Abstract
Background and Objectives
Delirium creates distinct emotional distress in patients and family caregivers, yet there are limited tools to assess the experience. Our objective was to develop separate patient and family caregiver delirium burden instruments and to test their content and construct validity.
Research Design and Methods
Two hundred forty-seven patients and 213 family caregivers were selected from an ongoing prospective cohort of medical-surgical admissions aged ≥70 years old. New patient and family caregiver delirium burden instruments were developed and used to measure the subjective experiences of in-hospital delirium. Delirium and delirium severity were measured by the Confusion Assessment Method (CAM) and CAM-Severity (long form).
Results
Both Delirium Burden (DEL-B) instruments consist of eight questions and are measured on a 0 – 40 point scale. Final questions had good clarity and relevancy, as rated by the expert panel, and good internal consistency (Cronbach’s α = .82–.86). In the cohort validation, Patient DEL-B (DEL-B-P) was 5.1 points higher and Family Caregiver DEL-B (DEL-B-C) was 5.8 points higher, on average, for patients who developed delirium compared to those who did not (p < .001). Test–retest reliability of DEL-B-C at baseline and 1 month was strong (correlation = .73). Delirium severity was mildly-moderately correlated with DEL-B-P (correlation = .34) and DEL-B-C (correlation = .26), suggesting contribution of other factors.
Discussion and Implications
We created instruments to reliably measure and evaluate the burden of delirium for patients and their family caregivers. Although additional validation is indicated, these instruments provide a key first step toward measuring and improving the subjective experience of delirium for patients and their families.
Keywords: Delirium burden, Distress, Instrument development, Family caregiver, Caregiver burden, Patient burden
Delirium, an acute decline of global cognitive functioning, is a common, serious, and costly complication of hospitalization for older adults, with incidence rates of 29%–64% (Inouye, Westendorp, & Saczynski, 2014; Marcantonio, 2018). Although prior research in delirium has focused on medical aspects of prevention and treatment, recent studies have underscored its human toll, particularly on patients and family caregivers (Finucane, Lugton, Kennedy, & Spiller, 2017; Fuller, 2016; Morandi et al., 2012; Partridge, Martin, Harari, & Dhesi, 2013). Delirium burden, defined here as the subjective experience of delirium for patients and family members, includes awareness of delirium symptoms, situational stress, and emotional response. Delirium is often distressing to patients and their family caregivers, and delirium burden may continue to affect emotional, psychological, and physical well-being long after the delirium episode resolves. A better understanding of delirium burden may help to quantify these important aspects of the lived delirium experience and to develop better strategies to support patients and their family caregivers during and after a delirium episode.
To date, there are limited tools to assess delirium burden. Family caregiver burden in delirium has been studied using the Caregiver Burden Scale (Zarit, Reever, & Bach-Peterson, 1980), the Caregiver Burden Inventory (Novak & Guest, 1989), and the Older Persons and Informal Caregivers Survey Minimum DataSet (Lutomski et al., 2013); however, these tools are not delirium specific, and do not fully capture the burden of delirium (Breitbart, Gibson, & Tremblay, 2002; Bruera et al., 2009; Grover, Ghosh, & Ghormode, 2015; Morandi et al., 2012) associated with its acuity, unpredictability, and unique symptoms, such as inattention, visual hallucinations, and delusions. Although caregiver burden has been examined extensively in dementia (Chiao, Wu, & Hsiao, 2015), these measures emphasize long-term emotional, physical, and economic strains, which are common in dementia care, but do not translate well to the acute situation of delirium. The Delirium Experience Questionnaire (DEQ) (Breitbart et al., 2002), a brief instrument developed to assess delirium recall and distress in patients, caregivers, and nurses has not yet been fully validated but represented an important advance.
Our objective was to develop two separate instruments to assess delirium burden among patients and family caregivers that would be sensitive to the acuity and unique features of delirium, and that could be applied in both clinical and research settings. We followed guidelines for instrument development, including identifying content domains and their operational definitions, generating items, expert review of items, and testing in a target population (DeVellis, 2017; Lynn, 1986). We hypothesized that delirium burden would be greater for both patients and family caregivers in patients with delirium compared to no delirium and would increase with increasing delirium severity; thus, we investigated convergent validity with delirium incidence and severity.
Design and Methods
Content Domains and Operational Definitions
Content domains were based on our previous qualitative work which identified three themes common to patients’ and family caregivers’ delirium experience: symptom burden, emotional burden, and situational burden (Schmitt et al., 2017). Symptom burden encompasses burden related to experiencing (patient) or observing (family) symptoms of delirium, including disorientation, hallucinations, delusions, impaired communication, memory problems, personality changes, and sleep disturbances. Emotional burden encompasses emotions associated with delirium, such as anger, frustration, fear, guilt, and helplessness. Situational burden is triggered by having to manage delirium and includes loss of control, lack of support (e.g., lack of knowledge or resources to care for a delirious patient), safety concerns, unpredictability, and feeling unprepared. Based on first-hand accounts from our qualitative interviews supplemented by literature review (Schmitt et al., 2017), these content domains formed the basis for designing delirium burden instruments for both groups.
Generating Items and Expert Review
Our study team systematically generated a potential list of experts to represent relevant disciplines and content expertise, who are widely recognized as experts in the field. Eight agreed to participate who provided multidisciplinary perspectives (Supplementary Table 1) with extensive expertise in delirium, as well as relevant clinical and methodological expertise. The expert panel was varied in terms of discipline and expertise (e.g., anesthesiology, critical care, geriatrics, hospital medicine, internal medicine, neurology, nursing, occupational therapy, psychiatry, surgery); educational training (two MD, one PhD, one PsyD, two BS, one RN, two MPH; several had multiple degrees and specialties); geographic location (five northeast, one southeast, two southwest); and gender (63% female).
We generated an initial set of 17 items for patients and 23 items for family caregivers that reflected the three content domains (Tables 3 and 4). We circulated the initial set of items for each instrument to the expert panel along with a content validity rating form (see Supplementary Figures 1–2). Experts rated each item on perceived relevance to delirium burden and on clarity using a 1–4 scale (not relevant/clear—very relevant/clear). Some items were repeated to present various wording options. We asked experts to select the five best items (“top 5”), to comment in an open-ended fashion on individual items or on the instrument as a whole, and to suggest additional items.
