Abstract
Objective
To determine the prevalence of American Heart Association and American College of Cardiology Foundation (AHA/ACCF) heart failure (HF) Stages after initiating potentially cardiotoxic chemotherapy.
Background
For individuals receiving potentially cardiotoxic chemotherapy, the frequency of transitioning from Stage A to more advanced HF Stages is not well described.
Methods
In 143 Stage A HF patients with breast cancer, lymphoma/leukemia, renal cell carcinoma, or sarcoma prior to and then three, six, and 12–24 months after initiating potentially cardiotoxic chemotherapy, we obtained blinded cardiovascular magnetic resonance measures of left ventricular ejection fraction (LVEF).
Results
Three months after initiating potentially cardiotoxic chemotherapy, 18.9% of patients transitioned from Stage A to Stage B HF. Eight-three percent and 80% of patients with Stage A HF at three months respectively exhibited Stage A HF at six and 12–24 months; 68% and 56% of those with Stage B HF at three months respectively exhibited Stage B HF at six and 12–24 months (p<0.0001 and p=0.026, respectively).
Conclusions
Transition from Stage A to Stage B or remaining in Stage A HF three months after initiating potentially cardiotoxic chemotherapy relates to longer term (six to 24-month post-treatment) assessments of HF Stage.
Keywords: anthracycline, trastuzumab, cardiotoxicity, HF Stages
Condensed Abstract
This study was performed to determine the prevalence of AHA/ACCF Stages A, B, C, and D HF three, six, and 12–24 months after initiating potentially cardiotoxic chemotherapy. In the first three months after initiating chemotherapy, nearly one-fifth of individuals transitioned from Stage A to Stage B HF, and the HF Stage at three months was likely to stay the same out to 24 months. Circumstances in addition to LVEF decline may be responsible for HF symptomatology, as these did not correlate well.
Introduction
Heart failure (HF) may occur after receipt of potentially cardiotoxic chemotherapy (1–14). The American Heart Association and American College of Cardiology Foundation (AHA/ACCF) identify those with cancer receiving treatment with potentially cardiotoxic agents as having Stage A HF, a designation indicating one is at risk for developing more advanced Stages (B-D) of HF (13). Although individuals receiving potentially cardiotoxic chemotherapy are classified as Stage A HF, the rate has not been well-described for progression to more advanced HF Stages for which other etiologies of HF often receive a therapeutic intervention (15).
Accordingly, this study was performed in individuals scheduled to receive potentially cardiotoxic chemotherapy to assess the change in HF Stage from pre-treatment to three, six, and 12–24 months post-initiation of treatment.
Methods
Study population and design
This study was approved by the Institutional Review Board of the Wake Forest School of Medicine, and all participants provided witnessed and written informed consent. The study was funded by the National Institutes of Health grants R33CA12196 and R01CA167821 and the Susan G. Komen Foundation grant BCTR07007769. Participants scheduled to receive potentially cardiotoxic chemotherapy for treatment of their cancer were recruited from the hematology/oncology outpatient clinics at the Comprehensive Cancer Center of the Wake Forest School of Medicine. We consecutively recruited 144 individuals with Stage A HF at baseline without a contraindication to CMR who agreed to participate for serial examinations prior to and three months after initiation of chemotherapy. Based on pre-designed lengths of longitudinal follow-up, 73 patients also were scheduled for a six-month follow-up visit, and 67 patients were scheduled for a visit one to two years after initiating their cancer treatment.
At the time of enrollment, demographic data including age, race, and gender were collected; height and weight were used to calculate body mass index (BMI). Prior cancer treatment and the cumulative doses of chemotherapy agents were recorded. Medication data (the use of potentially cardioprotective medications such as angiotensin-converting enzyme [ACE] inhibitors, beta-blockers, or statins), the presence of cardiovascular risk factors (coronary artery disease, hypertension, hyperlipidemia, diabetes, smoking history [current or previous]), and the type of cancer were recorded. The number of risk factors for each participant was calculated by assessing one point each for: BMI ≥ 30, hypertension, diabetes, coronary artery disease, hyperlipidemia, or smoking. For age, participants received zero points for age < 40, one point for age 40–60, and two points for age > 60. The maximum risk factor score was eight.
