Abstract
Background
Shift work has been associated with increased risk of age-related morbidity and mortality. Biological age, estimated using DNA methylation (DNAm), may quantify the biological consequences of shift work on the risk of age-related disease. We examined whether prior employment in shift-working occupations was associated with epigenetic age acceleration.
Methods
In a sample of non-Hispanic White women aged 35–74 (n = 2574), we measured DNAm using the Illumina Infinium Human450 BeadChip and calculated DNAm age using three established epigenetic clocks. Age-acceleration metrics were derived by regressing DNAm age on chronological age and predicting the residuals. Using linear regression, we estimated associations between shift work history and age acceleration. We also conducted an epigenome-wide association study using robust linear-regression models corrected with false discovery rate (FDR) q-values.
Results
Approximately 7% of women reported any shift work. Higher age acceleration was observed for a 1-year increase in overall [β = 0.11, 95% confidence interval (CI): 0.02–0.21] and night-specific shift work (β = 0.12, 95% CI: 0.03–0.21). The association was strongest for ≥10 years of night shift work (β = 3.16, 95% CI: 1.17–5.15). From the epigenome-wide association study, years of overall and night shift work were associated with DNAm at 66 and 85 CpG sites (FDR < 0.05), respectively. Years of night shift work was associated with lower methylation of a CpG in the gene body of ZFHX3 (cg04994202, q = 0.04), a gene related to circadian rhythm.
Conclusions
Shift work was associated with differential CpG site methylation and with differential DNAm patterns, measured by epigenetic age acceleration, consistent with long-term negative health effects.
Keywords: Shift-work schedule, DNA methylation, epigenomics, ageing, circadian rhythm
Key Messages
Night shift work and irregular hours are increasingly common in modern occupations and are known to be related to adverse health effects.
Working more than 10 years of shift work was associated with increased epigenetic age acceleration, a marker of biologic ageing, which has been related to morbidity and mortality.
Shift work was also associated with differentially methylated CpG sites across the epigenome, including a CpG in the circadian rhythm gene ZFHX3.
Introduction
It is increasingly common for occupations to require work during hours outside of the usual work day.1 Shift work, especially work involving rotating shifts or night shifts, has been associated with higher mortality2,3 as well as a higher incidence of age-related diseases such as cardiovascular disease4 and cancer.5 Molecular biomarkers of age may serve as risk markers for these outcomes.6–11 Shift work-related biologic changes may further be reflected in DNA methylation (DNAm) patterns, which are relevant for future disease risk.
Epigenetic age estimators, or ‘epigenetic clocks’, are calculated using DNAm values from specific CpGs throughout the genome and have been proposed as potential markers representing ageing processes.12,13 The first generation of clocks, developed by Hannum et al.12 and Horvath,13 were designed to predict chronological age. Imperfect correlations with chronological age led to the hypothesis that the residual variation may represent age-related biological variation and both of these clocks have been used to predict mortality.14–17 A more recent epigenetic clock, by Levine et al.,18 was constructed to predict PhenoAge, a biological age metric derived from nine clinical parameters, and chronological age, to predict age-related mortality. Individuals with higher biological age estimates compared with their chronological age are said to be ‘age-accelerated’ and initial studies suggest age acceleration is associated with a number of age-related outcomes, including stroke,19 cardiovascular disease,20 cancer21–23 and death.14–17 There is some evidence to suggest that socio-economic status, diet, body size and cigarette smoking may predict epigenetic age acceleration24, 25; however, prior studies have not considered occupational exposure to shift work.
It is biologically plausible that shift work may result in increased age acceleration, as it has been associated with a higher risk of age-related diseases2–5 and has been previously found to be related to altered gene-specific DNAm.26–29 Therefore, the primary goal of this study was to evaluate the hypothesis that shift work is associated with epigenetic age acceleration. Using an epigenome-wide association study (EWAS), we also examined the broader hypothesis that shift work is associated with genome-wide DNAm.
Methods
Study population
The Sister Study is a nationwide prospective cohort study of 50 884 women that was designed to investigate environmental and lifestyle risk factors for breast cancer.30,31 During 2003–09, women aged 35–74 with a sister with breast cancer were recruited throughout the USA, including Puerto Rico. At the time of enrolment, participants donated a fasting blood sample and completed a two-part computer-assisted telephone interview. Participants are contacted annually and complete detailed follow-up questionnaires every 2–3 years. Questionnaires included information on demographics, lifestyle factors such as smoking and alcohol intake, and occupational history. The Sister Study was approved by the NIEHS Institutional Review Board and the Copernicus Group IRB. Written informed consent was obtained from all study participants.
