Abstract
Background:
Perinatally HIV-infected adolescents (PHIVA) are an expanding population vulnerable to loss to follow-up (LTFU). Understanding the epidemiology and factors for LTFU is complicated by varying LTFU definitions.
Setting:
Asian regional cohort incorporating 16 pediatric HIV services across six countries.
Methods:
Data from PHIVA (aged 10-19 years) who received combination antiretroviral therapy (cART) 2007-2016 was used to analyze LTFU via: (i) an IeDEA method that determined LTFU as <90 days late for an estimated next scheduled appointment without returning to care; and (ii) the absence of patient-level data for >365 days prior to last data transfer from clinic sites. Descriptive analyses and competing-risk survival and regression analyses were used to evaluate LTFU epidemiology and associated factors when analyzed using each method.
Results:
Of 3,509 included PHIVA, 275 (7.8%) met IeDEA and 149 (4.3%) met 365-day absence LTFU criteria. Cumulative incidence of LTFU was 19.9% and 11.8% using IeDEA and 365-day absence criteria respectively. Risk factors for LTFU across both criteria included: age at cART initiation <5 years compared to age ≥5 years, rural clinic settings compared to urban clinic settings, and high viral loads compared to undetectable viral loads. Age 10-14 years compared to age 15-19 years was another risk factor identified using 365-day absence criteria but not IeDEA LTFU criteria.
Conclusion:
Between 12% and 20% of PHIVA were determined LTFU with treatment fatigue and rural treatment settings consistent risk factors. Better tracking of adolescents is required to provide a definitive understanding of LTFU and optimise evidence-based models of care.
Keywords: HIV, adolescent, loss to follow-up
INTRODUCTION
A key component to achieving the ‘90-90-90’ target set by the United Nations Programme on HIV/AIDS (UNAIDS) is the retention of HIV-infected individuals in care on combination antiretroviral therapy (cART).1 Perinatally HIV-infected adolescents (PHIVA) represent an expanding population particularly vulnerable to being lost to follow-up (LTFU) as they deal with a chronic disease during a complex period of psychosocial development.2–6 Furthermore, adolescents have to navigate transition from pediatric to adult HIV services, which poses significant challenges to their care continuum.7–9 Despite these widely acknowledged vulnerabilities, data regarding interventions to improve adolescent retention in care are limited.10 Existing data relating to LTFU are complicated by the various definitions for what constitutes being lost and the potential for return to care. However, in the absence of routinely collected information on scheduled clinic appointments analyses on LTFU rely on such agreed definitions, which has implications for interpreting data relating to the epidemiology and associated factors for LTFU to guide interventions.
Traditionally, analyses conducted within TREAT (Therapeutics Research, Education, and AIDS Training) Asia cohorts have defined LTFU as no observed visit for more than 365 days before the date of the last data transfer from clinic sites to a centralized database. This definition may not accurately depict specific clinic follow-up practices and is likely to provide a conservative estimate of LTFU. The International epidemiology Databases to Evaluate AIDS (IeDEA) consortium has developed a method designed to capture LTFU in the context of having no routinely collected data regarding a patient’s next scheduled clinic appointment.11 This uses patient and clinic visit schedules to estimate a next scheduled clinic appointment, and categorises patients who are more than 90 days late for their next estimated scheduled appointment as being LTFU.11 To date, this methodology has only been published in a largely adult sub-Saharan African cohort, and the validity of this methodology in other regions and in pediatric settings remains uncertain. However, the capacity to individualize follow-up schedules is appealing as it has the scope to provide a more precise assessment of LTFU compared to fixed-time definitions.
The purpose of this study is to analyze LTFU for PHIVA in the TREAT Asia pediatric cohort using both the IeDEA and traditional TREAT Asia definitions to compare the cumulative incidence and associated factors for LTFU and describe the characteristics of PHIVA who met one but not both LTFU criteria.
METHODS
The TREAT Asia pediatric HIV Observational Database (TApHOD) of IeDEA Asia-Pacific was established in 2007 as a regional collaboration evaluating HIV management and outcomes in Asian children and adolescents. The study involves the collection of data during routine HIV care across 16 pediatric HIV services in Asia (Cambodia=1, India=1, Indonesia=2, Malaysia=4, Thailand=5, and Vietnam=3), including demographics, HIV diagnosis, laboratory results, World Health Organization (WHO) clinical events, antiretroviral regimens and adverse events. Data are transferred to a centralized database at the Kirby Institute (University of New South Wales, Sydney, Australia) for management and statistical analysis. Full details of the collaboration are reported elsewhere.12 For this analysis, any PHIVA aged 10–19 years that ever received cART through the TApHOD network between January 2007 and December 2016 were included. Ethics approvals were obtained through the human research ethics committees at participant sites, Kirby Institute, and the coordination center at TREAT Asia/amfAR (Bangkok, Thailand).
