Abstract
Purpose
Age-related cataract (ARC) is the leading cause of visual impairment and blindness worldwide. The apoptosis of lens epithelial cells (LECs) induced by oxidative damage is a major contributing factor to ARC. Long noncoding RNAs (lncRNAs) play important roles in various biologic processes. We aimed to explore the role of glutathione peroxidase 3 (GPX3)-antisense (AS) in ARCs.
Methods
We extracted total RNAs from transparent and age-matched cataractous human lenses and detected lncRNA expression profiles using high-throughput RNA sequencing. The expression of GPX3-AS and GPX3 was detected by quantitative real-time PCR (qRT-PCR). Apoptotic proteins were detected by western blot and immunofluorescence. We treated SRA01/04 cells with H2O2 to mimic oxidative stress and induce cell apoptosis, which was analyzed by flow cytometry and TdT-mediated dUTP Nick-End Labeling (TUNEL) assay. The cell counting kit-8 (CCK-8) assay was used to detect the viability of SRA01/04 cells. The location of GPX3-AS was determined by fluorescence in situ hybridization (FISH) and cell nuclear and cytoplasmic RNA separation.
Results
The lncRNA GPX3-AS, which is located in the nuclei of LECs, was downregulated in cataractous human lenses compared with control lenses, and proapoptotic proteins were expressed at high levels in the anterior lens capsules of ARC tissues. An in vitro study suggested that GPX3-AS inhibited H2O2-induced SRA01/04 cell apoptosis. As GPX3-AS is transcribed from the AS strand of the GPX3 gene locus, we further revealed its regulatory role in GPX3 expression. GPX3-AS was positively correlated with GPX3 expression. In addition, GPX3-AS inhibited H2O2-induced SRA01/04 cell apoptosis by upregulating GPX3 expression.
Conclusions
In summary, our study revealed that GPX3-AS downregulated the apoptosis of LECs via promoting GPX3 expression, implying a novel therapeutic target for ARCs.
Introduction
Age-related cataract (ARC) is a common cause of visual impairment and blindness among elderly individuals worldwide [1]. Cataract surgery is currently the most effective therapeutic method for treating ARC [2]. However, surgery is invasive, and it inevitably brings multiple complications, including posterior capsule tearing and vitreous prolapse [3]. Furthermore, many people, especially those in developing countries, become blind from cataracts because of inadequate surgery availability or unaffordable surgical expenses. Therefore, ascertaining the determinant factor in cataract pathogenesis is crucial for reducing the cataract incidence and rate of blindness.
The pathogenesis of ARC is not completely understood. Substantial evidence shows that oxidative stress is a major predisposing factor in ARC [4-7]. H2O2 is a major reactive oxygen species (ROS) present in the aqueous humor and lens [8]. Cataract patients may have deficient defense systems, such as oxidative stress at the onset of the disease [9]. Such stress triggers the apoptosis of lens epithelial cells (LECs), thereby initiating cataract development [10,11]. Therefore, LEC apoptosis induced by oxidative stress appears to be a common cellular basis for the development of noncongenital cataracts [12].
Long noncoding RNAs (lncRNAs) are transcripts longer than 200 nucleotides with similar structures to protein-coding mRNAs, which have little or no protein-coding capability [13]. LncRNAs play regulatory roles in diverse biological processes, including cell apoptosis [14,15]. LncRNA dysfunction is involved in multiple human diseases, such as cancer, neurological problems, and cardiovascular diseases. Recently, several studies have confirmed that lncRNAs have a close relationship with ARCs [16-18]. To date, several isoforms of glutathione peroxidase (GPX) proteins have been identified. Of these, only GPX3, which scavenges H2O2 and peroxidized organic molecules to reduce systemic oxidative stress, is secreted [19]. Cortical neurons subjected to O2 deprivation and low glucose (ODLG) display a loss of mitochondrial respiration. However, despite ROS production, neither necrosis nor apoptosis occurs. The absence of cellular death is a consequence of increased antioxidants, such as superoxide dismutase-1 (SOD1) and GPX3 [20]. In addition, GPX3 has been shown to regulate the antioxidative effects of retinoic acid and promote the viability of human muscle stem cells [21].
The relationship between GPX3-antisense (AS) and ARC is unclear. In the present study, we identified the novel ARC-associated lncRNA GPX3-AS. We aimed to reveal the roles of GPX3-AS in ARC and seek a potential lncRNA-based therapeutic target.
Methods
Clinical sample collection
The transparent lens epithelium samples were collected from the patients with vitreoretinal diseases (no other ocular diseases or systemic diseases,5 for RNA sequence and 20 for qRT-PCR verification) and ARC patients (no other ocular diseases or systemic diseases,6 for RNA sequence and 60 for qRT-PCR verification). We divided ARC patients into three groups according to the location of the lens opacity: the age-related cortical cataract (ARCC), age-related nuclear cataract (ARNC), and age-related posterior subcapsular cataract (ARPC) groups, which had 20 patients each. The specific criteria can be referred to our previous studies [22]. All of them had their lenses removed at the Affiliated Hospital of Nantong University (Nantong, China) from January 2017 to June 2018 and the anterior lens capsules that had torn off during surgery were collected immediately.
This study protocol was approved by the Affiliated Hospital of Nantong University. All experiments were performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients prior to the study.
Tissue collection and RNA extraction
The collected samples were stored in liquid nitrogen. Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher, Waltham, MA). The yield of the RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher). LncRNA high-throughput sequencing analysis, which included labeling, hybridization, scanning, normalization, and data analysis, was performed by Ribobio Technology (Guangdong, China).