A case–cohort design was used to select Sister Study participants for DNA methylation analysis. The sample was limited to non-Hispanic White women with an available baseline blood sample and included 2878 women (1542 incident cases that arose during follow-up and a subcohort composed of a random sample of 1336, 74 who were also incident cases). Women were breast cancer free at the time of blood draw. These data were from data release version 5.0.1.
Exposure classification
At baseline, women self-reported detailed information about their current job as well as all past jobs that lasted for 2 years or longer. The earliest women to enroll completed a survey that did not obtain necessary shift work details and were excluded (n = 149). Participants were defined as shift workers if they responded both (i) ‘no’ to whether they worked ‘regular hours, that is, starting and stopping work at about the same time every day’, and (ii) ‘yes’ to whether they worked ‘rotating shifts’. Women were defined as ever shift workers if they reported at least one job for which they performed shift work. Night shift workers were defined as women whose shift ever included at least 1 hour between midnight and 2 in the morning. Total years of overall shift and night-specific shift work were summed across jobs.
DNAm
Details regarding DNA-processing procedures were previously reported.32 Briefly, genomic DNA was extracted from aliquots of whole blood drawn at enrolment using an automated system (Autopure LS, Gentra Systems) in the NIEHS Molecular Genetics Core Facility or using DNAQuik at BioServe Biotechnologies LTD (Beltsville, MD). One microgram of DNA from each woman was bisulphite-converted in 96-well plates using the EZ DNA Methylation Kit (Zymo Research, Orange County, CA). Methylation analysis was carried out at the NIH Center for Inherited Disease Research at Johns Hopkins University (Baltimore, MD). Samples were tested for completion of bisulphite conversion and converted DNA was analysed on Illumina HumanMethylation450 BeadChip following the manufacturer’s protocol. The arrays were analysed with high-throughput robotics to minimize batch effects. Methylation data preprocessing and quality control were completed using the ENmix R software package33 and has been detailed previously.32 The final number of CpGs was 423 500. β-values for methylation were determined using the fluorescence intensities for unmethylated (U) and methylated (M) alleles as follows: β = M/(M + U + 100). The β-values were transformed on the logit scale to be M-values, which were used in all statistical tests. After exclusions, the final sample size was 2574 women (including 1449 cases).
Statistical analysis
We considered shift work and night shift work status (ever/never) and years of overall shift work and night-specific shift work as continuous variables and categorized (never, ≤5 years, 5.1–10 years, >10 years). All models were adjusted for a priori selected covariates including case status (invasive breast cancer or ductal carcinoma in situ, no breast cancer), age at baseline (years, continuous), alcohol consumption (drinks per week, continuous), education (high school or less, some college or technical school, college degree or more) and smoking status (current, former, never). Body mass index (BMI), which was ascertained at baseline, was not included as a covariate, as we hypothesized that it may be on the causal pathway. However, we conducted sensitivity analyses including BMI in the adjustment set. We also evaluated the impact of removing cigarette smoking status from the model, as it is plausible it could also be on the causal path. We conducted a sensitivity analysis excluding women who developed breast cancer during follow-up (mean = 5.4 years, standard deviation = 2.8 years).
For the age acceleration analysis, we first calculated DNAm age using R code provided from the manuscripts of the clock developers.12,13,18 For each of the three epigenetic clocks (i.e. Hannum, Horvath and Levine), age acceleration was calculated by regressing DNAm age on chronological age and predicting the residuals. By using this method of taking the residuals, the metrics of age acceleration are not associated with chronological age. These metrics can be either positive (epigenetic age is older than chronological age) or negative. We used linear regression to evaluate the relationship between shift working history and age acceleration. We estimated blood cell composition (BCC) using the Houseman method to control for difference in methylation by blood cell type.34 We report our results both unadjusted and adjusted for BCC; adjustment for BCC in the linear regression model is hypothesized to represent an intrinsic measure of ageing.14 In analyses adjusting for BCC, we excluded B-cell proportion to avoid model overfitting and non-convergence, as the blood-cell proportions add up to 1; B-cell proportion was not significantly associated with chronological age and had the smallest amount of variation within the study population. We excluded any participants if the absolute value of their age-acceleration estimate was greater than four standard deviations from the mean (n = 10).