Table 3.
Expert Ratings of Patient Delirium Burden (DEL-B-P) Itemsa
| Burden theme | Items for expert review | Relevance | Clarity | Top 5b | Included/reason not included |
|---|---|---|---|---|---|
| Symptom Burden | 1. Unsure of where they were | 3.8 | 3.6 | 4 (50%) | Included |
| 2. Could not remember parts of the hospital stay | 3.4 | 3.9 | 2 (25%) | Included | |
| 3. Saw or heard things that were not really there | 3.6 | 3.9 | 3 (38%) | Included | |
| 4. Nightmares or vivid dreams that were intense or bothersome | 2.8 | 3.6 | 2 (25%) | Included | |
| 5. Difficulty communicating | 2.9 | 3.0 | 1 (13%) | Dropped due to low relevancy rating | |
| 6. Suddenly felt confused | 3.9 | 3.9 | 6 (75%) | Included | |
| Emotional Burden | 7. Felt they may never be normal again | 2.9 | 3.1 | 1 (13%) | Dropped due to low relevancy rating |
| 8. Thought that they would not get better | 3.3 | 3.8 | 2 (25%) | Included | |
| 9. Afraid of losing their mind | 3.8 | 3.6 | 6 (75%) | Included | |
| Situational Burden | 10. Felt the hospital staff did not listen to them | 2.6 | 3.0 | 1 (13%) | Dropped due to low relevancy rating |
| 11. Felt they were being scolded by hospital staff | 2.4 | 3.4 | 0 | Dropped due to low relevancy rating | |
| 12. Felt they were not being respected by hospital staff | 2.9 | 3.4 | 2 (25%) | Dropped due to low relevancy rating | |
| 13. Restricted from getting out of a bed or a chair with alarms or restraints | 3.1 | 3.5 | 4 (50%) | Included | |
| 14. Concerned of being/becoming a burden to others | 3.1 | 3.8 | 2 (25%) | Dropped due to non-specificity to delirium | |
| 15. Concerned about how they would function in the future | 3.0 | 3.4 | 2 (25%) | Dropped due to non-specificity to delirium |
Note: aThe instruments are copyright pending and will be made freely and publicly available at https://www.hospitalelderlifeprogram.org/delirium-instruments/.
bTop 5 refers to the n (%) of reviewers who selected the item in response to the question “If you were to develop a 5 item delirium burden questionnaire based on the above items, which would you choose?”
Table 4.
Expert Ratings of Family Caregiver Delirium Burden (DEL-B-C) Itemsa
| Burden theme | Items for expert review | Relevance | Clarity | Top 5b | Included/reason not included |
|---|---|---|---|---|---|
| Symptom Burden | 1. Loved one did not recognize caregiver | 3.5 | 4.0 | 2 (25%) | Included |
| 2. Loved one behaved in an upsetting way | 2.5 | 3.1 | 0 | Dropped due to low relevancy rating | |
| 3. Loved one saw or heard things that were not really there | 3.6 | 4.0 | 2 (25%) | Included | |
| 4. Loved one did not know where he/she was | 3.5 | 3.9 | 1 (13%) | Dropped due to length | |
| 5. Loved one experienced changes in memory and thinking (wording 1) | 3.8 | 3.8 | 2 (25%) | Included | |
| 6. Loved one experienced changes in memory and thinking (wording 2) | 3.8 | 3.4 | 2 (25%) | Dropped due to comparable item (#5) | |
| 7. Loved one became irritable or angry | 3.0 | 3.5 | 3 (38%) | Included | |
| 8. Loved one had trouble understanding | 2.8 | 3.5 | 1 (13%) | Dropped due to low relevancy rating | |
| 9. Difficulty understanding loved one | 3.0 | 3.4 | 0 | Dropped due to nonspecificity to delirium | |
| 10. Difficulty communicating with loved one | 2.6 | 3.4 | 1 (13%) | Dropped due to low relevancy rating | |
| 11. Feelings that his/her presence made loved one’s symptoms worse | 2.9 | 3.4 | 1 (13%) | Dropped due to low relevancy rating | |
| Emotional Burden | 12. Feelings of helplessness as a caregiver (wording 1) | 3.3 | 3.4 | 3 (38%) | Dropped due to comparable item (#13) |
| 13. Feelings of helplessness as a caregiver (wording 2) | 3.6 | 3.5 | 6 (75%) | Included | |
| 14. Concern about loved one’s future | 3.1 | 3.0 | 0 | Dropped due to nonspecificity to delirium | |
| 15. Concern about increased responsibilities as a caregiver | 3.9 | 3.8 | 4 (50%) | Included | |
| 16. Concern that loved one would lose their independence | 3.1 | 3.0 | 0 | Dropped due to nonspecificity to delirium | |
| 17. Loved one refused his/her help | 2.0 | 3.6 | 0 | Dropped due to low relevancy rating | |
| 18. Thoughts that loved one would not recover | 3.1 | 3.3 | 0 | Dropped due to nonspecificity to delirium | |
| 19. Concern that loved one would never be back to his/her usual self | 3.7 | 3.3 | 2 (25%) | Included | |
| Situational Burden | 20. Concern that the doctors couldn’t fix what was wrong | 2.9 | 3.4 | 1 (13%) | Dropped due to low relevancy rating |
| 21. Loved one demonstrated unsafe behaviors | 3.8 | 3.8 | 5 (63%) | Included | |
| 22. Feelings that loved one was not being taken seriously by the hospital staff | 2.8 | 3.1 | 1 (13%) | Dropped due to low relevancy rating |
Note: aThe instruments are copyright pending and will be made freely and publicly available at https://www.hospitalelderlifeprogram.org/delirium-instruments/.
bTop 5 refers to the n (%) of reviewers who selected the item in response to the question “If you were to develop a 5 item delirium burden questionnaire based on the above items, which would you choose?”
Finalization of Items
We averaged and evaluated experts’ rankings of relevance and clarity for each item. We did not consider items rated as less than moderately relevant (on average) for inclusion in the final instrument. We adjudicated the remaining items based on “top 5” ratings, qualitative feedback, and face validity, and modified final items based on expert suggestions. We used Cronbach’s α to assess internal consistency of items included in the final versions of both instruments. Following expert review, our research team consulted the field team for additional feedback. The field team suggested some minor wording changes for clarification that were incorporated into the final version (e.g., adding “while you were in the hospital” to clarify the timeframe of reference for some questions).
