Abstract
Microneedle-based skin allergen-specific immunotherapy (AIT) can benefit from adjuvants that can stimulate a stronger Th1 response against the allergen. We evaluated two stimulator of interferon genes (STING) agonists, namely, cyclic diguanylate monophosphate (c-di-GMP) and cyclic diadenylate monophosphate (c-di-AMP) as skin adjuvants using coated microneedles (MNs). For comparison, the approved subcutaneous (SC) hypodermic injection containing alum was used. Ovalbumin (Ova) was used as a model allergen. Ova-specific IgG2a antibody in serum, which is a surrogate marker for Th1 type immune response was significantly higher when STING agonists were used with coated MNs as compared to SC injection of Ova+alum in mice. In contrast IgG1 antibody, a surrogate marker for Th2 type immune response was at comparable levels in the MN and the SC groups. Restimulation of splenocytes with Ova produced higher levels of Th1 cytokines (IFN-γ and IL-2) in the STING agonists MN group as compared to the SC group. In conclusion, delivery of STING agonists into the skin using coated MNs activated the Th1 pathway better than SC- and MN-based delivery of alum. Thus, STING agonists could fulfill the role of adjuvants for skin AIT and even for infectious disease vaccines, where stimulation of the Th1 pathway is of interest.
Keywords: Allergy adjuvant, cutaneous allergen immunotherapy, microneedles, skin immunotherapy, STING adjuvants
1. Introduction
Allergen-specific immunotherapy (AIT) is an established method for the treatment of respiratory and hymenoptera venom allergies.1 The treatment involves subcutaneous (SC) hypodermic injections of increasing allergen amounts till tolerance is achieved.2 The high number of injections (50–80) administered over multiple years (3–5 or more) cut down patient-adherence rates dramatically.3 To replace the painful SC injections, we have recently proposed and demonstrated that allergens coated on micrometer-sized needles commonly known as microneedles (MNs) can be used to deliver the allergen into the skin.4,5 MNs are minimally invasive and painless6 and could be self-applied by patients if deemed safe. Coated MNs can easily penetrate the top barrier layer of the skin called the stratum corneum, and can directly deliver the allergen into the underlying epidermis and dermis regions.7
SC AIT is also associated with adverse reactions including fatal anaphylaxis.8 MNs also have the potential to improve this safety profile because MN length can be decreased to deliver the allergen primarily in to the epidermis, which is non-vascularized. The non-vascularize nature of epidermis can reduce systemic exposure of the allergen and hence the side effects.9
To further improve the efficacy and safety profile of AIT, different adjuvants are being evaluated.10 Adjuvant use can reduce the amount of allergen required, which can in turn improve safety. Furthermore, allergic patients have an established Th2 bias,11 thus selection of adjuvants that can steer the immune response to a Th1 bias can help to enhance the therapeutic effect of AIT and perhaps even reduce the duration of treatment.12 For SC AIT, aluminum salts (Alum) are licensed as adjuvants.13 Alum is however thought to have a Th2 bias and is associated with risk of local granuloma formation at injection sites.14 Thus, there is a need for other adjuvants for MN-based AIT. With this perspective, previously we have shown that CpG, a TLR-9 agonist with a Th1 bias can be used as an adjuvant in MN-based skin AIT for prevention of allergy progression in mice.5
In the current study we wanted to expand the repertoire of available Th1-biased adjuvants for MN-based AIT. Therefore, we characterized stimulator of interferon genes (STING) agonists, cyclic diguanylate monophosphate (c-di-GMP) and cyclic diadenylate monophosphate (c-di-AMP) as potential adjuvants. STING agonists have recently emerged as adjuvants,15 and they are known to induce a broad Th1/Th2/Th17 cellular activation,16 which could make them quite useful as adjuvants for AIT. To our knowledge, STING agonists have not been previously delivered as adjuvants into the skin using MNs. Therefore, in this study we have coated c-di-AMP and c-di-GMP on MNs along with ovalbumin (Ova) as a model allergen and delivered them to mouse skin. Subsequently we have characterized the systemic antibody responses, and Th1/Th2 cytokines secreted by splenocytes upon re-stimulation with Ova to assess their immune response bias.
2. Materials and Methods
2.1. Coating and delivery efficiency of MN patch
A MN patch with 57 individual micron-sized needles was fabricated thorough a wet etch process. Individual MNs on a patch were manually bent out of the plane to make them perpendicular to the base of the patch, and then coated using a micro-precision dip coater as previously described.4 The coating solution contained carboxymethylcellulose (CMC) (1%, w/v) (low viscosity, USP grade, CarboMer, San Diego, CA, USA) as a viscosity enhancer, and Lutrol F-68 NF (BASF, Mt. Olive, NJ, USA) as a surfactant.Ovalbumin (MP Biomedicals, OH, USA) with or without STING adjuvants, namely, c-di-AMP and c-di-GMP (VacciGrade, InvivoGen, CA, USA) were added to the coating solution.