RNA sequencing
A quality check of the input total RNA was performed by running an aliquot on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA) to confirm its integrity, and the RNA concentration was measured using an ultramicrospectrophotometer. A 3-μg quantity of RNA was selected as the initial amount of each sample from which to construct an lncRNA library, following removal of the rRNA in the sample using Ribo-Zero™ Gold kits (Cat. No. GZG122; Epicenter, Madison, WI). Different index tags were selected according to the New England Biolabs (NEB) Next Ultra database direct operating protocol (NEB Next Ultra Directional RNA Library Prep kit for Illumina; Cat. No. E7760; NEB, Ipswich, MA). The specific steps of library construction were as follows: First, the Ribo-Zero™ Gold kit was used to remove rRNA. Then, fragmentation buffer was added to the reaction system to fragment the RNA into short segments, and the short fragments were used as templates for first-strand cDNA synthesis using random six-base primers (random hexamers). This was followed by second-strand cDNA synthesis using DNA polymerase I and RNaseH. The cDNA fragments were then subjected to 3′-end repair, the addition of a single “A” base, and the ligation of Illumina multiplexing adapters. The products were purified and enriched by PCR to create the final cDNA sequencing library. During PCR, the thermocycling conditions were as follows: step 1, 65 °C for 15 min; step 2, 30 °C for 10 min, 42–50 °C for 45 min, and 95 °C for 5 min.
Data processing
The raw data were filtered to provide high-quality, clean reads, and a follow-up analysis was conducted. The data processing steps were as follows: i) removal of contaminated reads, ii) removal of low-quality reads, and iii) removal of reads containing >5% bases with nitrogen content [23].
LncRNA analysis
Only lncRNAs were analyzed in the present study. According to the characteristics of the lncRNA, a series of strict filters were used. The filter conditions were a length of ≥200 bp, an exon number of ≥2, and minimum coverage of ≥3 transcription reads. Known mRNA transcripts, known noncoding RNA transcripts, homologous transcripts, and transcripts with protein-coding capability were removed.
Read comparison
In this study, we used TopHat software version 2.0.12 (Center for Computational Biology, Johns Hopkins, Baltimore, MD) with the default parameters to compare the reference sequence to hg19 [24].
Differential expression analysis
The cataract and normal samples from each group were subjected to differential expression analysis using DESeq2. Differential expression analysis was performed following standardization and variance estimation. The standards used when screening the differences in mRNA and lncRNA expression were p<0.001, a false discovery rate (FDR) of <0.001, and a |log2 ratio| >2 [25].
Cell lines and treatment
The SRA01/04 (Jennio Biotech Company, Guangzhou, China) human lens epithelial cell (HLEC) line was grown in Dulbecco’s modified Eagle medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Gibco), penicillin (100 U/ml), and streptomycin (100 mg/ml). The cells were maintained at 37 °C in a humidified incubator in 5% CO2. At 80–90% confluence, the cells were exposed to H2O2 (200 µM) for 24 h to mimic oxidative stress.
Total RNA was extracted from SRA01/04 cells with TRIzol reagent (Invitrogen), and quantitative real-time PCR (qRT-PCR) was performed with the powerUP SYBR Green Master Mix (Cat. No. A25742, Applied Biosystems, Foster City, CA). Relative gene expression was calculated using the 2-△△CT method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control. The AS oligos (ASOs) and overexpressed plasmid were purchased from RiboBio (Guangzhou, China) and GenePharma (Suzhou, China; Table 1), respectively. Cell transfection was performed with Lipofectamine 2000 (Invitrogen).
Table 1. The detailed information on the ASO and plasmids used in this study.
| Product catalog number | Product name | Forward (5’-3’) |
|---|---|---|
| lnc6180329053341 |
ASO-h-NONHSAT204874.1_001 |
GGTATCAGTTAGAGCAGAAC |
| lnc6180329053354 |
ASO-h-NONHSAT204874.1_002 |
GAACTGGAGAGAAAGGGTTG |
| lnc6180329053428 |
ASO-h-NONHSAT204874.1_003 |
GCCTTGAGTGATAGGAAAGG |
| siB161011044323 |
Scramble siRNA |
GGCUCUAGAAAAGCCUAUGCdTdT |
| stB0006520A |
GPX3 siRNA1 |
CCATGAAGGTTCACGACAT |
| stB0006520B |
GPX3 siRNA2 |
GTGGCACCATTTACGAGTA |
| stB0006520C | GPX3 siRNA3 | TCCCTAATTTCCAGCTCTT |
Short tandem repeat analysis
Nineteen short tandem repeat (STR) loci plus the gender-determining locus, amelogenin, were amplified using the commercially available EX20 Kit from AGCU (Cat. No. 600,010, Suzhou, China). A sample from the cell line was processed using the ABI Prism® 3500 Genetic Analyzer. The data were analyzed using GeneMapper® ID-X v 1.2 software (Applied Biosystems, Suzhou, China). Appropriate positive and negative controls were run and confirmed for each sample submitted.
Cell viability assays
Cell viability was examined by Cell Counting Kit-8 (CCK-8) assay (Cat. No. CK04–500T, Dojindo Laboratories, Japan). Cells were seeded in 96-well plates at a density of 4 × 104 cells/well. After transfection or treatment, 10 µl of CCK-8 solution was added to each well, and the cells were incubated at 37 °C for 2 h. Finally, the absorbance at 450 nm was calculated.
Flow cytometry analysis
After H2O2 treatment or transfection, SRA01/04 cells were washed twice with PBS (in 800ml distilled H2O. 8g of NaCl; 0.2g of KCl; 1.44g of Na2HPO4; 0.24g of KH2PO4) and collected for apoptosis assay. Apoptosis was examined by annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) labeling with an apoptosis detection kit (Cat. No. AD10–50T, Dojindo Laboratories). The samples were analyzed within 60 min by flow cytometry (BD Biosciences) after incubation for 15 min at room temperature (RT) in the dark.
Western blot analysis
Proteins from human lens tissue and SRA01/04 cell lysates were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride (PVDF) membrane. Primary mouse antibodies against caspase-3 (Abcam, UK), BCL2 associated X(Bax), B-cell lymphoma-2(Bcl-2), and GAPDH (Proteintech), as well as a secondary antibody, horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG; Sigma), were used. Band intensities were quantified by Quantity One software. The protein levels were normalized to the GAPDH level.
