Abstract
Objective:
Despite potential for dependence and adverse neurological effects, long-term benzodiazepine (BZD) use is common among persons living with HIV (PLWH). As PLWH are at risk for CNS dysfunction, we retrospectively examined the association between BZD use and HIV-associated neurocognitive impairment (NCI).
Methods:
306 PLWH underwent comprehensive neurobehavioral evaluations. Current BZD use (BZD+) was determined via self-report. Using propensity scores, 153 BZD- individuals were matched to 153 BZD+ participants on demographics and medical comorbidities. Multiple regression models examined NCI and demographically-adjusted neurocognitive T-scores as a function of BZD status, adjusting for estimated premorbid ability, current affective symptoms, and nadir CD4 count. Secondary analyses explored neurocognitive correlates of positive BZD urine toxicology screens (TOX+) and specific BZD agents.
Results:
Median duration of BZD use was 24 months. Current BZD use related to higher likelihood of NCI (OR=2.13, p=0.003) and poorer global (d=−0.28, p=0.020), processing speed (d=−0.23, p=0.047), and motor T-scores (d=−0.32, p=0.008). Compared to BZD-/TOX-, BZD+/TOX+ exhibited additional decrements in executive function (d=−0.48, p=0.013), working memory (d=−0.49, p=0.011), and delayed recall (d=−0.41, p=0.032). For individual agents, diazepam, lorazepam, and alprazolam were most strongly associated with NCI (ORs>2.31).
Discussion:
BZD use may elevate risk for NCI in PLWH, potentially through diffuse neurocognitive slowing and acute compromise of recall and higher-order capacities. These effects are robust to psychosocial and HIV-specific factors and occur in comparison to a tightly-matched BZD- group. Prospective and interventional studies should evaluate causal associations between NCI and BZD use and explore treatment alternatives to BZDs in PLWH.
Keywords: benzodiazepines, neuroHIV, HIV-associated neurocognitive disorders, executive functions, memory, processing speed
INTRODUCTION
Despite the success of antiretroviral (ARV) medication in extending longevity, people living with HIV (PLWH) have elevated rates of medical and neuropsychiatric comorbidities that may contribute to CNS dysfunction1-4. HIV-associated neurocognitive impairment (NCI), which is estimated to impact roughly 50% of PLWH5, is characterized by a fronto-striatal pattern of neurocognitive deficits that can compromise real-world skills such as driving6,7 and medication management8,9. An indirect consequence of this comorbidity burden is the extensive use of non-ARV medications prescribed to treat comorbid conditions or provide symptom relief. Although there is increased awareness that polypharmacy can contribute to adverse health outcomes in PLWH10, the neurocognitive correlates of specific pharmacotherapies among PLWH remains poorly understood.
Benzodiazepines (BZDs), primarily indicated as sedatives and anxiolytics, are widely-used medications yet generally understudied in the context of neurocognition. Consistent with national trends demonstrating a 31% surge in BZD use between 2002–201411, an estimated 30.5 million (12.5%) American adults use BZDs each year12. Given the high prevalence of anxiety and sleep disorders in PLWH13,14, the ubiquity of BZD use is particularly notable among PLWH. It has been reported that PLWH have higher rates of BZD use (24%) than HIV-uninfected individuals (19%) among a nationally representative clinical sample15. Moreover, unpublished data collected over a 10-year period at the University of California San Diego (UCSD) Owen HIV Clinic indicates that 42% of patients will, at some point during their medical care, be prescribed a BZD. BZDs have potential for abuse16 due to their activity in reward circuitry and also carry known adverse effects on the CNS that can acutely impair motor coordination and awareness17. These CNS effects of BZD use are particularly concerning in PLWH because of their potential to exacerbate HIV- and comorbidity-related neurobehavioral dysfunction and increase risk for falls and traffic accidents18,19. For similar reasons, BZD use is discouraged in other neurologically vulnerable clinical populations, such as the elderly20,21 and patients with substance use disorders22.
The present study examined the association between current BZD use and neurocognitive impairment (NCI) among PLWH. We hypothesized that, in comparison to a well-matched group of BZD non-using (BZD-) individuals, those who report BZD use (BZD+) would exhibit a higher rate of NCI characterized by deficits across multiple neurocognitive domains. In order to isolate the neurocognitive correlates of recent BZD use among BZD+ users, we also compared the neurocognitive profiles of BZD+ users with positive urine toxicology results (BZD+/TOX+) to users with negative urine toxicology results (BZD+/TOX-) and the BZD- reference group. As hypothesized above, we expected BZD use to adversely impact neurocognition regardless of toxicological status, yet neurocognitive deficits would be most pronounced among BZD users with evidence of recent use (BZD+/TOX+ group).