Definitions
The adolescent age range, and period of adolescence, was defined as 10–19 years of age. Year of enrolment referred to the calendar year at which participants were first enrolled in care at a TApHOD clinic site. Orphan status was based on having both biological parents either deceased or alive. Combination antiretroviral therapy was defined as an antiretroviral regimen incorporating at least three antiretroviral agents of any class. Switch to a subsequent cART regimen was defined as either a change in antiretroviral drug class, the addition of a new antiretroviral class, or a change in at least two antiretroviral agents. Using IeDEA methodology,11 the estimated next scheduled clinic appointment date was calculated by adding the interval between the participant’s last two clinic visits to their last clinic visit date. To account for extreme variations in visit interval, if the participant’s visit interval fell outside the 25th-95th percentile range for their clinic’s visit interval based on year of antiretroviral therapy the median visit interval for that clinic and year of antiretroviral therapy was added to the participant’s last clinic date to obtain their estimated next scheduled appointment date.11 Loss to follow-up using IeDEA criteria (IeDEA LTFU) was defined as being more than 90 days late for an estimated next scheduled clinic appointment and did not return to care prior to date of last data transfer from clinic sites, with the date of LTFU defined as 90 days after the estimated next scheduled clinic appointment date.11 Traditional TREAT Asia LTFU criteria was defined as more than a 365-day absence of data prior to date of last data transfer from clinic sites (365-day absence LTFU), with the date of LTFU was defined as 365 days following the last clinic visit. Transfer to another HIV service was identified through clinic sites reporting transfer of the participant to another HIV service. HIV viral suppression was defined as site-reported “undetectable” HIV viral load. The beginning of follow-up (baseline) was either the participant’s 10th birthday or first clinic visit for those who commenced care after their 10th birthday. The end of the study period was defined as the date of last data transfer from clinic sites to the centralized database, which incorporated data up to December 2017 to permit at least a possible 365 days of observation for adolescents who ever received cART from 2007 to 2016.
Statistical analysis
Descriptive analyses were used to report demographic and clinical characteristics at baseline and last clinic visit for the whole study population, PHIVA who met IeDEA LTFU criteria, PHIVA who met 365-day absence LTFU criteria, and PHIVA who met one but not both LTFU criteria. For CD4, HIV viral load, and WHO clinical stage values, the most recent result within a 365-day period prior to baseline and last clinic visit were reported. Competing-risk (Fine and Gray method)13 survival analyses, with death as a competing risk, were used to determine the cumulative incidence of being LTFU using both IeDEA and 365-day absence criteria. The period of observation began at baseline and finished at either the date of last data transfer from clinic sites if remained in care, date of transfer, date of death, or date of LTFU. Participants who remained in care at 20 years of age were censored on the day prior to their 20th birthday. A competing-risk regression analysis (Fine and Gray method),13 with death as a competing risk, was used to determine the subdistribution hazard ratio (SHR) and adjusted SHR (aSHR) with 95% confidence intervals (CI) for factors associated with LTFU using both IeDEA and 365-day absence criteria. Covariates included age, sex, clinic setting, orphan status, primary caregiver, age at cART initiation, CD4 count, HIV viral load, WHO clinical stage, and cART regimen (first or subsequent). Age, CD4 count, and HIV viral load were analysed as time-updated variables. Covariates with a p-value of <0.2 on univariate analysis were included in multivariate analyses. Multivariate analyses were conducted in a stepwise fashion maintaining covariates that retained a p-value of 0.05. For variables that did not remain significant in the multivariate analyses, aSHRs were obtained by including and removing each insignificant variable to the final model. Unknown or missing data were included in the analyses as a separate category within each variable. A sensitivity competing-risk regression analysis (Fine and Gray method)13 was also conducted, with death and transfer as competing risks, to evaluate factors associated with LTFU using IeDEA and 365-day absence criteria. All statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, Texas, US).
RESULTS
There were 3,650 PHIVA enrolled in care within the TApHOD network between 1st January 2007 and 31st December 2016, of which 3,509 (96.1%) had received cART during this period and were included in the study. Of the 3,509 PHIVA included in the study 2,865 (81.7%) were enrolled in care prior to ten years of age and 644 (18.4%) were enrolled in care at ten years of age or older. The median period between baseline and last reported clinic visit for the entire study population was 5.3 [interquartile range (IQR) 3.2, 7.8] years. There were 141 (4.0%) PHIVA who did not receive cART during the study period and were excluded from the study. There was a similar proportion of males in the excluded and included populations (44.7% vs 49.3%, Chi-square test p=0.28); but the excluded population had a higher proportion managed in a rural clinic setting (34.8% vs 6.1%, Chi-square test p<0.001), had a lower baseline median CD4 count (532 cells/μL vs 700 cells/μL, Kruskal-Wallis test p<0.001), and a lower proportion of undetectable baseline HIV viral loads (17.0% vs 34.3%, Chi-square test p<0.001) compared to the included population.
