Abstract
One of the greatest challenges in treating acute myeloid leukemia (AML) is chemotherapy refractory disease. Previously, we demonstrated a novel mechanism whereby AML-induced endothelial cell (EC) activation leads to subsequent leukemia cell adherence, quiescence and chemoresistance, identifying activated ECs as potential mediators of relapse. We now show mechanistically that EC activation induces the secretion of interleukin-8 (IL-8) leading to significant expansion of non-adherent AML cells and resistance to cytarabine (Ara-C). Through crystallography and computational modeling, we identified a pocket within IL-8 responsible for receptor binding, screened for small molecules that fit within this pocket, and blocked IL-8 induced proliferation and chemo-protection of AML cells with a hit compound. Results from this study show a new therapeutic strategy for targeting the sanctuary of an activated leukemia microenvironment.
Keywords: interleukin-8, endothelial cell, acute myeloid leukemia, vascular niche, microenvironment, chemoresistance
1.0. INTRODUCTION
Despite achieving initial morphologic remission after chemotherapy, 60-80% of patients with acute myeloid leukemia (AML) suffer from disease relapse [1]. Even with recent drug approvals by the FDA, the majority of AML patients will die of relapsed or refractory leukemia. Thus, there is an urgent need for new therapeutics for people with AML.
AML is highly dependent on its microenvironment for its survival and growth [2]. Studies have shown that interaction with soluble factors, stromal cells and extracellular matrix (ECM) in the bone marrow (BM) is essential in maintaining AML [2-4]. Previously, we demonstrated that endothelial cells (ECs) serve as sanctuary sites for refractory AML [5, 6].
Mechanistically, AML cells activate ECs and lead to a positive feedback loop involving the adhesion of a subset of AML cells to activated ECs, quiescence of AML cells, and resistance to chemotherapeutic agents such as cytarabine and anthracyclines that are cornerstones to clinical regimens [7]. Experiments with semipermeable membranes have also shown the ability of ECs to enhance the proliferation of non-adherent AML cells through paracrine action [8, 9]. What has yet to be fully understood are the molecular mechanisms by which AML-activated ECs protect and enhance the growth of AML cells. By identifying these mechanisms, new therapeutic strategies can be devised.
EC activation is an inflammatory response that alters resting EC behavior resulting in the increased production of various soluble factors involved in the general immune response [10, 11]. Interestingly, one of the most highly secreted factors is interleukin (IL)-8, a factor which has been shown to significantly affect AML cell proliferation [12-14]. In these studies, we directly demonstrate that AML-induced EC activation results in the increased production of IL-8 that enhances AML cell proliferation and chemoresistance. Studies aimed at blocking IL-8 signaling, utilizing crystallography and computational modeling, resulted in the identification of an IL-8 small molecule inhibitor able to abrogate enhanced growth and chemoresistance to Ara-C. Other studies have shown that IL-8 is overexpressed is AML patient samples and blockade of IL-8 receptor (CXCR2) activity through knockdown or pharmacologic approaches resulted in decreased proliferation of AML further suggesting an important role of this axis in AML progression [15]. Overall, these findings suggest that IL-8 production by EC activation plays a central role in: 1) the growth and survival of AML, 2) the supportive effects of ECs observed in AML and 3) identifies IL-8 inhibition as a potential method to augment chemotherapy.
2.0. MATERIALS AND METHODS
2.1. Cell lines and Primary Cells
Human umbilical vein endothelial cells (HUVECs; Lonza, Walkersville, MD; C2517A) were cultured using EGM-2 MV media (Lonza; CC-3202). KG-1 human leukemia cells (ATCC, Manassas, VA; CCL-246) were grown in IMDM (Hyclone, Fisher Scientific, Hanover Park, IL; SH30228.01) plus 20% FBS.
Bone marrow endothelial cells (BMECs) were freshly isolated from healthy human bone marrow (Lonza). Briefly, mononuclear cells were isolated from fresh bone marrow samples using Ficoll separation (GE Healthcare, Chicago, IL; GE17-1440-02) and plated in collagen-coated plates. Adherent cells were collected and cultured using EGM-2 MV media supplemented with 10% FBS to generate the BMECs.
2.2. AML-EC Co-cultures
Co-cultures were established in 6 or 12-well plates to study the relationship between AML and ECs. HUVECs and BMECs were first grown to 60-80-% confluency then 0.5 to 1 ×106 KG-1 cells were added. Co-cultures were maintained in base media supplemented with 10% FBS.
2.3. Conditioned Media Studies
Activated EC conditioned media (CM) was prepared by collecting the supernatant from contact co-cultures after 48-hours of incubation. Non-activated CM was collected from HUVEC or BMEC alone cultures. CM was mixed with base EBM media (without supplements) at a 50/50 ratio by volume prior to use.
To test the effects of the CM on leukemia cell growth, 5×105 KG-1 cells were grown in EBM base media supplemented with the different CMs. As controls, KG-1 cells were also grown with non-activated CM alone or 100% EBM. To measure growth, cells were enumerated every other day over 8-days or exposed to proliferation assays.
2.4. Analysis of Proliferative Status and Viability
To assess the proliferative status of AML cells in our co-cultures, BrdU uptake levels were quantified. Briefly, co-cultured cells were collected and incubated with BrdU as previously described [7]. Cells were then stained with anti-CD45-APC (555485) and anti-BrdU-FITC (51-33284X, both from BD Biosciences, San Jose, CA, USA) and analyzed by flow cytometry using a FACSCanto II (BD Biosciences) and FACSDiva software. Alternatively, proliferation studies were performed using CellTiter Blue (Promega, Madison, WI; G8080) or XTT cell proliferation assay (ATCC; 30-1011K) as per the manufacturer’s protocols.
To assess viability, AML cells were harvested after 24-hour of treatment and stained with anti-CD45-V450 (560367), Annexin V-FITC (556419) and 7-AAD (555815) for flow cytometric analysis.
2.5. Drug treatments
Cells grown alone or in co-culture with ECs were treated with cytarabine (Ara-C) (Sigma-Aldrich, St Louis, MO; C6645) at a final concentration of 20μM in 0.1% DMSO for a period of 24 to 48-hours.
For in vitro IL-8 inhibitor screening, the top 19 compounds with the lowest energy scores were obtained from the National Cancer Institute/Developmental Therapeutics Program (NCI/DTP) Open Chemicals Repository 2007 plate. AML cells (in monoculture or co-culture) were treated with each compound for 24-hours at a final concentration of 100μM in 0.01% DMSO.
2.6. E-selectin Expression Analysis
Co-cultures were established and after 24 to 48-hours, ECs were harvested and stained using anti-E-selectin-APC (551144) and anti-CD105-PE (560839; both from BD Biosciences) then analyzed by flow cytometry. The levels of E-selectin expression were determined and used to quantify EC activation [10].
2.7. Cytokine Analysis
Cell culture supernatants were analyzed for the concentration of IL-8 utilizing the VersaMAP Custom Multi-Analyte Profiling Development System (R&D Systems, Minneapolis, MN) and BioPlex array reader equipped with Bio-Plex software (Bio-Rad, Hercules, CA). Values were extrapolated from a standard curve.
