Abstract
Background:
Human IL-32 is a polyfunctional cytokine that was initially reported to inhibit HIV-1 infection. However, recent data suggest that IL-32 may enhance HIV-1 replication by activating the HIV-1 primary targets, CD4+ T-cells. Indeed, IL-32 is expressed in multiple isoforms, some of which are pro-inflammatory while others are anti-inflammatory.
Setting and Methods:
Here, we aimed to determine the relative expression of IL-32 isoforms and to test their inflammatory nature and potential to induce HIV-1 production in latently-infected cells from virologically-suppressed HIV-infected individuals. IL-32 and other cytokines were quantified from plasma and supernatant of CD4+ T-cells by ELISA. Transcripts of IL-32 isoforms were quantified by qRT-PCR in PBMCs. The impact of recombinant human IL-32 isoforms on HIV-1 transcription was assessed in CD4+ T-cells from HIV-1+cART+ individuals by qRT-PCR.
Results:
All IL-32 isoforms were significantly upregulated in HIV-1+cART+ compared to HIVneg individuals with IL-32β representing the dominantly expressed isoform, mainly in T-cells and NK-cells. At the functional level, while IL-32γ induced typical pro-inflammatory cytokines (IL-6 and IFN-γ) in TCR-activated CD4+ T-cells, IL-32α showed an anti-inflammatory profile by inducing IL-10 but not IL-6 or IFN-γ. However, IL-32β showed a dual phenotype by inducing both pro- and anti-inflammatory cytokines. Interestingly, consistent with its highly pro-inflammatory nature, IL-32γ, but not IL-32α or IL-32β, induced HIV-1 production in latently-infected CD4+ T-cells isolated from cART-treated individuals.
Conclusions:
Our data report on the differential expression of IL-32 isoforms and highlight the potential role of IL-32, particularly the γ isoform, in fueling persistent inflammation and transcription of viral reservoir in HIV-1 infection.
Keywords: HIV-1, IL-32, Inflammation, HIV-1 reservoir
Introduction
Combined Anti-Retroviral Therapy (cART) has greatly diminished mortality and morbidity in people living with HIV (PLWH) by suppressing viral replication to undetectable levels. This remarkable control of viral infection underlies the significant decline in AIDS-related comorbidities and the increased life expectancy1,2. However, non-AIDS co-morbidities remain common within the cART-treated PLWH, which clearly compromises the quality of life of individuals living with HIV3. Among key mediators of these non-AIDS co-morbidities are chronic immune activation and inflammation, which are not normalized even after prolonged therapy4,5. Chronic perturbations in the cytokine network, particularly in proinflammatory cytokines such as TNF-α, IFN-α/β and IL-6, contribute to the compromised immunity in the cART era6,7,8. While the contribution of these inflammatory mediators to HIV pathogenesis and disease progression is relatively well documented9, the role of novel cytokines known to be dysregulated during infection such as the human interleukin 32 (IL-32) remains elusive. IL-32 was initially reported as a pro-inflammatory mediator that induces the production of other inflammatory cytokines such as TNF-α, IL-8, IL-1β, IFN-γ and IL-610,11,12. Early studies on IL-32 showed that this cytokine is upregulated in HIV infection and is associated with innate antiviral responses through the induction of type I interferon (IFN-α/β) responses, which is supported by an increase in HIV replication upon inhibition of endogenous IL-32 expression13,14. However, recent data revealed that certain isoforms such as the IL-32γ may enhance HIV replication by increasing CD4+ T-cell activation and susceptibility to infection15.
Furthermore, in untreated HIV-infected individuals, high levels of IL-32 are associated with inflammation, immune suppression and disease progression11,16. Interestingly, Smith et al.,16 showed that IL-32 is highly expressed in the lymphoid tissues and that the gamma isoform significantly induces the immunosuppressive enzyme Indoleamine 2,3-dioxygenase (IDO1)16. In turn, increased IDO1 expression is associated with impaired intestinal mucosal immunity, immune exhaustion and disease progression17,18. This is in line with our recent data showing an association between IL-32 expression and disease progression in cART-naïve HIV-infected slow progressors11. These studies suggest that IL-32 may indeed exert multiple functions in HIV infection that might be explained by the existence of multiple IL-32 isoforms (α, β, γ, δ, ε, D, ζ, η, sm and θ), which are produced by alternative splicing12,19,20. Interestingly, while IL-32γ induces a number of pro-inflammatory cytokines including TNF-α and IFN-γ10, IL-32β specifically induces the anti-inflammatory cytokine IL-10 by monocyte-derived DCs 21,22,23. Moreover, the functions of IL-32γ on the induction of TNF-α through the activation of NF-κB and p38 MAPK, is counteracted by the newly identified IL-32θ isoform that diminishes these effects through the interaction and blockade of PKCδ, an upstream activator of both NF-κB and MAPK24. IL-32θ also inhibits the PMA-induced expression of IL-1β through the interaction with PKCδ25.
Considering the yet poorly documented expression and functions of IL-32 isoforms in the context of HIV infection, we sought in the current study to identify the dominantly expressed IL-32 isoforms and to investigate their functional consequences on persistent inflammation and residual HIV transcription during cART treatment.
Methods
Study population
Plasma and peripheral blood mononuclear cells (PBMCs) were collected from HIV-infected individuals and non-infected controls enrolled in the Canadian HIV and Aging Cohort Study (CHACS)26. Samples from the baseline visits (study entry) of n=107 HIVneg individuals, n=379 HIV+ virologically-suppressed individuals receiving cART and n=46 HIV+ viremic individuals were analyzed. Demographic and clinical parameters for the study participants are shown in Supplemental Table 1. Written informed consent was obtained from all participants included in this study. The study was approved by Institutional Review Boards (IRB) of the Centre Hospitalier de l’Université de Montréal Research Center (Ethical approval #: CE.11.063) and the participating centers.