METHODS
Participants
This cross-sectional, retrospective study analyzed data from the baseline visit of PLWH enrolled in NIH-funded, Institutional Review Board-approved research studies coordinated by the UCSD HIV Neurobehavioral Research Program (HNRP). All visits took place between 1999 and 2017. For the present study, participants were excluded if they: 1) met DSM-IV criteria for dependence within the last 5 years or abuse within the last 12 months for any substances with the exception of alcohol and cannabis, which were not exclusionary in some parent studies due to their high prevalence in this population; 2) had a diagnosis of psychotic or mood disorder with psychotic features, neurological, or medical condition that may significantly impact neurocognition, such as traumatic brain injury, stroke, epilepsy, or advanced liver disease; 3) displayed evidence of a possible learning or developmental disability as indicated by a low verbal IQ of < 70, estimated by the Third or Fourth edition of the reading subtest of the Wide Range Achievement Test (WRAT)23; and 4) had urine toxicology evidence of recent use of illicit drugs (except marijuana) or positive Breathalyzer test for alcohol on the day of testing. After applying exclusion criteria, 153 BZD+ participants were identified from the pool of available data (n = 1444). Using propensity score matching (see Statistical Analysis), a well-matched group of 153 participants who did not report BZD use (BZD-) and were TOX- were identified from the remaining data (n = 1291) as a comparison group.
Benzodiazepine Exposure Assessment
Details of medication use were assessed via self-report using a structured, clinician-administered questionnaire. The BZD+ group consisted of participants reporting active use of prescribed BZDs. Urine toxicology was assessed by lateral flow immunoassay using the Rapid Response Multi-Drug Test Panel (BTNX). In this assay, the detection limit for BZDs is 300 ng/ml. We defined recent BZD use as those with a TOX+ result for BZD above the limit of detection the day of their study visit. Table 1 shows BZD use characteristics, including the duration of current BZD use, the distribution of individual agents and prevalence of TOX+ results. It is important to note that because TOX+ results do not differentiate between specific agents and that specific agents differ with respect to half-life, TOX+ results cannot be used to estimate time since last use. Generally, short-to-intermediate acting agents may be detected in urine up to 3 to 5 days after exposure while longer acting agents may be detected as long as 4 weeks after exposure; however, other factors such as duration of use, dose, and individual metabolism can prolong or shorten the window of detection24.
Table 1.
Benzodiazepine (BZD) use characteristics (n = 153)
| Duration of current BZD use (months), median [IQR]a | 24 [7-61] |
| Positive toxicology screen (TOX+), n (%) | 33 (21.6%) |
| Individual Agents | |
| Clonazepam, n (%)b | 44 (28.8%) |
| Lorazepam, n (%) | 40 (26.1%) |
| Alprazolam, n (%) | 39 (25.5%) |
| Temazepam, n (%)c | 30 (19.6%) |
| Diazepam, n (%)c | 17 (11.1%) |
| Flurazepam, n (%) | 2 (1.3%) |
| Triazolam, n (%) | 1 (<1%) |
| Estazolam, n (%) | 1 (<1%) |
n = 83
TOX+ rates were lower in clonazepam users (12.1% TOX+, OR=0.28, 95% CI [0.09, 0.84], p=.011)
TOX+ rates were higher in temazepam (33.3% TOX+, OR=2.66, 95% CI [1.11, 6.37], p=.032) and diazepam users (64.7% TOX+, OR=9.50, 95% CI [3.18, 28.38], p<0.001)
Neuromedical Evaluation
All participants underwent a comprehensive neuromedical assessment and venipuncture. HIV infection was diagnosed by enzyme-linked immunosorbent assay (ELISA) with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, syphilis serology, hepatitis C virus (HCV) antibody and current CD4+ T-cell counts (flow cytometry) were performed at each site’s certified clinical laboratory. Levels of HIV viral load in plasma were measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN), with a lower limit of quantitation (LLQ) of 50 copies/ml. HIV viral load was considered undetectable below the LLQ of 50 copies/ml. A subset of participants (n = 158) completed the AIDS Clinical Trials Group 4-day adherence self-report questionnaire to assess nonadherence to any ARV medications over the past four days25. Nonadherence was defined as report of any missed dose over the last four days.
Neurocognitive Evaluation
Participants completed a validated, comprehensive battery of neurocognitive tests covering neurocognitive domains commonly impacted in HIV5: verbal fluency, executive function, processing speed, learning, delayed recall, working memory, and motor skills. Raw test scores were converted to demographically-adjusted standard T-scores (mean of 50 and standard deviation of 10) that corrected for the effects of age, education, sex and race/ethnicity, as appropriate26-28. The demographically-corrected T-scores were averaged across all tests to derive a global T-score, and averaged within each neurocognitive ability area to create domain-specific mean T-scores. In accordance with Frascati criteria for HIV-associated neurocognitive disorders, neurocognitive impairment was classified using the standardized and well-validated clinical ratings method, which defines NCI as showing at least mild impairment in two or more of the seven neurocognitive domains29,30. The dichotomous NCI classification (impaired vs. unimpaired) and continuous global and domain-specific neurocognitive T-scores served as primary outcomes.
Neuropsychiatric Evaluation
The fully-structured computer-based Composite International Diagnostic Interview (CIDI)31 was administered to determine DSM-IV diagnoses of current and lifetime substance use disorders (SUD) and Major Depressive Disorder (MDD). In order to assess current affective symptoms, participants completed the Beck Depression Inventory (BDI)32 version one or two (n = 306) and the Tension/Anxiety subscale of the Profile of Mood States33 (POMS; n = 169), a measure of affective distress validated in PLWH34. The Tension/Anxiety subscale is a 9-item subscale that asks participants to rate past-week feelings such as “anxious” and “nervous.” Higher Tension/Anxiety subscale scores reflect higher levels of anxiety-related distress.