2.8. Quantitative Real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed on HUVECs that were activated by co-culture with KG-1 for 24-hours. Following activation, HUVECs were sorted using a BD FACSAria (BD Biosciences) based on EC specific staining with CD105 [5]. Isolated HUVECs were then subjected to RNA extraction and first strand synthesis using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). The reactions were performed using TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA). The following primers were used: IL-8 (Hs00174103_m1, Thermo Fisher Scientific). Data detection was performed using the Strategene qRT-PCR instrument software (Agilent Technologies, Santa Clara, CA). All data was calculated based on β-actin endogenous control levels.
2.9. Protein expression analysis
Protein expression was performed using Western blot analysis. Briefly, cells were lysed in RIPA buffer including Halt protease inhibitor (Fisher Scientific, Hanover Park, IL; 87785) and subjected to electrophoresis using 12% polyacrylamide gels (Bio-Rad, Hercules, CA; 4568044). Proteins were transferred onto a 0.45 μm polyvinylidene difluoride (PVDF) membrane (Fisher Scientific, Hanover Park, IL; IPVH0010). Membranes were blocked in 5% BSA and immunoblotted with Akt (Cell Signaling Technologies, Danvers, MA; 9272), 4E-BP1 (Cell Signaling Technology; 9644), p-4E-BPl (Cell Signaling Technologies; 9451), p-Akt (Ser473) (Affinity; AF0016), and GAPDH (Life Technologies; 398600). HRP-conjugated secondary antibodies (Cell Signaling Technologies; 7074) were used, and protein levels were visualized using enhanced chemiluminescence (ECL) (Bio-Rad; 1705060)
2.10. Crystallization of Human Interleukin IL-8
Recombinant IL-8 isolated and purified from Pichia pastoris [16] was concentrated to 10 mg/ml with equal volumes of Hampton Crystal Screen Cryo 1 (HR2-121) and 2 (HR2-122) (Hampton Research, CA). Large crystals formed in 0.2M Ammonium acetate, 0.085M Sodium citrate tribasic dihydrate pH 5.6, 30% w/v Polyethylene glycol 4,000 and 15% v/v Glycerol. Single crystals were flash cooled and stored in liquid nitrogen prior to data collection at the National Synchrotron Light Source beamline X6A.
2.11. Data reduction and structure determination of Human Interleukin IL-8
X-ray data was reduced with DENZO and SCALEPACK. The 2.0 Å crystal structure of human IL-8 expressed in E. coli, PDB 3IL8, was used for molecular replacement with X-ray data from pichia pastoris rIL-8 crystals. SHELXL was used to refine the molecular replacement model to 1.0 Å, PDB 5D14 and 0.95 Å, PDB 4XDX.
2.12. Molecular docking to select IL-8 binding compounds
We mapped the site of IL-8 presumed to be involved in receptor binding based on previous studies with IL-8/CXCR2 binding [17]. This site was localized at a solvent accessible pocket formed at the interface of two IL-8 subunits that form the dimer. We used molecular docking to select compounds with the potential to bind this site. To prepare the site for docking, all water molecules were removed and protonation of IL-8 was done with SYBYL (Tripos). The molecular surface of the structure was explored using sets of spheres to describe potential binding pockets. The sites selected for molecular docking were defined using the SPHGEN program (generates a grid of points that reflect the shape of the selected site) and filtered through CLUSTER. The CLUSTER program groups the selected spheres to define the points that are used by DOCK6 to match potential ligand atoms with spheres. Intermolecular AMBER energy scoring, contact scoring, and bump filtering were implemented in the DOCK program algorithm. Atomic coordinates for 139,735 small molecules in the National Cancer Institute Developmental Therapeutics Program 2007 library (NCI/DTP) of drug-like compounds were positioned in each structural pocket in 1000 different orientations and scored based on predicted polar and nonpolar interactions. The most favorable orientation and scores (contact and electrostatic) were calculated. PYMOL was used to generate graphic images.
2.13. Statistics
Statistical differences were calculated using the Student t test. The reported values represent the mean ± SEM. A p value ≤ 0.05 was considered to be significant.
3.0. RESULTS
3.1. Leukemia Cell Mediated Endothelial Cell Activation affects the Proliferative Status of Leukemia Cells in vitro
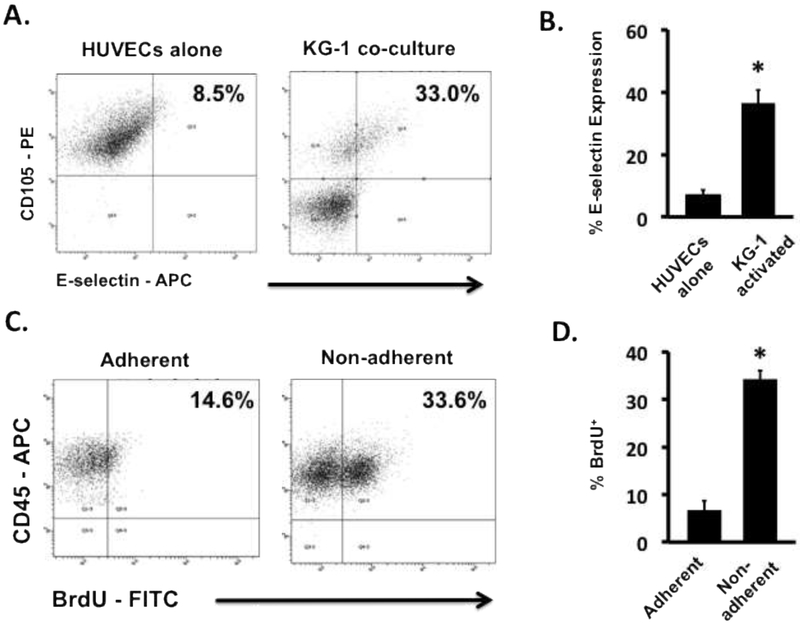
We and others have demonstrated the ability of AML cells to initiate EC activation [7, 18]. To confirm the ability of AML cells to activate ECs, co-cultures of KG-1 cells with HUVECs were prepared. The results indicated a significant increase in E-selectin, a potent biomarker of EC activation, expression on ECs in co-cultures compared to monoculture ECs (Figures 1A and B). We previously observed that AML cells that adhere to activated ECs adopt a quiescent state [7]. In these studies, we observed a similar outcome based on BrdU uptake (Figure 1C), however; the proliferative status of the non-adherent AML cells remained active (Figure 1D). Having shown the effects of EC activation on adhesion and quiescence of AML cells as well as its potential role in leukemia relapse, we now focused our efforts on examining the role of EC activation on the growth of the non-adherent AML cell sub-population.
Figure 1. Leukemia cells activate resting ECs resulting in altered leukemia cell proliferation.
