Abstract
Purpose
This study was conducted to analyze the clinical features associated with the pathogenic variants of ABCA4 in Korean patients with inherited retinal dystrophies (IRDs).
Methods
We enrolled patients with IRDs who visited a tertiary referral hospital and identified the pathogenic variants of ABCA4 through targeted gene panel sequencing and whole exome sequencing. We analyzed the clinical characteristics and phenotypic spectrum according to genotype.
Results
Eleven patients (from nine families) with IRDs and pathogenic variants in ABCA4 were included. Eight patients (from seven families) with Stargardt disease (STGD), two (from one family) with cone-rod dystrophy (CRD), and one with early-onset retinitis pigmentosa (RP) were included. Two heterozygous mutations were identified in eight families, and one variant was found in a patient with fundus flavimaculatus. Two variants, p.Gln294Ter and p.Gln636Lys, were associated with severe phenotypes, such as early-onset RP and CRD. Four novel pathogenic variants, p.Gln636Lys, p.Ile1114del, p.Thr1117Ala, and p.Asn1588Tyr, were identified. p.Gln294Ter, p.Leu1157Ter, and p.Lys2049ArgfsTer12 were repeatedly detected in Koreans with ABCA4-associated retinal diseases (ABCA4-RD).
Conclusions
Various pathogenic variants of ABCA4, including four novel variants, were identified, and ABCA4-RD exhibited various phenotypes and disease severities in a Korean IRD cohort. These findings will be useful for understanding the clinical features of ABCA4-RD and ethnicity-specific variants in East Asians.
Introduction
ABCA4, which is located at 1p22.1, encodes the ATP-binding cassette sub-family A member 4, a retina-specific protein that is exclusively expressed in the outer segments of photoreceptors [1]. This transmembrane protein facilitates the removal of toxic retinoid compounds from photoreceptor cells [2,3]. ABCA4 is composed of 50 exons, and many variants of this gene have been reported [4]. Heterogeneous retinal dystrophies, including Stargardt disease (STGD1; OMIM 248200), cone-rod dystrophy (CRD3; OMIM 604116), retinitis pigmentosa (RP19; OMIM 601718), and early-onset severe retinal dystrophy (OMIM 248200), are associated with recessive mutations in ABCA4 [5]. Numerous studies have reported phenotypic and allelic heterogeneity and variable severity in ABCA4-associated retinopathies (ABCA4-associated retinal diseases, ABCA4-RD). More than 800 disease-associated ABCA4 variants have been identified [1], and the deleterious effects of these variants, including missense, nonsense, splice-site, insertion-deletion, and frameshift mutations [6], have been associated with disease onset and phenotypic severity [7]. However, studying the genotype–phenotype correlation remains challenging because most patients harbor compound heterozygous mutations in ABCA4, and phenotypic variations have been observed among family members with the same mutations.
Moreover, more than half of such mutations have been detected only once, and the most frequent disease-associated ABCA4 variants have been described only in approximately 10% of STGD patients [8]. This low detection rate of identical variations makes it difficult to validate their pathogenicity and to evaluate their clinical impacts and genotype–phenotype correlations. Thus, the extent to which each variant contributes to the etiology of ABCA4-RD in relation to other factors, such as age, lifestyle, environmental factors, and genetic modifiers, remains unknown. Furthermore, several studies have identified frequent ethnic group-specific ABCA4 variants [5,8-11]. Ethnic differences and variant diversity cause highly variable ABCA4-RD phenotypes. However, clinical and genotypic data for ABCA4-RD in Asian populations are insufficient [1,5,12,13].
In this study, we analyzed the phenotypic spectrum and genotypes in 11 patients from 9 families with ABCA4-RD. We also identified four novel pathogenic variants of ABCA4 and report the variants that have been repeatedly detected in Koreans.
Methods
This study was conducted as part of a study to identify pathogenic variants in patients with inherited retinal dystrophies (IRDs) registered at Seoul National University Bundang Hospital between January 2009 and January 2018. Of these patients, those with pathogenic variants of ABCA4 (11 patients from 9 families) were selected for the analysis. This study was conducted at Seoul National University Bundang Hospital. Institutional Review Board approval was obtained (IRB no. B-1105–127–014 and no. B-1901–519–103), and written informed consent was obtained from all the participants. The study protocols adhered to the tenets of the Declaration of Helsinki and its later amendments.
Clinical investigations
Clinical diagnoses and classifications were based on patient age at the time of symptom onset, visual acuity, and findings from fundus examinations, autofluorescence (AF), optical coherence tomography (OCT), and electroretinograms (ERGs). All patients underwent comprehensive ophthalmic examinations, including slit-lamp and dilated fundus examinations. Vision was assessed by measuring best-corrected visual acuity (BCVA). BCVAs were measured using a decimal chart and converted to Snellen equivalents. Spectral domain-OCT (SD-OCT) scans and fundus autofluorescence (FAF) imaging were conducted using a confocal scanning laser ophthalmoscope (SPECTRALIS HRA+OCT; Heidelberg Engineering, Heidelberg, Germany). The areas of definitely decreased autofluorescence (DDAF) and questionably decreased autofluorescence (QDAF) were measured and analyzed in FAF images according to a previous report [14]. When AF of the optic disc was defined as 100% black, DDAF was defined as more than 90% black, and QDAF was defined as 50 to 90% black. The size of the area was semi-automatically evaluated using the area-calculating module built in Spectralis HRA + OCT. Full-field ERGs were performed for all patients, and all procedures complied with the guidelines and recommendations of the International Society for Clinical Electrophysiology of Vision standard available at the time of recording.