Statistical Analysis
Comparing neurocognitive outcomes by exposure status (e.g., BZD+ vs. BZD-) in a retrospective observational analysis is challenging because participants are not randomized to exposure conditions. Consequently, the association between exposure status and neurocognition can be confounded by exposure status differences in factors known to impact neurocognitive outcomes. Propensity score matching selects control cases (i.e., BZD-) among a larger set of control cases that are well-matched to exposure cases in order to reduce group discrepancies and improve comparison of outcomes in retrospective observational studies35. We used the MatchIt package36 in R statistical software (version 3.4.4, R Foundation for Statistical Computing, Vienna, Austria) to perform nearest neighbor 1:1 propensity score matching to balance demographic characteristics (i.e., age, sex, education, race/ethnicity), estimated premorbid verbal IQ (i.e., WRAT), and medical comorbidities (i.e., hypertension, hyperlipidemia, diabetes, and hepatitis C) between BZD+ and BZD- groups. Adequate balancing of covariates across BZD exposure status was confirmed by examination of absolute standardized differences, which indicated standardized differences of ≤0.10 for each balancing variable37. HIV disease and treatment parameters were not considered for propensity matching and were alternatively considered for multivariable regression analyses (see below) in order to examine whether BZD use related to NCI independent of disease severity.
BZD status differences in demographics, neuropsychiatric, and HIV disease and treatment variables were examined using ANOVAs, Wilcoxon tests, and Chi-square statistics as appropriate. Cohen’s d statistics for continuous variables and odds ratios (OR) for binary variables are presented for estimates of effect size of group comparisons. To evaluate the effect of BZD exposure on neurocognition, multivariable regressions modelled NCI (logistic regression) and domain-specific T-scores (separate linear regressions) as a function of BZD status, covarying for WRAT scores (in order to partial out the effects of education quality on neurocognition38) and group differences (p<.10) in current affective distress and nadir CD4. Although current alcohol and cannabis use disorders also differed by BZD status at a trend-level (p<.10), their overall prevalence was small and they were therefore not included as covariates. Alternatively, a sensitivity analysis examined the association between NCI and BZD use in participants without current alcohol and cannabis use disorders. Given that the BDI data were available on more participants and strongly correlated with the POMS Tension/Anxiety subscale (rs =0.75, p < .0001), BDI was chosen to model affective distress in the primary multivariable regression analyses in order to optimize sample size and avoid multicollinearity. Separate analyses covarying for the POMS Tension/Anxiety scores instead of the BDI demonstrated the same pattern of results as the primary analyses (data not presented). Sensitivity analyses also examined BZD status differences in neurocognition among the subset of participants with undetectable plasma HIV viral load to determine if differences persisted in this clinically-relevant group.
To determine the neurocognitive correlates of recent BZD use, T-score linear regression analyses were conducted with three groups: 1) BZD- reference group (each of whom was also TOX-), 2) BZD+/TOX- and 3) BZD+/TOX+. To explore the impact of individual BZD agents on neurocognition, logistic regressions examined the association between probability of NCI and individual BZD agents in comparison to the BZD- reference group. Regression analyses were performed using JMP Pro version 12.0.1 (JMP®, Version <12.0.1>, SAS Institute Inc., Cary, NC, 1989–2007).
RESULTS
Participant Characteristics
The entire sample of 306 PLWH was 89% male and 78% non-Hispanic White with a mean age of 45.1 years (range: 19–70) and mean education of 13.9 years. Participant characteristics by BZD status are presented in Table 2. By design, groups were well-matched with respect to demographics and medical comorbidities. Groups were mostly comparable on HIV disease and treatment characteristics, with the exception of BZD+ participants trending toward lower nadir CD4 counts than BZD- participants (p=0.051). ARV therapy-induced immune reconstitution was evident across the entire sample as the majority of participants were on ARV medication (76%) and had markedly higher current CD4 counts (median=412 cells/mm3) compared to nadir CD4 counts (median=168 cells/mm3). Half the sample (50%) had detectable levels of plasma viral RNA at a limit of detection of 50 copies/ml.
Table 2.