(A) Representative flow cytometry plots showing E-selectin levels on KG-1 activated HUVECs and non-activated HUVEC controls. (B) The levels of E-selectin expression on the surface of ECs showed significant increases when activated with KG-1 cells in co-culture. * p < 0.05 (C) Representative flow cytometry plots showing BrdU uptake by adherent and non-adherent AML cells in contact co-cultures of HUVECs and KG-1 cells. (D) BrdU uptake in non-adherent KG-1 cell populations was significantly higher in comparison to adherent populations indicating a proliferative phenotype. * p < 0.05
3.2. EC Activation Enhances AML Cell Growth
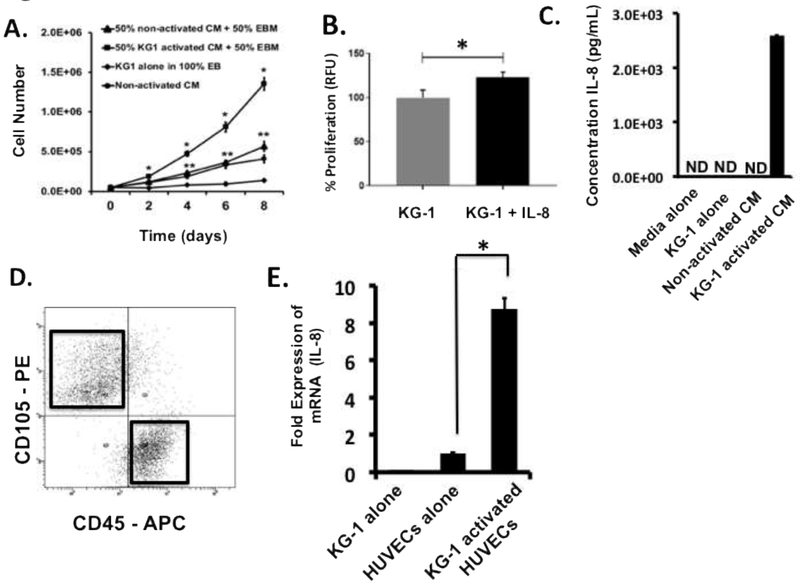
Previous studies have shown that soluble factors released by ECs enhance the growth and survival of leukemia cells in vitro [8], however; the mechanism initiating this response has not been elucidated. To determine if EC activation is responsible for initiating this synergistic intercellular response, experiments were performed wherein AML cells were grown in media supplemented with CM generated from both activated and non-activated ECs. Our results clearly demonstrated that supplementing base media (EBM) with CM from activated ECs (50/50 ratio by volume) resulted in significantly increased KG-1 cell growth when compared to KG-1 cells grown in EBM supplemented (also 50/50) with non-activated CM (Figure 2A). KG-1 cells grown with 100% CM from non-activated ECs or with base EBM media alone also did not grow as well as cultures with activated CM (Figure 2A). Interestingly, cells grown with non-activated CM with or without EBM grew better than EBM alone intimating that non-activated ECs can produce factors that enhance KG-1 proliferation but not as significantly as activated ECs. The data clearly demonstrate that CM from AML activated ECs contains key soluble factors capable of significantly enhancing AML cell growth and that EC activation is a necessary trigger to initiate the synergy between AML and ECs.
Figure 2. Leukemia activated ECs secrete IL-8 which enhances leukemia cell expansion.
(A) Growth curves of KG-1 cells grown in different media are shown. Supplementing base EBM media with 50% (by volume) activated CM induces significant levels of KG-1 cell growth in comparison to all other cultures tested including those supplemented with non-activated CM. Cells from each culture cohort were enumerated every 2-days over an 8-day culture period. * p < 0.05 versus all other cultures; ** p < 0.05 versus 100% EBM cultures. (B) KG-1 proliferation was significantly enhanced when media was supplemented with IL-8. (C) Fresh, unfrozen supernatants from non-activated and activated co-cultures were evaluated for the production of IL-8 by ELISA. Higher IL-8 concentrations were observed in activated CM. As controls, supernatants from KG-1 alone cultures and pure EGM-2 media were analyzed. Values were extrapolated from standard curves with linear detection limits of 10–3300 pg/mL. ND indicates non-detectable levels. (D) Flow cytometry-based sorting was used to isolate ECs from activating co-cultures. Flow cytometry plots identify gates established for sorting. Representative plots are shown. ECs were isolated based on CD105 (PE) expression while KG-1 cells were identified using CD45 (APC). (E) Sorted ECs were analyzed for mRNA expression levels for IL-8 using qRT-PCR. Non-activated ECs and KG-1 cells alone were analyzed as negative controls. * p < 0.05 versus ECs alone
3.3. EC Activation Results in Increased Production of Soluble Factors that Promote AML Proliferation
Previous studies have reported elevated levels of several soluble factors in the serum of leukemic patients many of which have been linked to AML cell growth in vitro [8, 19-23]. Interestingly, among these factors is IL-8 which is also specifically produced during the normal EC activation immune response [10]. Initial studies were performed to determine the direct effect of IL-8 on AML proliferation. Following exposure to IL-8 for 24-hours, KG-1 cells were analyzed using a proliferation assay which showed that IL-8 supplementation was able to significantly enhance AML cell proliferation (Figure 2B). Next, to directly demonstrate that AML induced EC activation results in the production of IL-8, we quantitatively measured its production by activated and non-activated ECs using ELISA. The results demonstrated a significant presence of IL-8 in CM from AML activated ECs in comparison to CM from non-activated ECs (Figure 2C). Fresh media and CM from KG-1 cells alone had undetectable levels of IL-8 (Figure 2C). While it has been shown that primary AML cells and various AML cell lines can produce IL-8 in vitro [8, 24], the KG-1 cell line produces low to non-detectable quantities of this factor making them an ideal control leukemia cell in these experiments [25, 26]. These findings demonstrate that AML-induced EC activation is necessary for the production of IL-8. The observation that non-activated ECs did not produce this supportive factor further highlights the importance of EC activation as an essential initial step to enable ECs to support the growth of AML [5, 8, 27-31].
To confirm our ELISA analysis and demonstrate that IL-8 production was targeted to activated ECs, we next measured mRNA expression of this soluble factor in purified activated and non-activated ECs using qRT-PCR. Immediately prior to analysis, activated ECs were sorted from KG-1 containing co-cultures using flow sorting based on CD105 and CD45 expression patterns (Figure 2D). The results confirmed our findings with ELISA demonstrating increased mRNA expression of IL-8 in activated ECs in comparison to non-activated EC controls (Figure 2E). Analysis of purified KG-1 cells from co-cultures was also performed to determine if AML cells were a source of IL-8. Our data demonstrated that KG-1 cells do not express detectable levels of IL-8 intimating that activated ECs were the main source of this factor in our system (Figure 2E).