Measurements of soluble IL-32, IL-6, IL-10 and IFNγ proteins
Plasma levels of human IL-32 (total pool of isoforms) were quantified using the human IL-32 ELISA kit (R&D System Inc., Cat # DY3040-05) as per manufacturer’s recommendations on the available samples from baseline visits (study entry) of HIV+ and HIVneg individuals. Samples of both HIV+ and HIVneg were inactivated using disruption buffer (DB) [PBS; 0.05%, Tween-20, 2.5% Triton X-100, 0.02% thimerosal and 1% Trypan blue] with a ratio of 1:4 (DB:Sample). IL-10, IFNγ and IL-6 were measured using human ELISA sets from R&D and PeproTech (Cat #DY-217B-05, 900-M27 and 900-K16, respectively) according to the supplier’s protocol. Cell-associated IL-32 protein was measured as previously described11.
Quantification of IL-32 by Real-time quantitative SYRR Green PCR
Total RNA was isolated from PBMCs using the RNeasy plus mini kit from Qiagen (Catalog No. 74134) as per manufacturer’s protocol. Quantification of IL-32 isoforms (α, β, γ, D, ε and θ) was carried out using one-step SYBR Green quantitative real-time PCR, which was performed on the LightCycler 480 II (Roche) apparatus using QIAGEN reagents (RNase-Free DNase Set (50); Cat. No. 79254). Real-time qRT-PCR was performed in duplicates using 25ng RNA per reaction. No-template controls were included in parallel for each gene master mix. Gene expression for each individual isoform was normalized to the housekeeping gene β-glucuronidase. Primer sets for IL-32α, β, ε (C) and β-glucuronidase have been described earlier 27, while primer sets for IL-32 γ and θ were designed based on γ and θ transcript reference sequences as summarized in Supplemental Table 2.
Cell sorting
To study the expression of IL-32 isoforms in different cell types, we sorted T-cells, B-cells, NK cells and monocytes from total PBMCs of HIV positive and HIV negative subjects using flow cytometry (BD FACSAria; BD Biosciences). Briefly, cell Surface staining was performed with fluorochrome-conjugated antibodies from BD Biosciences; Pacific Blue™ Mouse Anti-Human CD3 (Clone UCHT1, Cat # 558117), Alexa Fluor® 700 Mouse Anti-Human CD4 (Clone SK3, Cat # 566318), APC-H7 Mouse anti-Human CD8 (Clone SK1, Cat # 560179), APC Mouse Anti-Human CD14 (Clone M5E2, Cat # 555399), PerCP-Cy™5.5 Mouse Anti-Human CD19 (Clone HIB19, Cat # 561295), PE Mouse Anti-Human CD56 (Clone B159, Cat # 561903) and FITC Mouse Anti-Human CD16 (Clone 3G8, Cat # 555406). Average purity of sorted cells was of >95% as determined by post sorting FACS quality control analysis.
Isolation and stimulation of CD4+ T-cells
Total CD4+ T-cells were isolated from PBMCs by negative selection using EasySep Human CD4+ T-cell Enrichment Kit (StemCell® Cat # 19052) according to the supplier’s protocol. Purity of isolated CD4+ T-cells were on average 99%. Stimulation of purified cells took place in pre-coated plates with anti-CD3 antibodies (1μg/ml) (BD biosciences®, Cat # 555329) and soluble anti-CD28 antibodies (0.5μg/ml) (BD biosciences®, Cat # 5555726) with or without 500ng IL-32 isoforms from R&D (IL-32α # 3040-IL-050, IL-32β cat# 6769-IL-025 and IL-32γ cat# 4690-IL-025/CF). Cells were cultured at 37°C for 2 days and secreted cytokines in the supernatant were measured by ELISA.
HIV-1 production in primary CD4+ T-cells from cART-treated individuals
CD4+ T-cells were isolated from PBMCs of virologically-suppressed individuals by negative magnetic selection (StemCell). Cells (4-5x106 cells per experimental condition) were cultured in the presence of antiretrovirals (ARVs) (200nM lamivudine, 200nM raltegravir). Cells were stimulated with 250-500 ng/mL of IL-32α, IL-32β or IL-32γ or with Dynabeads Human T-expander CD3/CD28 (Invitrogen) at a concentration of 1 bead per cell as a positive control. Medium was harvested at day 6 post-stimulation and used for quantification of viral particles and replaced with fresh medium containing ARVs and IL-32 when appropriate. Freshly collected cell-culture supernatants were centrifuged for 1 hour at 25,000 g to pellet HIV particles. Viral RNAs were extracted using the Qiamp viral RNA kit (Qiagen) and quantified using an ultrasensitive semi-nested real-time reverse transcription–polymerase chain reaction (RT-PCR) with a detection limit of a single copy of HIV RNA per PCR reaction. Briefly, extracted viral RNA was reverse transcribed and subjected to 16 cycles of amplification followed by dilution and a second amplification by a nested real-time PCR for 40 cycles on the Rotor-Gene Q using primers and probes summarized in Supplemental Table 3. In all experiments, serial dilutions of HIV particles (LAI strain) in culture medium were processed in parallel of experimental samples and used as quantification standards.
Statistical analysis
Differences between groups were considered statistically significant at values of p <0.05. Kruskal-Wallis and Dunn’s subtest were used to analyze more than one group, non-parametric Mann-Whitney was used for unpaired samples, Wilcoxon for paired samples and nonparametric Spearman test was used for correlation studies. All analyses were performed using Prism 7 software (GraphPad, San Diego, CA, USA).
