Abstract
Background
Maternal antidepressant use in pregnancy has been associated with both shorter gestational length and child anxiety. We employed paternal antidepressant use as a negative-control exposure to indirectly assess whether confounding by genetic or shared familial environmental factors associated with depression may explain these associations.
Methods
The study sample came from the population-based Norwegian Mother and Child Cohort Study (MoBa) that recruited participants from 1999 to 2008. We included 70 959 families where the father completed a questionnaire about medication use in the 6 months prior to pregnancy. In 42 511 infants who completed the 3-year follow-up, we computed Z-scores for the anxiety domain of the Child Behavior Checklist. We used linear and logistic regression to assess the association between paternal antidepressant use, gestational age at birth and child anxiety.
Results
Antidepressants were used by 1.1% (n = 755) of fathers. Paternal antidepressant use was not associated with gestational age at birth [β = 0.63 days, 95% confidence interval (CI) –1.56, 0.31] whereas it was positively associated with a child anxiety symptom Z-score and high anxiety symptoms (odds ratio 1.33, 95% CI 0.90, 1.97) in unadjusted analyses. This association was attenuated when controlling for maternal and paternal history of depression and other measured factors (odds ratio 1.14, 95% CI 0.76, 1.69).
Conclusions
These results support the suggested effect of maternal use of antidepressants in pregnancy on shorter gestation; however, they suggest familial confounding could explain the association between maternal use of antidepressants and anxiety traits in the offspring.
Keywords: Antidepressants, drug safety, negative controls, paternal exposure, pregnancy, pharmacoepidemiology, Norwegian Mother and Child Cohort Study, MoBa
Key Messages
Paternal antidepressant use may be an appropriate negative-control exposure for maternal antidepressant use and offspring outcomes.
The null association between paternal antidepressant use and gestational age at birth supports the suggested effect of maternal use of antidepressants in pregnancy on shorter gestation.
The finding of a positive association between paternal antidepressant use and offspring anxiety suggests familial confounding could explain the association between maternal use of antidepressants and anxiety traits in the offspring.
Introduction
Multiple studies have reported that maternal antidepressant use in pregnancy is associated with short- and long-term adverse pregnancy outcomes including shorter duration of pregnancy1,2 and neurodevelopmental problems in children.3–5 Whether such associations are due to the effects of in utero exposure to the drugs or to confounding by maternal characteristics, genetic predisposition or family environment is difficult to disentangle. Confounding control is particularly challenging when studying the effects of prenatal exposures on outcomes that occur in childhood. In this setting, where unmeasured genetic and family environment characteristics may be strong confounders, the sibling design may have unique advantages over a traditional cohort design.6,7 Associations between antidepressants and gestational length remained and between antidepressants and anxiety at 3 years strengthened after restriction to siblings that were discordant with respect to maternal use of antidepressants in pregnancy.1–3 Whereas this may suggest robust effects, the unique limitations inherent in sibling-controlled studies including an increased risk of exposure misclassification bias and potential amplification of the effects of non-shared confounders might also explain these associations.
Therefore, to assess the likelihood that observed associations between maternal use of medications during pregnancy and neurodevelopmental outcomes reflect true causal effects, rather than confounding, other approaches should be considered. The robustness of results in observational studies can be assessed by conducting multiple sensitivity analyses with different limitations and potential biases to corroborate or refute the evidence for a causal effect.8 One of these approaches is the use of negative-control exposures.9,10
We assessed the likelihood of residual confounding in observational studies on the effect of maternal use of antidepressants during pregnancy on the risk of shorter gestational length and child anxiety using paternal exposures as a negative control.11,12 The rationale was that an association between paternal use of antidepressants in the 6 months before pregnancy with these adverse outcomes in the children would suggest residual confounding, assuming paternal use of antidepressants does not have a direct biological effect on offspring.
Methods
We used data from the Norwegian Mother and Child Cohort Study (MoBa)—a prospective population-based study carried out by the Norwegian Institute of Public Health.13 Participants from all over Norway were recruited via postal invitation from 1999 to 2008 at around the 17-week ultrasound. Women consented to participate in 41% of pregnancies. The cohort includes children from over 112 000 pregnancies in 95 200 mothers with 75 200 participating fathers. The current study is based on quality-assured data from self-reported questionnaires (Q) (version 9, released for research October 2015) as well as from the Medical Birth Registry of Norway (MBRN). We included singleton, live-born pregnancies where information from the father, mother and child was available at baseline (Q1, QF), at delivery (MBRN) and in a sub-sample, where information about child outcomes at 3 years was available (Q6).