3.4. Small molecule inhibitor to inhibit IL-8 – receptor interactions
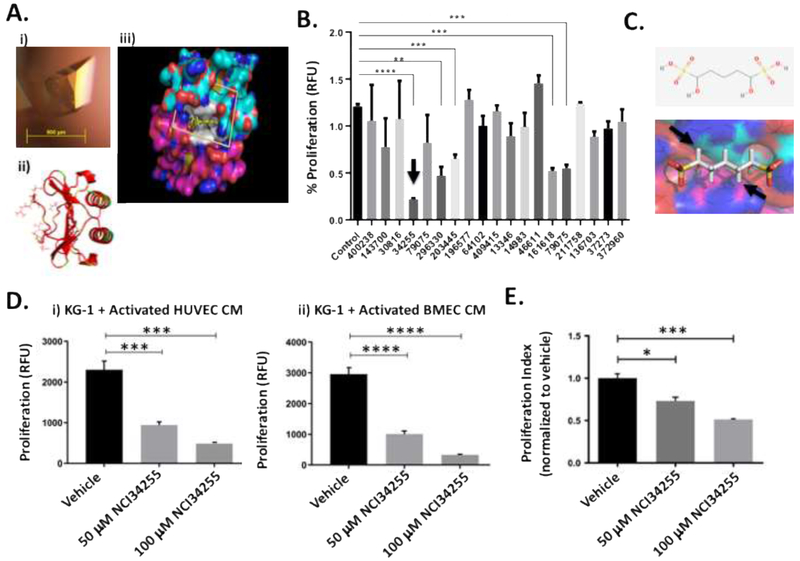
There is increasing interest in the design and testing of IL-8 inhibitors since IL-8 signaling has been shown to be involved in cancer cell proliferation, tumor angiogenesis and metastasis [32]. Since AML activated ECs secrete IL-8, potentially explaining enhanced AML cell growth, we hypothesized that compounds that block IL-8’s ability to interact with its receptors would abrogate these responses. The 2.0 angstrom (A) crystal structure and NMR solution structures of IL-8 were determined and provided insight into the structural framework of IL-8 and the surfaces involved in receptor binding (Figure 3 Ai). The IL-8 monomer consists of three antiparallel β-strands connected to a long alpha helix corresponding to carboxy terminal residues 57-72 (Figure 3 Aii). IL-8 has been shown to be dimeric in solution and in the crystal structure, stabilized by residues in the first β-strand (residues 23-29) in each molecule, forming a 6 stranded β-sheet in the dimer. Although the sites on IL-8 involved in receptor binding are not completely understood, site directed mutagenesis studies implicated positions at the homodimer interface between two IL-8 subunits (Figure 3 Aiii). To determine if the structural pocket was targetable, we used the 0.95 Å crystal structure of human IL-8 as the basis for selection of candidate small molecules by molecular docking.
Figure 3. Identification of small molecule inhibitor of IL-8 that can significantly reduce leukemia cell expansion.
(A) Representative IL-8 crystal grown using the vapor diffusion hanging drop method. IL8 crystal yielding complete X-ray diffraction data set to 0.95 Å grown in 0.17 M Ammonium acetate, 0.085 M Sodium citrate tribasic dihydrate pH 5.6, 20% w/v Polyethylene glycol 4,000 and 15% v/v Glycerol (i). The IL-8 dimer is shown as a ribbon diagram in red. Side chains are shown for residues implicated in receptor binding based on mutagenesis studies (ii). The molecular surface of the IL-8 dimer is shown with blue for nitrogen, red for oxygen, cyan for carbon in the (top IL-8 subunit) and magenta for carbon (bottom IL-8 subunit). Residues implicated in receptor binding are shown in white. The scoring grid for molecular docking is shown as a box. 139,735 NCI/DTP compounds were docked into the binding site in silico to predict the binding affinity for IL-8 (iii). (B) Docking studies identified 19 small molecules capable of binding IL-8’s receptor binding site. Treatment of KG-1 cells with these molecules identified 5 that were able to significantly decrease KG-1 proliferation. The best hit molecule is identified with an arrow. ** p < 0.01; *** p < 0.001; **** p < 0.0001 (C) The best hit molecule was NCI34255 which interferes with binding at the homodimeric interface of two IL-8 sub-units (see arrows). (D) KG-1 cells were grown in CM from KG-1 activated ECs (HUVECs and BMECs) supplemented with NCI34255. The presence of NCI34255 was able to significantly decrease IL-8 induced cell proliferation contrary to the enhanced proliferation observed in previous CM studies. *** p < 0.001; **** p < 0.0001 (E) Supplementation of co-cultures comprising KG-1/BMECs with NCI34255 showed significant decreases in non-adherent KG-1 cell proliferation indicating the ability of NCI34255 to overcome activated EC-generated IL-8 signaling. * p < 0.05; *** p < 0.001
3.5. NCI34255 inhibits IL-8 and reduces the growth of AML cells
Using the site identified as the receptor binding site on IL-8, we screened 139,735 small molecule compounds from the NIH NCI/DTP. Following docking studies to measure affinity between the compounds and the receptor binding site, these compounds were ranked based on overall energy scores. Proliferation studies using the top 19 compounds (Supplemental Table 1) revealed that 5 of the 19 compounds tested could significantly reduce KG-1 proliferation (Figure 3B). Of these the compound with the highest inhibitory activity was 1,5-dihydroxy-1,5-pentanedisulfonic acid (NCI34255). NCI34255 interfered with IL-8-receptor interaction by binding at the homodimeric interface of two IL-8 sub-units (Figure 3C) with a highly favorable docking score of −28.9 as computed by AUTODOCK. To test the effect of the IL-8 inhibitor NCI34255 on AML cells, KG-1 cells that were grown in activated EC CM were exposed to NCI34255 at different concentrations (50 and 100 μM) and proliferation assays were performed. The results demonstrated that NCI34255 significantly abrogated IL-8 effects resulting in significantly decreased KG-1 proliferation at both concentrations tested (Figure 3 Di). To further confirm these effects, we also performed experiments using an additional, more clinically relevant, EC source that was derived from human bone marrow (BMECs). In similar studies with BMEC CM, we observed that NCI34255 was able to decrease KG-1 cell proliferation in the presence of BMEC CM in comparison to non-treated controls (Figure 3 Dii). Finally, experiments were then conducted to test NCI34255 using our co-culture system. Here, BMECs were cultured in the presence of KG-1 cells to allow for EC activation and subsequent IL-8 production. Co-cultures were then treated with NCI34255 and non-adherent KG-1 cells analyzed for proliferation. The results show that NCI34255 was able to significantly decrease KG-1 growth even in the presence of activated ECs that were a continual source of IL-8 (Figure 3E). The data suggests that NCI34255 is an effective inhibitor of the IL-8 pathway and can prevent enhanced AML cell growth due to activated EC-derived IL-8.
3.6. Inhibition of IL-8 significantly increases AML response to cytarabine
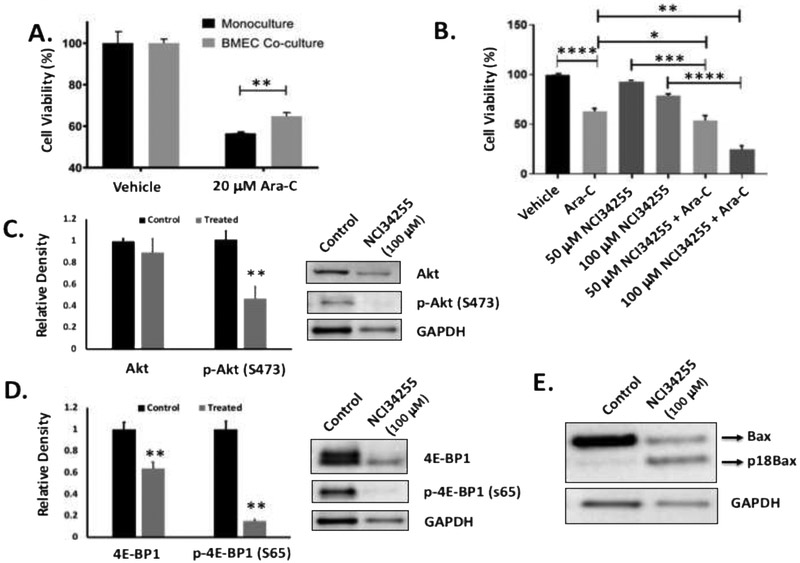
We next investigated how NCI34255 affected chemotherapy treatment. Interestingly, initial studies demonstrated that co-culture of KG-1 cells with BMECs conferred chemoprotective effects on non-adherent AML cells (Figure 4A). While Ara-C treatment was still able to decrease cell viability in all cohorts, the numbers of viable KG-1 cells was significantly higher in co-cultures in comparison to KG-1 alone monocultures. To determine if the protective effects were modulated by activated EC-generated IL-8, we next tested whether or not NCI34255 treatment could enhance Ara-C induced killing. Here KG-1 cells were co-cultured with ECs and treated with Ara-C alone, NCI34255 alone or in combination. The results showed that the combination of Ara-C and NCI34255 induced significantly higher cytotoxicity than Ara-C or NCI34255 alone (Figure 4B). As expected, Ara-C alone induced significant apoptosis of KG-1 cells while NCI34255 alone did not at the concentrations tested. This data supports the conclusion that EC generated IL-8 confers chemoprotective effects and that IL-8 inhibition through the use of NCI34255 can significantly improve AML response to Ara-C.