Determination of left ventricular ejection fraction
All participants underwent CMR imaging with 1.5-Tesla Magnetom Avanto Scanner (Siemens, Erlangen, Germany) prior to and a combination of three, six, and 12 to 24 months after initiating chemotherapy. CMR imaging was chosen to assess LVEF due to its accuracy and prior use in NIH funded initiatives such as the Multi-Ethnic Study of Atherosclerosis (16). LVEF measurements were obtained via previously published methods using cine white blood steady-state free precession techniques with a 256 × 128 matrix, a 40-cm field of view, a 10-ms repetition time (TR), a 4-ms echo time (TE), a 20-degree flip angle, an 8-mm thick slice with a 2-mm gap, and a 40-ms temporal resolution (16). All images were analyzed by readers blinded to all identifiers via an unpaired-blinded read.
Definition of HF Stages
All participants were Stage A HF, by definition, at enrollment and stratified into Stages A through D HF at each subsequent visit. Stage A HF was defined as those participants at risk for HF with a preserved left ventricular ejection fraction (i.e., an LVEF ≥ 53%) (2,13,15). Stage B HF was defined as CMR imaging evidence of LV dysfunction as seen by a LVEF decline to an absolute value <50% or a LVEF decline to <53% due to an absolute drop in LVEF of at least 10%. Stage C HF was defined as CMR evidence of LV dysfunction as defined in Stage B and current or past symptoms of HF requiring the new administration of heart failure therapy that included a) angiotensin converting enzyme or receptor blockers, b) beta-blockers, or c) oral or intravenous diuretics administered during a hospitalization, within an observation unit, or in an outpatient setting. Stage D HF was defined as those who met criteria for Stage C but required specialized advanced HF treatment during a hospitalization, including device therapy for support of LV performance (2,13,15).
Heart failure quality of life determination
At each visit, participants were asked to complete the Minnesota Living with Heart Failure Questionnaire (MLHFQ), a well-studied survey to assess quality of life in HF outpatients with questions rated on a six-point Likert scale where a score of zero signifies no impact and a score of five has a very significant impact on quality of life (17). We evaluated a five-question subset from the 21 questions asked during the MLHFQ survey that specifically inquired about symptoms of HF including: a) leg swelling, b) fatigue, c) low energy, d) dyspnea on exertion, and e) shortness of breath. As a result, participants could experience a maximum score of 25.
Statistical Analysis
Counts and percentages were reported for all categorical variables. Means and standard errors were reported for all continuous variables. Chi square and Fisher’s exact tests were used to test for associations between categorical variables (risk factors) and the transition from Stage A to other Stages of HF. From these tests, odds ratios (OR) were estimated to examine the relationship between each risk factor and the transition from Stage A to Stage B HF. These ORs and corresponding 95% confidence intervals were calculated and compared by creating Forest plots that examined each risk factor independently. Two sample Student’s t-tests were performed to examine the associations between continuous variables and the transition from Stage A to Stage B HF. All analyses assessed HF transition from baseline to three, six, and 12–24 months after initiating chemotherapy. Next, correlation analyses were performed to examine the relationship between changes in LVEF and MLHFQ subset scores at each time point. Logistic regression models were fit to assess longitudinally the relationship between the transistion from HF stage from baseline to 3 months predicted the HF stage observed at 6 months or 12–24 months. P-values ≤ 0.05 were considered to be statistically significant. Statistical analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Of the 144 subjects enrolled, one individual did not return for the three-month visit; demographic data for the 143 remaining subjects are shown in Table 1.
Table 1.