Study sample characteristics by benzodiazepine (BZD) group
| BZD+ (n=153) |
BZD- (n=153) |
p | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 45.0 (8.77) | 45.1 (10.97) | 0.942 |
| Sex (male), n (%) | 134 (87.6%) | 139 (90.8%) | 0.461 |
| Education (years), mean (SD) | 13.9 (2.48) | 13.9 (2.93) | 0.817 |
| WRAT, mean (SD) | 101.9 (11.96) | 101.7 (11.97) | 0.920 |
| Race/ethnicity | 0.817 | ||
| Non-Hispanic White, n (%) | 117 (76.5%) | 121 (79.1%) | |
| Black, n (%) | 19 (12.4%) | 19 (12.4%) | |
| Hispanic, n (%) | 9 (5.9%) | 9 (5.9%) | |
| Asian, n (%) | 3 (2.0%) | 2 (1.3%) | |
| Other, n (%) | 5 (3.3%) | 2 (1.3%) | |
| Psychiatric | |||
| Lifetime Major Depressive Disorder, n (%) | 99 (64.7%) | 99 (64.7%) | 1.000 |
| Current Major Depressive Disorder, n (%) | 47 (30.7%) | 30 (19.6%) | 0.035 |
| Beck Depression Inventory-II, median [IQR] | 17 [9, 26] | 12 [4, 21] | <0.001 |
| Profile of Moods States- Tension/Anxiety subscale, median [IQR] | 13 [7, 23] | 10 [6, 17] | 0.021 |
| Antidepressants | |||
| On SSRI, n (%) | 45 (29.4%) | 40 (26.1%) | 0.523 |
| On SNRI, n (%) | 10 (6.5%) | 7 (4.6%) | 0.453 |
| On Tricyclic, n (%) | 14 (9.1%) | 11 (7.2%) | 0.531 |
| On Atypical, n (%) | 29 (19.0%) | 19 (12.4%) | 0.115 |
| Substance Use | |||
| Alcohol | |||
| Lifetime Alcohol Use Disorder, n (%) | 81 (52.9%) | 82 (53.6%) | 1.000 |
| Current Alcohol Use Disorder, n (%) | 9 (5.9%) | 2 (1.3%) | 0.061 |
| Average Drinks/Drinking Day in Last 12 | 3.2 (3.21) | 3.5 (3.30) | 0.626 |
| Months, mean (SD)a | |||
| Lifetime Cannabis Use Disorder, n (%) | 49 (32.0%) | 48 (31.4%) | 1.000 |
| Current Cannabis Use Disorder, n (%) | 7 (4.6%) | 2 (1.3%) | 0.091 |
| Average Grams/Day of Use in Last 12 Months, mean (SD)b | 0.40 (0.39) | 0.56 (0.55) | 0.230 |
| Other Substance Use | |||
| Lifetime Cocaine Use Disorder, n (%) | 45 (29.4%) | 38 (24.8%) | 0.440 |
| Lifetime Methamphetamine Use Disorder, n (%) | 38 (24.8%) | 41 (26.8%) | 0.794 |
| Lifetime Opioid Use Disorder, n (%) | 14 (9.2%) | 9 (5.9%) | 0.386 |
| Lifetime Tobacco Use, n (%)c | 64 (77.1%) | 91 (76.5%) | 0.916 |
| Current Tobacco Use, n (%)c | 64 (35.3%) | 91 (34.9%) | 0.959 |
| Medical Comorbidities | |||
| Hepatitis C, n (%) | 32 (20.9%) | 27 (17.6%) | 0.562 |
| Diabetes, n (%) | 13 (8.5%) | 9 (5.9%) | 0.507 |
| Hypertension, n (%) | 39 (25.5%) | 38 (24.8%) | 1.000 |
| Hyperlipidemia, n (%) | 26 (17.0%) | 23 (15.0%) | 0.755 |
| HIV Disease Characteristics | |||
| AIDS Diagnosis, n (%) | 109 (71.2%) | 96 (62.7%) | 0.145 |
| Estimated years of infection, median [IQR] | 10.7 [5.3, 15.7] | 11.6 [5.4, 16.6] | 0.826 |
| Nadir CD4 count, median [IQR] | 136 [23, 264] | 180 [60, 300] | 0.051 |
| Current CD4 count, median [IQR] | 407 [214, 599] | 432 [243, 626] | 0.419 |
| On ARV medication, n (%) | 114 (74.5%) | 118 (77.1%) | 0.689 |
| ARV medication nonadherence, n (%)d | 5 (7.0%) | 8 (9.2%) | 0.622 |
| Detectable plasma virus, n (%) | 71 (46.4%) | 85 (55.6%) | 0.137 |
Note. WRAT = Wide Range Achievement Test Reading subtest; ARV = antiretroviral; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor
n = 125
n = 61
n = 202
n = 158
Groups were also comparable on historical neuropsychiatric diagnoses, including remote substance use disorders and lifetime Major Depressive Disorder. As expected, the BZD+ group had significantly higher levels of current affective distress, as evidenced by higher rates of current Major Depressive Disorder (p=0.035) as well as higher POMS Tension/Anxiety (p=0.021) and BDI scores (p<0.001). However, rates of current antidepressant use did not significantly differ between BZD+ and BZD- participants.
Benzodiazepine Use and Neurocognition
The rate of NCI was significantly higher in BZD+ participants (75%) compared to BZD- participants (56%, p<0.001). After adjusting for nadir CD4 count, WRAT scores and BDI scores, BZD+ participants were 2.13 times more likely to be classified with NCI than BZD- participants (OR=2.13, 95%CI [1.29, 3.56], p=0.003). Higher BDI scores (OR=1.02, 95%CI [1.00, 1.05], p=0.043) and lower WRAT scores (OR =0.97, 95%CI [0.95, 0.99], p=0.002) also significantly increased the likelihood of NCI, while there was no significant association between nadir CD4 counts and NCI (OR=1.00, p=0.140). BZD status differences on adjusted mean neurocognitive T-scores are reported in Table 3. Compared to BZD- participants, BZD+ participants exhibited poorer neurocognition across all domains, with statistically significant differences in global function (d=−0.28, p=0.020), processing speed (d=−0.23, p=0.047) and motor skills (d=−0.32, p=0.008).
Table 3.