Blood sampling and DNA collection
A professional nurse for clinical research collected blood from the patients’ antecubital fossa vein after alcohol disinfection and stored blood in EDTA bottles. DNA was extracted using a DNA extraction kit (QIAamp DNA Blood Maxi Kit, Qiagen, Hilden, Germany) for targeted exome sequencing. DNA sampling and basic ophthalmic examinations were also performed for the family members of the patients who showed no visual symptoms.
Sequencing and variant identification
ABCA4 variants were identified using two methods: whole-exome sequencing for the samples from patients H75 and H830, and targeted gene panel sequencing for the samples from the other patients. Both methods covered all 50 exons and exon-intron boundaries of ABCA4. For targeted gene panel sequencing, pre-capture libraries (Illumina, Inc., San Diego, CA) were prepared, and the capture process (Roche NimbleGen, Madison, WI) was performed according to the manufacturer’s protocols. SureSelect Human All Exon V6 (Agilent Technologies, Santa Clara, CA) was used for exome capture in whole-exome sequencing. All captured libraries were sequenced using the Illumina HiSeq 2000 platform (paired-end, average depth ≥100x). Burrows-Wheeler Aligner was used to align the sequence reads in the human reference sequence (hg19, GRCh37). Genome analysis toolkit (GATK) packages, SAMtools, and Dindel were used for variant calling, local realignment, and variant recalibration. ANNOVAR was used for variant annotation. NextGENe software (SoftGenetics, State College, PA) was also used for the analysis. We compared the allele frequencies of all variants to the 1000 Genome database, Exome Variant Server of the NHLBI Exome Sequencing Project, and a genome aggregation database (gnomAD; Cambridge, MA) to filter out the common variants. Exonic or splicing variants with allele frequencies <0.1 in the 1000 Genome database and the gnomAD browser (accessed: February 12, 2019) were used for variant prioritization. Prediction software programs CADD, Polyphen, SIFT, and MutationTaster were used to evaluate the pathogenicity of the novel ABCA4 variants. Sanger sequencing was performed to validate the candidate variants in additional familial samples.
Results
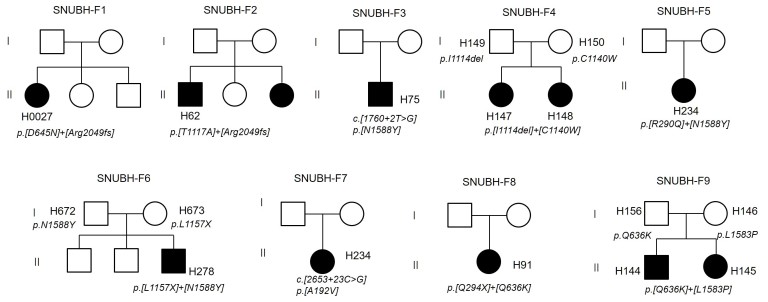
In this study, 11 patients (7 females and 4 males from 9 families) with pathogenic variants of ABCA4 were evaluated (Table 1). The median BCVA was 20/320 and ranged from the ability to distinguish hand motions to 20/20 vision. The median age at the onset of symptoms was 11 years (range: 4–48 years). Eight STGD patients from seven families, two CRD patients from one family, and one patient with early-onset RP were involved. Two heterozygous mutations were identified in eight unrelated individuals, and one variant was found in a patient (H830) with fundus flavimaculatus. All patients exhibited autosomal recessive or isolated inheritance patterns in their pedigrees (Figure 1). For three families (F4, F6, and F9), the parents’ DNA was collected and sequenced.
Table 1. Clinical features of ABCA4-associated retinopathies in Korean patients.
| Family |
Patients |
Sex |
Age |
Age at onset (years) |
Clinical diagnosis |
CDS variants |
Protein variation |
BCVA (Snellen) |
Fundus, FAG findings |