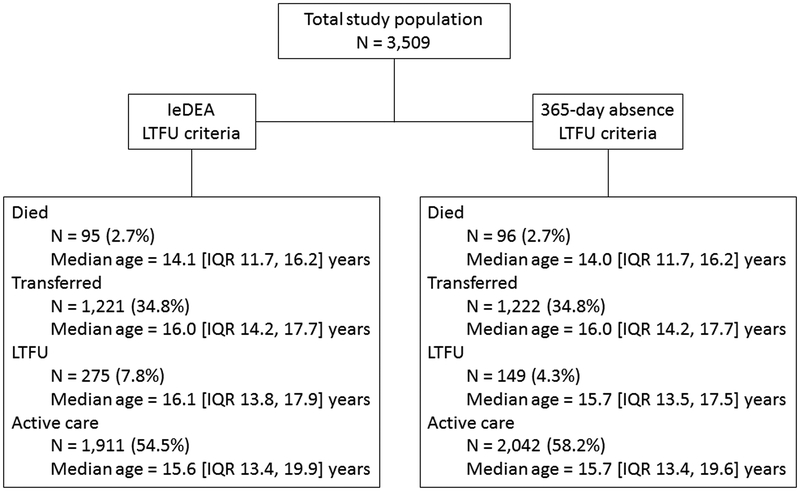
Figure 1 outlines the outcomes of the study population using IeDEA and 365-day absence LTFU criteria. Using IeDEA LTFU criteria, there were 275 (7.8%) PHIVA who were LTFU at a median age of 16.1 [IQR 13.8, 17.9] years. There was a total 19,326.2 person-years observation, resulting in an IeDEA LTFU rate of 1.42 [95%CI 1.26, 1.60] per 100 person-years. Using 365-day absence LTFU criteria, there were 149 (4.3%) PHIVA LTFU at a median age of 15.7 [IQR 13.5, 17.5] years. There was a total 19,411.7 person-years observation, resulting in a 365-day absence LTFU rate of 0.77 [95%CI 0.65, 0.90] per 100 person-years. Table 1 summarises the demographic and clinical characteristics at baseline and last clinic visit for the total study population, PHIVA who met IeDEA LTFU criteria, and PHIVA who met 365-day absence LTFU criteria.
Figure 1. Outcomes for study population using IeDEA and 365-day absence loss to follow-up criteria.
IeDEA = International epidemiology Databases to Evaluate AIDS. LTFU = loss to follow-up.
Table 1.
Characteristics of study population at baselinea and at last clinic visit for total population, and cohorts meeting IeDEA and 365-day loss to follow-up criteria.
| Total population N=3,509 |
IeDEA LTFU cohort N=275 |
365-day absence LTFU cohort N=149 |
||||
|---|---|---|---|---|---|---|
| Baselinea | Last visit | Baselinea | Last visit | Baselinea | Last visit | |
| Median age (years) [range] | 10 [10,18.9] | 15.7 [10.0, 20.0] | 10 [10, 16.0] | 15.5 [10.1, 19.5] | 10 [10, 15.0] | 15.5 [10.1, 18.8] |
| Median period between baseline and last visit (years) [IQR] | 5.3 [3.2, 7.8] | 5.0 [2.8, 7.0] | 5.0 [2.7, 6.6] | |||
| Sex | ||||||
| Male | 1,731 (49.3) | 145 (52.7) | 79 (53.0) | |||
| Country | ||||||
| Cambodia | 549 (15.7) | 32 (11.6) | 21 (14.1) | |||
| India | 162 (4.6) | 32 (11.6) | 27 (18.1) | |||
| Indonesia | 125 (3.6) | 15 (5.5) | 6 (4.0) | |||
| Malaysia | 257 (7.3) | 33 (12.0) | 15 (10.1) | |||
| Thailand | 1,592 (45.4) | 148 (53.8) | 67 (45.0) | |||
| Vietnam | 824 (23.5) | 15 (5.5) | 13 (8.7) | |||
| Clinic setting | ||||||
| Urban | 1,935 (55.1) | 151 (54.9) | 64 (43.0) | |||
| Semi-urban | 1,360 (38.8) | 86 (31.3) | 55 (36.9) | |||
| Rural | 214 (6.1) | 38 (13.8) | 30 (20.1) | |||
| Calendar period at enrolment in care | ||||||
| <2002 | 326 (9.3) | 29 (10.6) | 20 (13.4) | |||
| 2002–2006 | 1,615 (46.0) | 129 (46.9) | 76 (51.0) | |||
| 2007–2011 | 1,303 (37.1) | 88 (32.0) | 42 (28.2) | |||
| 2012–2016 | 265 (7.6) | 29 (10.6) | 11 (7.4) | |||
| Orphan | ||||||