Baseline Characteristics and Therapy Distribution
| Age (years) | |
| < 40 | 26 (18.2) |
| 40–60 | 70 (49.0) |
| > 60 | 47 (32.9) |
| Gender | |
| Male | 40 (28.0) |
| Female | 103 (72.0) |
| Race | |
| White | 117 (81.8) |
| Black | 25 (17.5) |
| Other | 1 (< 1) |
| LVEF at baseline (%) | 60.6 ± 0.5 |
| Risk factors for HF | |
| BMI ≥ 30 | 63 (44.1) |
| HTN | 60 (42.0) |
| Smoking | 35 (24.5) |
| HLD | 35 (24.5) |
| Diabetes | 23 (16.1) |
| CAD | 7 (4.9) |
| Average number of risk factors | 2.7 ± 0.1 |
| Cardioprotective medications | |
| Beta-blocker | 21 (14.7) |
| ACE inhibitor or ARB | 38 (26.6) |
| Statin | 34 (23.8) |
| Type of cancer | |
| Breast cancer | 77 (53.8) |
| Lymphoma | 47 (32.9) |
| AML | 9 (6.3) |
| Sarcoma | 7 (4.9) |
| Renal cell carcinoma | 3 (2.1) |
| Prior cancer treatment (all regimens) | 24 (16.8) |
| Prior anthracycline treatment | 17 (11.9) |
| Chemotherapy treatment regimen | |
| Cyclophosphamide | 90 (62.9) |
| Anthracycline | 85 (59.4) |
| Trastuzumab | 45 (31.5) |
| Paclitaxel | 40 (28.0) |
| Docetaxel | 23 (16.1) |
| Other* | 13 (9.1) |
Total participants, n = 143. Values are mean ± SE or number n (%).
Other includes lapatinib (n=4), sunitunib (n=3), bortezomib (n=2), bevacizumab (n=1), pertuzumab (n=1), gemtuzumab (n=1), mitomycin (n=1)
BMI = body mass index; HTN = hypertension; HLD = hyperlipidemia; CAD = coronary artery disease; ACE inhibitor = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; AML = acute myelogenous leukemia.
At three months, 18.9% of participants transitioned to Stage B HF. This number slightly increased to 30.1% at six months and was 23.9% at 12–24 months (Figure 1). No participant met criteria for Stage C or D HF throughout the study (at three, six, or 12–24 months). The distribution of HF Stages and risk factors across the study times points are shown in Table 2. With the exception of a history of smoking (p=0.001 at 12–24 months), age, gender, race, and the risk factor score that included BMI ≥ 30, coronary artery disease, hypertension, diabetes, hyperlipidemia (p=0.053 to 1.0) were not clearly associated with the transition from Stage A to B HF. Patients concurrently receiving cardioprotective medications for conditions like hypertension or pre-existing coronary artery disease, such as ACE inhibitors, statins, and beta-blockers did not experience an increased association with a transition from Stage A to a more advanced HF Stage at any time point (p = 0.365 to 1.0).
Figure 1 – Frequency of HF Stages at 3, 6, and 12–24 Months.
This bar graph shows the percent of participants in each Stage of HF at baseline (left-most bar), three months, six months, and 12–24 months (right-most bar).
Table 2.