Full field ERG findings |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | ID | (years) | Right | Left | |||||||
| F1 |
H27 |
F |
28 |
NA |
STGD |
c.6146delA |
p.Lys2049ArgfsTer12 |
20/250 |
HM |
Flecks, macular atrophy |
Normal |
| |
|
|
|
|
|
c.1933G>A |
p.Asp645Asn |
|
Dark choroid |
||
| F2 |
H62 |
M |
23 |
11 |
STGD |
c.6146delA |
p.Lys2049ArgfsTer12 |
20/500 |
20/320 |
Flecks, macular atrophy |
Normal |
| |
|
|
|
|
|
c.3349A>G |
p.Thr1117Ala |
|
Dark choroid |
||
| F3 |
H75 |
M |
21 |
14 |
STGD |
c.4762A>T |
p.Asn1588Tyr |
20/500 |
20/200 |
Flecks, macular atrophy |
Normal |
| |
|
|
|
|
|
c.1760+2T>G |
Splice site |
|
Dark choroid |
||
| F4 |
H147 |
F |
28 |
11 |
STGD |
c.3420C>G c.3342_3344delCAT |
p.Cys1140Trp |
20/500 |
20/500 |
Flecks, macular atrophy |
Cone↓, Rod ↓ |
| |
|
|
|
|
|
|
p.Ile1114del |
|
Dark choroid, BM hole |
||
| F4 |
H148 |
F |
20 |
NA |
STGD |
c.3420C>G |
p.Cys1140Trp |
20/320 |
20/500 |
Flecks, macular atrophy |
Cone↓ |
| |
|
|
|
|
|
c.3342_3344delCAT |
p.Ile1114del |
|
Dark choroid |
||
| F5 |
H234 |
F |
22 |
16 |
STGD |
c.3470T>G |
p.Leu1157Ter |
20/125 |
20/125 |
Macular atrophy |
Rod↓↓ |
| |
|
|
|
|
|
c.869G>A |
p.Arg290Gln |
|
Dark choroid |
||
| F6 |
H278 |
M |
11 |
11 |
STGD |
c.4762A>T |
p.Asn1588Tyr |
20/63 |
20/100 |
Flecks |
Normal |
| |
|
|
|
|
|
c.3470T>G |
p.Leu1157Terr |
|
Macular atrophy |
||
| F7 |
H830 |
F |
51 |
48 |
FF |
c.575C>T |
p.Ala192Val |
20/20 |
20/20 |
Flecks |
Rod↓ |
| F8 |
H91 |
F |
18 |
4 |
RP |
c.1906C>A |
p.Gln636Lys |
CF |
HM |
Extensive CR atrophy |
No cone & rod response |
| |
|
|
|
|
(early-onset) |
c.880C>T |
p.Gln294Ter |
|
Diffuse pigmentation |
||
| F9 |
H144 |
F |
15 |
5 |
CRD |
c.4748T>C |
p.Leu1583Pro |
20/500 |
20/200 |
Macular atrophy |
Cone↓↓, Rod↓↓ |
| |
|
|
|
|
|
c.1906C>A |
p.Gln636Lys |
|
|
|
|
| F9 |
H145 |
M |
19 |
4 |
CRD |
c.4748T>C |
p.Leu1583Pro |
20/200 |
20/500 |
Macular atrophy |
Cone↓↓, Rod↓↓ |
| c.1906C>A | p.Gln636Lys |
||||||||||
BCVA, best-corrected visual acuity; STGD, Stargardt disease; FF, fundus flavimaculatus; RP, retinitis pigmentosa; CRD, cone-rod dystrophy; BM, Bruch’s membrane; CR, chorioretinal; HM, hand motion; CF, counting finger. p.R290Q in H234, a patient with STGD features, is a rare variant (allele frequency: 3.995e-6 (%) in gnomAD) and has not been reported. However, the pathogenicity of this variant was predicted as benign by most prediction programs, including polyphen, SIFT, and MutationTaster.
Figure 1.
Pedigrees of ABCA4-associated retinopathies. SNUBH-F1–F7 were diagnosed as Stargardt disease (STDG). SNUBH-F8 (H91) showed retinitis pigmentosa (RP), and SNUBH-F9 members (H144 and H145) showed cone-rod dystrophies (CRDs). The pathogenic variants of SNUBH-F4, F6, and F9 were also confirmed with their parents’ genotypes.
Retinal phenotypes and visual function
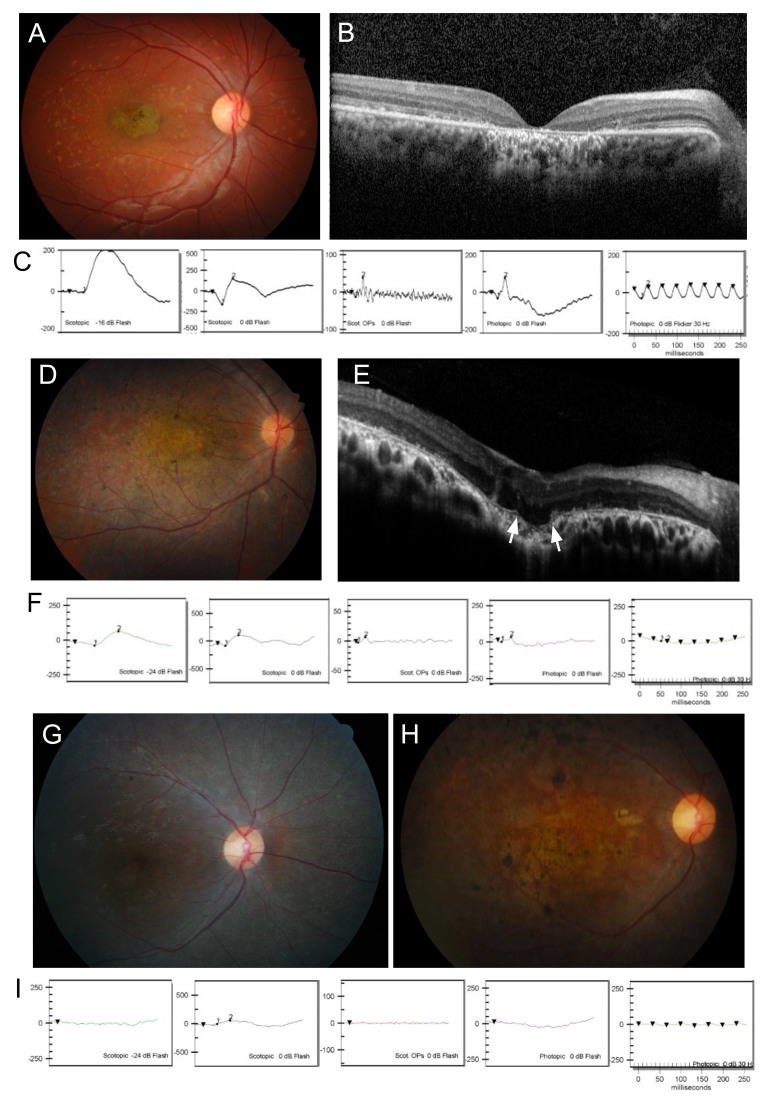