Results
Persistent upregulation of IL-32 under cART
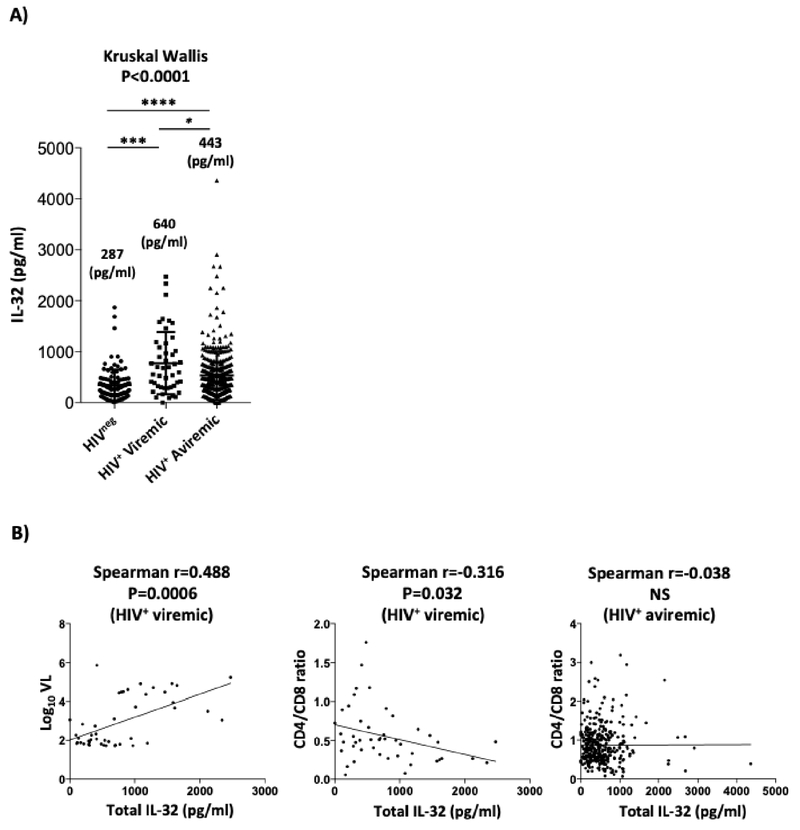
Our earlier longitudinal studies on a limited number of HIV+ subjects from the Montreal Primary HIV-infected Cohort showed that IL-32 is highly expressed during the primary phase of HIV infection and is not normalized after a year of treatment with cART11. In the current study, we investigated the expression of IL-32 in HIV-infected individuals receiving cART for extended periods of time (average: 12.7±6.7 years) by measuring plasma IL-32 levels (total pool of all IL-32 isoforms). IL-32 levels remained significantly elevated (p<0.0001) in HIV-infected individuals (n=379) (IQR: 437.4pg/ml, Q1-Q3: 229.9-667.3 pg/ml) compared to non-infected controls (n=107) (IQR: 254.14 pg/ml, Q1-Q3: 162.5-416.7 pg/ml). However, these levels were significantly lower compared to HIV+ viremic individuals (n=46) (IQR: 783, Q1-Q3: 301.6-1085.2pg/ml), Figure 1A. As we have previously shown11, IL-32 levels positively correlated with HIV viral load (r=0.488, p=0.0006) and negatively with the ratio CD4/CD8 (r=−0.316, p=0.032) in the HIV+ viremic individuals (Figure 1B, left and middle panels, respectively). However, this association between IL-32 and CD4/CD8 ratio was lost in virologically-suppressed individuals (Figure 1B, right panel).
Figure 1: High levels of IL-32 in plasma from cART-treated HIV+ individuals.
Shown are (A) plasma levels of total IL-32 in HIVneg (n=107), HIV+ viremic individuals (n=46), and HIV+ virologically-suppressed on cART individuals (n=379). Numbers on the graph refer to the median levels of IL-32 in plasma of each group expressed as pg/ml. (B) correlation between total IL-32 levels and Log10 viral load (VL) (left panel), CD4/CD8 ratios (middle panel) in viremic individuals (n=46) and CD4/CD8 ratios in cART-treated individuals (right panel). Data were analyzed by the non-parametric Kruskal-Wallis and Dunn’s sub-test for multiple comparisons in A. Spearman correlation test was used to assess the significance of correlations between IL-32 and HIV VL or CD4/CD8 ratio in B. NS, non-significant (p>0.05).
Dominant expression of IL-32β isoform
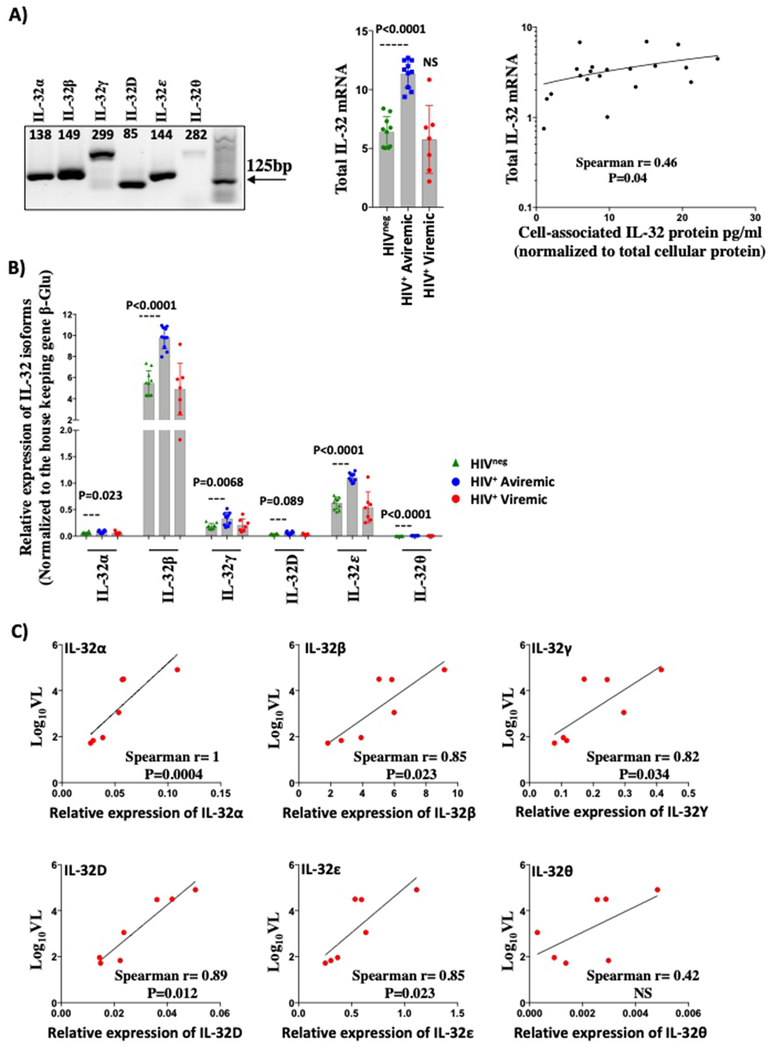
Our data showed that circulating levels of total IL-32 remain elevated in HIV+ cART-treated individuals. However, as IL-32 is expressed in different isoforms for which specific antibodies are currently unavailable and since these isoforms have distinctive functions19, it was important to identify the expression of these individual transcripts. Therefore, we developed isoform-specific RT-PCRs to quantify individual IL-32 isoforms (α, β, γ, ε, and θ in addition to the new uncharacterized isoform D, NCBI Reference # NM_001012636.1) in PBMCs from both HIV+ and HIVneg individuals (amplification of the rest of the reported isoforms was unsuccessful). Our RT-PCR strategy is depicted in Supplemental Figure 1. As shown in Figure 2A (left panel), the amplified complementary DNA corresponded to the expected spliced transcripts of the different IL-32 isoforms and was further confirmed by DNA sequencing (data not shown). Similar to the total pool of IL-32 protein, the total pool of IL-32 mRNA (a sum of relative expression of all isoforms amplified in PBMCs) was significantly higher in HIV+cART+ aviremic participants (n=10) compared to non-infected controls (n=10) (Figure 2A, middle panel, p<0.0001). However, we observed a high variability in IL-32 mRNA expression in HIV+ viremic participants (n=7) (likely due to the high level of cell death of activated and infected cells). As expected, total IL-32 mRNA levels positively and significantly correlated with the total pool of cell-associated IL-32 protein, measured by ELISA from total cell lysates (Figure 2A right panel, n=20, r=0.46, p=0.04). At the single isoform level, IL-32β was the dominant isoform in HIVneg (in green) and both HIV+ virologically-suppressed individuals (in blue) and HIV+ viremic participants (in red) (Figure 2B). Interestingly, with the exception of the IL-32D, all of the IL-32 isoforms were expressed at significantly higher levels in HIV+ cART treated individuals compared to HIVneg controls (p=0.023, p<0.0001, 0.0068, 0.089, p<0.0001, and <0.0001 for α, β, γ, D, ε, and θ, respectively). Furthermore, in the HIV+ viremic participants , these isoforms positively correlated with the HIV viral load (Figure 2C).