To assess the delivery efficiency of coated MNs in mice skin, Ova was conjugated with N-hydroxysuccinamide (NHS)-activated fluorescein dye (Thermo Scientific, Rockford, IL, USA), mixed with STING agonist c-di-GMP or c-di-AMP in 1:1 ratio and then coated on MN patches. The delivery efficiency of coated MN patches was determined as previously described using calibrated fluorescence spectroscopy.17–19 Fluorescein-labelled Ova served a dual purpose, first it permitted direct measurement of Ova concentration, and second it served as an internal standard for measuring STING agonist concentration since Ova and STING agonists were mixed in a 1:1 ratio (mass). In brief, coated MN patches (n=3) were dissolved into 1 ml of PBS solution to obtain the Ova amount coated on patches. Another set of coated MN patches was inserted in to mice skin (n=3) and held for 3 min. For MN application, mice were anesthetized with isoflurane. Their flank was shaved with an electrical hair trimmer. Hair removing cream was next applied for few seconds and cleaned. The area was next washed with lukewarm water to fully remove the cream, and then dried gently with the help of a paper towel. After MN patch application, patches were removed and placed in 1 ml PBS solution to obtain the amount of Ova left on the patch. A cotton swap pre-soaked in PBS solution was gently rubbed on skin surface and placed back into 1 ml of PBS solution to quantify the amount of Ova left on the skin surface. Finally, the delivered amount was obtained by subtracting Ova left on skin and patches from the amount of Ova available on unused coated MN patches. The concentration of fluorescein-conjugated Ova was quantified using spectrofluorometer (Cary Eclipse, Agilent Technologies, CA, USA) at an excitation of 480 nm and an emission of 530 nm.
2.2. Mice and immunization schedule
Balb/c female mice, 8–10 weeks old, obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA) were used in the experiments. Experimental protocols were approved by the Institutional Animal Care and Use Committee, Texas Tech University (TTU).
Ova and STING adjuvant were each coated at an amount of 25μg on the MNs. Mice were immunized at day 0 (d0) and boosted on day 28 (d28). Coated MN patches were applied on hairless skin and held in place for 3 min.
2.3. Measurement of gene expression
Healthy mice were immunized with coated MNs. Six hours later, skin samples from application sites were harvested in Trizol reagent (Invitrogen, Carlsbad, CA), and immediately stored in liquid nitrogen until use. Total RNA was isolated from these samples using Trizol reagent and cDNA was synthesized from 1μg of total RNA using a high capacity cDNA synthesis kit (Invitrogen, Carlsbad, CA). Quantitative reverse transcription polymerase chain reaction (qRT-PCR). RT-PCR was performed with PowerUp™ SYBR® Green master mix (Invitrogen, Carlsbad, CA) by using QuantStudio 3 Real-Time PCR System (Applied Biosystems, Foster City, CA). RNA was normalized to the expression levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and relative expression was calculated with the 2−ΔΔCt method. The primer sequences used in qRT-PCR were customized by Integrated DNA Technologies, Inc, CA. The sequences of primers were as follows; GAPDH (sense 5’-TCCAGTATGACTCCACTCAC-3’, antisense 5’-GGCTAAGCAGTTGGTGGT-3’), IRF-3 (sense 5’- GTGCCTCTCCTGACACCAAT-3’, antisense 5’- TCGAACTCCCATTGTTCCTC-3’) and IFN-γ (sense 5’-CACGGCACAGTCATTGAAAG-3’, antisense 5’- GCTGATGGCCTGATTGTCTT-3’).
2.4. Antibody analysis
To assess the antibody response, blood was collected at different time points: before first dose (at d0), before booster dose (d28), at d60 and d180. Anti-Ova IgG, IgG1, IgG2a and IgE antibodies were analyzed in these samples using ELISA.20
2.5. Splenocyte culture
Spleens were harvested at the end of the study and splenocytes were cultured in triplicates at a concentration of one million cells per well in 96 well plates for 72 h with (i) Ova (200 μg/ml), (ii) culture medium as a negative control, or (iii) a positive control of 5μg/ml of concanavalin A (Con A) (Sigma Aldrich, MO, USA). RPMI (Gibco, Life Technologies, USA). Cell culture media was supplemented with fetal bovine serum (FBS) and penicillin-streptomycin antibiotics. Supernatants of cultured cells were collected after 14 h for IL-2 measurement and after 72 h for measurement of IFN-γ, IL-4 and IL-5 cytokines. Cytokines were analyzed through sandwich ELISA. For each group, cytokine levels after stimulation with media alone were subtracted from Ova-stimulated wells.