Clinically, all patients exhibited mild to severe phenotypes consistent with the described spectrum for ABCA4-RD. Patients with STGD showed typical features: a “beaten bronze metal” appearance in their macula and bilateral atrophic macular changes with or without diffuse pisciform flecks (Figure 2A, Figure 3, and Table 1). Patient H234 showed only bull’s-eye lesions without flecks. Fundus fluorescein angiography revealed a dark choroid sign in some patients. Multiple hyper-autofluorescent dots observed at earlier ages changed into larger hypo-autofluorescent lesions during follow-up periods. Outer retinal foveal atrophy and loss of the inner ellipsoid zone were observed after OCT scanning (Figure 2B). Patients with STGD showed a normal or subnormal response in their scotopic and photopic ERGs (Figure 2C). A patient with fundus flavimaculatus (H830) showed yellowish flecks in their RPE with no other anatomic or functional changes. Early clinical features in H144 and H145 of the F9 family appeared in the form of CRD. The cone response significantly decreased, while the rod response remained relatively unchanged on the ERG, and the fundus examination revealed macular dystrophy without flecks (Figure 2D,F). After five years, the retinal degenerative areas progressed beyond the macula, and bony spicule pigments appeared similar to those observed in RP. The most severe phenotype characterized by early-onset and extensive atrophy in the entire fundus was observed in patient H91 (Figure 2G–I). The BCVAs for H91 were 20/63 in the right eye and 20/200 in the left eye, and 20 prism diopters of the exotropia and nystagmus were observed at the age of nine years, when the patient first visited the hospital. During the next five years, retinal degeneration rapidly progressed, and severe macular atrophy and pigmentary changes were observed. At 18 years, BCVAs were lower (ability to count fingers through the right eye and respond to hand motions through the left eye) than those in CRD and STGD, and both rod and cone responses on ERGs were extinct. In patients with STGD, the DDAF and QDAF areas were not directly correlated with visual acuities, visual fields, or ERG response (Table 2). As shown in Figure 3 and Table 2, the DDAF and QDAF areas in patient H27 were relatively small, but the BCVA in her left eye was hand motions; on the contrary, the DDAF and QDAF areas in patients H75 and H278 were large, but their BCVAs were relatively high. All STGD patients showed central scotomas of less than five degrees to as much as 20 degrees in their visual fields.
Figure 2.
Clinical features of ABCA4-associated retinopathies. A, D, G, and H: Fundus photography. B and E: Optical coherence tomography (OCT). C, F, and I: Electroretinograms (ERGs). A, B, and C: The ophthalmologic examination of patient H147 showed typical features of STDG. Atrophy of photoreceptors and RPE in the macula is shown on fundus photography and OCT. D, E, and F: Patient H145 showed CRD with macular degeneration and reduced cone response. The white arrows indicate choroidal excavation and defect of the Bruch’s membrane. G and H: Fundus examination of patient H91 at 10 and 19 years of age, respectively. I: Both the cone and the rod responses on the ERG at the age of 10 years were strikingly decreased, suggesting typical features of RP.
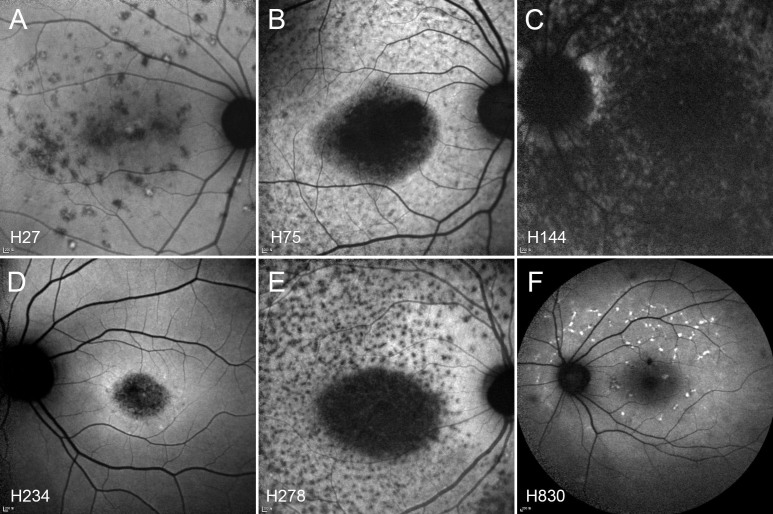
Figure 3.
Fundus autofluorescence (FAF) images. A: Multiple hypo-autofluorescence dots without a bull’s-eye pattern in patient H27. B and E: Typical FAF images of STGD, with round macular hypo-autofluorescence and multiple dots (H75 and H278). C: Hypo-autofluorescence on the entire macula in a patient with CRD (H144). D: Bull’s-eye maculopathy without flecks (H234). F: FAF images in a patient with fundus flavimaculatus (H830).