Neurocognitive T-scores by benzodiazepine (BZD) group
| BZD+ (n=153) | BZD- (n=153) | ||
|---|---|---|---|
| Domain | Mean (SD) | Mean (SD) | Cohen’s d |
| Global | 43.2 (6.60) | 45.4 (6.74) | −0.28* |
| Verbal Fluency | 45.8 (8.61) | 47.7 (9.66) | −0.16 |
| Executive Function | 43.1 (8.87) | 44.8 (9.84) | −0.14 |
| Processing Speed | 44.8 (9.26) | 47.5 (9.44) | −0.23* |
| Learning | 40.4 (8.08) | 42.2 (8.87) | −0.18 |
| Recall | 41.1 (9.09) | 42.6 (10.10) | −0.11 |
| Working Memory | 44.8 (9.10) | 46.6 (8.59) | −0.18 |
| Motor | 41.4 (11.13) | 45.0 (9.69) | −0.32** |
Note. Effect size estimates are adjusted for covariates.
p < .01;
p <. 05
To focus on a clinically relevant subgroup in the post-cART era (n=150), we re-examined BZD status differences on neurocognitive outcomes among participants with undetectable plasma levels of HIV RNA (i.e., <50 copies/ml). Consistent with the total sample analyses, BZD+ aviremic participants had significantly higher rates of NCI (OR=2.12, p=0.041) and lower global (d=−0.39, p=0.021), processing speed (d=−0.40, p=0.018) and motor T-scores (d=−0.43, p=0.012) than BZD- aviremic participants. Additionally, BZD use was associated with poorer learning T-scores (d=−0.34, p=0.044) in this aviremic subgroup. BZD use also remained a significant risk factor for NCI (OR=1.96, p=.012) in the sensitivity analysis in participants without current alcohol and cannabis use disorders (n = 287).
Positive Benzodiazepine Toxicology and Neurocognition
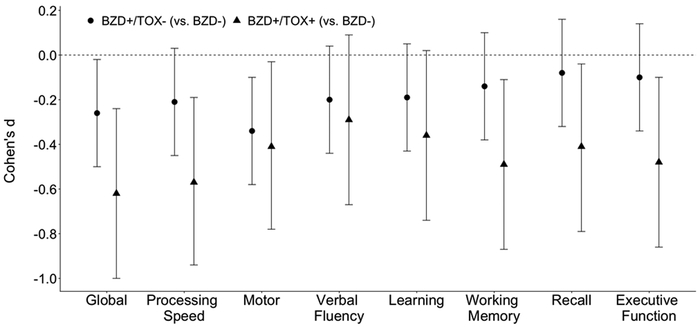
Additional neurocognitive deficits emerged when comparing BZD+/TOX- and BZD+/TOX+ participants to the BZD- reference group (see Figure 1). Consistent with the broader BZD+ vs. BZD- analyses, both TOX groups exhibited poorer global function (BZD+/TOX+: d=−0.62, p=0.001; BZD+/TOX-: d=−0.26, p=0.031), processing speed (BZD+/TOX+: d=−0.57, p=0.003; BZD+/TOX-: d=−0.21, p=0.079), and motor skills (BZD+/TOX+: d=−0.41, p=0.035; BZD+/TOX-: d=−0.34, p=0.006) compared to the BZD- group. Additionally, the BZD+/TOX+ group exhibited significant decrements in working memory (d=−0.49, p=0.011), delayed recall (d=−0.41, p=0.032), and executive function (d=−0.48, p=0.013) as compared to the BZD- group. With respect to TOX group comparisons, BZD+/TOX+ participants had significantly poorer executive function than BZD+/TOX- participants (d=−0.40, p=0.043).
Figure 1.
Both BZD+ groups (i.e., BZD+/TOX− and BZD+/TOX+) exhibited poorer neurocognition than the BZD− reference group. Estimates reflect each BZD+ group minus the BZD− reference group such that lower effect sizes indicate that the BZD+ group has lower T-scores for that domain. Statistically significant differences (i.e., p < 0.05) exist when the 95% confidence interval bars do not cross the dashed horizontal line (i.e., Cohen’s d = 0).
Individual Agents and Neurocognitive Impairment
In comparison to the BZD- group, the probability of NCI was significantly higher among individuals with prescriptions for diazepam (OR=3.73, 95%CI [1.03, 13.52], p=0.026), lorazepam (OR=2.76, 95%CI [1.23, 6.18], p=0.009), and alprazolam (OR=2.32, 95%CI [1.06, 5.09], p=0.029), with a nonsignificant trend in the association of clonazepam use and NCI (OR=1.90, 95%CI [0.93, 3.93], p=0.073). A smaller increased risk of NCI with temazepam use did not reach statistical significance (OR=1.38, 95%CI [0.62, 3.10], p=0.429).
DISCUSSION
In the present study, we found that among PLWH, BZD users were more than twice as likely to be classified with NCI than non-users, including in the subset of aviremic participants. BZD users showed poorer global function, with slower psychomotor speed and information processing as the primary deficits. Those with very recent BZD use, indicated by positive urine toxicology, exhibited additional decrements in executive function, working memory, and delayed recall. The observed neurocognitive deficits among BZD users were not attributable to any single agent, suggesting that BZDs confer risk for NCI as a broad class of medications.