2.6. Statistical analysis
Statistical analysis was conducted using Graphpad Prism 6 software (CA, USA). All ELISA experiments were run in triplicate and two-way ANOVA test was used to compare the response between the groups at different time points while one-way ANOVA test was applied for statistical calculations between the groups. Significance was considered for p < 0.05 for a 95% confidence interval.
3. Results
3.1. Allergen and STING coated MN patch
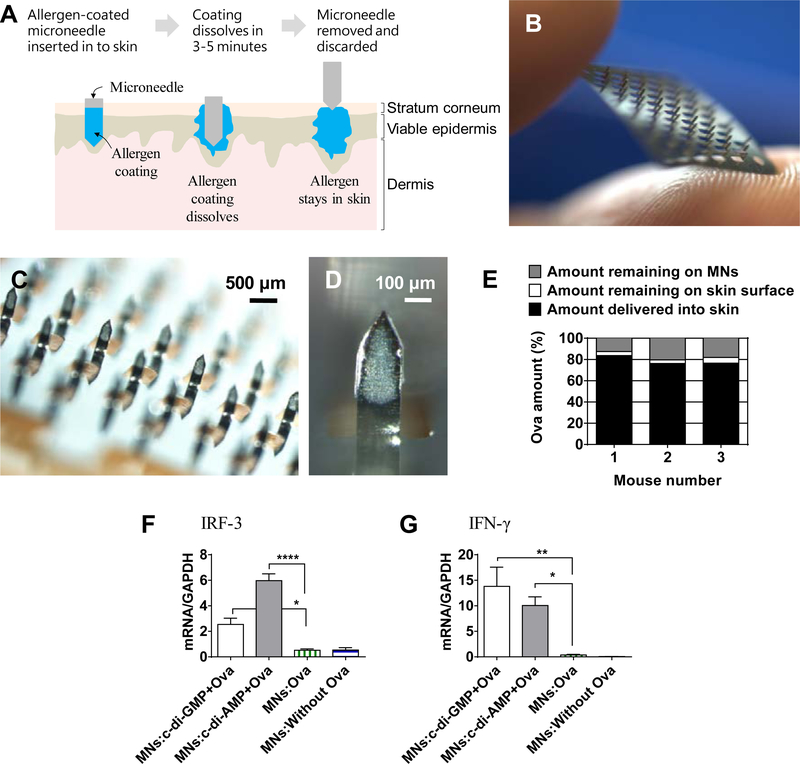
The concept of using an allergen-coated MN patch for skin AIT is shown in Figure 1A. A MN patch with 57 individual needles (Figure 1B) was prepared and used for skin immunization. Through precision coating, Ova with or without the STING adjuvants was successfully coated on to MNs of a patch (Figure 1C). Of note, the coatings were localized just on the MN shafts without contaminating the MN patch flat substrate. Figure 1D is a zoomed-in image of a single MN of the patch, which shows that coatings were evenly produced without bare spots. Using calibrated fluorescence spectroscopy, it was found that 78.8% (±4.2) of coated Ova was delivered in to the skin, while 4.2% (±1.1)
Figure 1.
Allergen-coated microneedles. (A) Schematic showing the concept of allergen-coated microneedles for skin allergen-specific immunotherapy. (B) Digital photograph of an uncoated microneedle patch containing micron-sized needles. (C) Stereomicroscope image of Ova-coated MN patch. (D) Zoomed stereomicroscope image of a single microneedle coated with Ova. (E) In vivo delivery efficiency of MNs coated with Ova+STING adjuvant in mice skin. Gene expression analysis of (F) IRF-3, and (G) IFN-γ in skin of mice immunized with Ova and STING using coated MNs. Error bars denote ± SEM. * p < 0.05, ** p < 0.01, and **** p < 0.0001
Ova was found on the skin, and 17.0% (±4.0) Ova remained on the MN surface (Figure 1E). Since Ova and the STING adjuvant were mixed in a 1:1 ratio, then by considering Ova as the internal standard, delivery efficiency of STING adjuvant is considered to be the same as Ova.
To determine whether STING adjuvants were activating the STING pathway, expression of IRF-3 and IFN-γ in skin was quantified. IRF-3 and IFN-γ expression was significantly higher in the groups receiving the STING adjuvants (Figure 1F and 1G).
3.2. Serum antibody analysis
Mice were immunized on d0 and boosted on d28 (Figure 2A). Mice were divided into 6 groups with 5 mice per group (Figure 2B): (i) MNs coated with just the coating formulation but without Ova (MNs:Without Ova), (ii) MNs coated with Ova in coating formulation (MNs:Ova), (iii) MNs coated with c-di-GMP and Ova in coating formulation (MNs:c-di-GMP+Ova), (iv) MNs coated with c-di-AMP and Ova in coating formulation (MNs:c-di-AMP+Ova), (v) MNs coated with alum and Ova in coating formulation (MNs:Alum+Ova), and (vi) subcutaneous alum+Ova injection (SC:Alum+Ova).