Figure 2: Expression of IL-32 isoforms in HIV+ individuals.
A) PCR products (amplicons) for each individual isoform analyzed on agarose gel (1.5%) (left panel). Numbers above the DNA fragments represent the size, in base pairs, of each isoform. Middle panel: Relative expression of total IL-32 mRNA (sum of relative expression of 6 IL-32 isoforms) in PBMCs from HIVneg (n=10, in green), HIV+ cART-treated (n=10, in blue) and HIV+ viremic participants (n=7 in red). Right panel: Correlation between cell-associated total IL-32 mRNA and cell-associated IL-32 protein (measured by ELISA on total PBMCs lysate) (n= 20). B) Relative expression of individual IL-32 isoforms in total PBMCs from HIVneg (n=10, in green), HIV+ cART-treated individuals (n=10, in blue) and HIV+ viremic participants (n=7, in red). C) Correlations between Log10 HIV viral load (Log10VL) and relative expression of individual IL-32 isoforms in viremic individuals (n=7). Data were analyzed by non-parametric Mann-Whitney test in A (middle panel) and B, whereas Spearman correlation was used for data in A (right panel) and C. β-glu: β-glucuronidase.
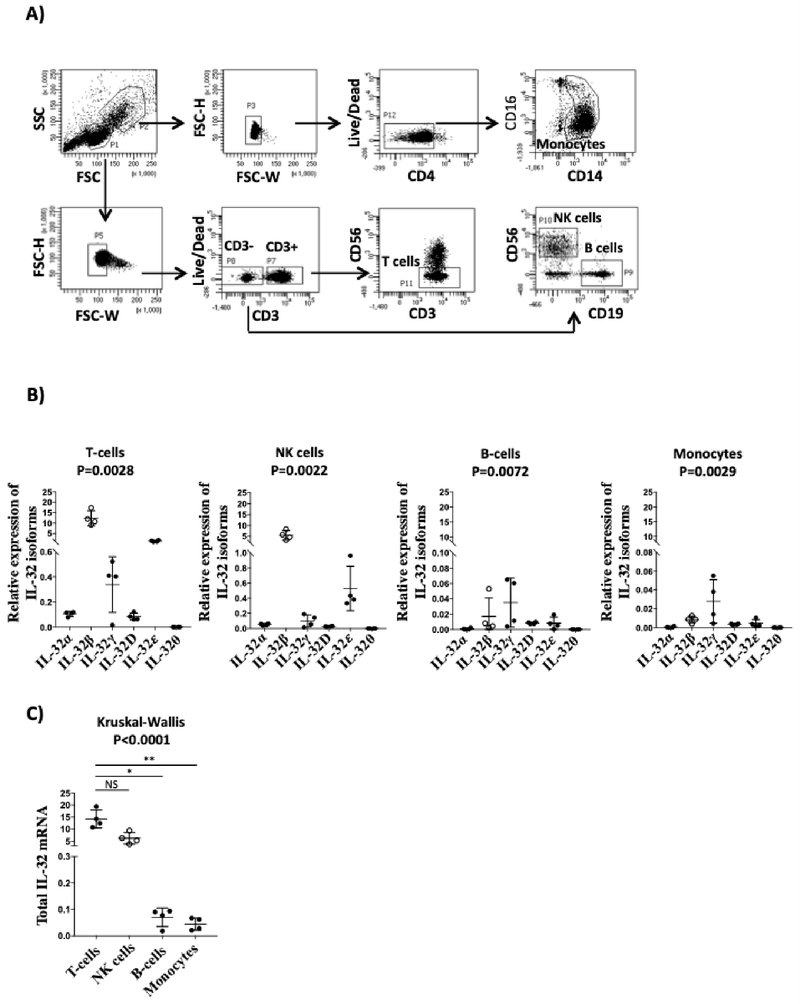
As these measures were performed in total PBMCs, we next aimed to determine the expression of individual IL-32 isoforms in different cell subsets to identify the major cellular source of IL-32. We used classical phenotypic lineage markers for T-cells (CD3+CD56neg), NK cells (CD3negCD19negCD56+(hi/lo)), B-cells (CD3negCD56negCD19+), and monocytes (CD3negCD4loCD14+CD16+/−) to isolate these cell subsets by flow cytometry-based cell sorting from n=4 individuals (Figure 3A). Quantification of IL-32 isoforms in these cells showed that IL-32β was the dominant isoform in the former two cell types followed by IL-32ε and IL-32γ then IL-32α, IL-32D and IL-32θ (Figure 3B). However, IL-32γ, the most pro-inflammatory isoform, was the dominant one in monocytes and B cells. Finally, by pooling all IL-32 isoforms together, T-cells and NK cells showed the highest expression levels of IL-32 compared to B cells or monocytes (Figure 3C). Of note, within the T-cell population, both CD4 and CD8 cells showed similar levels of IL-32 isoform expression (data not shown).