Fluorescence in situ hybridization
To detect GPX3-AS expression in SRA01/04 cells, Cyanine 3 Dye (Cy3)-labeled probes for GPX3-AS, 18S, and U6 (RiboBio, Guangzhou, China) were used for fluorescence in situ hybridization (FISH). Briefly, SRA01/04 cells were fixed with 4% paraformaldehyde at RT for 15 min, permeabilized in 0.5% Triton X-100 for 10 min on ice, and then treated with prehybridization buffer for 30 min at 37 °C. Next, we hybridized the cells with Cy3-labeled probes in a moist, dark chamber at 37 °C. After 12 h, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI). Finally, the FISH results were obtained by a confocal microscope (SP8, Leica, Germany).
TdT-mediated dUTP Nick-End Labeling (TUNEL) staining
An In Situ Cell Death Detection Kit (TUNEL fluorescence FITC kit, Cat. No. 11,684,795,910, Roche) was used to detect cell apoptosis. SRA01/04 cells were placed in 24-well culture plates. After treatment, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 on ice for 5 min. Then, 50 μl of TUNEL reaction mixture was added to the samples and incubated at 37 °C for 60 min. The nuclei were stained with DAPI. TUNEL staining was assessed via fluorescence microscopy (Leica, Germany).
Nuclear and cytoplasmic cellular RNA isolation
Nuclear and cytoplasmic fractions were segregated using a protein and RNA isolation system (PARIS) Kit (Cat. No. AM1556, Life Technologies). Cells were washed twice with PBS, resuspended in precooled cell fractionation buffer (300 μl), and incubated for 10 min on ice. Following centrifugation for 5 min at 500 ×g and 4 °C, the supernatant (cytoplasm) and pellet (nucleus) were segmented. RNA was isolated from both the cytoplasmic and nuclear fractions by a binding and washing procedure. The RNA was prepared for the following reverse transcription reaction, and GPX3-AS was verified using qRT-PCR. U6 RNA and GAPDH mRNA were selected as the nuclear and cytoplasmic control transcripts, respectively.
Immunofluorescence
SRA01/04 cells were fixed with 4% paraformaldehyde for 20 min at RT after treatment and then permeabilized with 0.5% Triton X-100, blocked with bovine serum albumin (BSA), and incubated with primary antibody for GPX3 (Proteintech) at 4 °C overnight. After washing with PBS, the LECs were incubated with the corresponding DyLight 488-conjugated secondary antibodies (EarthOx, San Francisco, CA). Cellular nuclei were counterstained with DAPI. Immunofluorescence was observed with a fluorescence microscope (Leica).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) was applied for statistical analysis. The Student t-test was used for comparisons between groups. All presented data were based on biologic triplicates. Data are shown as the mean ± standard error of the mean (SEM), and p<0.05 indicates statistical significance.
Results
LncRNA GPX3-AS decreases and proapoptotic proteins increase in the anterior lens capsules of ARC tissues
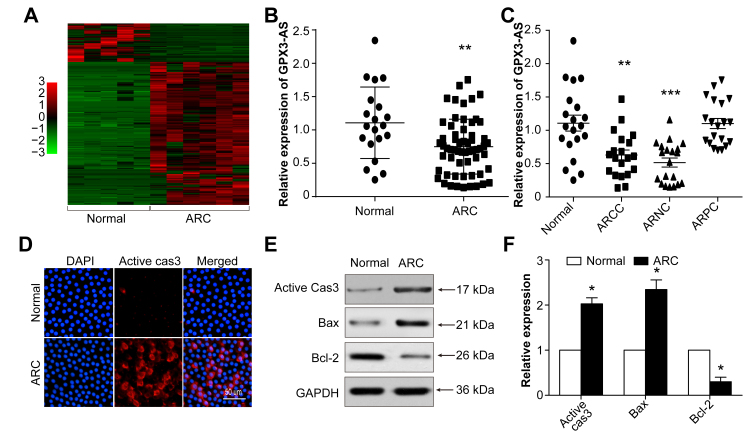
To identify ARC-associated lncRNAs, we analyzed RNA sequencing data from six anterior lens capsules from cataract patients and five matched normal capsular membranes. A total of 231 differentially expressed lncRNAs, including 49 downregulated and 182 upregulated lncRNAs, were detected (Figure 1A). GPX3-AS (NONCODE Transcript ID: NONHSAT204874.1; NONCODE Gene ID: NONHSAG091317.1) was decreased in the anterior lens capsules of ARCs compared with its expression in normal tissues, as shown by the sequencing results and verified to be consistent with the sequencing results of the expanded samples by qRT-PCR (Figure 1B). Subgroup analysis performed to identify which ARC subtype was mostly correlated with GPX3-AS suggested that GPX3-AS expression was most downregulated in the ARCC and ARNC groups (Figure 1C). We also measured apoptosis-related protein expression in the anterior lens capsules by immunofluorescence and western blot. The expression levels of active caspase-3 and Bax were upregulated, while Bcl-2 was decreased (Figure 1D-F). These data imply that GPX3-AS may be correlated with cell apoptosis during cataract formation.
Figure 1.
Long noncoding RNA (lncRNA) glutathione peroxidase 3–antisense (GPX3-AS) is decreased and apoptosis-related proteins are increased in the anterior lens capsules of age-related cataract (ARC) tissue. A: A heat map sequence analysis shows differentially expressed lncRNAs in the anterior lens capsules of transparent (n = 5) and ARC lenses (n = 6). B: GPX3-AS levels in the anterior lens capsules of the normal and ARC groups were detected by quantitative real-time PCR (qRT-PCR). *p<0.05 versus normal group. C: GPX3-AS levels in the normal, age-related cortical cataract (ARCC), age-related nuclear cataract (ARNC), and age-related posterior subcapsular cataract (ARPC) groups were detected by RT-PCR. *p<0.05 versus normal group. D: Anterior lens capsules were stained for active caspase-3 (red). The nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bar = 100 μm. E, F: Active caspase-3, BCL2 associated X(Bax), B-cell lymphoma-2(Bcl-2) expression in the anterior lens capsules of normal and cataract tissues was detected by western blot. *p<0.05 versus each normal group. n = 6 per group.