Figure 2.
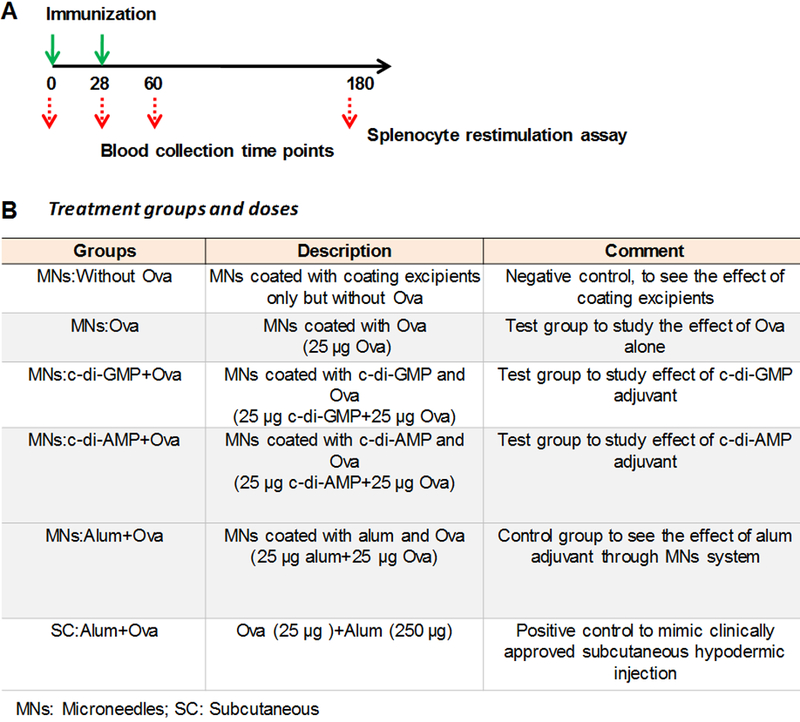
Immunization schedule and treatment groups. (A) Immunization and sample collection schedule. (B) The different treatment groups, and vaccination doses.
Anti-Ova IgG response is shown in Figure 3A. After one dose, on d28, low anti-Ova IgG response was seen in the MNs STING groups (MNs:c-di-GMP+Ova & MNs:c-di-AMP+Ova) and other control groups (SC:Alum+Ova, MNs:Alum+Ova & MNs:Ova). As expected, the control MNs:Without Ova group did not show any IgG response because this group did not receive Ova but just the coating excipients. After the second dose, on d60, anti-Ova IgG response increased considerably in all the groups that received Ova. No significant difference was observed between the MNs STING groups (MNs:c-di-GMP+Ova & MNs:c-di-AMP+Ova) and the SC:Alum+Ova group or MNs:Alum+Ova group. Even MNs:Ova group that had no adjuvants stimulated a strong IgG response. The IgG response persisted till six months (d180) post immunization, indicating the capability of STING adjuvants and the SC route to induce a long term immune response.
Figure 3.
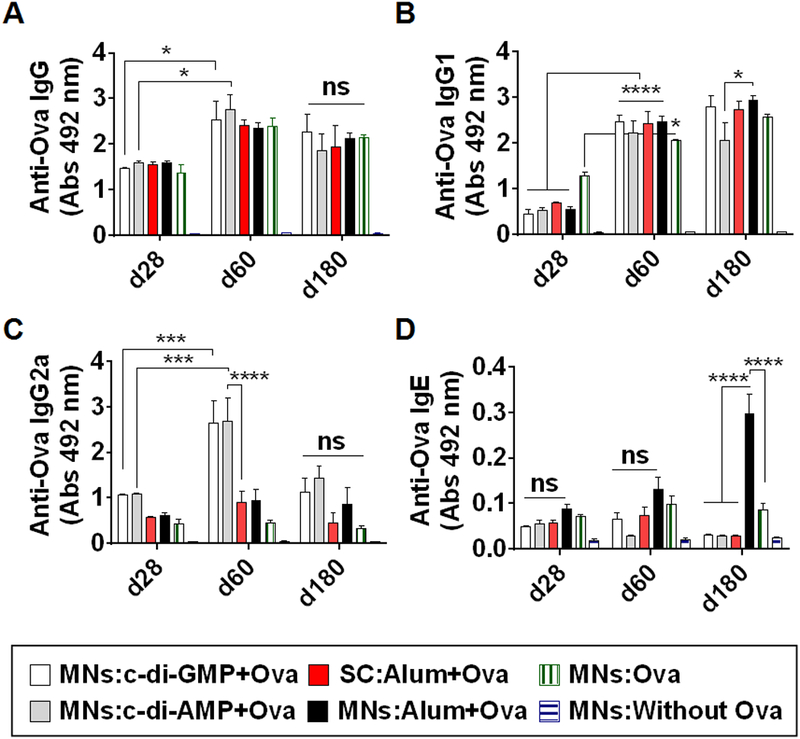
Anti-Ova response in serum at different time points. (A) anti-Ova IgG response at 1:100 serum dilution, (B) anti-Ova IgG1 response at 1:100 serum dilution, (C) anti-Ova IgG2a response at 1:100 serum dilution, and (D) IgE response at 1:20 serum dilution. ELISA was used to measure anti-Ova antibody responses in the form of optical density at 492 nm. Individual mice serum was used in analysis. Error bars denote ± SEM. * p < 0.05, *** p < 0.001, **** p < 0.0001, and ns: not significant.