Table 2. Area of decreased autofluorescence and functional measurement.
| Patients |
DDAF (mm2) |
QDAF (mm2) |
Visual field |
Dark-adapted 0.01 ERG |
Light-adapted 3 ERG |
||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Right | Left | Right | Left | (method) | Rod amp | Rod IT | Cone amp | Cone IT |
| H27 |
0.13 |
0 |
0.13 |
1.68 |
≤10° CS (GVF) |
1.93 |
0.79 |
2.03 |
0.97 |
| H62 |
NA |
NA |
NA |
NA |
NA |
1.12 |
0.97 |
1.5 |
1.05 |
| H75 |
6.84 |
6.61 |
12.3 |
13.34 |
≤20° CS (GVF) |
1.75 |
0.99 |
0.72 |
0.98 |
| H147 |
NA |
NA |
NA |
NA |
≤10° CS (GVF) |
0.57 |
1.08 |
0.75 |
1 |
| H148 |
NA |
NA |
NA |
NA |
≤5° CS (GVF) |
1.02 |
1.14 |
0.7 |
1.04 |
| H234 |
0 |
0 |
2.67 |
2.46 |
≤5° CS (HVF) |
0.48 |
1.02 |
1.29 |
0.92 |
| H278 |
0.58 |
0.44 |
8.19 |
8.08 |
≤10° CS (HVF) |
3.04 |
1.03 |
4.8 |
0.95 |
| H830 |
0 |
0 |
0 |
0 |
Normal |
0.84 |
1.12 |
2.49 |
0.92 |
| H91 |
NA |
NA |
NA |
NA |
Extrafoveal VF island |
0 |
0 |
0 |
0 |
| H144 |
EA |
EA |
EA |
EA |
No detected |
0.43 |
1.25 |
0.64 |
0.83 |
| H145 | EA | EA | EA | EA | No detected | 0.41 | 1.29 | 0.53 | 1.09 |
DDAF, definitely decreased autofluorescence; QDAF, questionably decreased autofluorescence; GVF, Goldmann visual field test (III4e); HVF, Humphery visual field 24–2; amp, the ratio of b-wave amplitude compared to the normative value; IT, the ratio of b-wave implicit time compared to the normative value; NA, not applicable; CS, central scotoma; EA, the entire macula
Analysis of ABCA4 variants in Korean patients
In our analysis, 13 rare variants of ABCA4 were identified, and four novel variants, p.Gln636Lys, p.I1114del, p.Thr1117Ala, and p.Asn1158Tyr, were observed (Table 3). All the novel variants were predicted to be deleterious mutations by the pathogenicity prediction program. The novel variant p.Gln636Lys was present in each patient with RP and CRD. In-frame deletion p.Ile1114del was found in two siblings of the F4 family with a known pathogenic variant, p.Cys1140Trp. Novel variant p.Asn1588Tyr was repeatedly present in two different STGD families. Two patients commonly harbored variant c.6146delA (p.Lys2049ArgfsTer12), which has not been uploaded in ClinVar or other databases but was recently reported in two Korean patients, suggesting that it is frequently present in Korean STGD with ethnic specificity [12]. Two known variants, c.3342_3344delCAT (p.Ile1114del) and c.3349A>G, (p.Thr1117Ala), were also identified repeatedly in different families. All variants were typically predicted to be deleterious except two missense variants, p.Ala192Val and p.Arg290Gln. The previously reported p.Ala192Val variant showed low allele frequency (2.131e-4) and was predicted to be a disease-causing variant in MutationTaster but not in Polyphen or SIFT. Despite its rarity, variant p.R290Q was predicted as benign by Polyphen, SIFT, and MutationTaster and showed a low Phred score (7.2) in CADD.
Table 3. Profiles of pathogenic variants in Korean families with ABCA4-associated retinopathies.
| No. exon /intron | Nucleotide change | Protein variant / annotation | No. family† | Disease | Polyphen (score) | SIFT (score) | Mutation taster (ENST00000370225) | CADD Phred score (GRCh37-v1.4)‡ | Allele frequency (%) in gnomAD¶ | Clinical significance (ClinVar) |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 |
c.575C>T |
p.Ala192Val |
1 |
FF |
Benign |
Tolerated (0.58) |
Disease causing |
19.32 |
0.000213 |
Not provided§ |
| -0.353 |
(58/272,116) |
|||||||||
| 8 |
c.880C>T |
p.Gln294Ter |
1 |
RP |
NA |
NA |
Disease causing |
35 |
None |
Pathogenic |
| 12 |
c.1760+2T>G |
Splice site |
1 |
STGD |
NA |
NA |
NA |
23.1 |
None |
Pathogenic |
| 13 |
c.1906C>A |
p.Gln636Lys |
2 |
RP, CRD |
Possibly damaging |
Deleterious |
Disease causing |
26 |
None |
Novel |
| -0.634 |
0 |
|||||||||
| 13 |
c.1933G>A |
p.Asp645Asn |
1 |
STGD |
Probably damaging, (0.954) |
Deleterious (0) |
Disease causing |
28.4 |
1.99E-05 |
Not provided |
| (5/251,094) |
||||||||||
| 23 |
c.3342_3344delCAT |
p.Ile1114del |
1 |
STGD |
NA |
NA |
Disease causing |
NA |
None |
Novel |
| 23 |
c.3349A>G |
p.Thr1117Aal |
1 |
STGD |
Damaging |
Deleterious (0) |
Disease causing |
26.5 |
None |
Novel |
| 23 |
c.3420C>G, |
p.Cys1140Trp, |
1 |
STGD |
Probably damaging, (0.997) |
Deleterious (0) |
Disease causing |
25.8 |
None |
Reported#18 |
| 23 |
c.3470T>G, |
p.Leu1157Ter, |
2 |
STGD |
NA |
NA |
Disease causing |
43 |
None |
Reported12 |
| 33 |
c.4748T>C |
p.Leu1583Pro |
1 |
CRD |
Possibly damaging, (0.878) |
Deleterious (0) |
Disease causing |
25.9 |
7.95E-06 |
Not provided |
| (2/251,468) |
||||||||||
| 33 |
c.4762A>T, |
p.Asn1588Tyr |
2 |
STGD |
Probably damaging, (0.962) |
Deleterious (0.04) |
Disease causing |
26.7 |
None |
Novel |
| 44 | c.6146delA, | p.Lys2049ArgfsTer12 | 2 | STGD | NA | NA | Disease causing | NA | None | Reported12 |
†No. family, the number of families with the same variant; ‡CADD, https://cadd.gs.washington.edu/; ¶gnomAD, https://gnomad.broadinstitute.org/ §Not provided, reported but clinical significance was not determined; #Reported, recently reported but no information in ClinVar or other database.