Information on paternal antidepressant use was collected in the fathers’ questionnaire (QF) that included assessment of medication use in the 6 months prior to pregnancy. Our primary exposure of interest was paternal self-reported use of antidepressants at any time in the 6 months prior to pregnancy, coded according to the Anatomical Therapeutic Chemical (ATC) classification system (ATC N06A). A previous comparison of data from the Norwegian Prescription Registry with paternal self-reported medication use in MoBa found that agreement between self-report and prescription data on antidepressant use among fathers (κ = 0.74) was similar to what was previously reported among mothers (κ = 0.72).14,15 We also considered paternal use of selective serotonin reuptake inhibitor (SSRI) antidepressants (N06AB) as a secondary exposure of interest.
The outcome gestational age at birth was obtained from MBRN data. For this outcome, we excluded multiples (twins, triplets, quadruplets) and pregnancies with congenital malformations as we would expect a shorter duration of pregnancy to be driven by these conditions.
The outcome child anxiety was assessed by the anxiety domain of the Child Behavior Checklist (CBCL), completed by mothers when the child was approximately 36 months of age. The CBCL has been validated in Norway16 and the internalizing domain of the short version administered in MoBa at 36 months was validated against the full scale administered in a sample at 6 years (correlation 0.79) and other short-scale time points (correlations 0.90 for 18 months and 0.86 for 5 years).17 The anxiety domain was based on three items in the CBCL (‘Clings to adults or too dependent’, ‘Gets too upset when separated from parents’ and ‘Too fearful or anxious’), scored as 1 = not true, 2 = somewhat or sometime true or 3 = very true or often true. We computed the average across the three domains and allowed for subjects missing one item to be included. Thus, for this analysis, we excluded subjects who were missing the 3-year follow-up questionnaire or more than one item of the CBCL anxiety domain. We computed standardized Z-scores [mean = 0, standard deviation (SD) = 1] and T-scores (mean = 50, SD = 10) based on the distribution of scores in all subjects who completed a 3-year follow-up (i.e. not restricted to those with father’s participation) (mean = 1.2, SD = 0.3). Our primary outcome was the continuous Z-score. In sensitivity analyses, we considered a dichotomous outcome of high anxiety symptoms as T-score ≥70 (which corresponded to a report of 2 or 3 on each CBCL anxiety sub-domain item), used in psychology research to classify the outcome of clinical importance.18
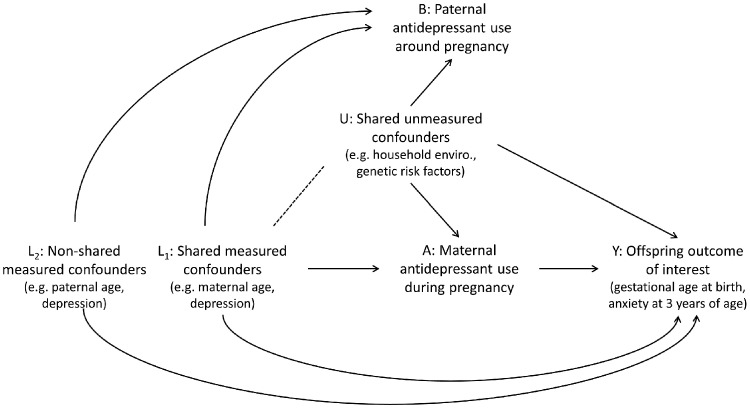
Relevant confounders were selected a priori and informed by a directed acyclic graph (DAG) (Figure 1) and were similar to those previously used for the maternal exposure. The structure and notation of the DAG were modelled after the causal diagram proposed by Lipsitch et al. for an ideal negative-control exposure.9 Data on confounders came from MoBa questionnaires (maternal and paternal) and MBRN data. Maternal self-reported antidepressant use in pregnancy was based on any use reported in questionnaires at approximately 17 and 30 gestational weeks and 6 months post-partum.15 Measured confounders included the maternal factors [maternal age (continuous), parity (0, 1, 2, 3, ≥4), maternal depression before or during pregnancy (yes, no), maternal anxiety before or during pregnancy (yes, no), maternal lifetime history of major depression (yes, no), maternal education (less than university, completed university or higher), any maternal smoking in pregnancy (yes, no), any maternal alcohol use in pregnancy (yes, no)] and paternal factors [paternal age (<30, 30–34, ≥35), paternal lifetime history of major depression (yes, no) and symptoms of anxiety and depression (Z-score of the eight-item version of the Hopkins Symptom Checklist; SCL-8)].