For the EWAS, to examine associations between shift work and CpG site DNAm, we conducted robust linear regression. We adjusted for the same confounders listed above, as well as BCC, technical variation and batch. To correct for multiple testing, we estimated the false discovery rate (FDR).35 We considered whether any of the genes identified to have statistically significant CpG sites in relation to shift work were associated with circadian rhythm using gene ontology (GO: 0007623). We considered whether any of the observed statistically significant CpGs overlapped with those used to calculate the epigenetic age measures. We determined statistical significance at q < 0.05. All analyses were conducted using Stata (version 14.2, College Station) and R.
Results
Shift work was reported by 175 women (7%), with 120 (5%) reporting night shift work (Table 1 ). Among shift workers, the median duration of shift work was approximately 5 years, with 22% working more than 10 years. Shift workers and non-shift workers were similar in age at blood draw but shift workers were slightly more likely to have higher educational attainment and were more likely to be current smokers.
Table 1.
Study participant baseline characteristics by shift-working status, Sister Study 2003–09
| Ever shift work |
||||
|---|---|---|---|---|
| No (N = 2399) |
Yes (N = 175) |
|||
| N | (%) | N | (%) | |
| Age at time of blood draw, years | ||||
| ≤45 | 218 | 9 | 22 | 13 |
| 46–55 | 760 | 32 | 63 | 36 |
| 56–65 | 924 | 39 | 56 | 32 |
| >65 | 497 | 21 | 34 | 19 |
| Education | ||||
| < High-school degree or equivalent | 384 | 16 | 14 | 8 |
| Some college/technical school | 755 | 31 | 62 | 35 |
| College or post-college grad | 1260 | 53 | 99 | 57 |
| Alcohol intake (drinks/week) | ||||
| None | 394 | 16 | 27 | 15 |
| ≤1 | 901 | 38 | 69 | 39 |
| 1.1–4 drinks | 553 | 23 | 37 | 21 |
| ≥5 drinks | 551 | 23 | 42 | 24 |
| Smoking status | ||||
| Never | 1262 | 53 | 83 | 47 |
| Past | 969 | 40 | 75 | 43 |
| Current | 168 | 7 | 17 | 10 |
| Years of any shift work | ||||
| 0 years | 2399 | 100 | ||
| ≤5 years | 86 | 49 | ||
| 5.1–10 years | 50 | 29 | ||
| >10 years | 39 | 22 | ||
| Ever night-shift work | ||||
| Never | 2399 | 100 | 55 | 31 |
| Ever | 120 | 69 | ||
| Years of night-shift work | ||||
| 0 years | 2399 | 100 | 55 | 31 |
| ≤5 years | 59 | 34 | ||
| 5.1–10 years | 29 | 17 | ||
| >10 years | 32 | 18 | ||
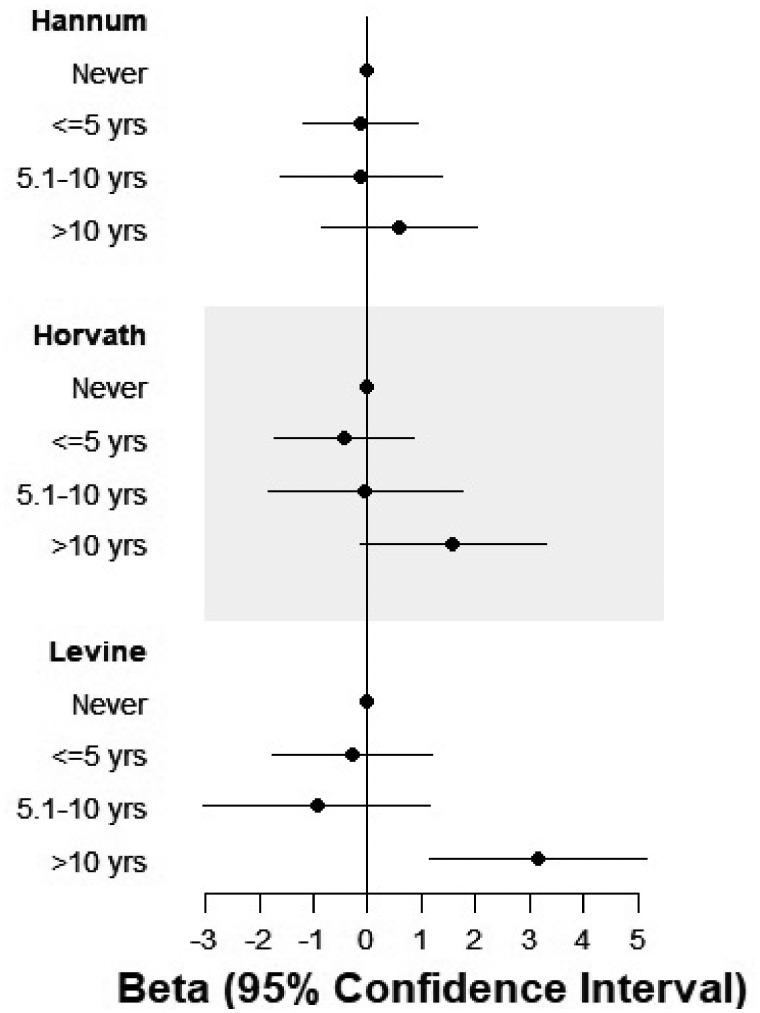
Using the Levine clock,18 years of both overall and night-specific shift work were associated with increased age acceleration (Table 2 ). The β-values presented here represent the mean difference in age acceleration in years. For example, for each year of overall shift work, we observed epigenetic age acceleration equivalent to 1.2 months [β = 0.09, 95% confidence interval (CI): 0.01–0.18, p = 0.02]. This association was stronger for each year of night shift work (β = 0.12, 95% CI: 0.03–0.21, p = 0.008). Using the Levine clock, we found that those who worked more than 10 years in shift working or night shift occupations showed the greatest amount of age acceleration (overall shift work: β = 2.36, 95% CI: 0.56–4.17, p = 0.01; night shift work β = 3.16, 95% CI: 1.17–5.15, p = 0.002). Inclusion of the a priori