| No | 2,449 (69.8) | 2,395 (68.3) | 184 (66.9) | 178 (64.7) | 97 (65.1) | 95 (63.8) |
| Yes | 800 (22.8) | 854 (24.3) | 65 (23.6) | 71 (25.8) | 38 (25.6) | 40 (26.9) |
| Missing/unknown | 260 (7.4) | 260 (7.4) | 26 (9.5) | 26 (9.5) | 14 (9.4) | 14 (9.4) |
| Primary caregiver | ||||||
| Immediate family | 1,369 (39.0) | 1,643 (46.8) | 93 (33.8) | 122 (44.4) | 42 (28.2) | 67 (45.0) |
| Extended family | 834 (23.8) | 1,230 (35.1) | 68 (24.7) | 95 (34.6) | 34 (22.8) | 49 (32.9) |
| Foster care | 292 (8.3) | 389 (11.1) | 35 (12.7) | 45 (16.4) | 19 (12.8) | 22 (14.8) |
| Missing/unknown | 1,014 (28.9) | 247 (7.0) | 79 (28.7) | 13 (4.7) | 54 (36.2) | 11 (7.4) |
| CD4 count (cells/μL)b | ||||||
| Median [IQR] | 700 [416, 977] | 644 [447, 860] | 737 [482, 1002] | 627 [427, 861] | 707 [417, 965] | 560 [333, 778] |
| ≥500 | 2,101 (59.9) | 1,896 (54.0) | 171 (62.2) | 150 {54.6) | 87 (58.4) | 65 (43.6) |
| 350–499 | 319 (9.1) | 394 (11.2) | 21 (7.6) | 28 (10.2) | 11 (7.4) | 21 (14.1) |
| 200–349 | 233 (6.6) | 218 (6.2) | 17 (6.2) | 24 (8.7) | 12 (8.1) | 17 (11.4) |
| <200 | 407 (11.6) | 246 (7.0) | 24 (8.7) | 22 (8.0) | 12 (8.1) | 14 (9.4) |
| Missing/unknown | 449 (12.8) | 755 (21.5) | 42 (15.3) | 51 (18.6) | 27 (18.1) | 32 (21.5) |
| Viral load (copies/mL)b | ||||||
| Median log [IQR]c | 4.0 [2.7, 4.9] | 3.5 [2.1, 4.7] | 3.5 [2.2, 4.8] | 3.9 [2.4, 4.7] | 3.5 [2.5, 4.9] | 4.3 [3.1, 4.7] |
| Undetectable | 1,205 (34.3) | 1,866 (53.2) | 125 (45.5) | 120 (43.6) | 55 (36.9) | 48 (32.2) |
| Detectable <1,000 | 182 (5.2) | 254 (7.2) | 17 (6.2) | 19 (6.9) | 8 (5.4) | 7 (4.7) |
| 1,000–9,999 | 110 (3.1) | 121 (3.5) | 9 (3.3) | 15 (5.5) | 5 (3.4) | 10 (6.7) |
| ≥10,000 | 300 (8.6) | 252 (7.2) | 17 (6.2) | 29 (10.6) | 9 (6.0) | 18 (12.1) |
| Missing/unknown | 1,712 (48.8) | 1,016 (29.0) | 107 (38.9) | 92 (33.5) | 72 (48.3) | 66 (44.3) |
| WHO clinical stageb | ||||||
| I/II | 3,252 (92.7) | 3,431 (97.8) | 252 (91.6) | 267 (97.1) | 13 (92.0) | 145 (97.3) |
| III/IV | 257 (7.3) | 78 (2.2) | 23 (8.4) | 8 (2.9) | 12 (8.1) | 4 (2.7) |
| Age at cART initiation (years) | ||||||
| Median [IQR] | 7.0 [4.4, 9.8] | 6.8 [4.0, 9.4] | 7.4 [5.0, 10.3] | |||
| <5 | 1,048 (29.9) | 91 (33.1) | 38 (25.5) | |||
| 5–9 | 1,655 (47.2) | 129 (46.9) | 70 (47.0) | |||
| ≥10 | 806 (23.0) | 55 (20.0) | 41 (27.5) | |||
| cART regimens | ||||||
| 0 | 625 (17.8) | 0 | 43 (15.6) | 0 | 33 (22.2) | 0 |
| 1 | 2,385 (68.0) | 2,490 (71.0) | 181 (65.8) | 177 (64.4) | 91 (61.1) | 101 (67.8) |
| ≥2 | 499 (14.2) | 1,019 (29.0) | 51 (18.6) | 98 (35.6) | 25 (16.8) | 48 (32.2) |
Values n (%) unless otherwise specified.