Figure 1.
Causal diagram. Adapted with permission from Wolters Kluwer Health, Inc.: Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:38388. https://journals.lww.com/epidem.
Statistical analysis
We used linear regression to assess the association between paternal antidepressant use and both gestational age at birth in days (unstandardized) and child anxiety at 3 years in units of SD (Z-scores), respectively. For all analyses, the comparison group is children of unexposed fathers. In sensitivity analyses, we used logistic models to estimate the association between exposure and high symptoms of anxiety, T-score ≥70. We used robust standard errors to account for the potential for fathers with more than one child. We used multiple imputation with chained equations to impute 20 complete datasets to address missing confounder information19 and censoring weights to address possible selection bias from incomplete follow-up of children at 3 years.20 We computed censoring weights in each imputed dataset and then weighted subjects according to the inverse probability of censoring, based on measured covariates (see online supplementary material, available as Supplementary data at IJE online). We carried out corresponding analyses for maternal antidepressant use to compare the magnitude of the associations for maternal and paternal antidepressant use in this study sample.
Ethics
The establishment and data collection in MoBa obtained a license from the Norwegian Data Inspectorate and approval from the Regional Committee for Medical Research Ethics. The current study (application 2015/2103) was approved on 12 December 2015 by the Regional Committee for Medical Research Ethics (Region South-East). The study was pre-approved by the MoBa steering committee.
Results
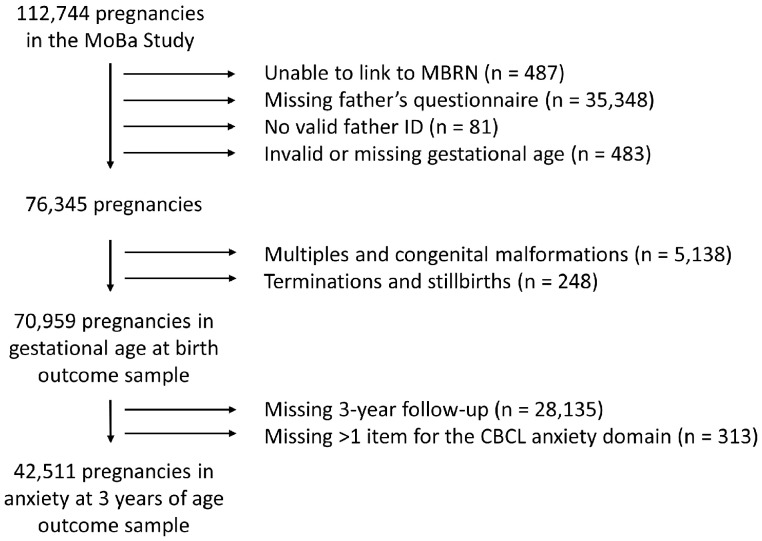
There were 70 959 pregnancies among 62 276 fathers available for the analysis of gestational length and 42 511 children among 37 719 fathers for the analysis of child anxiety at 3 years (Figure 2). Among the 70 959 pregnancies, 1.1% of fathers used antidepressants in the 6 months before pregnancy (n = 755, of whom 553 used SSRIs) and 1.0% of mothers used antidepressants in pregnancy (n = 686, of whom 553 used SSRIs). In 37 pregnancies, both the father and the mother were exposed to antidepressants, suggesting that having a partner who used an antidepressant increased the likelihood of using an antidepressant approximately 5-fold. The mean gestational age at birth was 279.5 days (SD = 12.2).
Figure 2.
Flow diagram of study sample selection.