Both the final Patient Delirium Burden (DEL-B-P) and Family Caregiver Delirium Burden (DEL-B-C) instruments (Table 1) have eight two-level questions where if the answer to the stem question (whether a certain delirium burden feature was experienced or not) was positive, a follow-up question was answered on a 0–4 scale about how upsetting/distressing the experience was (not at all upsetting/distressing—extremely upsetting/distressing) for the respondent. This format is analogous to that used in the Neuropsychiatric Inventory-Questionnaire (Cummings et al., 1994; Kaufer et al., 1998). For each pair of questions, we rated a Delirium Burden (DEL-B) item as zero if the respondent reported that the patient (or family caregiver, as relevant) did not experience the particular DEL-B item, if the respondent was uncertain or refused to answer that question. If patients or family caregivers refused to answer all questions, they were not included in analyses. For each item, a score of 1 indicates that the patient or family caregiver confirmed that they experienced the DEL-B item but it was “not at all distressing.” Scores of 2–5 indicate both that they experienced the DEL-B item and that it was distressing, with higher numbers indicating greater distress. Scores were summed across the eight paired items, yielding a minimum possible score (least burdensome) of 0 and a maximum (most burdensome) of 40. DEL-B items that were endorsed but were self-reported as “not at all distressing” contributed to the total score because awareness of these experiences is part of the subjective experience of delirium and because individuals may vary widely in their likelihood of reporting any feelings of distress.
Table 1.
Patient Delirium Burden (DEL-B-P) and Family Caregiver Burden Delirium (DEL-B-C) Instrument Elements
| Patient items (DEL-B-P) |
|---|
| • Unsure of where they were |
| • Could not remember parts of the hospital stay |
| • Saw or heard things that were not really there |
| • Nightmares or vivid dreams that were intense or bothersome |
| • Suddenly felt confused |
| • Thought that they would not get better |
| • Afraid of losing their mind |
| • Restricted from getting out of a bed or a chair with alarms or restraints |
| Family caregiver items (DEL-B-C) |
| • Loved one did not recognize caregiver |
| • Loved one experienced changes in memory and thinking |
| • Loved one saw or heard things that were not really there |
| • Loved one became irritable or angry |
| • Feelings of helplessness as a caregiver |
| • Concern about increased responsibilities as a caregiver |
| • Concern that loved one would never be back to his/her usual self |
| • Loved one demonstrated unsafe behaviours |
Note: The instruments are copyright pending and will be made freely and publicly available at https://www.hospitalelderlifeprogram.org/delirium-instruments/.
Responses from the expert panel were generally positive for both sets of burden questions, with above-average ratings for both relevance and clarity, and clear suggestions for improvement; therefore, we did not perform a second round of review.
Testing in a Target Population
We assessed the DEL-B-P and DEL-B-C instruments in surgical and medical patients and their family caregivers utilizing a large subset of the Better Assessment of Illness (BASIL) study conducted at Hebrew SeniorLife and Beth Israel Deaconess Medical Center, whose Institutional Review Boards approved this study. BASIL is an ongoing prospective, observational study of 352 surgical and medical patients with the primary goal of developing and testing measures of delirium severity. Eligibility criteria for patients included age ≥ 70 years, ability to communicate in English, and expected hospital stay of >2 days. We excluded patients for: imminently terminal condition; recent alcohol dependence, history of schizophrenia or psychosis, severe deafness; and nonverbal condition (e.g., aphasic, intubated). Patients with dementia were included. We screened participants for their capacity to consent using a standard Capacity for Informed Consent instrument and requested written consent from all participants and/or their proxies. Patients who were unable to communicate or interact with an interviewer were excluded from the study, which included some patients with advanced dementia.
The BASIL study consists of: an initial in-hospital assessment; daily hospital interviews; follow-up interviews at 5–10 days (phone), 1 and 12 months postdischarge (in-person); and chart abstraction. We administered the final DEL-B instruments to patients and their family caregivers during the 1-month visit. To assess retest reliability, we administered the DEL-B-C to a subset of family caregivers at the in-hospital assessment. We did not do so among patients because we did not expect reliable responses during active delirium.
In addition to the burden instruments, we collected data on delirium status, delirium severity, cognitive function, and other clinical characteristics and outcomes. We assessed delirium status and severity by the Confusion Assessment Method (CAM) and CAM-Severity (CAM-S) long form. The CAM (Inouye et al., 1990) is a standardized approach for detecting delirium with high sensitivity (94%–100%), specificity (90%–95%), and reliability (Inouye et al., 1990; Wei, Fearing, Sternberg, & Inouye, 2008). The CAM-S is a score created from the 10 features of the full CAM instrument and ranges from 0 to 19, with higher scores indicating more severe delirium (Inouye et al., 2014). We assessed both CAM-S peak, the highest single CAM-S rating during hospitalization, using prespecified cutpoints (Vasunilashorn et al., 2018) and CAM-S sum, which is the sum of CAM-S scores on all hospital days, on a continuous scale (Vasunilashorn et al., 2016).
To test convergent validity, we first compared DEL-B scores for patients who did and did not develop delirium, and second, examined the correlation between DEL-B and delirium severity. We hypothesized that DEL-B would be higher for patients who developed delirium and would be associated with greater delirium severity. Differences between DEL-B scores by delirium status were assessed by t-tests. We assessed associations between CAM-S sum score and DEL-B (both continuous measures) by Spearman rank correlations (rho) and CAM-S peak tertiles (categorical variable) and DEL-B by analysis of variance. All analyses were conducted in StataIC (StataCorp, 2017).
Results
Cohort Characteristics
Table 2 describes the characteristics of the 267 patients from the BASIL study who completed the DEL-B-P instrument and/or the DEL-B-C instrument.
Table 2.