Figure 3: Expression of IL-32 isoforms in primary T-cells, NK, B-cells and monocytes.
A) Shown is the flow cytometry-based cell sorting gating strategy using lineage markers to isolate the different cell types from PBMC as follows: T-cells (CD3+CD56neg), B-cells (CD3negCD56negCD19+), NK cells (CD3negCD19negCD56+(hi/lo)) and monocytes (CD3negCD4loCD14+CD16+/−). B) Shown is the ex vivo relative expression of the individual IL-32 isoforms (normalized to the house keeping gene β-glucuronidase) in T, NK, B-cells and monocytes sorted from PBMCs of n=4 (n=2 HIV+ and n=2 HIVneg individuals). C) Comparison between the relative expression of IL-32 mRNA (sum of all IL-32 isoforms) in the different cell types described in A. Data were analyzed by the non-parametric Kruskal-Wallis for multiple comparisons and Dunn’s sub test in B and C.
Differential functions of IL-32 isoforms
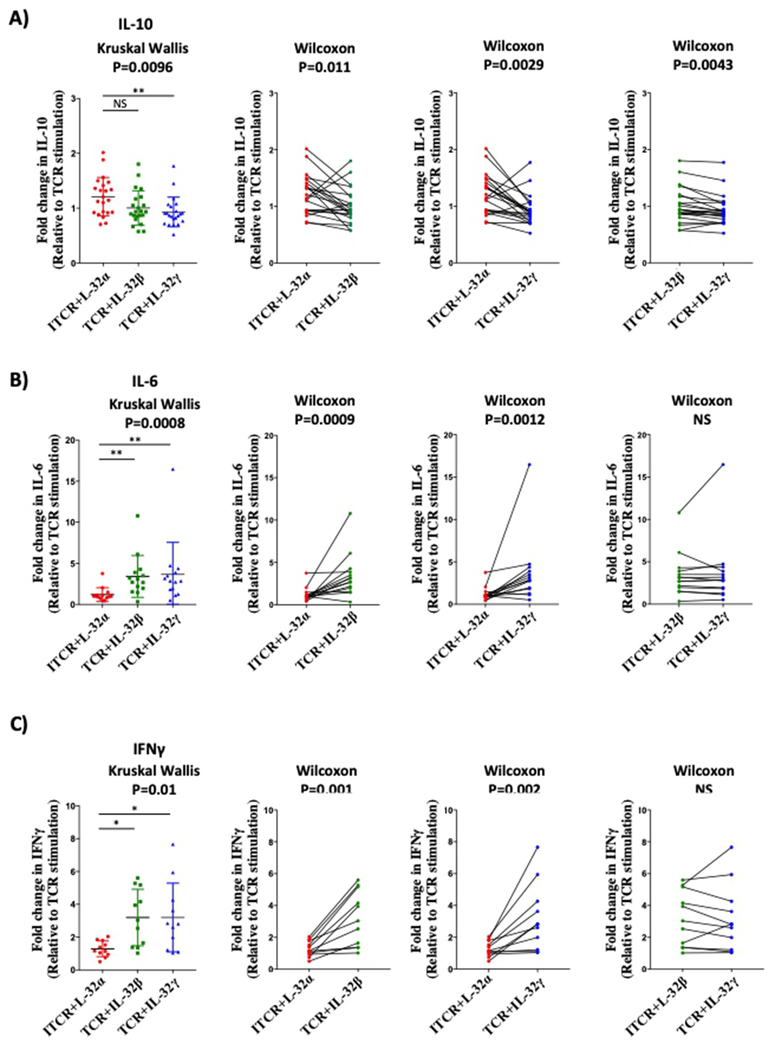
Since we identified T-cells as the major source of IL-32, we aimed to investigate the response of this subset to IL-32 isoform proteins (IL-32α, β and γ; the only three available recombinant proteins for IL-32). Primary CD4+ T-cells isolated from uninfected controls were activated through the TCR in the presence of IL-32α, β, or γ as described earlier11 and anti- and pro-inflammatory cytokines were quantified in the supernatants. IL-32α and IL-32β but not IL-32γ induced IL-10 production in TCR-activated CD4+ T-cells (Figure 4A, left panel, n=22, p=0.0096, data are normalized to the levels of IL-10 induced by TCR stimulation alone from each donor). Paired analysis from the same donor cells showed that IL-32α induced higher levels of IL-10 compared to IL-32β and IL-32γ (Figure 4A, left and middle panels, p=0.011 and p=0.0029, respectively) and that IL-32β had a higher capacity to induce IL-10 compared to IL-32γ (Figure 4A, right panel, p=0.0043). In contrast to IL-10, IL-6 and IFNγ productions were induced by IL-32β and IL-32γ but not by IL-32α (Figure 4B, n=14, P=0.0008 and Figure 4C, n=11, p=0.01, respectively). Altogether, these results are in agreement with earlier reports describing IL-32γ as a potent inducer of proinflammatory cytokines compared to IL-32α28, and show for the first time that IL-32α, and to a lower extent IL-32β, may favor an anti-inflammatory response by inducing IL-10 from activated CD4+ T-cells.
Figure 4: Differential induction of pro and anti-inflammatory cytokines by recombinant human IL-32α, β, and γ isoforms.
Purified CD4+ T-cells were stimulated with plate-coated anti-CD3 (1μg/ml) and soluble anti-CD28 (0.5μg/ml) in the presence or absence of IL-32α, IL-32β or IL-32γ (500ng/ml) for 48h. A) IL-10 production by TCR-stimulated cells in the presence of IL-32α, IL-32β or IL-32γ (Left panel, n=22). Middle and right panels: matched pair analysis for IL-10 production by IL-32 isoforms. B) IL-6 production (n=14) and C) IFN-γ production (n=11) as in A. Data were analyzed by the non-parametric Kruskal-Wallis for multiple comparisons (A, B and C left panels) and Wilcoxon for matched pair analysis (A, B and C, middle and right panels).