Distribution of HF Stages by Demographics
| 3 Months | 6 Months | 12–24 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage A (n = 116) | Stage B (n = 27) | p-value | Stage A (n = 51) | Stage B (n = 22) | p-value | Stage A (n = 51) | Stage B (n = 16) | p-value | |
| Age | 0.326 | 0.637 | 0.261 | ||||||
| < 40 | 19 (16.4) | 7 (25.9) | 8 (15.7) | 5 (22.7) | 10(19.6) | 3 (18.8) | |||
| 40–60 | 60 (51.7) | 10 (37.0) | 24 (47.1) | 11 (50.0) | 21 (41.2) | 10 (62.5) | |||
| > 60 | 37 (31.9) | 10 (37.0) | 19 (37.3) | 6 (27.3) | 20 (39.2) | 3 (18.8) | |||
| Gender | 0.831 | 0.249 | 0.053 | ||||||
| Male | 32 (27.6) | 8 (29.6) | 8 (15.7) | 6 (27.3) | 10 (19.6) | 7 (43.8) | |||
| Female | 84 (72.4) | 19 (70.4) | 43 (84.3) | 16 (72.7) | 41 (80.4) | 9 (56.3) | |||
| Race | 0.221 | 0.400 | 0.101 | ||||||
| White | 96 (82.8) | 21 (77.8) | 43 (84.3) | 19 (86.4) | 44 (86.3) | 11 (68.8) | |||
| Black | 20 (17.2) | 5 (18.5) | 8 (15.7) | 2 (9.1) | 7 (13.7) | 4 (25.0) | |||
| Other | 0 (0.0) | 1 (3.7) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 1 (6.3) | |||
| Risk factors for HF | |||||||||
| BMI ≥ 30 | 49 (42.2) | 14 (51.9) | 0.365 | 20 (39.2) | 10 (45.5) | 0.619 | 25 (49.0) | 6 (37.5) | 0.420 |
| CAD | 6 (5.2) | 1 (3.7) | 1.000 | 2 (3.9) | 2 (9.1) | 0.579 | 2 (3.9) | 0 (0.0) | 1.000 |
| HTN | 50 (43.1) | 10 (37.0) | 0.565 | 26 (51.0) | 9 (40.9) | 0.429 | 19 (37.3) | 5 (31.3) | 0.662 |
| Diabetes | 19 (16.4) | 4 (14.8) | 1.000 | 9 (17.6) | 5 (22.7) | 0.613 | 6 (11.8) | 4 (25.0) | 0.234 |
| HLD | 29 (25.0) | 6 (22.2) | 0.762 | 12 (23.5) | 4 (18.2) | 0.762 | 15 (29.4) | 4 (25.0) | 1.000 |
| Smoker | 26 (22.4) | 9 (33.3) | 0.235 | 18 (35.3) | 7 (31.8) | 0.774 | 5 (9.8) | 8 (50.0) | 0.001§ |
| ≥ 2 risk factors* | 84 (72.4) | 20 (74.1) | 0.861 | 37 (72.5) | 16 (72.7) | 0.987 | 38 (74.5) | 13 (81.3) | 0.581 |
| Cardioprotective medications | |||||||||
| ACE inhibitor | 31 (26.7) | 7 (25.9) | 0.933 | 17 (33.3) | 5 (22.7) | 0.365 | 14 (27.5) | 4 (25.0) | 1.000 |
| Beta-blocker | 18 (15.5) | 3 (11.1) | 0.765 | 12 (23.5) | 3 (13.6) | 0.529 | 6 (11.8) | 1 (6.3) | 1.000 |
| Statin | 28 (24.1) | 6 (22.2) | 0.833 | 11 (21.6) | 4 (18.2) | 1.000 | 14 (27.5) | 4 (25.0) | 1.000 |
Values are number (%n).
BMI = body mass index, CAD = coronary artery disease, HTN = hypertension, HLD = hyperlipidemia, ACE = angiotensin converting enzyme
Refer to Table 1 for risk factor determination.
p-values ≤ 0.05 were considered significant.
On average, the LVEF declined from 60.6±0.5% at baseline to 56.7±0.6%, 53.7±0.8%, and 56.9±0.9% at three, six, and 12–24 months, respectively (p<0.001 for all; Figure 2A–C). Averaged scores from the MLHFQ subset increased from 4.3±0.4 at baseline to 7.6±0.5 at three months (p<0.001) and 7.0±0.7 at six months (p<0.001), but then were similar at 4.0±0.6 at 12–24 months (p=0.748). Change in MLHFQ subset scores correlated poorly with change in LVEF at any time point (R2 = 0.0028 to 0.0216 for all; Figure 3A–C).
Figure 2A-C – Relationship of LVEF Score at Baseline and 3, 6, and 12–24 Months.