CBCL, child behaviour checklist; MBRN, Medical Birth Registry of Norway.
Compared with non-users, the crude association between paternal antidepressant use and gestational age at birth was –0.63 days [95% confidence interval (CI) –1.56, 0.31] (Table 1). When we adjusted for maternal antidepressant use and confounders related to it, the estimate was –0.45 days (95% CI –1.39, 0.49) and, when we further adjusted for confounders that may be unique to the association with paternal antidepressant use (paternal factors), the estimate was –0.18 days (95% CI –1.13, 0.78). The associations were similarly null for paternal SSRI exposure. Adjusted estimates suggested pregnancies exposed to maternal antidepressant use were on average approximately 2 days shorter (see online supplementary material, available as Supplementary data at IJE online).
Table 1.
Association between paternal antidepressant use and gestational age and child anxiety
| Outcome (units) | Exposure | Unadjusted model | Adjusted model: maternal factors only | Adjusted model: maternal and paternal factors |
|---|---|---|---|---|
| Gestational age (days), β (95% CI), N = 70 959 | Antidepressant | –0.63 (–1.56, 0.31) | –0.45 (–1.39, 0.49) | –0.18 (–1.13, 0.78) |
| SSRI | –0.23 (–1.21, 0.75) | –0.07 (–1.05, 0.91) | 0.21 (–0.78, 1.20) | |
| Anxiety Z-score (SD), β (95% CI), N = 42 511 | Antidepressant | 0.13 (0.03, 0.23) | 0.10 (0.00, 0.21) | 0.06 (–0.04, 0.17) |
| SSRI | 0.17 (0.04, 0.29) | 0.15 (0.02, 0.27) | 0.11 (–0.02, 0.23) | |
| High anxiety symptoms (yes/no), OR (95% CI) | Antidepressant | 1.33 (0.90, 1.97) | 1.23 (0.83, 1.83) | 1.14 (0.76, 1.69) |
| SSRI | 1.70 (1.13, 2.55) | 1.60 (1.06, 2.42) | 1.49 (0.99, 2.25) |
Maternal factors include maternal antidepressant use in pregnancy, maternal age (continuous), parity (0, 1, 2, 3, ≥4), maternal self-reported depression before or during pregnancy (yes/no), maternal self-reported anxiety before or during pregnancy (yes/no), maternal lifetime history of major depression (yes/no), maternal education (less than university/completed university or higher), any maternal smoking in pregnancy (yes/no) and any maternal alcohol use in pregnancy (yes/no). Maternal and paternal factors model additionally includes paternal age (<30, 30–34, ≥35) and paternal lifetime history of major depression (yes/no). CI, confidence interval; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; OR, odds ratio.
Among the 42 511 children included in the neurodevelopmental outcome analysis, 303 (0.7%) had one missing item from the CBCL anxiety domain questions. One percent of children had a father who used an antidepressant medication before pregnancy (n = 418, of whom 309 used SSRIs), whereas 0.9% had a mother who used an antidepressant during pregnancy (n = 381, of whom 303 used SSRIs) and 19 had two parents who used antidepressants. The mean Z-score was –0.02 (SD = 0.98) and 2178 (5.1%) children had high anxiety scores corresponding to T-scores ≥70. The crude association between paternal antidepressant use and child anxiety Z-score indicated that the anxiety scores were 0.13 SD (95% CI 0.03, 0.23) higher among children of exposed compared with unexposed fathers (Table 1) and 0.27 SD (95% CI 0.16, 0.39) higher for exposed mothers. After adjusting for maternal exposure and confounders, the association for paternal use moved to 0.10 SD (95% CI 0.00, 0.21) and, after additional adjustment for paternal factors, the difference attenuated further to 0.06 SD (95% CI –0.04, 0.17). The estimates for maternal exposure in these cohort-based analyses were also attenuated after adjustment for maternal factors. In particular, maternal self-reported anxiety, lifetime history of major depression, and lower education and paternal lifetime history of major depression had the strongest associations with the anxiety score. The paternal exposure association was somewhat stronger for SSRIs and especially with the dichotomous outcome high symptoms of anxiety where the OR remained elevated for the fully adjusted model (OR 1.49, 95% CI 0.99, 2.25). In a sensitivity analysis with further adjustment for the Z-scores of the anxiety and depression domains of the paternal SCL-8, the OR was further attenuated, but remained elevated (OR 1.36, 95% CI 0.90, 2.07). The maternal estimates went from OR ≥2 in the crude to ∼1.2 after adjustment.