Figure 4. IL-8 induces chemoresistance which is abrogated using NCI34255.
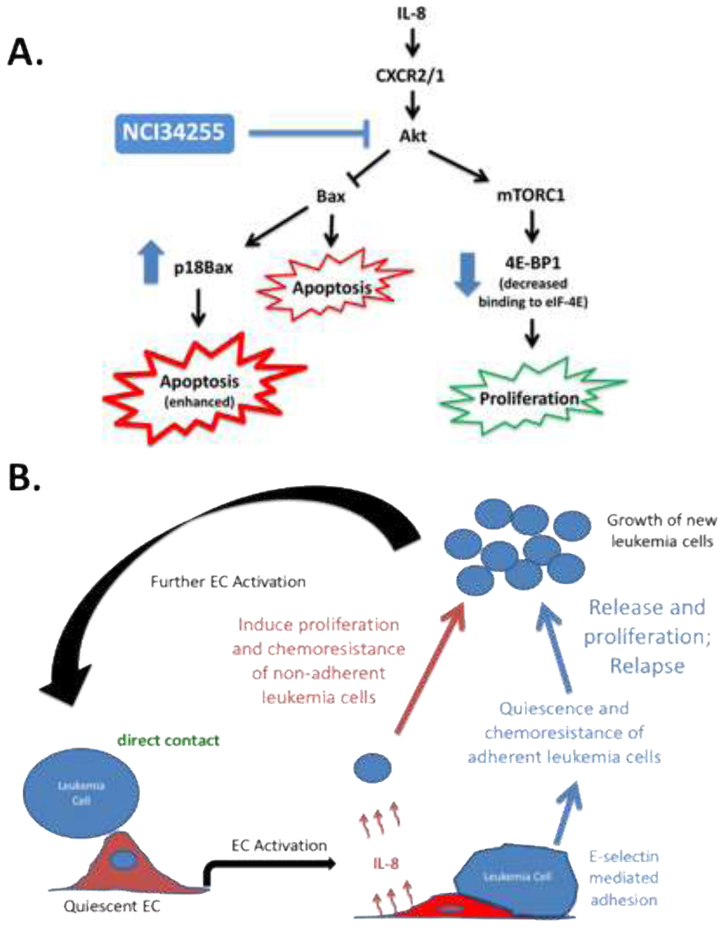
(A) The response of KG-1 cells in monoculture or non-adherent KG-1 cells in co-culture to Ara-C was tested and showed that KG-1 cells are significantly less sensitive to Ara-C when cultured with ECs. ** p < 0.01 (B) Co-cultures were treated with Ara-C, NCI34255 or a combination of both. Significant decreases in cell viability was observed when Ara-C alone treated groups were compared to Ara-C + NCI34255 treated groups demonstrating that NCI34255 was able to augment Ara-C and enhance apoptotic responses. NCI34255 alone treated groups did affect cell viability in comparison to vehicle controls. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 (C, D) Analysis of Akt activity in non-adherent KG-1 cells collected from EC co-culture treated with NCI34255 showed significantly decreased levels of phosphorylated Akt in comparison to non-treated controls (C). A similar analysis also showed a significant decrease in phosphorylated 4E-BP1 a downstream target of mTORC1 in the same cell population (D). ** p < 0.01 (E) Bax expression in KG-1 cells isolated from co-cultures exposed to NCI34255 treatment was assessed. The results showed that Bax is cleaved in response to NCI34255 forming the apoptosis enhancing truncated form, p18Bax.
3.7. NCI34255 effects on AML proliferation and resistance to Ara-C is through Akt Signaling
To further understand how NCI34255 decreases AML proliferation and increases AML response to Ara-C, we next performed studies to analyze the effects of NCI34255 on downstream IL-8 signaling. In these experiments, NCI34255 effects on the Akt pathway were analyzed due to the known dual effects of this pathway on cell proliferation and chemoresistance and the fact that this pathway is known to be constitutively active in 50-70% of AML cases [33]. Here, co-cultures with KG-1 and BMECs were first established and then subsequently treated with NCI34255. Following treatment, non-adherent KG-1 cells were collected and analyzed by Western blotting. The results showed a significant decrease of phosphorylated Akt (S743) in treated KG-1 cells in comparison to non-treated controls indicating that IL-8 inhibition using NCI34255 prevented activation of AKT (Figure 4C). Next, we performed analysis on specific downstream effectors of Akt involved in both cell proliferation and apoptosis. Akt signaling through mTOR (specifically mTORC1) is known to affect cell proliferation [34]. To test if decreased Akt activity could affect this pathway, we measured the levels of phosphorylated 4E-BP1, a downstream effector of mTORC1, and showed a significant decrease in phosphorylation compared to controls (Figure 4D). This decrease may explain the low levels of KG-1 proliferation measured in NCI34255 treated cultures. To determine how NCI34255 was able to enhance response to Ara-C treatment, we analyzed its effect on the pro-apoptotic protein Bax as Bax is known to be directly inhibited by Akt [35]. Analysis of total Bax expression levels showed no change in comparison to untreated cells (data not shown), however; there was a significant increase in the amount of truncated Bax (18kDa; p18Bax) generated after treatment (Figure 4E). This is an interesting finding since p18Bax has been shown to sensitize cellular response to apoptosis, thereby enhancing the overall effects of chemotherapy in a variety of cancer models [36, 37]. Overall, these results indicate that inhibition of IL-8 induced signaling through Akt is a possible mechanism of the dual effects NCI34255 has on decreasing AML proliferation and enhancing KG-1 response to Ara-C.
4.0. DISCUSSION
In this study we demonstrate that AML cell-induced EC activation is essential to initiate the synergistic effects seen between AML cells and ECs. Once ECs were activated by AML cells, they secreted IL-8 resulting in enhanced proliferation and chemoresistance of AML cells. Without EC activation or in the presence of an IL-8 inhibitor (NCI34255) these supportive effects were abrogated highlighting the importance of IL-8 in these processes. Our studies agree with others who have shown the role of EC-secreted factors in enhancing the proliferation and survival of AML cells in vitro [5, 8, 27-31]. Data from our study extend our understanding of the AML microenvironment by showing a novel mechanism wherein AML cell-induced EC activation is the initiating step and identifying the important role of IL-8 in these supportive processes.