Burden Sample Patient Characteristics
| Patient characteristics | Delirium burden sample (N = 267) |
|---|---|
| Age – mean (SD) | 80.3 (6.8) |
| Sex – n (% female, self-reported) | 152 (57%) |
| Nonwhite – n (%) | 45 (17%) |
| Education – mean (SD) | 14.6 (2.9) |
| Charlson score – n (%) | |
| 0 | 71 (27%) |
| 1 | 57 (21%) |
| 2+ | 139 (52%) |
| Dementia – n (%) | 79 (30%) |
| Any ADL impairment – n (%) | 204 (77%) |
| Any IADL impairment – n (%) | 108 (60%) |
| Delirium – n (%) | 59 (22%) |
| CAM-S peak score – n (%) | |
| 0–2 | 113 (42%) |
| 3–7 | 121 (45%) |
| 8–19 | 33 (12%) |
| CAM-S sum score – mean (SD) | 9.0 (9.4) |
| Caregiver relationship to patient – n (%) | |
| Spouse/Partner | 75 (32%) |
| Son/Daughter | 112 (47%) |
| Son/Daughter in law | 3 (1%) |
| Grandchild | 6 (3%) |
| Brother/Sister | 14 (6%) |
| Nephew/Niece | 9 (4%) |
| Cousin | 1 (<1%) |
| Other relative | 1 (<1%) |
| Friend/Neighbor | 16 (7%) |
| Paid employee or caretaker | 1 (<1%) |
| Caregiver lives with patient – n (%) | 109 (45%) |
Note: Patient delirium burden and Caregiver delirium burden samples are partially overlapping; 247 patients and 213 family caregivers completed the burden assessment at Month 1; 193 patient-caregiver dyads completed both burden instruments. Missing data: Information on education was only available in 264 patients, any ADL impairment in 264, any IADL impairment in 181, Caregiver relationship to patient in 238, and Caregiver lives with patient in 238. All other variables had no missing data for the 267 patients with patient and/or caregiver burden data. ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living, Dementia = proxy Informant Questionnaire of Decline in Elderly (IQCODE) score ≥ 3.5 or chart-based diagnosis of Alzheimer’s disease or dementia.
Content Validity
Patients: DEL-B-P Items
On average, experts deemed all DEL-B-P items at least somewhat relevant (≥2 points) and at least moderately clear (≥3 points) (Table 3). All of the final DEL-B-P items were scored as “top 5” by at least two reviewers and had clarity and relevancy ratings ≥ 3.25 except for two items (Table 3). Item 4 (concerning nightmares or vivid dreams) and Item 13 (concerning physical restraints) had high clarity scores but average relevancy scores of 2.75 and 3.13, respectively. However, we retained these items because of their “top 5” ratings and clinical judgment of the study research team. Cronbach’s α was .86 for the final DEL-B-P instrument, indicating good internal consistency.
Family Caregivers: DEL-B-C Items
On average, the experts deemed all DEL-B-C items at least somewhat relevant (≥2 points) and at least moderately clear (≥3 points) (Table 4). Expert panel members expressed concern that some items would be burdensome in patients with dementia as well; thus, we prioritized the choice of final items based on specificity to delirium. All of the final DEL-B-C burden questions were scored as “top 5” by at least two reviewers and had relevancy and clarity ratings ≥3.25. Cronbach’s α was .82 for the final DEL-B-C instrument, indicating good internal consistency.
Delirium Burden in Target Population
Patients: DEL-B-P in Target Population
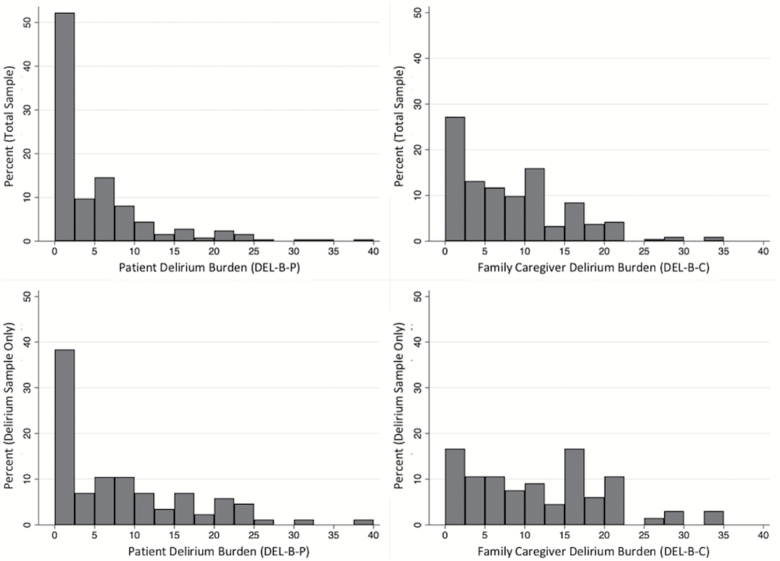
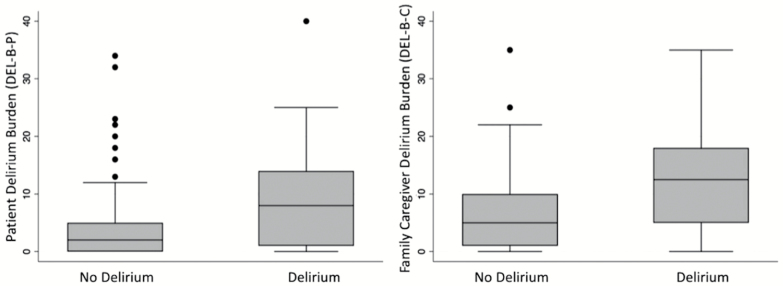
We assessed the DEL-B-P instrument in 247 BASIL patients who completed the 1-month interview; only one patient refused to answer all of the burden questions. The DEL-B-P instrument took 3 minutes on average (range 1–16 min) to administer. DEL-B-P scores spanned the full possible range (0–40). The median DEL-B-P score in the total sample (delirium and nondelirium combined) was 2 (interquartile range [IQR] = 0–7) out of 40 possible points (Figure 1). The median and IQR for DEL-B-P was 8 (IQR 1–14) in delirious patients compared to a median of 2 (IQR 0–5) in nondelirious patients (Figure 2). On average, DEL-B-P was 5.1 points (95% confidence interval [CI]: 3.3–7.0; p < .001) higher in patients who developed delirium compared to those who did not.
Figure 1.
Histograms of patient delirium burden (DEL-B-P, left) and family caregiver delirium burden (DEL-B-C, right) instruments assessed at 1-month post-hospitalization. The total sample is displayed in the top panel, and the subsample of delirium patients only are displayed in the bottom panel. All data displayed is from the 1-month assessment.