covariates in our statistical model resulted in point estimates that were similar to the unadjusted estimates (data not shown), although the results were slightly strengthened after adjustment for BCC (shown in Table 2). Using the Hannum and Horvath clocks, we also observed positive associations with age acceleration in relation to 10 or more years of shift work, although estimates were attenuated and CIs included the null value. Across all three clocks, there was no evidence of age acceleration for women who experienced shift work for fewer than 10 years (Figure 1 ). In sensitivity analyses, we considered statistical models with and without adjustment for breast cancer incidence and limited to women who were not diagnosed with breast cancer during the follow-up period. Results for years of shift work and night shift work were similar in an analysis limited to women who remained breast cancer free during follow-up (Supplementary Table 1, available as Supplementary data at IJE online). Including BMI in the adjustment set did not appreciably change the results and neither did removing smoking status from the model (data not shown).
Table 2.
Shift work and epigenetic age-acceleration metrics,a Sister Study 2003–09
| Hannum (2013) |
Horvath (2013) |
Levine (2018) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Unadjusted for BCC | Adjusted for BCC | Unadjusted for BCC | Adjusted for BCC | Unadjusted for BCC | Adjusted for BCC | |
| Shift work | ||||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Ever | 0.28 (–0.41, 0.98) | 0.30 (–0.33, 0.93) | 0.27 (–0.50, 1.04) | 0.35 (–0.41, 1.10) | 0.20 (–0.74, 1.14) | 0.34 (–0.54, 1.22) |
| Duration | ||||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≤5 years | 0.61 (–0.36, 1.59) | 0.59 (–0.29, 1.47) | 0.54 (–0.54, 1.62) | 0.60 (–0.46, 1.66) | –0.02 (–1.33, 1.29) | 0.12 (–1.11, 1.35) |
| 5.1–10 years | –0.42 (–1.69, 0.85) | –0.27 (–1.41, 0.88) | –0.85 (–2.26, 0.55) | –0.79 (–2.17, 0.59) | –0.95 (–2.66, 0.75) | –0.86 (–2.46, 0.74) |
| >10 years | 0.46 (–0.98, 1.89) | 0.40 (–0.89, 1.69) | 1.10 (–0.49, 2.69) | 1.25 (–0.31, 2.81) | 2.16 (0.23, 4.09) | 2.36 (0.56, 4.17) |
| Per year of shift work | 0.02 (–0.05, 0.08) | 0.02 (–0.04, 0.08) | 0.04 (–0.03, 0.12) | 0.05 (–0.02, 0.12) | 0.08 (0.00, 0.17) | 0.09 (0.01, 0.18) |
| Night shift work | ||||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Ever | –0.03 (–0.87, 0.80) | 0.07 (–0.68, 0.82) | 0.07 (–0.85, 1.00) | 0.20 (–0.70, 1.10) | 0.33 (–0.79, 1.45) | 0.48 (–0.57, 1.53) |
| Duration | ||||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≤5 years | –0.37 (–1.54, 0.80) | –0.12 (–1.18, 0.94) | –0.63 (–1.93, 0.67) | –0.44 (–1.71, 0.84) | –0.61 (–2.18, 0.97) | –0.28 (–1.75, 1.20) |
| 5.1–10 years | –0.14 (–1.80, 1.52) | –0.12 (–1.61, 1.38) | –0.03 (–1.87, 1.81) | –0.05 (–1.85, 1.75) | –0.73 (–2.96, 1.50) | –0.94 (–3.03, 1.15) |
| >10 years | 0.68 (–0.90, 2.27) | 0.59 (–0.83, 2.01) | 1.46 (–0.29, 3.20) | 1.59 (–0.13, 3.30) | 3.01 (0.88, 5.14) | 3.16 (1.17, 5.15) |
| Per year of night-shift work | 0.02 (–0.05, 0.09) | 0.02 (–0.04, 0.09) | 0.06 (–0.02, 0.13) | 0.06 (–0.01, 0.14) | 0.11 (0.02, 0.21) | 0.12 (0.03, 0.21) |