Differential impact of IL-32 isoforms on HIV production in CD4+ T-cells from cART-treated individuals
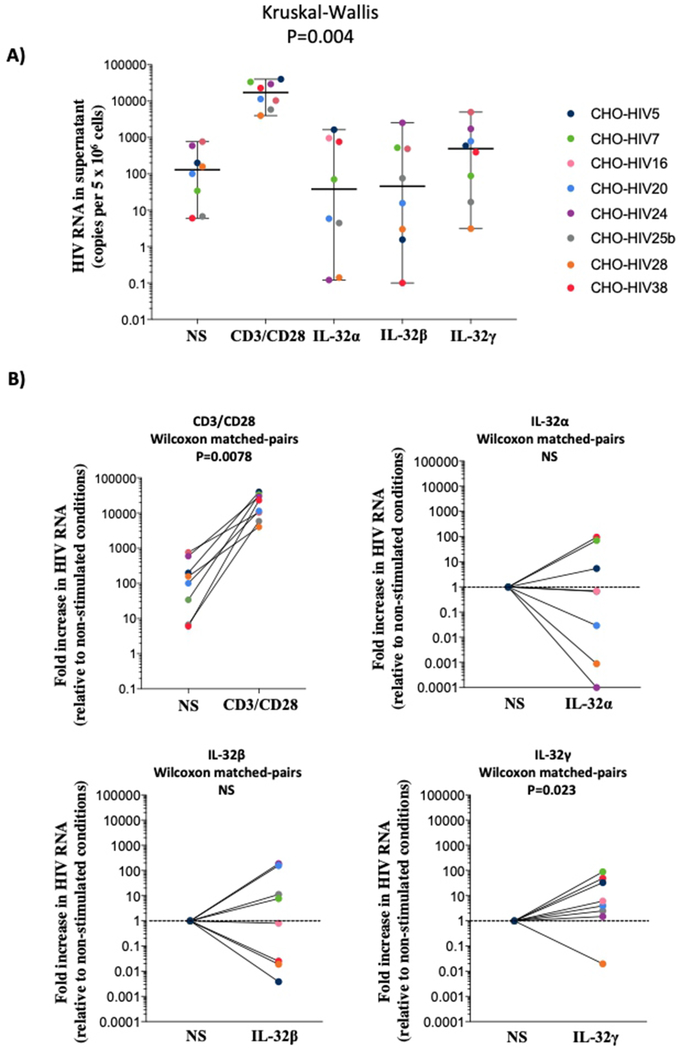
Our results showed that IL-32 isoforms modulate the production of pro and anti-inflammatory cytokines by CD4+ T-cells. Since this compartment harbors the major HIV reservoir under cART29 and given the recent reports showing that IL-32 enhances HIV replication in vitro15, we investigated the potential of IL-32 at inducing the residual HIV production in latently infected CD4+ T-cells. Residual HIV production under cART may further contribute to the persistent immune activation and inflammation seen in HIV-1 infection30,31. We investigated the potential impact of the dominantly expressed IL-32β isoform together with IL-32α and IL-32γ. CD4+ T-cells were isolated from peripheral blood of cART successfully treated HIV+ individuals with undetectable viremia (n=8) and exposed to the three IL-32 isoforms. Quantification of HIV particles was performed in culture supernatant after 6 days of activation. As shown in Figure 5A, HIV-1 production was readily detected under conditions of T-cell receptor stimulation with anti-CD3 and anti-CD28 antibodies. Both IL-32α and IL-32β treatment enhanced baseline levels of HIV production in cells from only 3 out of 8 individuals (Figure 5B, upper right and lower left panels). In contrast, IL-32γ significantly increased HIV production in samples from 7 out of 8 individuals (Figure 5B, lower right panel, matched pair analysis, p=0.023). Together, these data showed that consistent with its proinflammatory nature, IL-32γ has a significant potential to sustain HIV production in CD4+ T-cells from cART-treated individuals.
Figure 5: HIV-1 production in CD4+ T-cells from HIV-1+ cART-treated aviremic individuals (n=8) in response to IL-32 isoforms.
Cells were activated with either Dynabeads Human T-expander CD3/CD28 at a concentration of 1 bead per cell (used as positive control for the efficacy of HIV-1 reactivation assay) or with IL-32 recombinant proteins (250-500ng/ml). A) HIV-1 RNA quantified by qRT-PCR from supernatant of CD4+ T-cells (expressed as HIV-1 copies per 5 x 106 treated or untreated cells) at day 6 post stimulation. NS represents non-stimulated control cells from the same individuals. B) Matched pair analyses for cells from the same individual shown in A, stimulated with TCR (upper left panel), IL-32α (upper right panel), IL-32β (lower left panel) and IL-32γ (lower right panel) relative to non-stimulated (NS) cells. Data were analyzed by the non-parametric Kruskal-Wallis for multiple comparisons in A and matched pair analysis was done by Wilcoxon test in B.
Discussion
IL-32 is expressed in different isoforms with a multitude of functions that can promote either pro- or anti-inflammatory milieu21,22. The balance between these individual isoforms may dictate the overall impact of IL-32 on persistent inflammation in HIV infection. For instance, dominant expression of IL-32γ, the most active and pro-inflammatory isoform28 could be deleterious for the immune response. Therefore, IL-32γ may undergo multiple splicing into shorter and less inflammatory isoforms such as IL-32α&β to prevent deleterious inflammatory effects19,32. Furthermore, smaller isoforms such as IL-32θ were also shown to counteract the inflammatory nature of IL-32γ by specifically inhibiting TNFα production24. In the current study, we observed that IL-32 expression, both protein and RNA, remained higher in HIV-infected individuals receiving cART compared to non-infected controls. All IL-32 isoforms were upregulated in cells from HIV-infected individuals with IL-32β being the dominant isoform followed by IL-32ε and IL-32γ. Interestingly, in a clear contrast to IL-32γ that plays a potent proinflammatory role, the dominant IL-32β isoform was shown to have the potential to dampen inflammation as it induces expression of the anti-inflammatory cytokine IL-10 in monocytes and DCs11,22,23,11,28.