Either 10th birthday or first clinic visit if after 10th birthday.
Most recent value within prior 365 days.
Calculated only for detectable HIV viral loads.
cART = combination antiretroviral therapy. IeDEA = International epidemiology Databases to Evaluate AIDS. LTFU = loss to follow-up. WHO = World Health Organization.
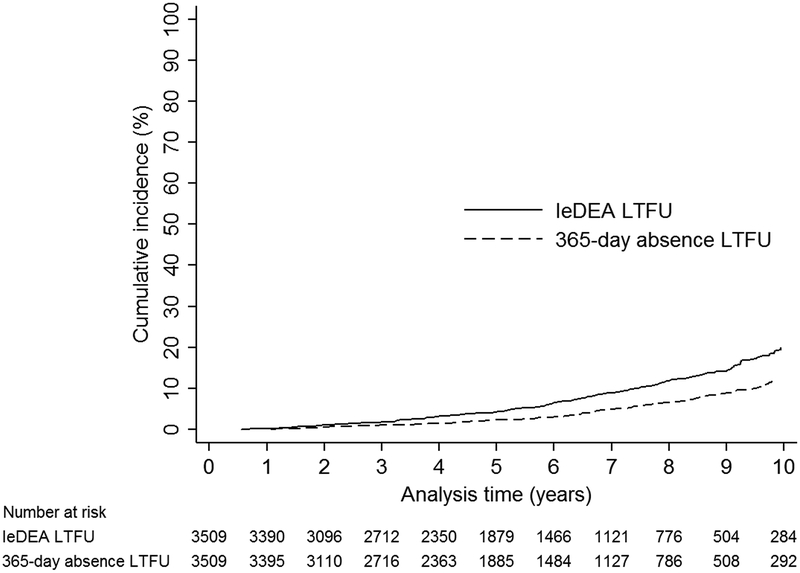
Figure 2 demonstrates the cumulative incidence of LTFU using IeDEA and 365-day absence criteria. Using IeDEA LTFU criteria, the 5-year cumulative incidence of LTFU was 4.2% [95%CI 3.5, 5.0] and the 10-year cumulative incidence of LTFU was 19.9% [95%CI 17.3, 22.7]. Using 365-day absence criteria, the 5-year cumulative incidence of LTFU was 2.3% [95%CI 1.7, 2.9] and the 10-year cumulative incidence of LTFU was 11.8% [95%CI 9.7, 14.2].
Figure 2. Cumulative incidence of loss to follow-up throughout adolescence for IeDEA and 365-day absence criteria.
IeDEA = International epidemiology Databases to Evaluate AIDS. LTFU = loss to follow-up.
Factors associated with LTFU
On multivariate competing-risk regression analysis (with death as a competing risk) the following factors were found to be associated with a higher risk of IeDEA LTFU: rural clinic settings compared to urban clinic settings (aSHR 1.9 [95%CI 1.2, 3.1]); and a last HIV viral load ≥10,000 copies/mL compared to an undetectable last HIV viral load (aSHR 1.9 [95%CI 1.4, 2.7]) (Table 2). A lower risk of IeDEA LTFU was associated with commencing cART at age ≥5 years compared to at age <5 years (age 5–9 years vs age <5 years aSHR 0.4 [95%CI 0.3, 0.6]; age ≥10 years vs age <5 years aSHR 0.3 [95%CI 0.2, 0.4]). Using 365-day absence LTFU criteria, the following factors were associated with a higher risk of LTFU: rural clinic settings compared to urban clinic settings (aSHR 3.0 [95%CI 1.6, 5.5]); and a last HIV viral load ≥1,000 copies/mL compared to an undetectable last HIV viral load (1,000–9,999 copies/mL vs undetectable aSHR 2.4 [95%CI 1.3, 4.2]; ≥10,000 copies/mL vs undetectable aSHR 2.3 [95%CI 1.5, 3.8]). A lower risk of LTFU was associated with current age 15–19 years compared to current age 10–14 years (aSHR 0.2 [95%CI 0.1, 0.5); and commencing cART age ≥5 years compared to age <5 years (age 5–9 years vs age <5 years aSHR 0.5 [95%CI 0.3, 0.8]; age ≥10 years vs age <5 years aSHR 0.5 [95%CI 0.3, 0.8]).
Table 2.