aEstimates derived from linear regression for the association between shift-working history and age acceleration, adjusted for breast-cancer status at follow-up (yes/no), and age (continuous), alcohol intake (continuous), smoking status (current/former/never) and education at enrolment (high-school degree or less/some college/bachelor’s or higher).
Figure 1.
Years of night shift work and epigenetic age acceleration estimates with adjustment for blood cell composition. Estimates derived from linear regression for the association between shift working history and age acceleration, adjusted for breast cancer status at follow-up (yes/no), and age (continuous), alcohol intake (continuous), smoking status (current/former/never) and education at enrolment (high-school degree or less/some college/bachelor’s or higher).
In the EWAS analysis, we observed 85 CpGs significantly associated with years of night-shift work (q < 0.05) and 66 CpGs significantly associated with years of overall shift work (q < 0.05) (Supplementary Figures 1 and 2 and Supplementary Tables 2 and 3, available as Supplementary data at IJE online). There were 36 CpGs significantly associated with both years of night shift work and years of overall shift work (q < 0.05) (Supplementary Table 4, available as Supplementary data at IJE online). In general, shift work was related to decreased methylation (72% of sites associated with night shift work and 68% of sites associated with overall shift work). No significant CpGs were observed in comparisons of ever vs never shift workers. The CpG with the lowest p-value for both overall and night specific shift work was in the gene body of ATXN7 (cg03997418). Higher methylation at this site was associated with shift work (β = 0.005 per year of night-shift work, p = 7.04 × 10–8 and q = 0.013).
We evaluated whether any of the significant CpGs included known circadian genes and identified ZFHX3 (cg04994202),36 which had significantly lower methylation with increasing years of night-shift work (β = –0.003, q = 0.03). Both of these CpG sites remained statistically significant when the analysis was limited to women who did not go on to develop breast cancer during the follow-up period. There was no overlap between the CpG sites identified from the EWAS and the sites used to calculate any of the epigenetic age metrics.
Discussion
We are the first to evaluate the association between epigenetic age acceleration and a history of occupational shift work. We observed that a history of shift work was associated with age acceleration as defined by the Levine epigenetic clock. The association was most notable for women who worked more than 10 years at a job involving any night shift work; these women had an estimated epigenetic age on average 3 years older than those who never participated in shift work. Although we also observed suggestive evidence of higher age acceleration in relation to >10 years of night shift work with the Horvath clock, these associations were not as apparent for the Hannum clock. In our EWAS, we observed several differentially methylated CpG sites in association with both years of overall and night-specific shift work. Our findings suggest that occupational shift work exposure may lead to a range of modifications in DNAm, including changes at specific CpG sites and epigenetic age.
