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. Journal of Animal Physiology and Animal Nutrition. 2017;101(4):605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Gonzales & Orlando (2008).Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutrition & Metabolism. 2008;5(17):1–13. doi: 10.1186/1743-7075-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil (2010).Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultén et al. (2002).Hultén C, Grönlund U, Hirvonen J, Tulamo R-M, Suominen MM, Marhaug G, Forsberg M. Dynamics in serum of the inflammatory markers serum amyloid A (SAA), haptoglobin, fibrinogen and α2-globulins during induced noninfectious arthritis in the horse. Equine Veterinary Journal. 2002;34(7):699–704. doi: 10.2746/042516402776250405. [DOI] [PubMed] [Google Scholar]
- Imamura et al. (2005).Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128(1):138–146. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Jacobsen & Andersen (2007).Jacobsen S, Andersen PH. The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Equine Veterinary Education. 2007;19(1):38–46. [Google Scholar]
- Jacobsen et al. (2006).Jacobsen S, Kjelgaard-Hansen M, Petersen HH, Jensen AL. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. The Veterinary Journal. 2006;172(2):315–319. doi: 10.1016/j.tvjl.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Jobin et al. (1999).Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. The Journal of Immunology. 1999;163(6):3474–3483. [PubMed] [Google Scholar]
- Jurenka (2009).Jurenka JS. Anti-inflammatory properties of Curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative Medicine Review. 2009;14(2):141–153. [PubMed] [Google Scholar]
- Kim et al. (2015).Kim SH, Kim KH, Kim H-K, Kim M-J, Back SH, Konishi M, Itoh N, Lee M-S. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia. 2015;58(4):809–818. doi: 10.1007/s00125-014-3475-6. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2000).Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, Seril DN, Yang CS. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutrition and Cancer. 2000;37(1):41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Kozutsumi et al. (1988).Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Laemmli (1970).Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin & Lin (1997).Lin Y-L, Lin J-K. (−)-Epigallocatechin-3-gallate blocks the induction of Nitric Oxide Synthase by down-regulating lipopolysaccharide-induced activity of transcription factor Nuclear Factor-κB. Molecular Pharmacology. 1997;52(3):465–472. doi: 10.1124/mol.52.3.465. [DOI] [PubMed] [Google Scholar]
- Lipiński et al. (2017).Lipiński K, Mazur M, Antoszkiewicz Z, Purwin C. Polyphenols in monogastric nutrition—a review. Annals of Animal Science. 2017;17(1):41–58. doi: 10.1515/aoas-2016-0042. [DOI] [Google Scholar]
- Marycz et al. (2018).Marycz K, Kornicka K, Szlapka-Kosarzewska J, Weiss C. Excessive endoplasmic reticulum stress correlates with impaired mitochondrial dynamics, mitophagy and apoptosis, in liver and adipose tissue, but not in muscles in EMS horses. International Journal of Molecular Sciences. 2018;19(1):1–20. doi: 10.3390/ijms19010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, Okuda & Miyazawa (1997).Nakagawa K, Okuda S, Miyazawa T. Dose-dependent incorporation of tea catechins, (-)-Epigallocatechin-3-gallate and (-)-Epigallocatechin, into human plasma. Bioscience, Biotechnology, and Biochemistry. 1997;61(12):1981–1985. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- Oliveira-Filho et al. (2011).Oliveira-Filho JP, Badial PR, Cunha PHJ, Peiro JR, Araujo JP, Divers TJ, Winand NJ, Borges AS. Lipopolysaccharide infusion up-regulates hepcidin mRNA expression in equine liver. Innate Immunity. 2011;18(3):438–446. doi: 10.1177/1753425911420181. [DOI] [PubMed] [Google Scholar]
- Schaap et al. (2013).Schaap FG, Kremer AE, Lamers WH, Jansen PLM, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95(4):692–699. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Siard, McMurry & Adams (2016).Siard MH, McMurry KE, Adams AA. Effects of polyphenols including curcuminoids, resveratrol, quercetin, pterostilbene, and hydroxypterostilbene on lymphocyte pro-inflammatory cytokine production of senior horses in vitro. Veterinary Immunology and Immunopathology. 2016:(173):50–59. doi: 10.1016/j.vetimm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Tacke (2012).Tacke F. Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo. Fibrogenesis & Tissue Repair. 2012;5(1):S27. doi: 10.1186/1755-1536-5-S1-S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros & Frank (2012).Tadros EM, Frank N. Effects of continuous or intermittent lipopolysaccharide administration for 48 h on the systemic inflammatory response in horses. American Journal of Veterinary Research. 2012;73(9):1394–1402. doi: 10.2460/ajvr.73.9.1394. [DOI] [PubMed] [Google Scholar]
- Vick et al. (2007).Vick MM, Adams AA, Murphy BA, Sessions DR, Horohov DW, Cook RF, Shelton BJ, Fitzgerald BP. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. Journal of Animal Science. 2007;85(5):1144–1155. doi: 10.2527/jas.2006-673. [DOI] [PubMed] [Google Scholar]
- Vinther et al. (2016).Vinther AML, Heegaard PMH, Skovgaard K, Buhl R, Andreassen SM, Andersen PH. Characterization and differentiation of equine experimental local and early systemic inflammation by expression responses of inflammation-related genes in peripheral blood leukocytes. BMC Veterinary Research. 2016;12(83):1–13. doi: 10.1186/s12917-016-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinther et al. (2015).Vinther AML, Skovgaard K, Heegaard PMH, Andersen PH. Dynamic expression of leukocyte innate immune genes in whole blood from horses with lipopolysaccharide-induced acute systemic inflammation. BMC Veterinary Research. 2015;11(134):1–11. doi: 10.1186/s12917-015-0450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan et al. (2018).Wan X-S, Wang X, Xiao J, Li X-K, Zhou H. Corrigendum to ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. BioMed Research International. 2018;2018 doi: 10.1155/2018/3218606. Article 3218606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2001).Wang Y, Kinzie E, Berger FG, Lim SK, Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Report. 2001;6(6):379–385. doi: 10.1179/135100001101536580. [DOI] [PubMed] [Google Scholar]
- Wein & Wolffram (2013).Wein S, Wolffram S. Oral bioavailability of quercetin in horses. Journal of Equine Veterinary Science. 2013;33(6):441–445. doi: 10.1016/j.jevs.2012.07.008. [DOI] [Google Scholar]
- Wheeler et al. (2004).Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. The Journal of Nutrition. 2004;134(5):1039–1044. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- Winkler et al. (2015).Winkler A, Gessner DK, Koch C, Romberg F-J, Dusel G, Herzog E, Most E, Eder K. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Archives of Animal Nutrition. 2015;69(6):425–441. doi: 10.1080/1745039X.2015.1093873. [DOI] [PubMed] [Google Scholar]
- Zabetian-Targhi et al. (2015).Zabetian-Targhi F, Mahmoudi MJ, Rezaei N, Mahmoudi M. Retinol Binding Protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Advances in Nutrition. 2015;6(6):748–762. doi: 10.3945/an.115.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang & Kaufman (2008).Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are presented as median (25./75. percentiles).
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.