Characteristics associated with loss to follow-up for IeDEA and 365-day absence criteria.
| IeDEA LTFU | 365-day absence LTFU | |||
|---|---|---|---|---|
| aSHR [95%CI] | p | aSHR [95%CI] | p | |
| Age (years) | ||||
| 10–14 | 1.0 | 1.0 | ||
| 15–19 | 0.6 [0.2, 1.4] | 0.23 | 0.2 [0.1, 0.5] | <0.001 |
| Sex | ||||
| Male | 1.0 | 1.0 | ||
| Female | 0.8 [0.6, 1.0] | 0.09 | 0.8 [0.5, 1.1] | 0.13 |
| Clinic setting | ||||
| Urban | 1.0 | 1.0 | ||
| Semi-rural | 0.8 [0.6, 1.1] | 0.14 | 1.1 [0.8, 1.7] | 0.50 |
| Rural | 1.9 [1.2, 3.1] | 0.01 | 3.0 [1.6, 5.5] | <0.001 |
| Orphan | ||||
| No | NS | NS | ||
| Yes | NS | NS | ||
| Primary carer | ||||
| Immediate family | 1.0 | 1.0 | ||
| Extended family | 0.9 [0.7, 1.3] | 0.60 | 0.9 [0.6, 1.4] | 0.64 |
| Foster care | 1.3 [0.9, 1.8] | 0.21 | 1.4 [0.8, 2.3] | 0.23 |
| Age at cART initiation | ||||
| <5 years | 1.0 | 1.0 | ||
| 5–9 years | 0.4 [0.3, 0.6] | <0.001 | 0.5 [0.3, 0.8] | 0.002 |
| ≥10 years | 0.3 [0.2, 0.4] | <0.001 | 0.5 [0.3, 0.8] | 0.003 |
| cART regimens | ||||
| 1 | 1.0 | NS | ||
| ≥2 | 1.1 [0.8, 1.4] | 0.65 | NS | |
| CD4 count (cells/μL) | ||||
| ≥500 | 1.0 | 1.0 | ||
| 350–499 | 0.9 [0.6, 1.4] | 0.74 | 1.6 [1.0, 2.5] | 0.05 |
| 200–349 | 1.1 [0.7, 1.8] | 0.78 | 1.2 [0.7, 2.4] | 0.50 |
| <200 | 0.8 [0.5, 1.5] | 0.49 | 0.8 [0.3, 1.9] | 0.60 |
| HIV viral load (copies/mL) | ||||
| Undetectable | 1.0 | 1.0 | ||
| Detectable <1,000 | 1.2 [0.8, 1.8] | 0.41 | 1.3 [0.7, 2.4] | 0.44 |
| 1,000–9,999 | 1.5 [0.9, 2.5] | 0.09 | 2.4 [1.3, 4.2] | 0.003 |
| ≥10,000 | 1.9 [1.4, 2.7] | <0.001 | 2.3 [1.5, 3.8] | <0.001 |
| WHO clinical stage | ||||
| I/II | NS | NS | NS | |
| III/IV | NS | NS | NS | |
aSHR = adjusted subdistribution hazard ratio. cART = combination antiretroviral therapy. CI = confidence interval. IeDEA = International epidemiology Databases to Evaluate AIDS. LTFU = loss to follow-up. NS = not significant on univariate analysis. WHO = World Health Organization.
In the sensitivity analysis, a multivariate competing-risk regression analysis (with death and transfer as competing risks), consistent factors including rural clinic settings compared to urban clinic settings and high last HIV viral loads compared to undetectable last HIV viral loads were associated with a higher risk of LTFU for both IeDEA and 365-day absence criteria. Similarly, commencing cART at age ≥5 years compared to at age <5 years was associated with a lower risk of LTFU for both IeDEA and 365-day absence criteria. Current age was the only factor between the primary and sensitivity regression analyses that differed, with current age 15–19 years compared to 10–14 years associated with a higher risk of LTFU using IeDEA criteria, and current age not found to be a significant factor using 365-day absence criteria (data available on request).
Discrepant LTFU population
There were 134 PHIVA who met IeDEA LTFU criteria but not 365-day absence LTFU criteria. Of these, 88 (65.7%) were age 15–19 years; 8 (6.0%) were managed in a rural clinic setting; 81 (60.5%) remained on their first cART regimen; 106 (79.1%) had a last CD4 count ≥500 cells/μL; and 93 (69.6%) had a last HIV viral load as undetectable. The median interval between their last clinic visit and estimated next scheduled appointment was 84 [IQR 78, 105] days; while the median interval between their last clinic visit and end of the study period was 239 [IQR 200, 273] days. There were two PHIVA who met 365-day absence LTFU criteria but not IeDEA LTFU criteria. Both PHIVA had been on cART for over 10 years, with their last CD4 counts ≥500 cells/μL and last HIV viral loads undetectable.