Discussion
We investigated the relationship between paternal pre-conceptional antidepressant use and both gestational length and child anxiety, considering the paternal exposure as a negative control for maternal exposure. We hypothesized that there may be an association with paternal exposure due to the presence of shared confounders, including unmeasured factors such as genetic predisposition to anxiety and depression, and economic and other household environmental factors that can be difficult to measure and could influence both maternal and paternal exposures. Some examples could include parental behaviours and attitudes towards parenting in a household where one parent has a mental health disorder. We found no association between paternal antidepressant use and gestational age at birth, which we might expect to be less affected by paternal depression. However, we did find that paternal exposure was associated with anxiety in the child at 3 years of age. After accounting for measured shared confounders (maternal factors), the association was attenuated and, with further adjustment for paternal age and lifetime history of depression, the association was close to null. These findings suggest that depression or anxiety in one of the parents can induce a positive association between use of antidepressants around the time of pregnancy and child anxiety. Given that adjustment for measured confounders tend to move the estimate downwards for the association between paternal antidepressant use and child anxiety, we expect further adjustment to move the estimate further towards the null. However, for the paternal association, since the fetus was not exposed to the drug, we believe the association must be due to confounding, thus supporting that the observed association in the mother is also due to residual confounding. Moreover, the adjusted OR of 1.36 for paternal SSRI use for potentially clinically relevant child anxiety symptoms suggests that we should be cautious in interpreting associations between maternal antidepressant use in pregnancy and offspring internalizing mental health outcomes, since this 36% increased risk reflects residual confounding that could also affect maternal estimates. The stronger associations for SSRIs could be due to chance or somewhat different indications for SSRIs and non-SSRI antidepressants.
The association between maternal antidepressant use and gestational length quantified here was consistent with prior studies suggesting an effect size of approximately 2–3 days shorter gestation.1,2 However, whereas we found a similar crude association in this MoBa sample to the previous sibling-based study, we found no association between maternal antidepressant use and child anxiety after control for confounding. In a prior report from MoBa, the adjusted estimate from a sibling-controlled analysis of maternal antidepressant use and 3-year anxiety CBCL score was β = 0.64 SD, whereas the crude was β = 0.44 SD. The crude estimate for the cohort-based analysis of the sibling sample was β = 0.22 (no adjusted reported), so our results are generally consistent with that study, but emphasize the need to for cautious interpretation.3
MoBa is a unique resource in which to explore the use of paternal medications as negative controls because fathers have actively participated in the study to report exposures in the period surrounding conception including prospective medication use. Furthermore, follow-up of children continues for years after birth and detailed child-development and behavioural screening instruments are administered over time. When we assess the association between paternal medication use and child outcome, then we can confidently assume that any association identified is not due to a causal effect of in utero exposure to the medication, i.e. crossing the placenta to affect the developing fetus. Therefore, this approach may complement the traditional sibling design to control for genetic and family environment confounding. Other data that may be useful for this approach include other pregnancy and birth cohorts that enrolled fathers, population registries where information from both parents may be available from linked prescription databases or insurance healthcare claims databases where members of the same family on a single plan share a common family enrolment identifier.
There is increasing interest in using paternal medication exposures as negative controls, when they are available. A study within the Swedish health registries assessed paternal exposure to antidepressant in the first trimester as a negative-control exposure for maternal use.21 They found a modest association between paternal antidepressant use and pre-term birth and stronger associations with autism and attention-deficit/hyperactivity disorder (ADHD). This, combined with the results from a sibling analysis that found no association for maternal use and autism or ADHD, strongly suggests that first-trimester antidepressant use does not cause autism or ADHD, but may result in pre-term birth.