Figure 2.
Delirium burden (DEL-B) by delirium status for patients (left) and family caregivers (right). Box plots display Patient Delirium Burden (DEL-B-P) and Family Caregiver Delirium Burden (DEL-B-C) data by delirium status (no delirium vs delirium) for data collected at the 1-month assessment.
Family Caregivers: DEL-B-C in Target Population
We administered the DEL-B-C instrument to 213 caregivers who completed the 1-month interview; only one caregiver refused to answer all of the burden questions. The DEL-B-C instrument took 2 min on average (range 1–10 min) to administer. DEL-B-C scores ranged from 0 to 35. The median score in the total sample was 7 (IQR = 2–12) out of 40 possible points (Figure 1). Family caregivers of delirious patients reported a median DEL-B-C score of 12.5 (IQR = 5–18) compared to a median of 5 (IQR = 1–10) in nondelirious patients (Figure 2). On average, DEL-B-C was 5.8 points (95% CI: 3.6–8.0; p < .001) higher in patients who developed delirium compared to those who did not.
Retest Reliability of DEL-B-C
In family caregivers who completed both the in-hospital and 1-month assessments (n = 143), DEL-B-C scores were strongly correlated (Spearman rho = .73, p < .0001; intraclass correlation coefficient [ICC] = .71, 95% CI: .63, .78), indicating good retest reliability (Koo & Li, 2016). We observed a trend that DEL-B-C tended to be higher at 1-month post-discharge than at the in-hospital assessment. However, a paired t test revealed that the mean difference of 0.6 points was not significant (95% CI: −1.5, 0.3, p = .18).
Correlation Between DEL-B-P and DEL-B-C
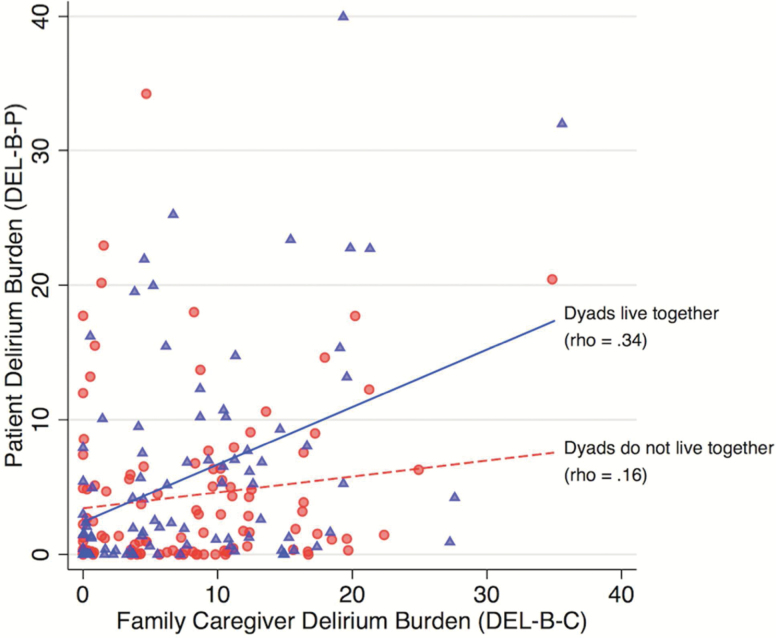
DEL-B-P at 1 month was modestly correlated with DEL-B-C at 1 month (Spearman’s rho = .28, p = .001). Since these correlations were lower than expected, we performed a supplemental analysis stratified by whether or not the family caregiver lived with the patient (Figure 3). Correlations were higher for patients and family caregivers who lived together (Spearman’s rho = .34, p = .001) versus those who did not (rho = .16, p = .10). However, these two correlations were not significantly different (z = 1.3, p = .20). On average, DEL-B-C was significantly higher than DEL-B-P by 2.9 points (95% CI: 1.6, 4.1); this was true for both patients and family caregivers who lived together (difference = 2.2, 95% CI: 0.4, 4.0) and those who did not (difference = 3.4, 95% CI: 1.7, 5.1).
Figure 3.
Correlation between Family Caregiver Delirium Burden (DEL-B-C) and Patient Delirium Burden (DEL-B-P). The correlation between DEL-B-P and DEL-B-C was stronger for caregiver-patient dyads who lived together (blue triangles/solid line) than for caregiver-patient dyads who did not live together (red circles/dashed line). Rho refers to Spearman’s rank order correlation coefficient.
Associations Between DEL-B and Delirium Severity
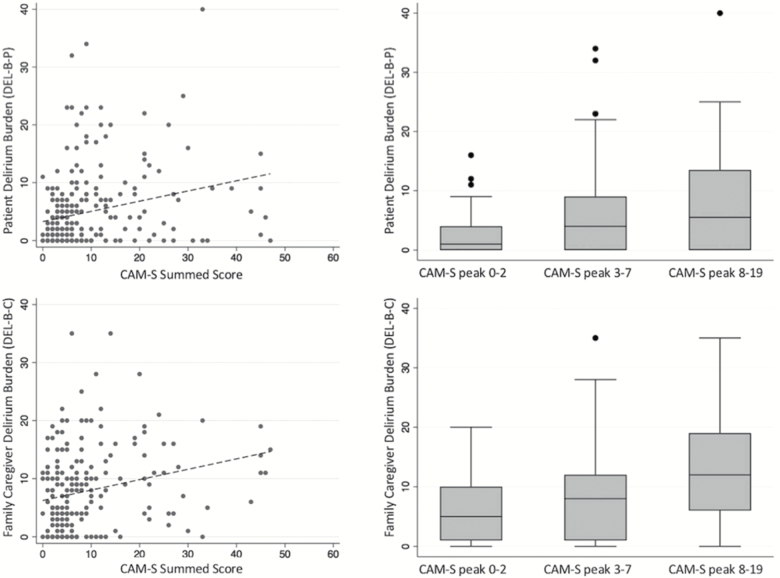
For both patients and family caregivers, higher delirium severity was associated with greater DEL-B (Figure 4).
Figure 4.
Association between delirium severity and patient (top) and family caregiver (bottom) delirium burden (DEL-B). Delirium severity was measured by CAM-S sum score (left, scatter plot) and CAM-S peak tertiles (right, box plot).