DISCUSSION
This study addresses the impact variations in LTFU definitions have on understanding the epidemiology and factors associated with LTFU and identifies consistent challenges to retaining Asian PHIVA in care. The IeDEA criteria provided a less conservative estimate of LTFU compared to the 365-day absence criteria, largely reflecting the shorter time period following a last clinic visit to be determined LTFU in a bid to provide a more individualized approach to determining LTFU. This raises concerns for overemphasising the true burden of LTFU using IeDEA criteria, however the 5-year cumulative incidence of LTFU using either method in our cohort was more conservative than the cumulative incidence of LTFU at age 15 years in South and South East Asia of 7.1% demonstrated by a recent multi-regional analysis that used a 365-day absence LTFU criteria.5 The lower cumulative incidence of LTFU in our analysis is likely due to only including adolescents who had received cART and having a more confined and contemporary study period (2007–2016 compared to 1999–2014).
The increased risk of LTFU associated with earlier age at cART initiation and poor virologic control consistent across both LTFU criteria raises concerns for treatment fatigue in our PHIVA cohort, and is in keeping with the identified relationship between retention in care and treatment adherence.10,14 Furthermore, there were only around 50% of the total PHIVA cohort with an undetectable HIV viral load within 12 months prior to their last clinic visit (and about 30% who had not had a HIV viral load test within the last 12 months). This identifies the need to develop models of care that address the various biopsychosocial components of chronic disease management in conjunction with the specific needs of adolescents to maintain the motivation to attend HIV health services and adherence to cART. There is emerging evidence for the role of adolescent-specific clinics, involving staff trained in adolescent health and adolescent peer-support programs, to improve retention in care and virologic outcomes in adolescents in sub-Saharan Africa.14–16 In addition, there are promising results for adolescent-specific gatherings, incorporating a holistic approach to well-being through health education, psychosocial support, socioeconomic empowerment, and recreational activities, that are run in conjunction with these adolescent-specific clinics.15,16 The higher risk of LTFU consistently identified with rural treatment settings in our cohort further advocates for a considered approach toward adolescent-friendly services outside of centralized specialist HIV services, but may also in part reflect differentiated models of cares for rural clinics attempting to access remote patients with infrequent attendance at the designated rural clinic.
Age was the only discrepant associated factor identified for LTFU. In the primary competing-risk regression analysis (with death as a competing risk), the younger adolescent age group were at higher risk of LTFU using 365-day absence criteria, which may reflect a survival bias associated with those who are retained in care into older adolescence continuing to being engaged in care. The fact age was not an associated factor using IeDEA LTFU criteria may in part be due to overestimating LTFU for clinically stable older adolescents who move to longer clinic intervals that would render them LTFU based on estimated scheduled clinic appointments derived from previous shorter clinic intervals. However, our sensitivity competing-risk regression analysis (with death and transfer as competing risks) using IeDEA criteria found that older adolescents were at higher risk of LTFU, while age was not a risk factor using 365-day absence criteria. These discrepancies highlight the impact of the transition period from adolescent to adult HIV care in analysing adolescent LTFU. Findings from adolescent cohorts in India17 and Malawi15 indicated a higher risk of LTFU for the older adolescent age group. However, the Indian cohort (which incorporated death as competing risk in the regression analysis) included only adolescents who had commenced antiretroviral therapy during adolescence with a median age at baseline of 13 years, and had a LTFU definition of missing three consecutive monthly clinic appointments;17 and the Malawi cohort (that did not incorporate competing risks in the regression analysis) had determined LTFU as not returning to clinic within two months after being expected to run out of antiretroviral therapy.15
The majority of PHIVA who met one but not both LTFU criteria had met IeDEA but not 365-day absence criteria, which was largely due to the shorter time period following a last clinic visit to meet IeDEA compared to 365-day absence LTFU criteria. These were relatively well and mainly older adolescents, which could reflect a change in practice for stable PHIVA from regular frequent clinic appointments to longer clinic intervals, the introduction of differentiated care models for stable PHIVA with less frequent formal clinic visits, or undocumented self-transfers (to either other pediatric or adult HIV services). Better tracking of adolescents, particularly as they navigate transition from pediatic to adult HIV services, is required to better understand LTFU and develop local evidence-based models of care to maintain an uninterrupted care continuum and optimize outcomes from adolescence to early adulthood.7–9 This is particularly the case for low- and middle-income countries, where health infrastructure is limited and current healthcare systems are not well adapted to managing the specific needs of an expanding population of HIV-infected adolescents.6,8
The limitations of this study include the observational nature of the study, involving variable periods of observation for each participant and the risk of incomplete and inconsistent data reporting. Also, this analysis only included adolescents who received cART and therefore more likely to be engaged in care, which could lead to an underestimation of LTFU in our overall PHIVA cohort. There was no tracing conducted to confirm LTFU, nor any prior tracing studies in our cohort to provide estimations to account for undocumented mortality and self-transfers. However, by taking a dual approach using relatively standard fixed-time criteria and a method that individually estimates LTFU from observational databases, this study provides a detailed and contemporary analysis of LTFU for PHIVA in Asia.