GPX3-AS is mainly localized in the SRA01/04 cell nucleus
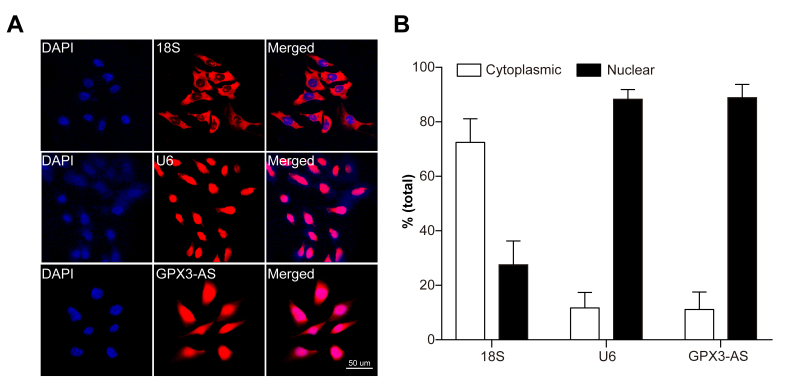
We identified the subcellular localization of GPX3-AS. RNA FISH and nuclear separation experiments showed that GPX3-AS was expressed in HLECs (SRA01/04 cells) and mainly localized in the nucleus (Figure 2A,B).
Figure 2.
Glutathione peroxidase 3–antisense (GPX3-AS) is mainly localized in SRA01/04 cell nuclei. A: The subcellular localization of GPX3-AS in SRA01/04 cells was detected by fluorescence in situ hybridization (FISH). U6 was used as a representative for nuclear localization, and 18S was used as a representative for cytoplasmic localization. B: Nuclear and cytoplasmic RNA isolation showed that GPX3-AS was mainly localized in the nuclei of SRA01/04 cells. The U6 and 18S mRNAs were selected as the nuclear and cytoplasmic control transcripts, respectively. n = 5 per group.
GPX3-AS expression decreases and SRA01/04 cell apoptosis increases following H2O2 exposure
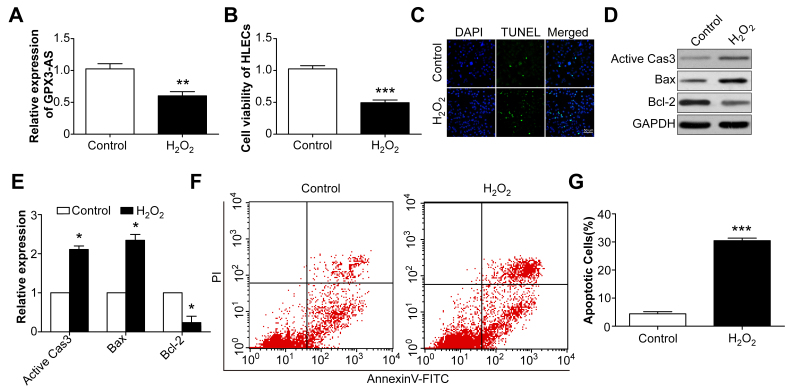
SRA01/04 cells were exposed to H2O2 (200 μM) for 24 h to mimic oxidative stress, following which, GPX3-AS levels decreased (Figure 3A). In addition, the SRA01/04 cell viability was inhibited by H2O2 treatment (Figure 3B). Correspondingly, the TUNEL assay showed that H2O2 induced SRA01/04 cell apoptosis (Figure 3C), and flow cytometry revealed increased apoptosis after H2O2 treatment (Figure 3F,G). Furthermore, western blotting showed that active caspase-3 and Bax were upregulated, while Bcl-2 was decreased (Figure 3D,E). The data suggest that H2O2 treatment induced a decrease in GPX3-AS levels and increased SRA01/04 cell apoptosis, implying a relationship between GPX3-AS and SRA01/04 cell apoptosis.
Figure 3.
Decreased glutathione peroxidase 3–antisense (GPX3-AS) expression and increased human lens epithelial cell (HLEC) apoptosis are induced by H2O2. A: SRA01/04 cells were treated with H2O2 for 24 h, and the GPX3-AS levels were detected by quantitative real-time PCR (qRT-PCR). **p<0.01 versus control group. B: Cell viability was detected by Cell Counting Kit-8 (CCK-8) assay. ***p<0.001 versus control group. C: TUNEL staining of SRA01/04 cells with or without H2O2 treatment. Scale bar = 50 μm. D, E: Active caspase-3, Bax, and Bcl-2 expression in SRA01/04 cells was detected by western blot. *p<0.05 versus each control group. F, G: Flow cytometry analysis of H2O2-induced apoptosis. ***p<0.001 versus control group. n = 5 per group.