Differences in the strength of the association for the index exposure and the negative control could be seen because of differences in the validity of self-reported medication use between mothers and fathers.22,23 If there were more misclassification among fathers, then we would expect a greater bias towards the null, which could spuriously produce the expected null risk estimates. One of the key strengths of this study was that both maternal and paternal antidepressant use had been validated, and agreement between self-reported and prescription records was similar for both.14,15 This gives us more confidence that the null association for gestational length is not attributable to more exposure misclassification in the negative control. We benefited from several strengths of the MoBa study, including the large sample size, which allowed us to investigate a rare exposure; the high quality of gestational age data, which is ultrasound-based and available in days (rather than weeks or dichotomized); and the long-term follow-up of children, which allowed us to investigate later neurodevelopmental outcomes. We had high-quality data on important confounders, including multiple measures of maternal and paternal depression and anxiety, with lifetime history of major depression, and standardized developmental assessments of the children.
The study may have limitations in its generalizability, since the participation in MoBa was only 41% at baseline and lower at 3 years. However, prior work has demonstrated an absence of selection bias for known exposure–outcome relationships20 and the selection-weighted and unweighted estimates for anxiety at 3 years were similar. Since the prior results came from the MoBa study and other cohorts in similar populations, the results should be a valid check for the presence of confounding bias in prior studies. Unfortunately, we cannot determine the magnitude of the residual confounding bias for maternal exposure estimates, but instead provide a qualitative assessment of confounding.9 However, a recent study in MoBa found that genetic transmission plays an important role in the association between maternal prenatal depression and offspring internalizing behaviour, which supports our interpretation of these findings.24 Furthermore, we have to assume that there is no direct effect of paternal exposure through e.g. the epigenetic influence of paternal genetic material in sperm or carrying drug in semen. Although we cannot rule this out as a possibility, no current literature supports a direct causal effect of paternal exposure and so we believe this assumption is plausible.25 However, even if this assumption did not hold, imperfect negative-control exposures may still be useful for causal inference,26 especially as part of a series of sensitivity analyses aimed at assessing the robustness of the findings.
In conclusion, these results increase our confidence in the suggested effect of maternal use of antidepressants in pregnancy on shorter gestation. However, our results suggest familial confounding for the effect of prenatal exposure to antidepressants on offspring internalizing mental health outcomes may bias estimates in the positive direction. Care-givers and healthcare personnel should support parents with mental health problems and their children, as these families may have extra challenges. Future medication safety studies should address causal questions by integrating results from several different approaches with different and unrelated key sources of potential bias. Paternal negative controls could be one of these approaches.
Funding
This work was supported by funding from the European Research Council starting grant ERC-STG-2014 DrugsInPregnancy (grant number 639377). Dr Jacqueline Cohen and Dr Sonia Hernández-Díaz were supported by the National Institute of Mental Health (R01 MH100216). Dr Eivind Ystrom was supported by the Research Council of Norway (grant number 262177). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract number N01-ES-75558), NIH/NINDS (grant numbers UO1 NS 047537-01 and UO1 NS 047537-06A1).
Supplementary Material
Acknowledgements
We are grateful to all the participating families in Norway who took part in this on-going cohort study. Parts of this work were presented as a poster at the 30th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Seattle, WA, 20–21 June 2017 and the 50th Annual Meeting of the Society for Epidemiologic Research, Seattle, WA, 21–23 June 2017, and as an oral presentation at the 33rd International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Montreal, Canada, 26–30 August 2017.
Conflicts of interest: Dr Cohen has received salary support from a research grant to Harvard T.H. Chan School of Public Health from GSK for unrelated work. Dr Hernández-Díaz’s institution has received research funding from GSK and Lilly for unrelated work, and salary support from the North American AED Pregnancy Registry; she has consulted for Roche. Drs Wood, Ystrom and Nordeng have no potential conflicts to declare.