Patients: Associations Between DEL-B-P and Delirium Severity
Greater DEL-B-P was correlated with greater delirium severity by CAM-S sum (Spearman’s rho = .31, p < .001), indicating moderate correlation. We also observed significant differences in DEL-B-P scores between tertiles of CAM-S peak scores (F = 13.1, p < .001). The average DEL-B-P score in the lowest tertile (n = 103) was 2.5 (SD = 3.2), middle tertile (n = 112) was 6.2 (SD = 7. 4), and highest tertile (n = 32) was 7.8 (SD = 9.2). Bonferroni-corrected pair-wise comparisons revealed that CAM-S peak tertiles 2 and 3 (i.e., higher CAM-S peak scores) had greater DEL-B-P scores compared to the lowest tertile (p < .005) but were not significantly different from each other (p = .64).
Family Caregivers: Associations Between DEL-B-C and Delirium Severity
Greater DEL-B-C was associated with greater patient delirium severity by CAM-S sum (Spearman’s rho = .27, p < .001), indicating a small to medium correlation. We also observed significant differences in DEL-B-C scores between tertiles of CAM-S peak scores (F = 9.9, p < .001). The average DEL-B-C score in the lowest tertile (n = 94) was 6.2 (SD = 5.6), middle tertile (n = 90) was 8.1 (SD = 7.1), and highest tertile (27) was 12.7 (SD = 8.3). Bonferroni-corrected pair-wise comparisons revealed that CAM-S peak tertile 3 (i.e., highest CAM-S peak scores) had greater DEL-B-C compared to tertiles 1 (p < .001) and 2 (p = .007), but tertiles 1 and 2 were not different from each other (p = .12).
Discussion and Implications
We have developed and tested new delirium burden measures designed to quantify the subjective experience of delirium for patients and their family caregivers. These instruments were feasible and brief (2–3 min on average) to administer and acceptable to patients and their families. Following recommended guidelines (DeVellis, 2017; Lynn, 1986), we identified and operationalized content domains, evaluated and refined items with input from an interdisciplinary expert panel, and prospectively tested the instruments in an appropriate target population of medical-surgical patients and their family caregivers.
Most patients and family caregivers reported a moderate level of burden if the patient developed delirium, but little to no burden if the patient did not develop delirium. About half the patient sample overall reported DEL-B-P scores of 2 or less; and about a quarter of the family caregiver sample overall reported DEL-B-C scores of 2 or less. By contrast, half of the patient sample with delirium reported DEL-B-P scores of 8 or higher, and half of the delirium patient caregiver sample reported DEL-B-C scores of 12.5 or higher. Interestingly, patient and family caregiver DEL-B scores showed only mild correlations. However, the correlation was stronger for patients and caregivers who lived together. Overall, the results suggest that patients and their family caregivers experience the burden of delirium differently, and that a burdensome experience for a family caregiver may not translate to a burdensome experience for a patient, and vice versa. Consistent with previous reports (Partridge et al., 2013), we also found that on average, DEL-B scores were higher in family caregivers than for patients. This may be in part because the delirium itself, or contributions from cognitive impairment, may have affected patients’ ability to accurately recall the event. Retest reliability of DEL-B-C was strong at 1 month (rho = .73); reliability of DEL-B-P could not be assessed since burden was not measured during hospitalization when a patient may have been experiencing an active delirium episode.
Finally, convergent validity was demonstrated: DEL-B scores were higher in patients who developed delirium compared to patients who did not develop delirium, and were correlated with delirium severity measures with small-moderate effect sizes. Although DEL-B appears to be at least somewhat related to the severity of the delirium episode, the experience of burden is incompletely explained by delirium severity alone, suggesting that additional factors contribute to the subjective experience of delirium. For instance, burden may be driven by delirium subtype (e.g., hypoactive vs hyperactive), emotional lability, functional impairments, incontinence, sleep disruption, comorbidity, socioeconomic or other factors which may influence the burdensome nature of the episode. An important area for future work will be to identify the predictors of delirium burden.
Strengths of this study include the robust approach to instrument development and testing, the prospective cohort utilized for testing, and the feasible measures that emerged. However, several important caveats of our study should be noted. First, burden is a complex construct that is difficult to capture in a single instrument; thus, important items may not have been included. Since we focused on distress, aspects such as physical demands and costs of care were not assessed. Furthermore, it is possible that a different expert panel would have led to selection of different items; we cannot exclude this potential source of bias despite our efforts for cross-disciplinary representation. Although we used a rigorous approach to select the final items for our instrument, we acknowledge that these burden instruments may not be comprehensive and important contributors to the delirium experience may not have been fully captured. Second, we assessed DEL-B at 1-month postdischarge, but it is quite possible that different results may have been generated at different timepoints. We found that the DEL-B-C instrument had acceptable test–retest reliability from hospitalization to 1 month later. The modestly higher scores at 1 month may have been influenced by many factors, including physical demands of caregiving not present during hospitalization, prolonged recovery period for patients, or post-traumatic stress. It is likely that even higher DEL-B scores would have been reported if patients and families had been interviewed during the peak of delirium; however, this was not feasible in the present study. The fact that so much burden is still reported at 1 month after the episode stresses the importance of the problem. Our data suggest that patients and family caregivers would benefit from support not only during and immediately after a delirium episode, but also some time after when they have had time to reflect on their experience. Future studies of test–retest at different intervals in both patients and caregivers are needed to better understand the reliability of these instruments. Third, the self-report of distress may differ by study population and factors such as age, sex, race, ethnicity, and country of origin (Knight & Sayegh, 2010; Napoles, Chadiha, Eversley, & Moreno-John, 2010; Pinquart & Sorensen, 2005; Scharlach et al., 2006). Moreover, burden may differ between patients with hyperactive compared to hypoactive delirium, with underlying dementia, or across different settings (e.g., surgery, intensive care, palliative care). Finally, it is likely that patients who cannot recall their delirium—due to delirium itself or other causes of cognitive impairment like dementia—will inaccurately report delirium burden. This is an important area for future investigation. Because our baseline assessment occurred after hospital admission, delirium and other factors could influence cognitive functioning, making it difficult to determine a patient’s true cognitive baseline. Future studies should further investigate whether prehospitalization factors, like dementia severity, are associated with delirium burden. In addition, future studies will be critical to examine the generalizability of these measures to other populations and settings.