Although there is growing evidence that epigenetic age acceleration is associated with negative health outcomes,6–11 little is known about how environment and lifestyle factors impact age acceleration. Shift work has been associated with increased risk of chronic disease4,5,37 and elevated death rates.2,3 The negative impacts of shift work are hypothesized to occur via multiple, complex mechanisms including disruption of circadian rhythms including altered sleep patterns.38 Shift working is also correlated with negative health behaviours such as obesity, increased calorie consumption and cigarette smoking.37 In the Women’s Health Initiative, symptoms of insomnia were associated with age acceleration defined using the Hannum clock, but not the Horvath clock.39 With our finding of an association between shift work and age acceleration, together these studies suggest that sleep disruption, which is common to both shift work and insomnia, may negatively impact biologic age.
Working more than 10 years of shift work, especially at night, was consistently associated with age acceleration across all three epigenetic clocks. However, our findings were strongest using the Levine clock,18 which was developed to predict PhenoAge, a biological age estimator that predicts age-related mortality. Our findings suggest that the clinical markers incorporated in the Levine epigenetic clock may better correlate with the deleterious health effects resulting from occupational shift work. The Levine clock was estimated using information on clinical parameters, including albumin, glucose and C-reactive protein, which have all been individually related to shift working status.40–42
We also observed that years of shift work was associated with methylation at a number of CpG sites across the genome independently of those used to calculate the epigenetic clocks. Our top CpG site in relation to both overall and night-specific shift work was in the gene Ataxin 7 (ATXN7). We also observed an association between night-shift work and a CpG in the circadian gene ZFHX3.36ATXN7 encodes a protein that participates in transcriptional regulation and has been found to be associated with susceptibility to and prognosis of tumours,43,44 including breast cancer.45ZFHX3 encodes a protein that, in addition to acting in circadian rhythm pathways, may function as a tumour suppressor by inhibiting oestrogen receptor function.46 Studies have previously shown that shift work, especially long-term night shift work, is associated with the risk of breast cancer,47 supporting a possible role for both ZFHX3 and ATXN7 in the shift work–breast cancer relationship. Both of these CpGs remained statistically significant when we restricted the analysis to those who did not go on to develop breast cancer during the follow-up period, suggesting these findings were not being driven by undiagnosed breast cancer.
Previous studies have also found evidence to suggest that shift work as well as acute sleep deprivation can influence DNAm. Shift workers had lower levels of CLOCK gene methylation levels and higher levels of CRY2 when using a genome-wide approach.29 Length of shift work has also been associated with methylation in the glucocorticoid receptor.48 Acute sleep deprivation altered the DNAm expression of circadian genes49 and genome-wide methylation.50,51 We did not observe any of these previously identified circadian genes to be statistically significant CpG sites (q < 0.05) in our study population, although we did observe one CLOCK CpG (cg25530661) to be related to night shift work (uncorrected p = 0.01).
Our population was limited to non-Hispanic White women who, by enrolment criteria of the parent study population, all have a family history of breast cancer. Although this would not likely alter the validity of our results, the associations reported here may not be applicable to all women. A benefit of our design is that we may have increased power to detect altered methylation at CpG sites relevant to breast cancer risk. Although long-term history of occupational shift work was assessed at the same time as the baseline blood sample collection, our findings were most notable for women who had worked shifts for greater than 10 years, suggesting that reverse causation is unlikely. Whereas we controlled for covariates we hypothesized to be related to both shift working history and methylation, there is the possibility that unmeasured confounding may affect our results. Our study participants recalled their shift working history at time of enrolment in the study. Although this may result in the possibility of misclassification, we only collected information on current jobs and any past jobs that lasted at least 2 years in order to maximize recall. Although we were not well powered to evaluate the impact of different scheduling, age acceleration may provide a useful intermediate marker to assess whether scheduling or other adjustments to rotating shifts can mitigate risks.
Blood is a convenient tissue to sample, but there may be different ageing profiles in different tissues. The Horvath clock was specifically designed to be robust across multiple tissue types so that findings in blood should reflect those in other target tissues. But the Levine and Hannum clocks were designed for blood and, although they are correlated with the Horvath clock, may be less predictive of age-related changes in other tissues.