We observed that AML-induced EC activation leads to significantly greater production of IL-8 by ECs. IL-8 was originally observed in AML in the 1990s [38]. Critical experiments subsequently demonstrated the pro-proliferative and anti-apoptotic effects of IL-8 on AML cells [12-14]. In vitro studies showed that stromal cells support leukemic myeloblast growth by stimulating the production of IL-8, while enhanced angiogenesis via IL-8 signaling has also been shown to be important for disease development and modulating therapeutic outcomes [14, 39-41]. In AML patients, transcript expression of IL-8 or one of its receptors (CXCR2) has prognostic importance [15, 42]. However, AML-EC cross-talk biology was assumed to be unidirectional with AML cells on the receiving end. Not appreciated until now is that AML cells instigate ECs to produce unwanted IL-8 through contact activation. Given these results, the poor prognosis associated with IL-8 may be caused by disease geography (i.e. AML/vascular association) and suggest that the IL-8 pathway represents a potential therapeutic target in the treatment of AML. Targeting the IL-8/CXCR1/2 axis is being tested in other cancers. In a phase Ib study (), patients with HER-2 negative metastatic breast cancer who had received more than three lines of cytotoxic chemotherapy and not known to be refractory to paclitaxel were treated with the CXCR1/2 inhibitor, reparixin, plus weekly paclitaxel [43]. Thirty-three patients were enrolled and there were no dose limiting toxicities. Of evaluable patients, 8/27 (30%) achieved a confirmed RECIST response. Most of the responding patients received the highest dose tested (1200 mg TID). Two patients showed durable responses of greater than 12 months. Repiraxin is now being tested in a randomized, double-blind, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (FRIDA trial; ).
To overcome the seemingly critical role of IL-8 in AML, we experimented with a strategy aimed at disrupting its receptor binding. Whereas past research focused on knocking-down or blocking CXCR2 activation in AML [15], we were cognizant of the fact that IL-8 is known to agonize both CXCR2 and CXCR1 receptors, both of which have been implicated in the progression of various cancers [44]. Thus, given IL-8’s receptor promiscuity, we sought to directly neutralize IL-8. However, our initial attempts to inhibit soluble IL-8 directly with antibodies did not prove successful (data not shown), likely due to the immense secretion of IL-8 by activated ECs. To overcome these obstacles, we carefully examined the IL-8 molecule with high-resolution crystallography and found an invagination within the protein representing a potential site wherein IL-8 physically interlocks with its receptors. Using high throughput computational methods and docking studies, we identified a series of small molecules (19 in total) that best fit inside within the IL-8 binding pocket. We then used in vitro assays to rank-order the compounds based on their ability to reduce AML cell proliferation. Interestingly, the best hit compound, NCI34255, displayed dual effects where treatment not only decreased AML proliferation but also enhanced AML cytotoxicity in the presence of Ara-C.
To mechanistically understand these findings, we investigated the Akt pathway, which is activated by IL-8 and has known effects on AML [33]. We initially found that AML cells treated with NCI34255 displayed a significant decrease in phosphorylated Akt at serine 473 (fully activated Akt [45]) and a concomitant decrease in phosphorylated 4E-BP1. In this state, 4E-BP1 is able to bind eIF-4E and prevent cell proliferation through inhibition of numerous proteins such as c-myc, MMP9, Bcl-2, and Mcl-1 [34, 46, 47]. These observations provide a mechanistic explanation for the decrease in AML cell proliferation upon treatment with NCI34255.
Akt has also been shown to directly prevent apoptosis by inhibiting pro-apoptotic molecules such as Bax, while enabling the function of anti-apoptotic proteins [48, 49]. Interestingly, analysis of Bax protein expression demonstrated that NCI34255 treatment produced a cleaved version of Bax. Several studies have shown that Bax can undergo cleavage via calpains to form an 18 kDa truncated version of Bax (p18Bax) [36, 50]. p18Bax has been shown to be more potent than full-length Bax (21 kDa) in the induction of apoptosis in part through increased efflux of cytochrome c [36]. Through such mechanisms, p18Bax enhances sensitivity of cancer cells to chemotherapy by essentially preparing the cells to enter apoptosis, a phenomenon referred to as mitochondrial priming [51, 52]. The observed increase in p18Bax levels in NCI34255 treated AML cells intimates that IL-8 inhibition ‘primes’ AML cells for apoptosis thus explaining why the combination of NCI34255 and Ara-C resulted in higher cell death in comparison to Ara-C alone. Surprisingly, we also observed that the level of total Bax was not significantly affected by NCI34255 treatment, even with IL-8 blockade and decreased Akt activity. This could be due to the milieu of other soluble factors produced in the activated EC co-cultures that may affect Bax. For example, activated ECs are known to produce high levels of the pro-inflammatory factor IL-6 [10]. Since IL-6 has been shown to affect Bax expression in various cell types through JAK/STAT [53, 54], we speculate that signaling through alternate pathways may have prevented observable changes in total Bax expression in response to NCI34255 treatment. Interestingly, maintenance of Bax expression may have allowed for the observed formation of truncated p18Bax. These results support the conclusion that inhibition of Akt pathway constituents using NCI34255 overcomes the AML promotive effects of IL-8 resulting in decreased cellular proliferation and enhancing sensitivity to chemotherapy (Figure 5A).
Figure 5.
(A) Schematic summarizing observed effects of NCI34255 on Akt induced proliferation and survival of AML cells. Treatment with NCI34255 reduces the effects of EC activation through Akt signaling (blue lines) resulting in decreased proliferation and enhanced apoptosis (in the presence of Ara-C) of AML cells. (B) The impact of EC activation on adherent and non-adherent AML cell populations is shown. Adherent cells become quiescent and chemoresistant implicating them in relapse. Non-adherent cells show significant expansion and chemoresistance following EC activation identifying them as the cellular source for enhanced leukemia cellularity. Therapies aimed at targeting this process may provide new avenues for the optimal treatment of patients with AML.
There is evidence that IL-8 blockade may also be useful for the treatment of specific AML subpopulations. Recently, the FDA has approved new drugs that target AML gene mutations IDH1 and IDH2. Mutations in IDH1/2 produce the oncometabolite R-2-hydroxyglutarate (R-2HG), which enhances NF-kB-dependent expression of IL-8 [55]. Thus, it is possible that IDH1/2 mutant AML may be more responsive to anti-IL-8 therapeutic strategies, including the strategy we demonstrate in this study. Interestingly, among AML patients, those with FLT3-ITD mutations have the highest IL-8 mRNA expression [42]. High IL-8 gene expression is also a prognostic marker of inferior survival outcomes in AML patients with FLT3-ITD. Thus, it is also conceivable that an anti-IL-8 treatment strategy, may be well suited for the treatment of the AML FLT3-mutant subpopulation.