Delirium is often distressing to patients and caregivers. Development of tools to measure different features contributing to delirium burden is a critical step toward improving support and management of delirium for patients and their family caregivers. Additional validation studies of these instruments are needed to examine predictive validity for important outcomes, such as quality of life, physical and emotional health, mortality, health care costs for both patients and their family caregivers, and to assure generalizability of these measures across diverse study populations and settings. Although interventions to reduce the risk of and severity of delirium should reduce delirium burden, our results suggest that interventions may need to focus more specifically on improving the lived experience of delirium for patients and their family caregivers. Burden from delirium is a tremendously important element to consider in the clinical management of delirium. Sustained emotional and psychological consequences from delirium are being increasingly recognized (Drews et al., 2015; Langan et al., 2017). Thus, burden should be carefully addressed as another important dimension of delirium and, ultimately, considered as an outcome for clinical trials and other intervention studies for delirium. We hope these instruments will lay the foundation for this important future work.
Funding
This work was supported by grants from the National Institutes of Health (R01AG044518 [S. K. Inouye/R. N. Jones], R24AG054259 [S. K. Inouye], K07AG041835 [S. K. Inouye], P01AG031720 [S. K. Inouye], R01AG030618 [E. R. Marcantonio], K24AG035075 [E. R. Marcantonio], and T32AG023480 [A. M. Racine]). S. K. Inouye holds the Milton and Shirley F. Levy Family Chair.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgment
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, and staff members who participated in the BASIL Study, including the teams at the BIDMC Hospital Medicine Service, Acute Geriatrics service, Department of General Surgery, and the Department of Orthopedic Surgery. This work is dedicated to the memory of Joshua Bryan Inouye Helfand and Bradley Yoshio Inouye.
The authors would like to especially thank the individual members of the BASIL Study Group team (presented in alphabetical order; individuals listed may be part of multiple groups, but are listed only once under major activity, listed in parentheses):
Expert Review Panel: Charles H. Brown, MD (Johns Hopkins); Sevdenur Cizginer, MD (Brown University); Diane Clark, PT, DScPT, MBA (University of Alabama); Joseph H. Flaherty, MD (St. Louis University); Anne Gleason, BS (HSL); Ann M. Kolanowski, PhD, RN (Penn State); Karen J. Neufeld, MD, MPH (Johns Hopkins University); Margaret G. O’Connor, PhD (BIDMC); Margaret A. Pisani, MD, MPH (Yale); Thomas Robinson, MD (University of Colorado); Joe Verghese, MB, BS (Albert Einstein); Heidi Wald, MD, MPH (University of Colorado); Sharon M. Gordon, PsyD (Vanderbilt)
Data Management and Statistical Analysis Team: Yun Gou, MA (HSL); Douglas Tommet, MPH (Brown University).
Field Team: Tatiana Abrantes, BS (HSL); Brett Armstrong, MPH (BIDMC); Sylvia Bertrand, BS (HSL); Angelee Butters, MA (BIDMC); Madeline D’Aquila, BS (HSL); Jacqueline Gallagher, MS (BIDMC); Jennifer Kettell, BS (HSL); Jacqueline Nee, BA (HSL); Katelyn Parisi, BA, (HSL); Margaret Vella, BS (HSL); Guoquan Xu, MD, PhD (HSL); Lauren Weiner, MA (BIDMC).
Co-Investigators: Tamara Fong, MD, PhD (HSL, BIDMC, HMS); Tammy Hshieh, MD (BWH); Edward R. Marcantonio, MD, SM (BIDMC, HMS); Annie Racine, PhD (HSL, HMS); Eva M. Schmitt, PhD (HSL); Dena Schulman-Green, PhD (Yale University); Patricia A.Tabloski, PhD, GNP-BC, FGSA, FAAN (Boston College); Thomas Travison, PhD (HSL, HMS)
Overall Principal Investigators (Multi PIs): Sharon K. Inouye, MD, MPH (Overall PI, HSL, BIDMC, HMS); Richard N. Jones, ScD (Brown University)
Contributor Information
BASIL Study Group:
Charles H Brown, Sevdenur Cizginer, Diane Clark, Joseph H Flaherty, Anne Gleason, Ann M Kolanowski, Karen J Neufeld, Margaret G O’Connor, Margaret A Pisani, Thomas Robinson, Joe Verghese, Heidi Wald, Sharon M Gordon, Yun Gou, Douglas Tommet, Tatiana Abrantes, Brett Armstrong, Sylvia Bertrand, Angelee Butters, Madeline D’Aquila, Jacqueline Gallagher, Jennifer Kettell, Jacqueline Nee, Katelyn Parisi, Margaret Vella, Guoquan Xu, Lauren Weiner, Tamara Fong, Tammy Hshieh, Edward R Marcantonio, Annie Racine, Eva M Schmitt, Dena-Green Schulman, Patricia A Tabloski, Thomas Travison, Sharon K Inouye, and Richard N Jones
References
- Breitbart W., Gibson C., & Tremblay A (2002). The delirium experience: Delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics, 43, 183–194. doi:10.1176/appi.psy.43.3.183 [DOI] [PubMed] [Google Scholar]
- Bruera E., Bush S. H., Willey J., Paraskevopoulos T., Li Z., Palmer J. L., … Elsayem A (2009). Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer, 115, 2004–2012. doi:10.1002/cncr.24215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao C. Y., Wu H. S., & Hsiao C. Y (2015). Caregiver burden for informal caregivers of patients with dementia: A systematic review. International Nursing Review, 62, 340–350. doi:10.1111/inr.12194 [DOI] [PubMed] [Google Scholar]
- Cummings J. L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D. A., & Gornbein J (1994). The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44, 2308–2314. doi:10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- DeVellis R. F. (2017). Scale development : Theory and applications (Fourth ed., Applied social research methods series ; v 26): Los Angeles: Sage publications. [Google Scholar]
- Drews T., Franck M., Radtke F. M., Weiss B., Krampe H., Brockhaus W. R., … Spies C. D (2015). Postoperative delirium is an independent risk factor for posttraumatic stress disorder in the elderly patient: A prospective observational study. European Journal of Anaesthesiology, 32, 147–151. doi:10.1097/EJA.0000000000000107 [DOI] [PubMed] [Google Scholar]