The women in this study population were free of breast cancer at the time of blood draw. However, some women were selected based on their case status during follow-up using a nested case–cohort design. To address this, we conducted a number of sensitivity analyses including analyses excluding breast cancer cases and considering the impact of including a variable for breast cancer case status in our statistical models. These sensitivity analyses did not alter the conclusions and therefore we chose to have all study participants remain in the study, adjusting for case status, to maximize our statistical power.
This study was the first to evaluate the relationship between shift work and age acceleration. Our study population had detailed assessment of occupational shift working history. Another strength of this study was the inclusion of multiple epigenetic clocks to evaluate age acceleration as well as an EWAS component to consider the impact of shift work on the epigenome more globally. The use of multiple epigenetic clocks is important, as prior studies have shown that associations with metabolic and inflammatory markers,52 cigarette smoking, socio-economic status18,25 and insomnia39 have not been consistent across clocks. The Levine clock has tended to show some stronger associations, which may be due to the inclusion of other mortality-associated serum biomarkers in the development of their predictive model.
In conclusion, these results support an association between shift work and epigenetic changes, observed for both age acceleration as well as more widely in DNAm patterns across the epigenome. This is an important occupational health issue as irregular work schedules become increasingly more prevalent1 and shift work has a number of established adverse health effects.2–5 A better understanding of the intermediate markers of disease-related risk, including DNAm, related to occupational exposures such as shift work may provide opportunities for intervention in the future. This study provides a quantitative assessment of the deleterious potential impact of shift work on longevity and health.
Funding
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005, Z01-ES049033, Z01-ES049032).
Conflict of interest: None declared.
Supplementary Material
References
- 1. Beers TM. Flexible schedules and shift work: replacing the 9-to-5 workday. Monthly Lab Rev 2000;123:33. [Google Scholar]
- 2. Gu F, Han J, Laden F, et al. Total and cause-specific mortality of US nurses working rotating night shifts. Am J Prevent Med 2015;48:241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jørgensen JT, Karlsen S, Stayner L, Hansen J, Andersen ZJ. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scand J Work Environ Health 2017;43:117–26. [DOI] [PubMed] [Google Scholar]
- 4. Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cherrie JW, Crawford JO, Davis A, et al. A review of the impact of shift-work on cancer: summary of the evidence for practitioners. Pol Pract Health Saf 2018;16:145–51. [Google Scholar]
- 6. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev 2006;127:240–48. [DOI] [PubMed] [Google Scholar]
- 7. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci 2013;68:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193–98. [DOI] [PubMed] [Google Scholar]
- 9. Holly AC, Melzer D, Pilling LC, et al. Towards a gene expression biomarker set for human biological age. Aging Cell 2013;12:324–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters MJ, Joehanes R, Pilling LC, et al. The transcriptional landscape of age in human peripheral blood. Nat Commun 2015;6:8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hertel J, Friedrich N, Wittfeld K, et al. Measuring biological age via metabonomics: the metabolic age score. J Proteome Res 2016;15:400–10. [DOI] [PubMed] [Google Scholar]
- 12. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 2013;49:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 2013;14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8:1844–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marioni RE, Shah S, McRae AF, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christiansen L, Lenart A, Tan Q, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell 2016;15:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics 2016;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, et al. Ischemic stroke patients are biologically older than their chronological age. Aging (Albany NY) 2016;8:2655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (Atherosclerosis Risk In Communities). Circ Genom Precis Med 2018;11:e001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging 2015;7:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durso DF, Bacalini MG, Sala C, et al. Acceleration of leukocytes’ epigenetic age as an early tumor and sex-specific marker of breast and colorectal cancer. Oncotarget 2017;8:23237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng Y, Joyce BT, Colicino E, et al. Blood epigenetic age may predict cancer incidence and mortality. EBioMedicine 2016;5:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horvath S, Erhart W, Brosch M, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA 2014;111:15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quach A, Levine ME, Tanaka T, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 2017;9:419–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobs DI, Hansen J, Fu A, et al. Methylation alterations at imprinted genes detected among long-term shiftworkers. Environ Mol Mutagen 2013;54:141–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhatti P, Zhang Y, Song X, et al. Nightshift work and genome-wide DNA methylation. Chronobiol Int 2015;32:103–12. [DOI] [PubMed] [Google Scholar]
- 28. Adams CD, Jordahl KM, Copeland W, et al. Nightshift work, chronotype, and genome-wide DNA methylation in blood. Epigenetics 2017;12:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu Y, Stevens RG, Hoffman AE, et al. Epigenetic impact of long-term shiftwork: pilot evidence from circadian genes and whole-genome methylation analysis. Chronobiol Int 2011;28:852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sister Study. Sister Study Response Rates for Annual and Detailed Follow-Up. 8 February 2016. https://sisterstudy.niehs.nih.gov/English/images/SIS-RespRatesFollowUps-website-20170908–508.pdf (10 October 2018, date last accessed).