A response-pattern similar to IgG was seen for anti-Ova IgG1 at d28 and d60. However at d180, MNs:Alum+Ova had higher IgG1 than MNs:c-di-AMP+Ova (Figure 3B). Interestingly however, a different response pattern was seen for anti-Ova IgG2a response (Figure 3C). On d28, all vaccinated groups showed low anti-Ova IgG2a response without any considerable difference between them. However, the response considerably increased on d60 in MNs:c-di-GMP+Ova (p=0.0002) and MNs:c-di-AMP+Ova (p<0.0001) groups in comparison to the SC:Alum+Ova group and MNs:Alum+Ova group, indicating the Th1 bias of the STING adjuvants over alum. MNs:c-di-GMP+Ova and MNs:c-di-AMP+Ova groups were not significantly different from each other (Figure 3C). The anti-Ova IgG2a response was low in the control MNs:Ova group at all time points (Figure 3C). Unlike IgG and IgG1 responses, IgG2a response decreased on d180 in all groups (Figure 3C), however, a trend of elevated IgG2a could be seen in groups receiving STING adjuvants.
Anti-Ova IgE response was low (<0.07 OD) in the STING adjuvant groups and the SC:Alum+Ova group, and it was similar to the negative control group of MNs:Without Ova that contained just the coating formulation (Figure 3D). This shows that co-delivery of STING adjuvants and the model allergen Ova into the skin using MNs is safe, and it should not exacerbate IgE mediated allergic conditions. However, at d180, the group MNs:Alum+Ova had a considerably higher anti-Ova IgE response than the other Ova immunized groups (Figure 3D).
3.3. Splenocytes culture analysis
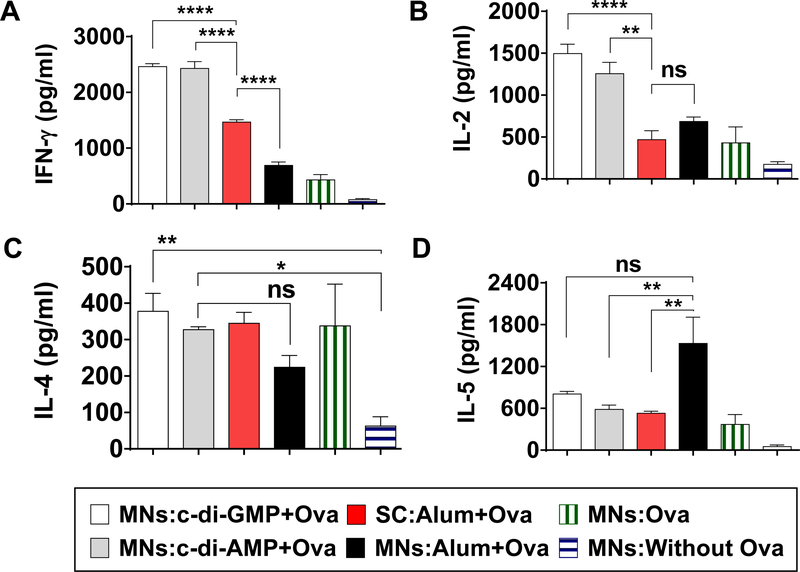
To further study the Th-bias induced by delivery of STING agonist into the skin using coated MNs, we re-stimulated the splenocytes with Ova and analyzed Th1 and Th2 cytokines in the culture medium. Up-regulation of Th1 type cytokines (IFN-γ and IL-2) was observed in STING adjuvant groups unlike the SC:Alum+Ova group (Figure 4A, 4B). In MNs:c-di-GMP+Ova and MNs:c-di-AMP+Ova groups, IFN-γ expression was significantly higher than the SC group (Figure 4A). Similarly, IL-2 expression was considerably higher in MNs:c-di-GMP+Ova and MNs:c-di-AMP+Ova groups in comparison to the SC:Alum+Ova group group (Figure 4B). No significant difference was observed between MNs:c-di-GMP+Ova, and MNs:c-di-AMP+Ova groups. The groups MNs:Alum+Ova and MNs:Ova showed low amount of Th1 cytokine secretion (Figure 4A, 4B). The Th2 cytokine, IL-4 was considerably higher in the MNs:c-di-GMP+Ova, MNs:c-di-AMP+Ova and SC:Alum+Ova group groups in comparison to the MNs:Without Ova group (Figure 4C). However, IL-5 cytokine expression was lower in MNs STING groups and SC:Alum+Ova group group as compared to the MNs:Alum+Ova group (Figure 4D). No significant difference was observed between MNs STING groups, and SC:Alum+Ova group (Figure 4D). In the positive control (Con A stimulation), cytokines expressions were significantly higher than Ova stimulation (data not plotted).