- Finucane A. M., Lugton J., Kennedy C., & Spiller J. A (2017). The experiences of caregivers of patients with delirium, and their role in its management in palliative care settings: An integrative literature review. Psycho-Oncology, 26, 291–300. doi:10.1002/pon.4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller V. (2016). Delirium recall - an integrative review. Journal of Clinical Nursing, 25, 1515–1527. doi:10.1111/jocn.13155 [DOI] [PubMed] [Google Scholar]
- Grover S., Ghosh A., & Ghormode D (2015). Experience in delirium: Is it distressing?The Journal of Neuropsychiatry and Clinical Neurosciences, 27, 139–146. doi:10.1176/appi.neuropsych.13110329 [DOI] [PubMed] [Google Scholar]
- Inouye S. K., van Dyck C. H., Alessi C. A., Balkin S., Siegal A. P., & Horwitz R. I (1990). Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine, 113, 941–948. doi:10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- Inouye S. K., Kosar C. M., Tommet D., Schmitt E. M., Puelle M. R., Saczynski J. S., … Jones R. N (2014). The CAM-S: Development and validation of a new scoring system for delirium severity in 2 cohorts. Annals of Internal Medicine, 160, 526–533. doi:10.7326/M13-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S. K., Westendorp R. G., & Saczynski J. S (2014). Delirium in elderly people. Lancet, 383, 911–922. doi:10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D. I., Cummings J. L., Christine D., Bray T., Castellon S., Masterman D., … DeKosky S. T (1998). Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The neuropsychiatric inventory caregiver distress scale. Journal of the American Geriatrics Society, 46, 210–215. doi:10.1111/j.1532-5415.1998.tb02542.x [DOI] [PubMed] [Google Scholar]
- Knight B. G., & Sayegh P (2010). Cultural values and caregiving: The updated sociocultural stress and coping model. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B, 5–13. doi:10.1093/geronb/gbp096 [DOI] [PubMed] [Google Scholar]
- Koo T. K., & Li M. Y (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15, 155–163. doi:10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan C., Sarode D. P., Russ T. C., Shenkin S. D., Carson A., & Maclullich A. M. J (2017). Psychiatric symptomatology after delirium: A systematic review. Psychogeriatrics: The Official Journal of the Japanese Psychogeriatric Society, 17, 327–335. doi:10.1111/psyg.12240 [DOI] [PubMed] [Google Scholar]
- Lutomski J. E., Baars M. A., Schalk B. W., Boter H., Buurman B. M., den Elzen W. P., … Consortium T.-M (2013). The development of the older persons and informal caregivers survey minimum dataset (topics-mds): A large-scale data sharing initiative. PLoS One, 8, e81673. doi:10.1371/journal.pone.0081673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn M. R. (1986). Determination and quantification of content validity. Nursing Research, 35, 382–386. doi:10.1097/ 00006199-198611000-00017 [PubMed] [Google Scholar]
- Marcantonio E. R. (2018). Delirium in hospitalized older adults. The New England Journal of Medicine, 378, 96–97. doi:10.1056/NEJMc1714932 [DOI] [PubMed] [Google Scholar]
- Morandi A., Rogers B. P., Gunther M. L., Merkle K., Pandharipande P., Girard T. D., … Hopkins R. O; VISIONS Investigation, VISualizing Icu SurvivOrs Neuroradiological Sequelae (2012). The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study*. Critical Care Medicine, 40, 2182–2189. doi:10.1097/CCM.0b013e318250acdc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoles A. M., Chadiha L., Eversley R., & Moreno-John G (2010). Reviews: Developing culturally sensitive dementia caregiver interventions: Are we there yet?American Journal of Alzheimer’s Disease and Other Dementias, 25, 389–406. doi:10.1177/1533317510370957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M., & Guest C (1989). Application of a multidimensional caregiver burden inventory. The Gerontologist, 29, 798–803. doi:10.1093/geront/29.6.798 [DOI] [PubMed] [Google Scholar]
- Partridge J. S., Martin F. C., Harari D., & Dhesi J. K (2013). The delirium experience: What is the effect on patients, relatives and staff and what can be done to modify this?International Journal of Geriatric Psychiatry, 28, 804–812. doi:10.1002/gps.3900 [DOI] [PubMed] [Google Scholar]
- Pinquart M., & Sörensen S (2005). Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: A meta-analysis. The Gerontologist, 45, 90–106. doi:10.1093/geront/45.1.90 [DOI] [PubMed] [Google Scholar]
- Scharlach A. E., Kellam R., Ong N., Baskin A., Goldstein C., & Fox P. J (2006). Cultural attitudes and caregiver service use: Lessons from focus groups with racially and ethnically diverse family caregivers. Journal of Gerontological Social Work, 47, 133–156. doi:10.1300/J083v47n01_09 [DOI] [PubMed] [Google Scholar]
- Schmitt E. M., Gallagher J., Albuquerque A., Tabloski P., Lee H. J., Gleason L., … Schulman-Green D (2017). Perspectives on the delirium experience and its burden: Common themes among older patients, their family caregivers, and nurses. The Gerontologist, gnx153-gnx153. doi:10.1093/geront/gnx153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp (2017). Stata statistical software: Release 15 (Version 15). College Station, TX: StataCorp LLC. [Google Scholar]
- Vasunilashorn S. M., Fong T. G., Albuquerque A., Marcantonio E. R., Schmitt E. M., Tommet D., … Inouye S. K (2018). Delirium severity post-surgery and its relationship with long-term cognitive decline in a cohort of patients without dementia. Journal of Alzheimer’s Disease, 61, 347–358. doi:10.3233/JAD-170288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S. M., Marcantonio E. R., Gou Y., Pisani M. A., Travison T. G., Schmitt E. M., … Inouye S. K (2016). Quantifying the severity of a delirium episode throughout hospitalization: The combined importance of intensity and duration. Journal of General Internal Medicine, 31, 1164–1171. doi:10.1007/s11606-016-3671-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. A., Fearing M. A., Sternberg E. J., & Inouye S. K (2008). The confusion assessment method: A systematic review of current usage. Journal of the American Geriatrics Society, 56, 823–830. doi:10.1111/j.1532-5415.2008.01674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit S. H., Reever K. E., & Bach-Peterson J (1980). Relatives of the impaired elderly: Correlates of feelings of burden. The Gerontologist, 20, 649–655. doi:10.1093/geront/20.6.649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.