Although the current literature on BZD use and neurocognition in PLWH is scarce, our findings align with a recent report from the Women’s Interagency HIV Study39. The results of this study demonstrated that anxiolytic use (primarily BZDs), which was significantly higher among HIV-seropositive women compared to HIV-seronegative women, moderated the effects of HIV on learning, such that HIV-related learning deficits only occurred within the anxiolytic group. Thus, these data provide some initial indication that current BZD use may exacerbate vulnerabilities to HIV-related neurocognitive compromise.
Compared to the limited number of studies examining the neurocognitive effects of BZDs in PLWH, there is a well-established body of evidence in HIV-uninfected cohorts suggesting that full restoration of neurocognitive function is not always accomplished following withdrawal from long-term BZD use. Current meta-analytical data from healthy adult samples links long-term use of BZDs to a constellation of neurocognitive deficits that can persist as long as 3.5 years post-withdrawal40. Specifically, current BZD users as well as prior users multiple years post-withdrawal exhibited working memory, processing speed, and recent memory (i.e., learning and recall) deficits, whereas psychomotor deficits emerged in recently withdrawn users. In our cohort of PLWH reporting current BZD use, we similarly observed deficits in working memory, recall, processing speed, and psychomotor speed (even in the absence of positive toxicology results). Although we additionally observed executive function deficits in BZD+/TOX+ individuals, whereas the aforementioned meta-analysis did not find poorer executive function in HIV-uninfected BZD users, our executive function composite T score includes the Trail Making Test B, which was categorized as divided attention in the meta-analysis and shown to be impaired in BZD users.
Notably, the magnitude of the BZD-related deficits in HIV-uninfected individuals in the Crowe and Stranks meta-analysis40 were substantially larger (Hedges’ g range: −0.71 to −1.35) than the modest effects (Cohen’s d range: −0.23 to −0.32) detected in the present study. In addition to study differences in the precision by which BZD exposure parameters were quantified (e.g., recency of use), the smaller effect sizes detected in the present study highlight the relevance of other HIV- and comorbidity-specific factors that explain meaningful variance in neurocognition among PLWH. Thus, the markedly elevated rate of NCI among BZD+ PLWH (75%) is most informative when considered alongside the already high rate of NCI (56%) in the BZD- reference group with comparable comorbidity burden. Nevertheless, our use of propensity score matching to balance groups on relevant demographic and clinical covariates enhances our ability to obtain ecologically valid estimates of the effect of BZD use in a clinically heterogeneous sample of PLWH.
An inherent complexity in evaluating the neurocognitive correlates of medication use retrospectively, as opposed to in a randomized clinical trial, is the potential confounding presence of comorbid conditions. In the context of BZD use and neurocognition, most research toward this end has focused on isolating the effects of BZDs from anxiety symptoms and found that long-term BZD users post-withdrawal exhibit impairments in memory and motor performance compared to controls with similar demographics and levels of anxiety41. Our results support the incremental validity of BZD use as a unique predictor of NCI above and beyond affective distress and established predictors, including nadir CD4 and estimated premorbid ability.
While our data does not permit for direct examination of biological pathways underlying the deleterious effects of BZDs on neurocognition, we can offer several potential neurobiological explanations. BZDs act primarily as gamma-aminobutyric acid A (GABAA) receptor agonists, thereby depressing CNS activity17. GABA is the main inhibitory neurotransmitter in the CNS and GABAA receptors are widely-distributed throughout cortical, subcortical, and cerebellar regions that support neurocognition. Given the pharmacodynamic tolerance associated with long-term BZD use and the neural ubiquity of GABA, BZD-mediated downregulation of GABAA receptors is likely to contribute to GABAergic dysfunction and a generalized slowing of psychomotor speed and information processing42,43. Disruption to GABA is also theorized to contribute to NCI in alcohol dependence44 and multiple sclerosis45-47, two conditions characterized by neurocognitive slowing and motor impairment. Our results demonstrating poorer executive function, working memory, and delayed recall among BZD+/TOX+ participants is consistent with prior neuroimaging research demonstrating a prominent role of GABA in supporting hippocampal and prefrontal cortex (PFC) function43,48-50. Notably, acute administration of lorazepam was associated with reductions in hippocampal and PFC activity during an fMRI-based task of learning and recall50.
BZDs may be particularly deleterious to neural health in the context of HIV-specific and comorbidity-associated (e.g., hepatitis C and substance use) hepatic vulnerabilities. Cytochrome P450 (CYP) enzymes are responsible for first-line metabolism of most BZDs through microsomal oxidation51, a process that is impaired in patients with hepatic dysfunction52. Lower CYP levels have been reported in PLWH53, potentially due to HIV-related proinflammatory downregulation of CYP activity and expression54. Inefficient metabolic clearance of BZDs extends the bioavailability of BZDs in the CNS55,56, which may exacerbate perturbation of the GABAergic system. Animal research and human neuropathological studies provide evidence of HIV-related GABAergic dysregulation, which has been proposed as a potential neuropathological mechanism contributing to HIV-associated neurocognitive disorders57,58. Long-term BZD use in HIV may augment stress upon the GABAergic system, resulting in compromised synaptic integrity, cerebrovascular abnormalities, and neurocognitive deficits58.