Figure 4.
Splenocyte culture supernatant analysis. Spleens were collected at end of the experiment and cultured for 72 h with medium alone as a negative control, 200 μg/ml Ova, or 5 μg/ml of concanavalin A as a positive control. Supernatant of cultured cells were collected after 14 h for IL-2, 72 h for IFN-, IL-4 and IL-5 analysis. Expression levels of (A) IFN-γ, (B) IL-2, (C) IL-4, and (D) IL-5 cytokines. Error bars denote ± SEM. * p < 0.05, ** p < 0.01, **** p < 0.0001, and ns: not significant. Values are presented after subtraction of media alone cytokine levels. Data for concanavalin A is not plotted.
4. Discussion
MNs are painless,6 and offer a simple approach for AIT. Our long-term goal is to establish a MN-based AIT as a viable treatment option that can substitute the SC allergy shots. To achieve this goal, it may be desirable to mix adjuvants with the allergen so that the immune response can be enhanced and steered towards the Th1 pathway. Since not many adjuvants have been characterized with respect to their delivery into the skin using MNs, in this work our objective was to characterize the Th1/Th2 bias produced by use of STING agonists as adjuvants for skin AIT. Study of the Th1/Th2 bias is important because it is known that a Th2-biased immune response against the allergen can initiate and maintain allergic inflammation in patients, while stimulation of a Th1 type immune response against the allergen can yield therapeutic effects.21 In this regard, to develop a MN-based AIT, it is important to deliver adjuvants into the skin that have a Th1 bias. We have previously shown that CpG is one such adjuvant.5 Recently STING agonists have emerged as a new class of adjuvants. When used as adjuvants via the intranasal and intramuscular routes they produce a broad Th1/Th2 stimulation.15, 16 A Th1 bias was also seen when a STING agonist, 2’3’- cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) was injected intradermally.22 Furthermore, cGAMP was also found to be more potent than CpG through intradermal injection.22 However, to our knowledge the adjuvant effect of STING agonists after delivery into the skin using MNs has not been previously studied.
Therefore, in this study we selected two STING agonists, c-di-AMP and c-di-GMP for comparison. We found that since both c-di-AMP and c-di-GMP were hydrophilic, they could be readily mixed with Ova and coated onto MN shafts of the patch with uniform coverage.
The coated MN patch reliably delivered significant amount of Ova allergen and STING adjuvants into the mouse skin. The delivery efficiency observed in this study was around 78% of the coated allergen. This is consistent with previous studies with coated MNs where 63% to 91% of coated protein or drug was delivered into skin or oral cavity mucosal tissue.17–19 STING activation causes activation of the transcription factor IRF-3. 23–25 Consistent with this, we found that delivery of STING adjuvants cAMP or cGMP into skin using coated MNs induced a significantly higher expression of IRF-3 gene in the skin as compared to when Ova alone was delivered. Moreover, it has recently been shown that STING activation increase IFN-γ production from CD4+ T cells.26 IFN-γ is an important Th-1-biasing cytokine. IFN-γ gene expression was also significantly higher when STING adjuvants were delivered into the skin.
After two doses, the c-di-AMP and c-di-GMP MN groups generated Ova-specific IgG and IgG1 antibodies at levels similar to those in the SC injection group, which contained alum as an adjuvant. This response persisted up to six months post immunization, suggesting the capacity of STING adjuvants to sustain longer term allergen specific immune response, which may in turn lead to longer duration desensitization. In mice, antibody subtype IgG2a is considered a surrogate marker for Th1 type immune response.27 When we measured anti-Ova IgG2a response it was significantly higher in the c-di-AMP and c-di-GMP MN groups as compared to the groups receiving alum as adjuvant via subcutaneous injection or coated MNs, indicating the Th1 bias of STING adjuvants over alum when delivered into the skin via MNs.