In conclusion, between 12% and 20% of PHIVA in our cohort are LTFU throughout adolescence, with consistency in the challenges associated with treatment fatigue and rural treatment settings identified irrespective of LTFU definition. Ongoing discrepancies in epidemiology and associated factors with varying LTFU definitions, particularly for adolescents as they navigate transition to adult HIV services, provides impetus for better tracking to provide a more definitive understanding of LTFU and establish evidence-based models of care to optimize outcomes for adolescents living with HIV.
ACKNOWLEDGEMENTS
The authors would like to thank Andreas Haas for his assistance in applying the IeDEA LTFU algorithm for this analysis. The TREAT Asia Pediatric HIV Network: PS Ly*, V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N Kumarasamy*, C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; A Kinikar*, V Mave, S Nimkar, BJ Medical College and Sassoon General Hospitals, Maharashtra, India; DK Wati*, D Vedaswari, IB Ramajaya, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati*, D Muktiarti, Cipto Mangunkusumo – Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; SM Fong*, M Lim, F Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff*, P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; TJ Mohamed*‡, MR Drawis, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy*, KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk*, V Sirisanthana, L Aurpibul, Department of Pediatrics, Faculty of Medicine, and Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; R Hansudewechakul*, P Ounchanum, S Denjanta, A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon*, P Kosalaraksa, P Tharnprisan, T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Puthanakit*, S Anugulruengkit, W Jantarabenjakul, R Nadsasarn, Department of Pediatrics, Faculty of Medicine and Research Unit in Pediatric and Infectious Diseases, Chulalongkorn University, Bangkok, Thailand; K Chokephaibulkit*†, K Lapphra, W Phongsamart, S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; KH Truong*, QT Du, CH Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do*, TM Ha, VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen*, DTK Khu, AN Pham, LT Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn*, JL Ross, T Suwanlerk, TREAT Asia/amfAR - The Foundation for AIDS Research, Bangkok, Thailand; MG Law*, A Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia; (*Steering Committee members; † Current Steering Committee Chair; ‡ co-Chair).
Conflicts of Interest and Source of Funding
A.W.B. received support from an Australian Government Department of Research Training Program Scholarship and has received grant support to his institution from Gilead. A.H.S. has received travel and grant support to her institution from ViiV HealthCare. There are no conflicts of interest for the remaining authors. This work was supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA) [Grant number U01AI069907], and the Australian Government Department of Health and Ageing. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
REFERENCES
- 1.The Joint United Nation Programme on HIV/AIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014. Available at: https://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed 17th Apirl, 2018.
- 2.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranzer K, Bradley J, Musaazi J, et al. Loss to follow-up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc. 2017;20(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults--seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 5.Slogrove AL, Schomaker M, Davies MA, et al. The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis. PLoS Med. 2018;15(3):e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14(7):627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey H, Cruz MLS, Songtaweesin WN, Puthanakit T. Adolescents with HIV and transition to adult care in the Caribbean, Central America and South America, Eastern Europe and Asia and Pacific regions. J Int AIDS Soc. 2017;20(Suppl 3):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahourou DL, Gautier-Lafaye C, Teasdale CA, et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: challenges, youth-friendly models, and outcomes. J Int AIDS Soc. 2017;20(Suppl 3):34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castelnuovo B, Mubiru F, Nakalema S, Twimukye A, Kiragga A. Describing the retention in care of human immunodeficiency virus-positive young adults who transition from adolescent to adult care. Int Health. 2018;10(4):318–320. [DOI] [PubMed] [Google Scholar]
- 10.Murray KR, Dulli LS, Ridgeway K, et al. Improving retention in HIV care among adolescents and adults in low- and middle-income countries: A systematic review of the literature. PloS One. 2017;12(9):e0184879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas AD, Zaniewski E, Anderegg N, et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21(2):e25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 14.Zanoni BC, Sibaya T, Cairns C, Lammert S, Haberer JE. Higher retention and viral suppression with adolescent-focused HIV clinic in South Africa. PloS One. 2017;12(12):e0190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKenzie RK, van Lettow M, Gondwe C, et al. Greater retention in care among adolescents on antiretroviral treatment accessing “Teen Club” an adolescent-centred differentiated care model compared with standard of care: a nested case-control study at a tertiary referral hospital in Malawi. J Int AIDS Soc. 2017;20(3):e25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izudi J, Mugenyi J, Mugabekazi M, et al. Retention of HIV-Positive Adolescents in Care: A Quality Improvement Intervention in Mid-Western Uganda. Biomed Res Int. 2018;2018:1524016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimkar S, Valvi C, Kadam D, et al. Loss to follow-up and mortality among HIV-infected adolescents receiving antiretroviral therapy in Pune, India. HIV Med. 2018;19(6):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]