Furthermore, IL-32β was also shown to limit the expression of IL-12 in monocyte-derived DCs23, which may diminish the inflammatory responses as IL-12 is a key mediator for Th1 cell development33. However, the impact of IL-32β on IL-10 and other cytokines production by T-cells (the major source of IL-32) was not yet known. Here we demonstrated that both IL-32β and IL-32α isoforms can induce IL-10 by activated T-cells, however IL-32α had a more profound effect. This effect of IL-32α combined with its moderate induction of IL-6 and IFNγ, compared to the two other isoforms, is consistent with earlier reports describing IL-32α as the least activatory isoform for proinflammatory cytokines11,28. This also suggests that IL-32α may function as an anti-inflammatory isoform, at least in the context of T-cell activation.
The specific impact of IL-32α and IL-32β on the induction of IL-10 is of particular interest given the key roles of IL-10 in regulating and balancing inflammatory responses and tolerance in vivo34. These later functions are dysregulated in HIV infection with higher and persistent levels of IL-10 likely contributing to immune suppression and disease progression. In this regard, we have previously shown that triggering of programmed cell death-1 (PD-1) on monocytes negatively impacts HIV-specific CD4 T-cell proliferative responses in an IL-10-specific fashion35. Therefore, expression of IL-10 must be fine-tuned in order to maintain a balance between efficient Ag-specific responses and control of inflammation. Indeed, the impact of at least IL-32β on IL-10 production seems to be tightly regulated by other isoforms of IL-32 such as the IL-32δ. This regulation is mediated by direct binding of IL-32δ to IL-32β leading to inhibition of IL-32β binding to PKCδ, a prerequisite for the induction of IL-1036,37. However, whether IL-32α-mediated IL-10 production is similarly regulated by other IL-32 isoforms and whether it correlates with or counteracts disease progression remains to be investigated. In this regard, one limitation of the current study is the use of only three IL-32 isoforms (α, β and γ; the only commercially available recombinant IL-32 proteins) in the activation assays. However, a better understanding for the IL-32 effects on cell activation and inflammation may require cell activation with a combination of the different isoforms to reflect the physiological conditions where all of these isoforms are expressed together and isoform-isoform interaction may likely impact the overall IL-32 functions36,37. Further studies are warranted to generate the different IL-32 isoform recombinant proteins to address these questions.
Another characteristic that differentiated IL-32 isoforms at the functional level was their capacity to modulate HIV production in primary CD4+ T-cells from successfully treated individuals. While IL-32γ showed a significant potential to enhance viral production from latent HIV-1 reservoirs, IL-32α and IL-32β failed to show consistent and significant impact. The variability in response to IL-32α and IL-32β may indicate an indirect impact of IL-32 on the production of HIV from latent reservoirs. In support of this hypothesis, recent reports by Palstra et al., 15 suggested that IL-32 might enhance HIV transcription and replication indirectly by promoting a proinflammatory environment conductive for HIV infection through the activation of CD4+ T cells. Meanwhile, CD4+ T-cell activation by IL-32 (as measured by cytokine production) shows donor-to-donor variability (Figure 4) that is likely mediated by donor-dependent activation threshold and/or differential expression of IL-32 receptor(s). Given the fact that IL-32 receptors are yet to be identified, these last probabilities remain hypothetical and further studies are warranted to validate them. It remains intriguing that while both IL-32β and IL-32γ can induce pro-inflammatory cytokines such as IL-6 and IFNγ in primary CD4+ T-cells in culture, they may exert opposite effects on viral transcription. A partial explanation for these observations may arise from the ability of IL-32β to induce IL-10, a cytokine associated with viral latency and inhibition of HIV-1 replication38,39. Although the ultimate goal towards an HIV-1 cure in the era of cART is the elimination of persistent viral reservoirs, exploiting the reactivation potential of proinflammatory cytokines such as IL-32γ is unlikely to be part of future cure strategies, given its relatively modest ability at inducing viral production from latently infected cells. In contrast, this modest, but detectable impact on HIV-1 transcription and production by IL-32γ may have detrimental clinical consequences in vivo as it may contribute to persistent inflammation under therapy40. This is supported by earlier reports clearly showing that cell-associated HIV RNA levels correlate with immune activation41. Furthermore, IL-32γ was shown to favor an inflammatory milieu with a high propensity to sensitize CD4+ T-cells for de novo HIV-1 infection15, which may further suggest a vicious cycle between HIV-1 infection, IL-32 expression, activation of HIV-1 production by cellular reservoirs and persistent inflammation. This persistent inflammation is likely to increase the risk for cardiovascular diseases, which are highly prevalent in the HIV+ population 42. In addition, IL-32γ was shown to suppress proper HIV-specific immune responses through the induction of IDO-1 expression in macrophages in gut and lymph nodes from HIV-infected individuals16. Interestingly, our data on the expression of IL-32 isoforms in different cell types showed that IL-32γ was the dominant isoform in monocytes; cells with highly plastic nature and potential to differentiate into inflammatory macrophages43. However, whether IL-32 can directly impact the differentiation of monocytes to inflammatory macrophages and whether the multiple isoforms of IL-32 can mediate distinctive differentiation phenotypes is not yet known.
In summary, our current data demonstrate that IL-32 levels remain significantly higher in HIV-1 infected and treated individuals with the IL-32β isoform being dominantly expressed. At the functional level, IL-32γ, as expected, shows a typical proinflammatory nature by inducing IL-6 and IFNγ, whereas IL-32α and IL-32β induce the anti-inflammatory cytokine IL-10 in activated T-cells. The proinflammatory nature of IL-32γ was consistent with its capacity to enhance HIV production from stable HIV reservoirs in CD4+ T-cells from individuals receiving cART. These data suggest that targeted blockade of IL-32 inflammatory isoforms, especially the IL-32γ, may represent an attractive approach to limit persistent inflammation and potentially decreasing HIV-1 residual viremia.
Supplementary Material
Acknowledgements
We would like to thank the BSL3 (Olfa Debbeche) and Flow Cytometry (Dominique Gauchat) platforms at the CRCHUM. We also thank Amélie Pagliuzza and Rémi Formentin for their technical help with the HIV reservoir assays and Stéphanie Matte and Daniel Tremblay-Sher for their help with administrative duties and cohort database. We are very thankful to all study participants.