In concert with our previous studies, we can now postulate a mechanism wherein EC activation governs the proliferation, survival and relapse of AML (Figure 5B). Here, AML cells initiate EC activation. Activated-ECs then induce the adhesion of a population of leukemia cells, which become quiescent and chemoresistant identifying these cells as potential mediators of relapse. The present results add a second scenario wherein activated ECs also produce soluble factors (IL-8) that enhance the proliferation and chemoresistance of non-adherent leukemia cells resulting in increased AML cellularity. Therefore, EC activation acts as a double-edged sword by generating microenvironments that support chemoresistance and relapse while concomitantly enhancing proliferation and growth. Now that we have identified IL-8 as a reactive cytokine from activated ECs and a cytokine that can be pharmacologically targeted, our next effort will be to scale the system to primary AML cells that secrete IL-8. A follow-up question will be whether the additional mass of IL-8 produced by both activated ECs and AML cells responds in a dose-dependent manner.
Overall, these findings identify EC activation-based processes as potential targets for the development of next generation therapies. In this context, the present results show that activated EC-generated IL-8 is an essential component in AML and directly blocking IL-8 may be a new therapeutic strategy to enhance AML patient outcomes.
Supplementary Material
HIGHLIGHTS.
AML cells activate endothelial cells (ECs) resulting in secretion of IL-8
Activated EC-derived IL-8 results in expansion and chemoresistance of AML cells
Inhibition of IL-8 decreases AML proliferation and enhances response to therapy
Effects of IL-8 inhibition are through Akt signaling pathways
5.0. ACKNOWLEDGEMENTS
NIH funding was provided to GJM (R15CA182889-01). CRC received funding from the Leukemia & Lymphoma Society, the Spanier Stem Cell Medicine Research and Education Foundation, the Harry T. Mangurian Foundation, and a Gatorade Foundation grant administered by the University of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 REFERENCES
- [1].Tallman MS, Gilliland DG, Rowe JM, Drug therapy for acute myeloid leukemia, Blood 106(4) (2005) 1154–63. [DOI] [PubMed] [Google Scholar]
- [2].Guerrouahen BS, Al-Hijji I, Tabrizi AR, Osteoblastic and vascular endothelial niches, their control on normal hematopoietic stem cells, and their consequences on the development of leukemia, Stem Cells Int 2011 (2011) 375857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Doan PL, Chute JP, The vascular niche: home for normal and malignant hematopoietic stem cells, Leukemia 26(1) (2012) 54–62. [DOI] [PubMed] [Google Scholar]
- [4].Kiel MJ, Radice GL, Morrison SJ, Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance, Cell Stem Cell 1(2) (2007) 204–17. [DOI] [PubMed] [Google Scholar]
- [5].Cogle CR, Goldman DC, Madlambayan GJ, Leon RP, Al Masri A, Clark HA, Asbaghi SA, Tyner JW, Dunlap J, Fan G, Kovacsovics T, Liu Q, Meacham A, Hamlin KL, Hromas RA, Scott EW, Fleming WH, Functional integration of acute myeloid leukemia into the vascular niche, Leukemia 28(10) (2014) 1978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Madlambayan GJ, Meacham AM, Hosaka K, Mir S, Jorgensen M, Scott EW, Siemann DW, Cogle CR, Leukemia regression by vascular disruption and antiangiogenic therapy, Blood 116(9) (2010) 1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pezeshkian B, Donnelly C, Tamburo K, Geddes T, Madlambayan GJ, Leukemia Mediated Endothelial Cell Activation Modulates Leukemia Cell Susceptibility to Chemotherapy through a Positive Feedback Loop Mechanism, PloS one 8(4) (2013) e60823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hatfield K, Ryningen A, Corbascio M, Bruserud O, Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts, Int J Cancer 119(10) (2006) 2313–21. [DOI] [PubMed] [Google Scholar]
- [9].Fielder PJ, Hass P, Nagel M, Stefanich E, Widmer R, Bennett GL, Keller GA, de Sauvage FJ, Eaton D, Human platelets as a model for the binding and degradation of thrombopoietin, Blood 89(8) (1997) 2782–8. [PubMed] [Google Scholar]
- [10].Zhang J, Defelice AF, Hanig JP, Colatsky T, Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury, Toxicol Pathol 38(6) (2010) 856–71. [DOI] [PubMed] [Google Scholar]
- [11].Ley K, Laudanna C, Cybulsky MI, Nourshargh S, Getting to the site of inflammation: the leukocyte adhesion cascade updated, Nat Rev Immunol 7(9) (2007) 678–89. [DOI] [PubMed] [Google Scholar]
- [12].Bruserud O, Glenjen N, Ryningen A, Effects of angiogenic regulators on in vitro proliferation and cytokine secretion by native human acute myelogenous leukemia blasts, Eur J Haematol 71(1) (2003) 9–17. [DOI] [PubMed] [Google Scholar]
- [13].Reikvam H, Hatfield KJ, Fredly H, Nepstad I, Mosevoll KA, Bruserud O, The angioregulatory cytokine network in human acute myeloid leukemia - from leukemogenesis via remission induction to stem cell transplantation, Eur Cytokine Netw 23(4) (2012) 140–53. [DOI] [PubMed] [Google Scholar]
- [14].Negaard HF, Iversen N, Bowitz-Lothe IM, Sandset PM, Steinsvik B, Ostenstad B, Iversen PO, Increased bone marrow microvascular density in haematological malignancies is associated with differential regulation of angiogenic factors, Leukemia 23(1) (2009) 162–9. [DOI] [PubMed] [Google Scholar]
- [15].Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L, Bhagat T, Bhattacharyya S, Ramachandra N, Bartenstein M, Pellagatti A, Boultwood J, Wickrema A, Yu Y, Will B, Wei S, Steidl U, Verma A, IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells, Blood 125(20) (2015) 3144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li H, Wang D, Xu A, Li S, Jin S, Wu D, High level expression and purification of active recombinant human interleukin-8 in Pichia pastoris, Protein Expr Purif 68(1) (2009) 60–4. [DOI] [PubMed] [Google Scholar]
- [17].Hebert CA, Vitangcol RV, Baker JB, Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding, J Biol Chem 266(28) (1991) 18989–94. [PubMed] [Google Scholar]
- [18].Stucki A, Rivier AS, Gikic M, Monai N, Schapira M, Spertini O, Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination, Blood 97(7) (2001) 2121–9. [DOI] [PubMed] [Google Scholar]
- [19].Russell NH, Autocrine growth factors and leukaemic haemopoiesis, Blood Rev 6(3) (1992) 149–56. [DOI] [PubMed] [Google Scholar]
- [20].Hsu HC, Lee YM, Tsai WH, Jiang ML, Ho CH, Ho CK, Wang SY, Circulating levels of thrombopoietic and inflammatory cytokines in patients with acute myeloblastic leukemia and myelodysplastic syndrome, Oncology 63(1) (2002) 64–9. [DOI] [PubMed] [Google Scholar]
- [21].Elbaz O, Shaltout A, Implication of Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) and Interleukin-3 (IL-3) in Children with Acute Myeloid Leukaemia (AML); Malignancy, Hematology 5(5) (2001) 383–388. [PubMed] [Google Scholar]
- [22].Tao M, Li B, Nayini J, Andrews CB, Huang RW, Devemy E, Song S, Venugopal P, Preisler HD, SCF, IL-1beta, IL-1ra and GM-CSF in the bone marrow and serum of normal individuals and of AML and CML patients, Cytokine 12(6) (2000) 699–707. [DOI] [PubMed] [Google Scholar]