Our study has several limitations. Although a considerable strength of our retrospective study design is the application of rigorous BZD exposure group matching to minimize the impact of comorbidities and demographic variables, our results may be influenced by unmeasured factors, such as the presence of sleep disorders. Although disrupted sleep can negatively impact neurocognition, it is noteworthy that the weakest association between BZDs and NCI occurred among those using temazepam, the only BZD analyzed in the present study that is primarily prescribed for sleep problems. Future studies should systematically evaluate the independent and interactive contributions of sleep disturbances and sedative/hypnotic medications (i.e., BZDs and “Z” drugs) to neurobehavioral health. Our BZD exposure assessment was dependent upon self-report and may therefore lack full precision. Utilization of electronic medical record (EMR) data would permit for a more nuanced evaluation of BZD use, however, EMR data are unlikely to provide the same depth of neurocognitive phenotyping that was available in the present study. The TOX+ results are informative with respect to the neurocognitive effects of recent BZD use, yet our toxicological analysis only serves as a proxy for recent use, as detectability is also influenced by other variables including the pharmacokinetics of specific agents, duration of use, and dosage. Having access to data on these additional factors, such as frequency of use, could further our understanding of the relationship between parameters of BZD exposure and neurocognition. Our cross-sectional and retrospective analysis cannot identify causal associations between BZD use and neurocognition. Thus, prospective longitudinal studies are warranted to elucidate the relationship between BZD exposure and incident neurobehavioral decline. Finally, the absence of an HIV-uninfected comparison group impacts our ability to isolate HIV-specific effects of BZD use. Nevertheless, our findings highlight the clinical value in identifying malleable pharmacological risk factors for NCI within PLWH, including among those that are virally-suppressed.
Although there is increased awareness that the utilization of non-ARV medications can reduce adherence to ARVs and exacerbate HIV-related depletion of organ system reserve, mitigating the iatrogenic consequences of pharmacotherapy among PLWH remains a challenge10. Taken together, our findings suggest that BZDs may elevate risk for NCI in PLWH, potentially through diffuse slowing of information processing and psychomotor activity as well as acute compromise of recall and higher-order capacities. These deleterious effects of BZD use are robust to psychosocial and HIV-specific factors and occur in comparison to a BZD- group tightly-matched on comorbidities and demographic factors germane to neurocognitive assessment. Importantly, BZD-related decrements in neurocognition were present in participants with undetectable viral loads, highlighting the salience of BZD effects even in those whose HIV is most successfully treated. Given the prevalence of BZD use and potential for adverse neuropsychiatric and neurocognitive effects, future work should aim to identify therapeutic alternatives to BZDs that effectively treat comorbid conditions yet support the preservation of neurobehavioral health in the increasingly older and neurologically vulnerable population of PLWH.
Acknowledgments
Conflicts of Interest and Source of Funding: This research was supported by the Translational Methamphetamine AIDS Research Center (TMARC) award P50DA026306, the HIV Neurobehavioral Research Center (HNRC) award P30MH062512, the California NeuroAIDS Tissue Network (CNTN) awards U01MH083506, R24MH59745, and U24MH100928, and the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study awards N01MH22005, HHSN271201000036C, and HHSN271201000030C. R.S. is supported by NIAAA award T32AA013525. The authors declare no conflicts of interest.
Footnotes
Poster to be presented at the National Academy of Neuropsychology (NAN) Annual Conference, San Diego, CA (2019, November).
REFERENCES
- 1.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- 2.Saloner R, Heaton RK, Campbell LM, et al. Effects of comorbidity burden and age on brain integrity in HIV. AIDS. 2019;33(7):1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson JH, Heaton RK, Patterson TL, et al. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer EJ, Thames AD. Neurobehavioral Manifestations of Human Immunodeficiency Virus/AIDS: Diagnosis and Treatment. Neurol Clin. 2016;34(1):33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, Clifford DB, Franklin DR Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcotte TD, Lazzaretto D, Scott JC, Roberts E, Woods SP, Letendre S. Visual attention deficits are associated with driving accidents in cognitively-impaired HIV-infected individuals. J Clin Exp Neuropsychol. 2006;28(1):13–28. [DOI] [PubMed] [Google Scholar]
- 7.Marcotte TD, Heaton RK, Wolfson T, et al. The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc. 1999;5(7):579–592. [DOI] [PubMed] [Google Scholar]
- 8.Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods SP, Moran LM, Carey CL, et al. Prospective memory in HIV infection: is “remembering to remember” a unique predictor of self-reported medication management? Arch Clin Neuropsychol. 2008;23(3):257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30(8):613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the Concomitant Prescribing of Opioids and Benzodiazepines, 2002–2014. Am J Prev Med. 2016;51(2):151–160. [DOI] [PubMed] [Google Scholar]
- 12.Blanco C, Han B, Jones CM, Johnson K, Compton WM. Prevalence and Correlates of Benzodiazepine Use, Misuse, and Use Disorders Among Adults in the United States. J Clin Psychiatry. 2018;79(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O’Cleirigh CM. Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clin Psychol Rev. 2017;51:164–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taibi DM. Sleep disturbances in persons living with HIV. J Assoc Nurses AIDS Care. 2013;24(1 Suppl):S72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wixson SE, Brouwer ES. Sex differences in benzodiazepine use in the HIV-infected population. AIDS Care. 2014;26(10):1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soyka M Treatment of Benzodiazepine Dependence. N Engl J Med. 2017;376(24):2399–2400. [DOI] [PubMed] [Google Scholar]