GPX3-AS inhibits H2O2-induced SRA01/04 cell apoptosis
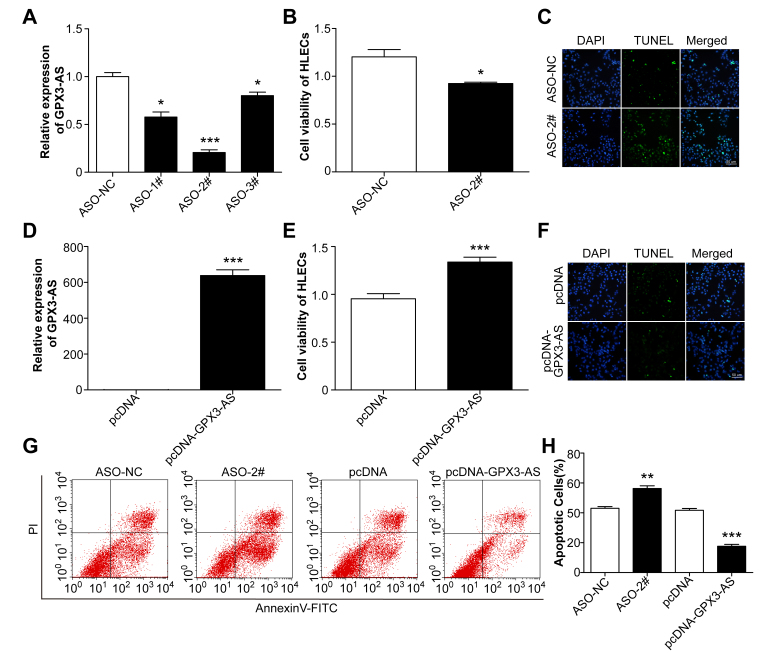
To verify the role of GPX3-AS in H2O2-induced SRA01/04 cell apoptosis, we used transfection to modulate the GPX3-AS levels in SRA01/04 cells. Endogenous GPX3-AS was downregulated in SRA01/04 cells transfected with three ASOs for human GPX3-AS; among them, ASO-#2 was the most efficient and was used for the subsequent experiments (Figure 4A). GPX3-AS was significantly upregulated in SRA01/04 cells transfected with the pcDNA-GPX3-AS plasmid (Figure 4D). Then, the cells were exposed to H2O2 for 24 h, and the viability of GPX3-AS knockdown cells decreased. After GPX3-AS was overexpressed, the opposite results were observed (Figure 4B,E). TUNEL staining showed that the proportion of apoptotic SRA01/04 cells increased following GPX3-AS knockdown, while GPX3-AS-overexpressing SRA01/04 cells exhibited less apoptosis (Figure 4C,F). The results of flow cytometry were similar to those of TUNEL staining (Figure 4G,H). Taken together, the findings indicated that GPX3-AS inhibited H2O2-induced SRA01/04 cell apoptosis.
Figure 4.
Glutathione peroxidase 3–antisense (GPX3-AS) inhibits H2O2-induced SRA01/04 cell apoptosis. SRA01/04 cells were transfected with AS oligo (ASO)-NC, ASO, pcDNA, or pcDNA-GPX3-AS for 24 h, and then the cells were exposed to H2O2 for an additional 24 h. A, D: Quantitative real-time PCR (qRT-PCR) was performed to detect GPX3-AS levels. (A) *p<0.05, ***p<0.001 versus ASO-NC group. (D) ***p<0.001 versus pcDNA group. B, E: Cell viability was detected by Cell Counting Kit-8 (CCK-8) assay. (B) * p<0.05 versus ASO-NC group. (E) ***p<0.001 versus pcDNA group. C, F: Apoptotic cells were analyzed by TUNEL staining. Scale bar = 50 μm. G, H: Flow cytometry analysis of SRA01/04 cell apoptosis. **p<0.01 versus ASO-NC group. ***p<0.001 versus pcDNA group. n = 5 per group.
GPX3-AS upregulates GPX3 expression
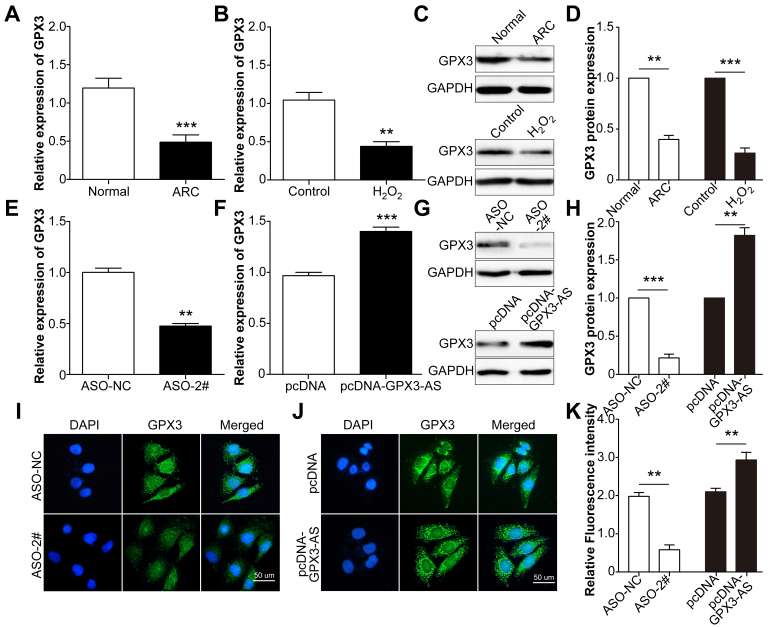
GPX3-AS is transcribed from the AS strand of the GPX3 gene locus 5q33.1 [26]. We assessed whether GPX3-AS affected GPX3 expression. We first measured GPX3 expression in both the anterior lens capsules of ARCs and SRA01/04 cells treated with H2O2. Like the expression of GPX3-AS, GPX3 expression also decreased (Figure 5A,B). GPX3 mRNA and protein levels increased in cells overexpressing GPX3-AS and decreased in cells following GPX3-AS knockdown (Figure 5C-F). These results suggested that GPX3 expression was upregulated by GPX3-AS.
Figure 5.
Glutathione peroxidase 3–antisense (GPX3-AS) upregulates GPX3 expression. A: GPX3 mRNA levels in the anterior lens capsules of the normal and age-related cataract (ARC) groups were detected by quantitative real-time PCR (qRT-PCR). ***p<0.001 versus normal group. B: SRA01/04 cells were treated with H2O2 for 24 h, and GPX3 mRNA levels were detected by qRT-PCR. **p<0.01 versus control group. C, D: GPX protein levels were detected in normal donors and ARC patients (upper lines) and control and H2O2-exposed SRA01/04 cells (lower lines). ***p<0.001 versus normal group. **p<0.01 versus control group. G, H: GPX protein levels were detected in AS oligo (ASO)-NC-transfected and ASO-#2-transfected SRA01/04 cells (upper lines) and pcDNA-transfected and pcDNA-GPX3-AS-transfected SRA01/04 cells (lower lines). ***p<0.001 versus ASO-NC group. **p<0.01 versus pcDNA group. SRA01/04 cells were transfected with ASO-NC, ASO, pcDNA, or pcDNA-GPX3-AS for 48 h, and qRT-PCR (E, F) and immunofluorescence (I, J, K) were conducted to detect GPX3 mRNA and protein levels, respectively. (C) **p<0.01 versus ASO-NC group. (D) ***p<0.001 versus pcDNA group. (K) **p<0.01 versus ASO-NC or pcDNA group. Scale bar = 50 μm. n = 5 per group.