Genotype–phenotype correlation
Patients who harbored the c.1760+2TG, c.1933G>A, c.3342_3344delCAT, c.3349A>G, c.3420C>G, c.3470T>G, c.4762A>T, and c.6146delA variants presented with typical STGD features—bilateral macular atrophy (bull’s-eye maculopathy) and blocked fluorescence—in fluorescein angiography. All variants in exon 23 of ABCA4 were associated with STGD in our cohort. One patient (H234) harboring the c.3470T>G (p.Leu1157Ter) and c.869G>A (p.Arg290Gln) variants showed bull’s-eye maculopathy without flecks. Two patients (H144 and H145) with CRD with c.1906C>A (p.Gln636Lys) and c.4748T>C (p.Leu1583Pro) showed a central defect in the RPE-Bruch’s membrane with choroidal excavation (Figure 2E). These patients also showed hypo-autofluorescence over the entire macula, consistent with fundus and OCT findings (Figure 3C). The patient with the p.Gln294Ter variant showed the early-onset RP phenotype, and the p.Gln636Lys variant was associated with early-onset RP and CRD in our cohort. Compared to patients with STGD, patients with RP and CRD showed a tendency to harbor at least one causative variant (p.Gln294Ter or p.Gln636Lys) in earlier exon regions (exons 8 and 13) of ABCA4 (Table 3). No other significant associations between genotypes, FAF, and visual function were found in our cohort.
Discussion
One of the most frequent genetic causes of retinal dystrophies is mutation in ABCA4, which encodes a retina-specific ABC transporter expressed in rod and cone photoreceptors [15]. ABCA4 mutations lead to the accumulation of toxic bisretinoid adducts of all-trans retinal within the photoreceptors and RPE [3,16]. In this study, all the STGD cases showed typical clinical features, with patients exhibiting symptoms in their teen years and visual acuity below 20/125 owing to the disease progression in their 20s. Although ABCA4-RD is considered to initiate in the vicinity of the macula and cause a decrease in central vision, the disease progresses to various extents and is not limited to the macular area; progression often occurs from the macula to the extramacular regions. Thus, RP-like disease patterns can be observed in patients with ABCA4 mutations, as observed in patient H91. This patient showed the most severe phenotype, characterized by aggressive, early-onset RP, and harbored an early nonsense mutation, p.Gln294Ter, in exon 8 of ABCA4 and a deleterious missense mutation from glutamine to lysine (p.Gln636Lys) in exon 13 of ABCA4. Glutamine is an amino acid with a polar uncharged side chain; the substitution of glutamine with lysine causes this side chain to be electrically charged. In this study, this mutation was found in both early-onset RP and CRD. On the basis of initial features and electrophysiologic findings, CRD was diagnosed in patients H144 and H145 who harbored the p.Gln636Lys mutation; however, the disease rapidly progressed beyond the macula, and profound destruction of both the rod and cone areas occurred, as previously observed in rapid onset ABCA4-RD [17]. Therefore, p.Gln294Ter and p.Gln636Lys are associated with rapidly progressive, early-onset ABCA4-RD with the severe phenotype.
In the gnomAD browser (accessed: February 13, 2019), 1,303 missense variants and 87 loss-of-function variants of ABCA4 were observed. ABCA4 encodes a large protein composed of 2,272 amino acids and more than 11 transmembrane domains, as well as an ATP-binding site, a Walker A/P loop, and D-, H-, and Q-loops (NP_000341.2). This complexity is associated with numerous variants, and the identification of disease-causing mutations can be challenging. Thus, categorizing new ABCA4 variants as pathogenic is likely highly important for molecular diagnosis in some patients. In this study, all four novel mutations (p.Gln636Lys, p.Ile1114del, p.Thr1117Ala, and p.Leu1588YTyr) were highly deleterious and are not listed in gnomAD, indicating that they have extremely low allele frequencies. Amino acids 1114 and 1117 in ABCA4 protein are included in the H-loop/switch region (1,114–1,120) and at the ATP-binding site (966–1,118), suggesting that deletion of isoleucine 1114 or substitution of threonine 1117 can result in a dysfunctional ABCA4 protein. Variant c.4762A>T (p.Asn1588Tyr) was detected in two different families with STGD, suggesting that this variant is the disease-causing mutation for STGD. Variant c.575C>T (p.Ala192Val) was predicted as a pathogenic variant through a FATHMM prediction (score 0.94) and MutationTaster. Moreover, in the same locus as p.Ala192Val but involving a different protein change, the Ala192Thr variant (alanine to threonine) in ABCA4 was reported previously in an individual with STGD who was compound heterozygous for the Ala192Thr variant and another variant [18,19]. However, p.Ala192Val was predicted to be benign by Polyphen and SIFT, and its allele frequency in gnomAD (58/272,116) was higher than that of other variants. Thus, p.Ala192Val may cause STGD; however, this could not be confirmed. Variants p.Cys1140Trp and p.Leu1157Ter are not present in the ClinVar database; however, they were identified in patients with STGD in recent reports as well as in two different families in our study, which suggests that they cause STGD [12,20].