The two STING agonists c-di-AMP and c-di-GMP did not provoke Ova specific IgE response in serum. This suggests that STING agonists when delivered via the skin are safe and should not stimulate allergic inflammation against the allergen. However, a significant amount of Ova-specific IgE was generated when alum was used as an adjuvant with MNs, which indicates the possibility that alum may cause skin sensitization. In contrast, when alum was used as an adjuvant via the subcutaneous route, insignificant IgE response was generated, suggesting that the route of administration can impact adjuvant effect.
Higher secretion of Th1 cytokines, IFN-γ and IL-2 was also seen in stimulated splenocyte cultures in groups of mice that received STING agonists as adjuvants via coated MNs. This, together with higher IgG2a secretion in serum suggests that STING agonists when delivered into the skin via MNs steer the immune response to a Th1 pathway.
Alum produced a predominantly Th2 biased response. Despite this well-known Th2 bias of alum, it is still widely used in clinics in Europe as an adjuvant for SC AIT.29 In contrast, STING adjuvants generated a stronger Th1 biased immune response as compared to alum. The STING agonists are known to stimulate both Th1 and Th2 responses16. Indeed, a Th2 response, evidenced by high IgG1 and Th1 cytokine secretion was also seen in our study. It is possible that the low Ova-specific IgE antibody that resulted from the use of STING adjuvants was due to their dual Th1/Th2 profile, while the significantly higher IgE response from alum (when delivered with MNs) resulted from the Th2 bias and lack of Th1 response from alum. IgE antibodies are implicated in allergic diseases, and thus their stimulation is not desirable.1, 2 Thus, the dual Th1/Th2 stimulation by STING agonists over the predominantly Th2 biased response of alum suggests that STING adjuvants might be of use in skin AIT.
The potential benefits and effects of STING adjuvants in skin AIT should be further studied in animal models of allergy. There are many cyclic-di-nucleotides that are STING agonists, and they show different grades of adjuvant effects.16 Here we have evaluated two of them, namely, c-di-AMP and c-di-GMP. Thus, in future studies, other STING agonists should be evaluated to identify the optimal STING agonist for use with MNs. It has also been shown that one of the STING agonist, namely, cGAMP is more potent than CpG through intradermal injection.22 Therefore, to identify the optimal adjuvant formulation for MN-based AIT the synergistic effect of mixing a STING agonist with CpG should also be investigated. STING is located in the cytoplasm anchored to the endoplasmic reticulum25. Despite the negative charge associated with the STING agonists, we saw that even though we used STING agonists in their soluble form, the STING pathway was still activated as evidenced by increase in IRF-3 gene expression. A similar phenomenon has been seen previously where a STING agonist both in its soluble and nanoparticle-encapsulated forms was able to enter the dendritic cells in vitro at equivalent levels.30 However, their study also showed that the in vivo targeting and efficacy was significantly improved by encapsulating STING agonist in a nanoparticle. Thus, formulating STING agonist in a nanoparticle form should be considered. Since Th1 immune responses are also involved in immunity against intracellular viral and bacterial pathogens28, the STING agonists could also be used as adjuvants to develop infectious disease vaccines based on MNs. A STING agonist ADU-S100™ (Aduro Biotech) is currently being tested in phase 1 clinical trials for cancer therapy (Clinicaltrials.gov IDs: NCT02675439 and NCT03172936), indicating the translational potential of STING agonists for human use.
5. Conclusion
This study demonstrated that STING agonists coated on MNs stimulate the Th1 and Th2 pathway and have potential for activation of long-term allergen specific immune responses. Thus, STING agonists coated on MNs may have potential as adjuvants for treatment or prevention of allergies. Since infectious disease vaccines can also benefit from the activation of Th1 pathway, STING agonists could be beneficial for the development of coated MN-based infectious disease vaccines as well.
Acknowledgement
This research was supported by the National Institutes of Health (NIH) [grant number AI121322].
Footnotes
Conflict of interest
HSG and AKS are co-inventors on a patent related to coated microneedles for allergen immunotherapy. HSG is also part of a startup company that is developing microneedles for food allergy immunotherapy. These potential conflicts of interest have been disclosed and are being managed by Texas Tech University.