GPX3-AS inhibits H2O2-induced SRA01/04 cell apoptosis by upregulating GPX3
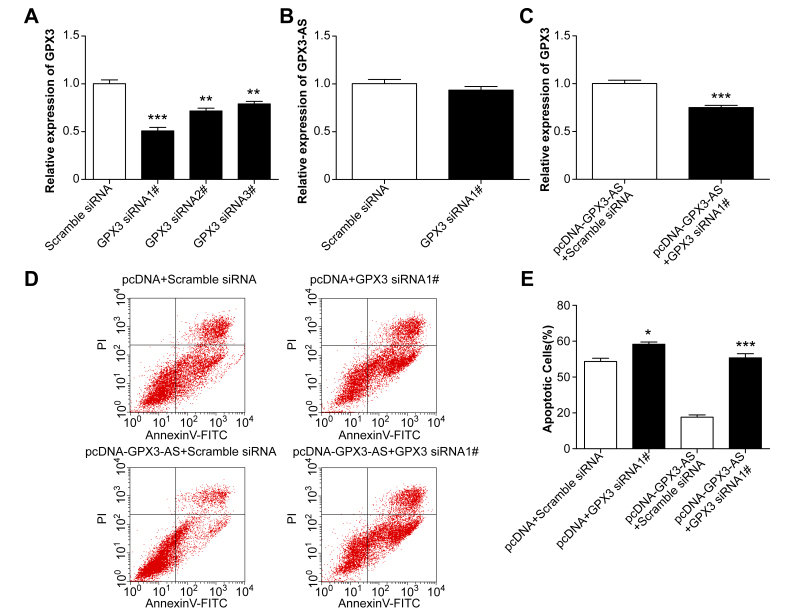
To further explore the role of GPX3 in the GPX3-AS-mediated antiapoptotic effect on SRA01/04 cells, the endogenous expression of GPX3 was remarkably decreased in SRA01/04 cells by transfection with GPX3 siRNA, and siRNA1 was selected for the subsequent study (Figure 6A). However, GPX3-AS expression did not change after GPX3 siRNA transfection, indicating that GPX3-AS is upstream of GPX3 (Figure 6B). Before exposure to H2O2, SRA01/04 cells were transfected with GPX3 siRNA in the absence or presence of pcDNA-GPX3-AS. Twenty-four hours after transfection, SRA01/04 cells were treated with H2O2 for 24 h, and cell apoptosis was then assessed by flow cytometry. Cotransfection with pcDNA-GPX3-AS and GPX3 siRNA significantly reversed GPX3 mRNA levels induced by pcDNA-GPX3-AS transfection (Figure 6C). Compared with the pcDNA and siRNA controls, there was little change in the apoptosis of SRA01/04 cells cotransfected with pcDNA-GPX3-AS and GPX3 siRNA (Figure 6D,E). Furthermore, GPX3 knockdown in SRA01/04 cells resulted in decreased cell viability and an increased cellular apoptosis ratio. These results suggest that GPX3-AS inhibits lens epithelial cell apoptosis by upregulating GPX3.
Figure 6.
Glutathione peroxidase 3–antisense (GPX3-AS) inhibits H2O2-induced SRA01/04 cell apoptosis by upregulating GPX3. A, B: SRA01/04 cells were transfected with scramble or GPX3 siRNA, and GPX3 mRNA and GPX3-AS levels were detected by quantitative real-time PCR (qRT-PCR). **p<0.01, ***p<0.001 versus scramble siRNA group. C: pcDNA-GPX3-AS and scramble siRNA or GPX3 siRNA were cotransfected into SRA01/04 cells. GPX3 mRNA levels were detected by qRT-PCR. ***p<0.001 versus pcDNA GPX3-AS plus scramble group. D, E: SRA01/04 cells were transfected with pcDNA plus scramble siRNA, pcDNA plus GPX3 siRNA1, pcDNA-GPX3-AS plus scramble siRNA or pcDNA-GPX3-AS plus GPX3 siRNA1 for 24 h, respectively. Then, they were exposed to H2O2 for 24 h. Apoptotic cells were detected by flow cytometry. *p<0.05 versus pcDNA plus scramble siRNA group; ***p<0.001 versus pcDNA-GPX3-AS plus scramble siRNA group. n = 5 per group.
Discussion
It is generally accepted that the apoptosis of lens epithelial cells is associated with the development of cataracts, and oxidative stress is regarded as a major cause of lens opacity [27]. Therefore, inhibiting LEC apoptosis and enhancing LEC antioxidant capacity is a promising strategy for the treatment of ARCs. In our study, we used H2O2-stimulated SRA01/04 cells for mimicking oxidative stress in vitro; these cells exhibited increased cell apoptosis, which is consistent with previous studies [8,28].
LncRNAs are a novel heterogeneous class of ncRNAs of more than 200 nucleotides in length and without protein-coding activity. They are important regulators of gene expression. The roles of lncRNA dysregulation in ocular diseases have been well verified [23]. In the present study, we used high-throughput RNA sequencing to obtain lncRNA expression profiles in cataract lenses and transparent lenses and found that GPX3-AS expression was significantly decreased in the cataract lenses compared with that in the transparent lenses. Our study demonstrated that GPX3-AS was mainly expressed in the nuclei of SRA01/04 cells (an HLEC line). By using SRA01/04 cells as an in vitro model to mimic oxidative stress in vivo, we determined that GPX3-AS levels were downregulated and associated with cell apoptosis. An in vitro study demonstrated that knockdown of the lncRNA GPX3-AS weakened cell viability and promoted apoptosis, while the overexpression of GPX3-AS enhanced cell viability and inhibited cell apoptosis. Moreover, the inhibition of cell apoptosis by GPX3-AS overexpression was reversed by GPX3 siRNA. Hence, GPX3-AS is a negative regulator of ARCs; the underlying mechanism of this regulation is the upregulation of GPX3 expression by GPX3-AS, which thereby reduces apoptosis in SRA01/04 cells.