References
- 1. Nezvalova-Henriksen K, Spigset O, Brandlistuen RE. et al. Effect of selective serotonin reuptake inhibitor (SSRI) exposure during pregnancy on birth weight and gestational age: a sibling-controlled cohort study. Int J Epidemiol 2016;45:2018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Viktorin A, Lichtenstein P, Lundholm C. et al. Selective serotonin re-uptake inhibitor use during pregnancy: association with offspring birth size and gestational age. Int J Epidemiol 2016;45:170–77. [DOI] [PubMed] [Google Scholar]
- 3. Brandlistuen RE, Ystrom E, Eberhard-Gran M. et al. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int J Epidemiol 2015;44:1397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skurtveit S, Selmer R, Roth C. et al. Prenatal exposure to antidepressants and language competence at age three: results from a large population-based pregnancy cohort in Norway. BJOG: Int J Obstet Gynaecol 2014;121:1621–31. [DOI] [PubMed] [Google Scholar]
- 5. Handal M, Skurtveit S, Furu K. et al. Motor development in children prenatally exposed to selective serotonin reuptake inhibitors: a large population-based pregnancy cohort study. BJOG: Int J Obstet Gynaecol 2016;123:1908–17. [DOI] [PubMed] [Google Scholar]
- 6. Wood ME, Lapane KL, van Gelder M. et al. Making fair comparisons in pregnancy medication safety studies: an overview of advanced methods for confounding control. Pharmacoepidemiol Drug Saf 2018;27:140–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frisell T, Oberg S, Kuja-Halkola R. et al. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology 2012;23:713–20. [DOI] [PubMed] [Google Scholar]
- 8. Lawlor DA, Tilling K, Davey Smith G.. Triangulation in aetiological epidemiology. Int J Epidemiol 2016;45:1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipsitch M, Tchetgen Tchetgen E, Cohen T.. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnold BF, Ercumen A, Benjamin-Chung J. et al. Brief Report: Negative controls to detect selection bias and measurement bias in epidemiologic studies. Epidemiology 2016;27:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magnus MC, Karlstad O, Haberg SE. et al. Prenatal and infant paracetamol exposure and development of asthma: the Norwegian Mother and Child Cohort Study. Int J Epidemiol 2016;45:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stergiakouli E, Thapar A, Davey Smith G.. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatr 2016;170:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magnus P, Birke C, Vejrup K. et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382–88. [DOI] [PubMed] [Google Scholar]
- 14. Cohen JM, Wood ME, Hernandez-Diaz S. et al. Agreement between paternal self-reported medication use and records from a national prescription database. Pharmacoepidemiol Drug Saf 2018;27:413–21. [DOI] [PubMed] [Google Scholar]
- 15. Skurtveit S, Selmer R, Odsbu I. et al. Self-reported data on medicine use in the Norwegian Mother and Child cohort study compared to data from the Norwegian Prescription Database. Nor J Epidemiol 2014;24:209–16.24646813 [Google Scholar]
- 16. Nøvik TS. Validity of the Child Behaviour Checklist in a Norwegian sample. Eur Child Adolesc Psychiatry 1999;8:247–54. [DOI] [PubMed] [Google Scholar]
- 17. Helland SS, Roysamb E, Wang MV. et al. Language difficulties and internalizing problems: Bidirectional associations from 18 months to 8 years among boys and girls. Dev Psychopathol 2018;30:1239–52. [DOI] [PubMed] [Google Scholar]
- 18. Achenback TM, Ruffle TM.. The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 2000;21:265–71. [DOI] [PubMed] [Google Scholar]
- 19. White IR, Royston P, Wood AM.. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 20. Nilsen RM, Vollset SE, Gjessing HK. et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 2009;23:597–608. [DOI] [PubMed] [Google Scholar]
- 21. Sujan AC, Rickert ME, Oberg AS. et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017;317:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol 2008;102:245–56. [DOI] [PubMed] [Google Scholar]
- 23. Sanderson E, Macdonald-Wallis C, Davey Smith G.. Negative control exposure studies in the presence of measurement error: implications for attempted effect estimate calibration. Int J Epidemiol 2018;47:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hannigan LJ, Eilertsen EM, Gjerde LC. et al. Maternal prenatal depressive symptoms and risk for early-life psychopathology in offspring: genetic analyses in the Norwegian Mother and Child Birth Cohort Study. Lancet Psychiatry 2018;5:808–15. [DOI] [PubMed] [Google Scholar]
- 25. Viktorin A, Levine SZ, Altemus M. et al. Paternal use of antidepressants and offspring outcomes in Sweden: nationwide prospective cohort study. BMJ 2018;361:k2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weisskopf MG, Tchetgen Tchetgen EJ, Raz R.. Commentary: on the use of imperfect negative control exposures in epidemiologic studies. Epidemiology 2016;27:365–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.