- [23].Tsimberidou AM, Estey E, Wen S, Pierce S, Kantarjian H, Albitar M, Kurzrock R, The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes, Cancer 113(7) (2008) 1605–13. [DOI] [PubMed] [Google Scholar]
- [24].Hatfield KJ, Hovland R, Oyan AM, Kalland KH, Ryningen A, Gjertsen BT, Bruserud O, Release of angiopoietin-1 by primary human acute myelogenous leukemia cells is associated with mutations of nucleophosmin, increased by bone marrow stromal cells and possibly antagonized by high systemic angiopoietin-2 levels, Leukemia 22(2) (2008) 287–93. [DOI] [PubMed] [Google Scholar]
- [25].Sutherland MK, Yu C, Lewis TS, Miyamoto JB, Morris-Tilden CA, Jonas M, Sutherland J, Nesterova A, Gerber HP, Sievers EL, Grewal IS, Law CL, Anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia, MAbs 1(5) (2009) 481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Steube KG, Meyer C, Drexler HG, Induction and secretion of the chemokines interleukin-8 and monocyte chemotactic protein-1 in human immature leukemia cell lines, Mol Cell Biol Res Commun 3(1) (2000) 60–5. [DOI] [PubMed] [Google Scholar]
- [27].Ayala F, Dewar R, Kieran M, Kalluri R, Contribution of bone microenvironment to leukemogenesis and leukemia progression, Leukemia 23(12) (2009) 2233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hussong JW, Rodgers GM, Shami PJ, Evidence of increased angiogenesis in patients with acute myeloid leukemia, Blood 95(1) (2000) 309–13. [PubMed] [Google Scholar]
- [29].Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE, Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients, Ann Oncol 15(1) (2004) 139–45. [DOI] [PubMed] [Google Scholar]
- [30].Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F, Resting and activated endothelial cells are increased in the peripheral blood of cancer patients, Blood 97(11) (2001) 3658–61. [DOI] [PubMed] [Google Scholar]
- [31].Rigolin GM, Mauro E, Ciccone M, Fraulini C, Sofritti O, Castoldi G, Cuneo A, Neoplastic circulating endothelial-like cells in patients with acute myeloid leukaemia, Eur J Haematol 78(5) (2007) 365–73. [DOI] [PubMed] [Google Scholar]
- [32].Waugh DJ, Wilson C, The interleukin-8 pathway in cancer, Clin Cancer Res 14(21) (2008) 6735–41. [DOI] [PubMed] [Google Scholar]
- [33].Deng L, Jiang L, Lin XH, Tseng KF, Liu Y, Zhang X, Dong RH, Lu ZG, Wang XJ, The PI3K/mTOR dual inhibitor BEZ235 suppresses proliferation and migration and reverses multidrug resistance in acute myeloid leukemia, Acta Pharmacol Sin 38(3) (2017) 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herschbein L, Liesveld JL, Dueling for dual inhibition: Means to enhance effectiveness of PI3K/Akt/mTOR inhibitors in AML, Blood Rev 32(3) (2018) 235–248. [DOI] [PubMed] [Google Scholar]
- [35].Yamaguchi H, Wang HG, The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change, Oncogene 20(53) (2001) 7779–86. [DOI] [PubMed] [Google Scholar]
- [36].Cao X, Deng X, May WS, Cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and a cathepsin-like protease may rapidly degrade p18 Bax, Blood 102(7) (2003) 2605–14. [DOI] [PubMed] [Google Scholar]
- [37].Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC, Oh YJ, Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2, J Neurochem 77(6) (2001) 1531–41. [DOI] [PubMed] [Google Scholar]
- [38].Tobler A, Moser B, Dewald B, Geiser T, Studer H, Baggiolini M, Fey MF, Constitutive expression of interleukin-8 and its receptor in human myeloid and lymphoid leukemia, Blood 82(8) (1993) 2517–25. [PubMed] [Google Scholar]
- [39].Bruserud O, Ryningen A, Wergeland L, Glenjen NI, Gjertsen BT, Osteoblasts increase proliferation and release of pro-angiogenic interleukin 8 by native human acute myelogenous leukemia blasts, Haematologica 89(4) (2004) 391–402. [PubMed] [Google Scholar]
- [40].Padro T, Ruiz S, Bieker R, Burger H, Steins M, Kienast J, Buchner T, Berdel WE, Mesters RM, Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia, Blood 95(8) (2000) 2637–44. [PubMed] [Google Scholar]
- [41].Aguayo A, Estey E, Kantarjian H, Mansouri T, Gidel C, Keating M, Giles F, Estrov Z, Barlogie M Albitar, Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia, Blood 94(11) (1999) 3717–21. [PubMed] [Google Scholar]
- [42].Kuett A, Rieger C, Perathoner D, Herold T, Wagner M, Sironi S, Sotlar K, Horny HP, Deniffel H Drolle M Fiegl, IL-8 as mediator in the microenvironment-leukaemia network in acute myeloid leukaemia, Sci Rep 5 (2015) 18411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, Perez RP, Kato G, Wicha M, Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer, Clin Cancer Res 23(18) (2017)5358–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K, The CXCL8-CXCR1/2 pathways in cancer, Cytokine Growth Factor Rev 31 (2016) 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Manning BD, Toker A, AKT/PKB Signaling: Navigating the Network, Cell 169(3) (2017) 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J, RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation, J Biol Chem 282(19) (2007) 14056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hsieh AC, Ruggero D, Targeting eukaryotic translation initiation factor 4E (eIF4E) in cancer, Clin Cancer Res 16(20) (2010) 4914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H, Ezzat A, Bavi P, Al-Kuraya KS, Role of phosphatidylinositol 3'-kinase/AKT pathway in diffuse large B-cell lymphoma survival, Blood 108(13) (2006) 4178–86. [DOI] [PubMed] [Google Scholar]
- [49].Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N, Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak, Mol Cell 16(5) (2004) 819–30. [DOI] [PubMed] [Google Scholar]
- [50].Wood DE, Newcomb EW, Caspase-dependent activation of calpain during drug-induced apoptosis, J Biol Chem 274(12) (1999) 8309–15. [DOI] [PubMed] [Google Scholar]
- [51].Gao G, Dou QP, N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death, J Cell Biochem 80(1) (2000) 53–72. [DOI] [PubMed] [Google Scholar]
- [52].Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, Deangelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A, Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy, Science 334(6059) (2011) 1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kaminski KA, Kozuch M, Bonda TA, Stepaniuk MM, Waszkiewicz E, Chyczewski L, Musial WJ, Winnicka MM, Effect of interleukin 6 deficiency on the expression of Bcl-2 and Bax in the murine heart, Pharmacol Rep 61(3) (2009) 504–13. [DOI] [PubMed] [Google Scholar]
- [54].Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA, Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs, Am J Respir Cell Mol Biol 29(4) (2003) 490–8. [DOI] [PubMed] [Google Scholar]
- [55].Chen JY, Lai YS, Tsai HJ, Kuo CC, Yen BL, Yeh SP, Sun HS, Hung WC, The oncometabolite R-2-hydroxyglutarate activates NF-kappaB-dependent tumor-promoting stromal niche for acute myeloid leukemia cells, Sci Rep 6 (2016) 32428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.