This study was supported by funds through the Canadian Institutes of Health Research, CIHR (PJT 148482), The Canadian Trial Network (CTN173) and the National Institutes of Health, NIH (1R01AG054324-01).
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References:
- 1.Legarth RA, Ahlstrom MG, Kronborg G, et al. Long-Term Mortality in HIV-Infected Individuals 50 Years or Older: A Nationwide, Population-Based Cohort Study. J Acquir Immune Defic Syndr. 2016;71(2):213–218. [DOI] [PubMed] [Google Scholar]
- 2.Smith DM, Salters KA, Eyawo O, et al. Mortality among people living with HIV/AIDS with non-small-cell lung cancer in the modern HAART Era. AIDS Care. 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 3.Hsu DC, Sereti I. Serious Non-AIDS Events: Therapeutic Targets of Immune Activation and Chronic Inflammation in HIV Infection. Drugs. 2016;76(5):533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull ME, Mitchell C, Soria J, et al. Monotypic low-level HIV viremias during ART are associated with disproportionate production of X4 virions and systemic immune activation. AIDS. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia H, Jiang W, Zhang X, et al. Elevated Level of CD4(+) T Cell Immune Activation in Acutely HIV-1-Infected Stage Associates With Increased IL-2 Production and Cycling Expression, and Subsequent CD4(+) T Cell Preservation. Front Immunol. 2018;9:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating SM, Golub ET, Nowicki M, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS. 2011;25(15):1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker JV, Neuhaus J, Duprez D, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS. 2011;25(17):2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy GA, Sieg S, Rodriguez B, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One. 2013;8(2):e56527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman ML, Shive CL, Nguyen TP, Younes SA, Panigrahi S, Lederman MM. Cytokines and T-Cell Homeostasis in HIV Infection. J Infect Dis. 2016;214 Suppl 2:S51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22(1):131–142. [DOI] [PubMed] [Google Scholar]
- 11.El-Far M, Kouassi P, Sylla M, et al. Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci Rep. 2016;6:22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH. Interleukin 32, inflammation and cancer. Pharmacol Ther. 2017;174:127–137. [DOI] [PubMed] [Google Scholar]
- 13.Nold MF, Nold-Petry CA, Pott GB, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181(1):557–565. [DOI] [PubMed] [Google Scholar]
- 14.Rasool ST, Tang H, Wu J, et al. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol Lett. 2008;117(2):161–167. [DOI] [PubMed] [Google Scholar]
- 15.Palstra RJ, de Crignis E, Roling MD, et al. Allele-specific long-distance regulation dictates IL-32 isoform switching and mediates susceptibility to HIV-1. Sci Adv. 2018;4(2):e1701729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AJ, Toledo CM, Wietgrefe SW, et al. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J Immunol. 2011;186(11):6576–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med.2(32):32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Far M, Halwani R, Said E, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5(1):13–19. [DOI] [PubMed] [Google Scholar]
- 19.Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine. 2012;60(2):321–327. [DOI] [PubMed] [Google Scholar]
- 20.Sloot YJE, Smit JW, Joosten LAB, Netea-Maier RT. Insights into the role of IL-32 in cancer. Semin Immunol. 2018;38:24–32. [DOI] [PubMed] [Google Scholar]
- 21.Kang JW, Park YS, Lee DH, et al. Interleukin-32delta interacts with IL-32beta and inhibits IL-32beta-mediated IL-10 production. FEBS Lett. 2013. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro-Dias F, Saar Gomes R, de Lima Silva LL, Dos Santos JC, Joosten LA. Interleukin 32: a novel player in the control of infectious diseases. J Leukoc Biol. 2017;101(1):39–52. [DOI] [PubMed] [Google Scholar]
- 23.Kang JW, Choi SC, Cho MC, et al. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128(1 Suppl):e532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MS, Kang JW, Jeon JS, et al. IL-32theta gene expression in acute myeloid leukemia suppresses TNF-alpha production. Oncotarget. 2015;6(38):40747–40761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MS, Kang JW, Lee DH, et al. IL-32theta negatively regulates IL-1beta production through its interaction with PKCdelta and the inhibition of PU.1 phosphorylation. FEBS Lett. 2014;588(17):2822–2829. [DOI] [PubMed] [Google Scholar]
- 26.Durand M, Chartrand-Lefebvre C, Baril JG, et al. The Canadian HIV and aging cohort study - determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: rationale and study protocol. BMC Infect Dis. 2017;17(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F, Wachi S, Liu H, Jung SS, August A IL-32B is the predominant isoform expressed under inflammatory conditions in vitro and in COPD. COPD Research and Practice. 2015;1(2). [Google Scholar]
- 28.Choi JD, Bae SY, Hong JW, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126(4):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18(7):981–989. [DOI] [PubMed] [Google Scholar]
- 31.Bull ME, Mitchell C, Soria J, et al. Monotypic low-level HIV viremias during antiretroviral therapy are associated with disproportionate production of X4 virions and systemic immune activation. AIDS. 2018;32(11):1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damen M, Popa CD, Netea MG, Dinarello CA, Joosten LAB. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis. 2017;264:83–91. [DOI] [PubMed] [Google Scholar]
- 33.Athie-Morales V, Smits HH, Cantrell DA, Hilkens CM. Sustained IL-12 signaling is required for Th1 development. J Immunol. 2004;172(1):61–69. [DOI] [PubMed] [Google Scholar]
- 34.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 35.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16(4):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang JW, Park YS, Lee DH, et al. Interleukin-32delta interacts with IL-32beta and inhibits IL-32beta-mediated IL-10 production. FEBS Lett. 2013;587(23):3776–3781. [PubMed] [Google Scholar]
- 37.Kang JW, Park YS, Lee DH, et al. Interaction network mapping among IL-32 isoforms. Biochimie. 2014;101:248–251. [DOI] [PubMed] [Google Scholar]
- 38.Vandergeeten C, Fromentin R, Chomont N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012;23(4-5):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arias JF, Nishihara R, Bala M, Ikuta K. High systemic levels of interleukin-10, interleukin-22 and C-reactive protein in Indian patients are associated with low in vitro replication of HIV-1 subtype C viruses. Retrovirology. 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254(1):326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.