References
- 1.Passalacqua G; Bagnasco D; Ferrando M; Heffler E; Puggioni F; Canonica GW Current insights in allergen immunotherapy. Ann. Allergy Asthma Immunol. 2018, 120, (2), 152–154. [DOI] [PubMed] [Google Scholar]
- 2.Cox L; Nelson H; Lockey R; Calabria C; Chacko T; Finegold I; Nelson M; Weber R; Bernstein DI; Blessing-Moore J; Khan DA; Lang DM; Nicklas RA; Oppenheimer J; Portnoy JM; Randolph C; Schuller DE; Spector SL; Tilles S; Wallace D Allergen immunotherapy: a practice parameter third update. J. Allergy Clin. Immunol. 2011, 127, (1 Suppl), S1–55. [DOI] [PubMed] [Google Scholar]
- 3.Senna G; Ridolo E; Calderon M; Lombardi C; Canonica GW; Passalacqua G Evidence of adherence to allergen-specific immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, (6), 544–8. [DOI] [PubMed] [Google Scholar]
- 4.Shakya AK; Gill HS A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy. Vaccine 2015, 33, (33), 4060–4. [DOI] [PubMed] [Google Scholar]
- 5.Shakya AK; Lee CH; Gill HS Cutaneous vaccination with coated microneedles prevents development of airway allergy. J. Control. Release 2017, August 15 pii: S0168–3659(17)30779–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill HS; Denson DD; Burris BA; Prausnitz MR Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, (7), 585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill HS; Prausnitz MR Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, (2), 227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James C; Bernstein DI Allergen immunotherapy: an updated review of safety. Curr. Opin. Allergy Clin. Immunol. 2017, 17, (1), 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senti G; von Moos S; Kündig TM Epicutaneous Immunotherapy for Aeroallergen and Food Allergy. Curr. Treat. Options Allergy 2014, 1, (1), 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klimek L; Schmidt-Weber CB; Kramer MF; Skinner MA; Heath MD Clinical use of adjuvants in allergen-immunotherapy. Expert Rev. Clin. Immunol. 2017, 13, (6), 599–610. [DOI] [PubMed] [Google Scholar]
- 11.Smarr CB; Bryce PJ; Miller SD Antigen-specific tolerance in immunotherapy of Th2-associated allergic diseases. Crit. Rev. Immunol. 2013, 33, (5), 389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis JN; Durham SR Adjuvants for allergen immunotherapy: experimental results and clinical perspectives. Curr. Opin. Allergy Clin. Immunol. 2004, 4, (6), 543–8. [DOI] [PubMed] [Google Scholar]
- 13.Jensen-Jarolim E Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ. J. 2015, 8, (1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelbruch M; Nuss B; Korner M; Kapp A; Kiehl P; Bohm W Aluminium-induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy 2000, 55, (9), 883–7. [PubMed] [Google Scholar]
- 15.Dubensky TW Jr.; Kanne DB; Leong ML Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines 2013, 1, (4), 131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libanova R; Becker PD; Guzman CA Cyclic di-nucleotides: new era for small molecules as adjuvants. Microb. Biotechnol. 2012, 5, (2), 168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y; Tao W; Krebs SJ; Sutton WF; Haigwood NL; Gill HS Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakya AK; Lee CH; Gill HS Cutaneous vaccination with coated microneedles prevents development of airway allergy. J Control Release 2017, August 15 pii: S0168–3659(17)30779–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakya AK; Lee CH; Gill HS Coated microneedle based cutaneous immunotherapy prevents Der p1 induced airway allergy in mice. J Allergy Clin Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y; Tao W; Krebs SJ; Sutton WF; Haigwood NL; Gill HS Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm. Res. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggi E T-cell responses induced by allergen-specific immunotherapy. Clin. Exp. Immunol. 2010, 161, (1), 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J; Li P; Wu MX Natural STING Agonist as an “Ideal” Adjuvant for Cutaneous Vaccination. J. Invest. Dermatol. 2016, 136, (11), 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber GN STING-dependent cytosolic DNA sensing pathways. Trends Immunol 2014, 35, (2), 88–93. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y; Chen ZJ STING Specifies IRF3 phosphorylation by TBK1 in the Cytosolic DNA Signaling Pathway. Science signaling 2012, 5, (214), ra20–ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber GN STING: infection, inflammation and cancer. Nature reviews. Immunology 2015, 15, (12), 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin B; Ilyukha V; Sorokin M; Buzdin A; Vannier E; Poltorak A Cutting Edge: Activation of STING in T Cells Induces Type I IFN Responses and Cell Death. J Immunol 2017, 199, (2), 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L; Gerth AJ; Peng SL CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur. J. Immunol. 2004, 34, (5), 1483–7. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L; Chong MM; Littman DR Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, (5), 646–55. [DOI] [PubMed] [Google Scholar]
- 29.Smarr CB; Bryce PJ; Miller SD Antigen-Specific Tolerance in Immunotherapy of Th2-Associated Allergic Diseases. Critical reviews in immunology 2013, 33, (5), 389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson MC; Crespo MP; Abraham W; Moynihan KD; Szeto GL; Chen SH; Melo MB; Mueller S; Irvine DJ Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. The Journal of Clinical Investigation 2015, 125, (6), 2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]