The regional and ethnic specificity of several variants has been reported [5]. The founder variant c.2588G>C (p.[Gly863Ala, Gly863del]) was reported to be associated with the European population, and the complex alleles c.[1622T>C;3113C>T] (p.[Leu541Pro;Ala1038Val]) and the variant c.2894A>G (p.Asn965Ser) were predominantly found in individuals of German and Danish descent, respectively [21-24]. Moreover, the c.6320G>A (p.Arg2107His) variant accounted for 19.3% of all disease-associated alleles in a large cohort of African-American patients with STGD [11]. Although several studies of the genotype spectrum and phenotypic correlation in Asian ABCA4-RD have recently been conducted, the data remain insufficient. Recently, c.859–9T>C was reported as a highly frequent variant in an Indian ABCA4-RD cohort [5]. Three novel ABCA4 mutations, IVS7–45_952delinsTCTGACC, IVS12+2T>G, and p.Arg2149Ter, were reported in Japanese patients with STGD [13]. Moreover, c.5646G>A (p.Met1882Ile) and c.3523–2A>G were recently identified as disease-causing novel variants in a Chinese STGD pedigree [25]. Such regional and ethnic specificities of the same variants were also observed in our cohort. In a recent report, three novel variants, c.880C>T (p.Gln294Ter), c.3470T>G (p.Leu1157Ter), and c.6146delA (p.Lys2049ArgfsTer12), were identified in Korean patients with STGD [12]. In the present study, the same variants were also found in patients with ABCA4-RD, despite the small number of people in the cohort. These variants have not previously been reported, and no information was available in gnomAD. Variant p.Gln294Ter induces early truncation of the ABCA4 protein, leading to a profound loss of function, and p.Leu1157Ter induces premature termination at the approximate midpoint of the ABCA4 protein. MutationTaster predicted that p.Lys2049ArgfsTer12 causes the resultant protein to undergo nonsense-mediated decay through selective degradation at the mRNA level. Two pathogenic variants, p.Arg2040Ter and p.Lys2056Ter, which induce early truncation of ABCA4 near the region of p.Lys2049ArgfsTer, have been identified as pathogenic variants of ABCA4-RD. Considering the rarity of p.Gln294Ter, p.Leu1157Ter, and p.Lys2049ArgfsTer12, as well as their repeated detection in Koreans with ABCA4-RD, these variants may be more frequent in Korean patients with ABCA4-RD than in patients of other ethnicities.
In summary, we described the clinical and genetic spectra of ABCA4-RD in Korean patients. Four novel variants, p.Gln636Lys, p.Ile1114del, p.Thr1117Ala, and p.Asn1588Tyr, were identified, and p.Gln294Ter, p.Leu1157Ter, and p.Lys2049ArgfsTer12 were frequently detected in a Korean population with ABCA4-RD. This information will be useful for understanding the clinical features of ABCA4-RD and ethnicity-specific variants in East Asians.
Acknowledgments
This study was supported by a research grant from the Seoul National University Bundang Hospital (02–2017–059 and 14–2018–019); Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science, and Technology (grant no. 2012R1A1A2006958); and NRF grant from the Ministry of Science, ICT, and Future Planning (MSIP; grant no. 2016R1D1A1B03934724, 2017R1A2B2011436, and 2019R1C1C1009345). The funding organizations had no role in the design or conduct of the study. Se Joon Woo (sejoon1@snu.ac.kr) and Sung Sup Park (sparkle@snu.ac.kr) are co-corresponding authors for this paper. Address for reprints: Department of Ophthalmology, Seoul National University Bundang Hospital, 173–82 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 13,620, Republic of Korea South Korea
References
- 1.Lin B, Cai XB, Zheng ZL, Huang XF, Liu XL, Qu J, Jin ZB. Clinical and genetic analyses reveal novel pathogenic ABCA4 mutations in Stargardt disease families. Sci Rep. 2016;6:35414. doi: 10.1038/srep35414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cideciyan AV, Swider M, Aleman TS, Tsybovsky Y, Schwartz SB, Windsor EA, Roman AJ, Sumaroka A, Steinberg JD, Jacobson SG, Stone EM, Palczewski K. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Genet. 2009;18:931–41. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quazi F, Lenevich S, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salles MV, Motta FL, Dias da Silva E, Varela P, Costa KA, Filippelli-Silva R, Martin RP, Chiang JP, Pesquero JB, Sallum JMF. Novel Complex ABCA4 Alleles in Brazilian Patients With Stargardt Disease: Genotype-Phenotype Correlation. Invest Ophthalmol Vis Sci. 2017;58:5723–30. doi: 10.1167/iovs.17-22398. [DOI] [PubMed] [Google Scholar]