According to their relative gene locations, lncRNAs are typically categorized into the five following classes: AS, intergenic, overlapping, intronic, and full lapping [29]. Among these categories of lncRNAs, AS lncRNAs, which are reverse complements of their endogenous sense counterparts, frequently do not encode proteins and comprise a substantial proportion of the entire long noncoding transcriptome [30-32]. Previous studies have shown that these natural AS transcripts play critical roles in multiple physiological and pathological processes, ranging from transcriptional regulation by gene promoter activation to posttranscriptional regulation by controlling mRNA stability and translation, through regulating sense gene expression [33,34]. For example, the AS lncRNAs keratin 7 (KRT7)-AS, fork head box C2 (FOXC2)-AS1 and zinc finger E-box binding homeobox 1 (ZEB1)-AS1 can alter the stability and expression of KRT7, FOXC2, and ZEB1, respectively [32,35,36]. Our study revealed that GPX3-AS upregulated GPX3 expression at both the mRNA and protein levels. KRT7-AS and KRT7 mRNA form a duplex RNA–RNA complex that protects KRT7 mRNA from degradation; thus, KRT7-AS upregulates KRT7 expression [36]. Therefore, we speculate that GPX3-AS could bind to GPX3 mRNA to promote GPX3 expression, although this is a question for future studies.
Several studies have demonstrated that the tumor-suppressive function of GPX3 in prostate, colon, and lung cancers is mediated by the induction of apoptosis [26,37,38]. However, high GPX3 expression is related to decreased overall patient survival, and GPX3 facilitates ovarian cancer cell viability by protecting cells from extracellular factors of oxidative stress [39]. Our study also found that GPX3 inhibited SRA01/04 cell apoptosis via protecting cells from oxidative stress. These data indicate dual roles for GPX3 in cell apoptosis depending on the cell type, oxidative stress status, and cell microenvironment. The detailed mechanisms underlying the inhibition of SRA01/04 cell apoptosis by GPX3 require further investigation. Taken together, our results suggest that GPX3-AS overexpression inhibits apoptosis in HLECs, possibly by upregulating GPX3 expression. The present study may provide a better understanding of lncRNA function in ARC development, and GPX3-AS could be a target for ARC therapies.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (no. 81770906).
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR. Vision Loss Expert Group of the Global Burden of Disease S. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.Tang Y, Wang X, Wang J, Huang W, Gao Y, Luo Y, Yang J, Lu Y. Prevalence of Age-Related Cataract and Cataract Surgery in a Chinese Adult Population: The Taizhou Eye Study. Invest Ophthalmol Vis Sci. 2016;57:1193–200. doi: 10.1167/iovs.15-18380. [DOI] [PubMed] [Google Scholar]
- 3.Bhagat N, Nissirios N, Potdevin L, Chung J, Lama P, Zarbin MA, Fechtner R, Guo S, Chu D, Langer P. Complications in resident-performed phacoemulsification cataract surgery at New Jersey Medical School. Br J Ophthalmol. 2007;91:1315–7. doi: 10.1136/bjo.2006.111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur J, Kukreja S, Kaur A, Malhotra N, Kaur R. The oxidative stress in cataract patients. J Clin Diagn Res. 2012;6:1629–32. doi: 10.7860/JCDR/2012/4856.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Ates O, Alp HH, Kocer I, Baykal O, Salman IA. Oxidative DNA damage in patients with cataract. Acta Ophthalmol. 2010;88:891–5. doi: 10.1111/j.1755-3768.2009.01583.x. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11:339–53. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su D, Hu S, Guan L, Wu X, Shi C, Yang X, Ma X. Down-regulation of GJA3 is associated with lens epithelial cell apoptosis and age-related cataract. Biochem Biophys Res Commun. 2017;484:159–64. doi: 10.1016/j.bbrc.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Liu Z, Ma L, Pei C, Qin L, Gao N, Li J, Yin Y. MiRNAs regulate oxidative stress related genes via binding to the 3′ UTR and TATA-box regions: a new hypothesis for cataract pathogenesis. BMC Ophthalmol. 2017;17:142. doi: 10.1186/s12886-017-0537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dairou J, Malecaze F, Dupret JM, Rodrigues-Lima F. The xenobiotic-metabolizing enzymes arylamine N-acetyltransferases in human lens epithelial cells: inactivation by cellular oxidants and UVB-induced oxidative stress. Mol Pharmacol. 2005;67:1299–306. doi: 10.1124/mol.104.009738. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y, Jiang H, Li H, Song W, Xia X. Alpha-A-Crystallin Protects Lens Epithelial Cell-Derived iPSC-Like Cells Against Apoptosis Induced by Oxidative Stress. Cell Reprogram. 2016;18:327–32. doi: 10.1089/cell.2016.0017. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZF, Zhang J, Hui YN, Zheng MH, Liu XP, Kador PF, Wang YS, Yao LB, Zhou J. Up-regulation of NDRG2 in senescent lens epithelial cells contributes to age-related cataract in human. PLoS One. 2011;6:e26102. doi: 10.1371/journal.pone.0026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding RNAs. Nat Immunol. 2017;18:962–72. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickard MR, Williams GT. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget. 2016;7:10104–16. doi: 10.18632/oncotarget.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gioia R, Drouin S, Ouimet M, Caron M, St-Onge P, Richer C, Sinnett D. LncRNAs downregulated in childhood acute lymphoblastic leukemia modulate apoptosis, cell migration, and DNA damage response. Oncotarget. 2017;8:80645–50. doi: 10.18632/oncotarget.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Song H, Chen L, Yang W, Nan K, Lu P. TUG1 promotes lens epithelial cell apoptosis by regulating miR-421/caspase-3 axis in age-related cataract. Exp Cell Res. 2017;356:20–7. doi: 10.1016/j.yexcr.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y, Dong LF, Zhou RM, Yao J, Song YC, Yang H, Jiang Q, Yan B. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. J Cell Mol Med. 2016;20:537–48. doi: 10.1111/jcmm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin X, Jin H, Shi Y, Guo Y, Zhang H. Long Non-Coding RNA KCNQ1OT1 Promotes Cataractogenesis via miR-214 and Activation of the Caspase-1 Pathway. Cell Physiol Biochem. 2017;42:295–305. doi: 10.1159/000477330. [DOI] [PubMed] [Google Scholar]