- 17.Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–223. [PMC free article] [PubMed] [Google Scholar]
- 18.Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: A systematic literature review. CNS Drugs. 2010;24(8):639–653. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Gutierrez MJ, Martinez-Cengotitabengoa M, Saez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: A systematic review. Maturitas. 2017;101:17–22. [DOI] [PubMed] [Google Scholar]
- 20.American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
- 21.Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guina J, Merrill B. Benzodiazepines I: Upping the Care on Downers: The Evidence of Risks, Benefits and Alternatives. J Clin Med. 2018;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson G, Robertson G. Wide Range Achievement Test-4 (WRAT-4). Lutz, FL: Psychological Assessment Resources Inc; 2006. [Google Scholar]
- 24.Kale N Urine Drug Tests: Ordering and Interpreting Results. Am Fam Physician. 2019;99(1):33–39. [PubMed] [Google Scholar]
- 25.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–266. [DOI] [PubMed] [Google Scholar]
- 26.Heaton RK, Taylor MJ, Manly J. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III In: Clinical interpretation of the WAIS-III and WMS-III. San Diego, CA, US: Academic Press; 2003:181–210. [Google Scholar]
- 27.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- 28.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33(7):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26(6):759–778. [DOI] [PubMed] [Google Scholar]
- 30.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Composite Diagnositic International Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 32.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation. 1996. [Google Scholar]
- 33.McNair DM. Manual profile of mood states. Educational & Industrial testing service; 1981. [Google Scholar]
- 34.Gold JA, Grill M, Peterson J, et al. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS Behav. 2014;18(6):1124–1132. [DOI] [PubMed] [Google Scholar]
- 35.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
- 36.Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42(8):1–28. [Google Scholar]
- 37.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casaletto KB, Cattie J, Franklin DR, et al. The Wide Range Achievement Test-4 Reading subtest “holds” in HIV-infected individuals. J Clin Exp Neuropsychol. 2014;36(9):992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin LH, Radtke KK, Eum S, et al. Cognitive Burden of Common Non-antiretroviral Medications in HIV-Infected Women. J Acquir Immune Defic Syndr. 2018;79(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowe SF, Stranks EK. The Residual Medium and Long-term Cognitive Effects of Benzodiazepine Use: An Updated Meta-analysis. Arch Clin Neuropsychol. 2018;33(7):901–911. [DOI] [PubMed] [Google Scholar]
- 41.Barker MJ, Greenwood KM, Jackson M, Crowe SF. An evaluation of persisting cognitive effects after withdrawal from long-term benzodiazepine use. J Int Neuropsychol Soc. 2005;11(3):281–289. [DOI] [PubMed] [Google Scholar]
- 42.Vinkers CH, Olivier B. Mechanisms Underlying Tolerance after Long-Term Benzodiazepine Use: A Future for Subtype-Selective GABA(A) Receptor Modulators? Adv Pharmacol Sci. 2012;2012:416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt-Wilcke T, Fuchs E, Funke K, et al. GABA—from Inhibition to Cognition: Emerging Concepts. Neuroscientist. 2018;24(5):501–515. [DOI] [PubMed] [Google Scholar]
- 44.Oscar-Berman M, Marinkovic K. Alcoholism and the brain: an overview. Alcohol Res Health. 2003;27(2):125–133. [PMC free article] [PubMed] [Google Scholar]
- 45.Cawley N, Solanky BS, Muhlert N, et al. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain. 2015;138(Pt 9):2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao F, Yin X, Edden RAE, et al. Altered hippocampal GABA and glutamate levels and uncoupling from functional connectivity in multiple sclerosis. Hippocampus. 2018;28(11):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao G, Edden RAE, Gao F, et al. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur Radiol. 2018;28(3):1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marenco S, Meyer C, van der Veen JW, et al. Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition. Neuropsychopharmacology. 2018;43(11):2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bañuelos C, Wołoszynowska-Fraser MU. GABAergic Networks in the Prefrontal Cortex and Working Memory. J Neurosci. 2017;37(15):3989–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lissek S, Golisch A, Glaubitz B, Tegenthoff M. The GABAergic system in prefrontal cortex and hippocampus modulates context-related extinction learning and renewal in humans. Brain Imaging Behav. 2017;11(6):1885–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008(182):335–360. [DOI] [PubMed] [Google Scholar]
- 52.Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy. 1996;16(1):49–57. [PubMed] [Google Scholar]
- 53.Jones AE, Brown KC, Werner RE, et al. Variability in drug metabolizing enzyme activity in HIV-infected patients. Eur J Clin Pharmacol. 2010;66(5):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–149. [DOI] [PubMed] [Google Scholar]
- 55.Basile AS, Pannell L, Jaouni T, et al. Brain concentrations of benzodiazepines are elevated in an animal model of hepatic encephalopathy. Proc Natl Acad Sci U S A. 1990;87(14):5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van der Kleijn E, Vree T, Baars A, Wijsman R, Edmunds L, Knop H. Factors influencing the activity and fate of benzodiazepines in the body. Br J Clin Pharmacol. 1981;11(S1):85S–98S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musante V, Summa M, Neri E, et al. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex. 2010;20(8):1974–1984. [DOI] [PubMed] [Google Scholar]
- 58.Buzhdygan T, Lisinicchia J, Patel V, et al. Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection. J Neuroimmune Pharmacol. 2016;11(2):279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]