- 31. Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The sister study: baseline methods and participant characteristics. Environ Health Perspect 2017;125:127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Brien KM, Sandler DP, Xu Z, Kinyamu HK, Taylor JA, Weinberg CR. Vitamin D, DNA methylation, and breast cancer. Breast Cancer Res 2018;20:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu Z, Niu L, Li L, Taylor JA. ENmix: a novel background correction method for Illumina HumanMethylation450 BeadChip. Nucleic Acids Res 2016;44:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform 2012;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003;100:9440–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parsons MJ, Brancaccio M, Sethi S, et al. The regulatory factor ZFHX3 modifies circadian function in SCN via an AT motif-driven axis. Cell 2015;162:607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramin C, Devore EE, Wang W, Pierre-Paul J, Wegrzyn LR, Schernhammer ES. Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med 2015;72:100–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 2013;17:273–84. [DOI] [PubMed] [Google Scholar]
- 39. Carroll JE, Irwin MR, Levine M, et al. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the women’s health initiative study. Biol Psychiatry 2017;81:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boogaard PJ, Caubo ME. Increased albumin excretion in industrial workers due to shift work rather than to prolonged exposure to low concentrations of chlorinated hydrocarbons. Occup Environ Med 1994;51:638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Proper KI, van de Langenberg D, Rodenburg W, et al. The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am J Prev Med 2016;50:e147–57. [DOI] [PubMed] [Google Scholar]
- 42. Thomas C, Power C. Shift work and risk factors for cardiovascular disease: a study at age 45 years in the 1958 British birth cohort. Eur J Epidemiol 2010;25:305–14. [DOI] [PubMed] [Google Scholar]
- 43. Gotoh M, Ichikawa H, Arai E, et al. Comprehensive exploration of novel chimeric transcripts in clear cell renal cell carcinomas using whole transcriptome analysis. Genes Chromosomes Cancer 2014;53:1018–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han C, Yu L, Liu X, et al. ATXN7 gene variants and expression predict post-operative clinical outcomes in hepatitis B virus-related hepatocellular carcinoma. Cell Physiol Biochem 2016;39:2427–38. [DOI] [PubMed] [Google Scholar]
- 45. Milne RL, Burwinkel B, Michailidou K, et al. Common non-synonymous SNPs associated with breast cancer susceptibility: findings from the Breast Cancer Association Consortium. Hum Mol Genet 2014;23:6096–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dong XY, Sun X, Guo P, et al. ATBF1 inhibits estrogen receptor (ER) function by selectively competing with AIB1 for binding to the ER in ER-positive breast cancer cells. J Biol Chem 2010;285:32801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fenga C. Occupational exposure and risk of breast cancer. Biomed Rep 2016;4:282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bollati V, Baccarelli A, Sartori S, et al. Epigenetic effects of shiftwork on blood DNA methylation. Chronobiol Int 2010;27:1093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cedernaes J, Osler ME, Voisin S, et al. Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J Clin Endocrinol Metabol 2015;100:2015–284. [DOI] [PubMed] [Google Scholar]
- 50. Nilsson EK, Bostrom AE, Mwinyi J, Schioth HB. Epigenomics of total acute sleep deprivation in relation to genome-wide DNA methylation profiles and RNA expression. OMICS 2016;20:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Skuladottir GV, Nilsson EK, Mwinyi J, Schioth HB. One-night sleep deprivation induces changes in the DNA methylation and serum activity indices of stearoyl-CoA desaturase in young healthy men. Lipids Health Dis 2016;15:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Irvin MR, Aslibekyan S, Do A, et al. Metabolic and inflammatory biomarkers are associated with epigenetic aging acceleration estimates in the GOLDN study. Clin Epigenetics 2018;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.