- 19.Benhar M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic Biol Med. 2018;127:160–4. doi: 10.1016/j.freeradbiomed.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Merino JJ, Roncero C, Oset-Gasque MJ, Naddaf A, Gonzalez MP. Antioxidant and protective mechanisms against hypoxia and hypoglycaemia in cortical neurons in vitro. Int J Mol Sci. 2014;15:2475–93. doi: 10.3390/ijms15022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Haddad M, Jean E, Turki A, Hugon G, Vernus B, Bonnieu A, Passerieux E, Hamade A, Mercier J, Laoudj-Chenivesse D, Carnac G. Glutathione peroxidase 3, a new retinoid target gene, is crucial for human skeletal muscle precursor cell survival. J Cell Sci. 2012;125:6147–56. doi: 10.1242/jcs.115220. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Zhou J, Zhang G, Wang Y, Kang L, Wu J, Chen J, Guan H. Relationship Between the Altered Expression and Epigenetics of GSTM3 and Age-Related Cataract. IOVS. 2016;57:4721–32. doi: 10.1167/iovs.16-19242. [DOI] [PubMed] [Google Scholar]
- 23.Sheng Y, He F, Lin JF, Shen W, Qiu YW. Tea and Risk of Age-Related Cataracts: A Cross-Sectional Study in Zhejiang Province, China. J Epidemiol. 2016;26:587–92. doi: 10.2188/jea.JE20150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZH, Jhaveri DJ, Marshall VM, Bauer DC, Edson J, Narayanan RK, Robinson GJ, Lundberg AE, Bartlett PF, Wray NR, Zhao QY. A comparative study of techniques for differential expression analysis on RNA-Seq data. PLoS One. 2014;9:e103207. doi: 10.1371/journal.pone.0103207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE, Coburn LA, Peek RM, Chaturvedi R, Wilson KT, Burk RF, Williams CS. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013;73:1245–55. doi: 10.1158/0008-5472.CAN-12-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XF, Hao JL, Xie T, Malik TH, Lu CB, Liu C, Shu C, Lu CW, Zhou DD. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. 2017;16:934–42. doi: 10.1111/acel.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong W, Zhu G, Li J, Yang X. LncRNA MALAT1 promotes the apoptosis and oxidative stress of human lens epithelial cells via p38MAPK pathway in diabetic cataract. Diabetes Res Clin Pract. 2018;144:314–21. doi: 10.1016/j.diabres.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Cai S, Jia Y, Qi C, Sun J, Zhang H, Wang F, Cao Y, Li X. Decoding Noncoding RNAs: Role of MicroRNAs and Long Noncoding RNAs in Ocular Neovascularization. Theranostics. 2017;7:3155–67. doi: 10.7150/thno.19646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Y, Ma G, Zhang Z, Hua Q, Chu H, Tong N, Yuan L, Qin C, Yin C, Zhang Z, Wang M. A novel antisense long noncoding RNA regulates the expression of MDC1 in bladder cancer. Oncotarget. 2015;6:484–93. doi: 10.18632/oncotarget.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magistri M, Faghihi MA, St Laurent G, 3rd, Wahlestedt C. Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends Genet. 2012;28:389–96. doi: 10.1016/j.tig.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FOXC2–AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66–75. doi: 10.1016/j.canlet.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 33.He A, He S, Li X, Zhou L. ZFAS1: A novel vital oncogenic lncRNA in multiple human cancers. Cell Prolif. 2019;52:e12513. doi: 10.1111/cpr.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zong X, Nakagawa S, Freier SM, Fei J, Ha T, Prasanth SG, Prasanth KV. Natural antisense RNA promotes 3′ end processing and maturation of MALAT1 lncRNA. Nucleic Acids Res. 2016;44:2898–908. doi: 10.1093/nar/gkw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su W, Xu M, Chen X, Chen N, Gong J, Nie L, Li L, Li X, Zhang M, Zhou Q. Long noncoding RNA ZEB1–AS1 epigenetically regulates the expressions of ZEB1 and downstream molecules in prostate cancer. Mol Cancer. 2017;16:142. doi: 10.1186/s12943-017-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang B, Song JH, Cheng Y, Abraham JM, Ibrahim S, Sun Z, Ke X, Meltzer SJ. Long non-coding antisense RNA KRT7-AS is activated in gastric cancers and supports cancer cell progression by increasing KRT7 expression. Oncogene. 2016;35:4927–36. doi: 10.1038/onc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Luo K, Tan LZ, Ren BG, Gu LQ, Michalopoulos G, Luo JH, Yu YP. p53-induced gene 3 mediates cell death induced by glutathione peroxidase 3. J Biol Chem. 2012;287:16890–902. doi: 10.1074/jbc.M111.322636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An BC, Choi YD, Oh IJ, Kim JH, Park JI, Lee SW. GPx3-mediated redox signaling arrests the cell cycle and acts as a tumor suppressor in lung cancer cell lines. PLoS One. 2018;13:e0204170. doi: 10.1371/journal.pone.0204170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worley B, Kim Y, Mardini J, Zaman R, Leon K, Walter V, Endres L, Phaeton R, Aird K, Hempel N. GPX3 supports ovarian cancer progression by manipulating the extracellular redox environment. Redox Biol. 2018;•••:101051. doi: 10.1016/j.redox.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]