- 5.Lee W, Schuerch K, Zernant J, Collison FT, Bearelly S, Fishman GA, Tsang SH, Sparrow JR, Allikmets R. Genotypic spectrum and phenotype correlations of ABCA4-associated disease in patients of south Asian descent. Eur J Hum Genet. 2017;25:735–43. doi: 10.1038/ejhg.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garces F, Jiang K, Molday LL, Stohr H, Weber BH, Lyons CJ, Maberley D, Molday RS. Correlating the Expression and Functional Activity of ABCA4 Disease Variants With the Phenotype of Patients With Stargardt Disease. Invest Ophthalmol Vis Sci. 2018;59:2305–15. doi: 10.1167/iovs.17-23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101:25–30. doi: 10.1136/bjophthalmol-2016-308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zernant J, Schubert C, Im KM, Burke T, Brown CM, Fishman GA, Tsang SH, Gouras P, Dean M, Allikmets R. Analysis of the ABCA4 gene by next-generation sequencing. Invest Ophthalmol Vis Sci. 2011;52:8479–87. doi: 10.1167/iovs.11-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JO, Hwang S, Kim WY, Park SJ, Kim SC, Park K, Lee B. Consortium HP-AS. Identification of ethnically specific genetic variations in pan-asian ethnos. Genomics Inform. 2014;12:42–7. doi: 10.5808/GI.2014.12.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsipi M, Tzetis M, Kosma K, Moschos M, Braoudaki M, Poulou M, Kanavakis E, Kitsiou-Tzeli S. Genomic screening of ABCA4 and array CGH analysis underline the genetic variability of Greek patients with inherited retinal diseases. Meta Gene. 2016;8:37–43. doi: 10.1016/j.mgene.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zernant J, Collison FT, Lee W, Fishman GA, Noupuu K, Yuan B, Cai C, Lupski JR, Yannuzzi LA, Tsang SH, Allikmets R. Genetic and clinical analysis of ABCA4-associated disease in African American patients. Hum Mutat. 2014;35:1187–94. doi: 10.1002/humu.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung Y, Choi SW, Shim SH, Song WK. Clinical and Genetic Characteristics Analysis of Korean Patients with Stargardt Disease Using Targeted Exome Sequencing. Ophthalmologica. 2019;241:38–48. doi: 10.1159/000490073. [DOI] [PubMed] [Google Scholar]
- 13.Fukui T, Yamamoto S, Nakano K, Tsujikawa M, Morimura H, Nishida K, Ohguro N, Fujikado T, Irifune M, Kuniyoshi K, Okada AA, Hirakata A, Miyake Y, Tano Y. ABCA4 gene mutations in Japanese patients with Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:2819–24. [PubMed] [Google Scholar]
- 14.Strauss RW, Munoz B, Ho A, Jha A, Michaelides M, Mohand-Said S, Cideciyan AV, Birch D, Hariri AH, Nittala MG, Sadda S, Scholl HPN. ProgStar Study G. Incidence of Atrophic Lesions in Stargardt Disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: Report No. 5. JAMA Ophthalmol. 2017;135:687–95. doi: 10.1001/jamaophthalmol.2017.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cideciyan AV, Swider M, Schwartz SB, Stone EM, Jacobson SG. Predicting Progression of ABCA4-Associated Retinal Degenerations Based on Longitudinal Measurements of the Leading Disease Front. Invest Ophthalmol Vis Sci. 2015;56:5946–55. doi: 10.1167/iovs.15-17698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsybovsky Y, Orban T, Molday RS, Taylor D, Palczewski K. Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter. Structure. 2013;21:854–60. doi: 10.1016/j.str.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Lee W, Zernant J, Schuerch K, Ciccone L, Tsang SH, Sparrow JR, Allikmets R. The Rapid-Onset Chorioretinopathy Phenotype of ABCA4 Disease. Ophthalmology. 2018;125:89–99. doi: 10.1016/j.ophtha.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch DG, Peters AY, Locke KL, Spencer R, Megarity CF, Travis GH. Visual function in patients with cone-rod dystrophy (CRD) associated with mutations in the ABCA4(ABCR) gene. Exp Eye Res. 2001;73:877–86. doi: 10.1006/exer.2001.1093. [DOI] [PubMed] [Google Scholar]
- 19.Webster AR, Heon E, Lotery AJ, Vandenburgh K, Casavant TL, Oh KT, Beck G, Fishman GA, Lam BL, Levin A, Heckenlively JR, Jacobson SG, Weleber RG, Sheffield VC, Stone EM. An analysis of allelic variation in the ABCA4 gene. Invest Ophthalmol Vis Sci. 2001;42:1179–89. [PubMed] [Google Scholar]
- 20.Zhang X, Ge X, Shi W, Huang P, Min Q, Li M, Yu X, Wu Y, Zhao G, Tong Y, Jin ZB, Qu J, Gu F. Molecular diagnosis of putative Stargardt disease by capture next generation sequencing. PLoS One. 2014;9:e95528. doi: 10.1371/journal.pone.0095528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maugeri A, van Driel MA, van de Pol DJ, Klevering BJ, van Haren FJ, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Dahl N, Brunner HG, Deutman AF, Hoyng CB, Cremers FP. The 2588G→C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet. 1999;64:1024–35. doi: 10.1086/302323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maugeri A, Klevering BJ, Rohrschneider K, Blankenagel A, Brunner HG, Deutman AF, Hoyng CB, Cremers FP. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67:960–6. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera A, White K, Stohr H, Steiner K, Hemmrich N, Grimm T, Jurklies B, Lorenz B, Scholl HP, Apfelstedt-Sylla E, Weber BH. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67:800–13. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg T, Klie F, Garred P, Schwartz M. N965S is a common ABCA4 variant in Stargardt-related retinopathies in the Danish population. Mol Vis. 2007;13:1962–9. [PubMed] [Google Scholar]
- 25.Zhang J, Qi A, Wang X, Pan H, Mo H, Huang J, Li H, Chen Z, Wei M, Wang B. Novel compound heterozygous mutations in ABCA4 in a Chinese pedigree with Stargardt disease. Mol Vis. 2016;22:1514–21. [PMC free article] [PubMed] [Google Scholar]