These spaghetti plots show change in LVEF and means at baseline and three months (Figure 2A), baseline and six months (Figure 2B), and baseline and 12–24 months (Figure 2C).
Figure 3A-C – Change in LVEF vs Change in MLHFQ Subset Score at 3, 6, and 12–24 months as compared to baseline.
The scatterplots graph the relationship between the change in LVEF (x-axis) from baseline and three (A), six (B), or 12–24 (C) months and the change in modified MLHFQ score (y-axis) in the same time period. Corresponding trendlines and R2 values are shown.
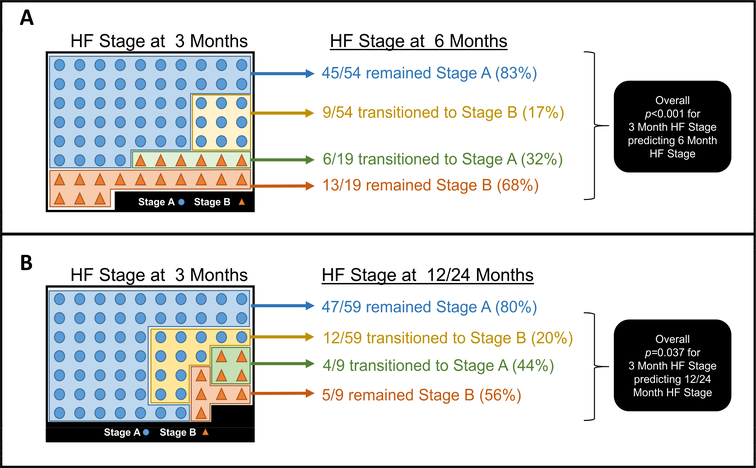
For participants who remained in Stage A HF three months after initiating chemotherapy, 83.3% remained in Stage A HF at six months and 79.7% at 12–24 months. Of those who developed Stage B HF at three months, 68% and 56% respectively remained in Stage B HF at six or 12–24 months. The odds ratio of remaining in Stage A or transitioning to Stage B HF at 3 months predicting Stage A or B HF was 10.8 (confidence interval from 3.3 to 36.1). This suggests nearly an 11 fold higher odds that the Stage at 3 months predicts an individual’s HF Stage at 6 months (p=0.0001). Similarly, the HF Stage at 3 months exhibited an odds ratio of 5.3 (confidence interval from 1.2 to 23.2) for forecasting the HF Stage at 12 to 24 months (p=0.026). Thus, these data indicate that for those with Stage A HF prior to initiating potentially cardiotoxic chemotherapy, there was a nearly 5.3 fold higher odds that a person’s HF Stage at 3 months would forecast their HF Stage at 12 to 24 months (Figure 4).
Figure 4 – Frequency of transitioning within HF Stages from 3 to 6 or 12–24 Months.
Panel A indicates the numbers of individuals at three months transitioning between HF Stage at six months, and Panel B demonstrates HF transitions from three to 12–24 months. Circles indicate those with Stage A HF and triangles indicate those with Stage B HF. As shown, HF Stage at three months predicts HF stage at six (p<0.0001) and 12–24 months (p<0.026). Corresponding odds ratios for 3 month HF Stage transition predicting 6 month HF stage is 10.8 (95% confidence interval 3.3 to 36.1) and for 3 month HF Stage transition predicting 12–24 month HF stage is 5.3 (95% confidence interval 1.2 to 23.2).
Table 3 displays the treatment-related changes in the Stage of HF between baseline and three, six, and 12–24 months. Five out of nine (56%) participants with acute myelogenous leukemia (AML) progressed from Stage A to Stage B HF (p=0.012) at three months. By six months, this change was no longer present. Cumulative trastuzumab dose and the combination of an anthracycline plus trastuzumab contributed to transition to a more advanced HF Stage at the six month time point (p=0.014 for anthracycline plus trastuzumab; p=0.022 for cumulative trastuzumab dose). Patients receiving anthracyclines identified a rate of 22.0% transitioning to Stage B HF three months into treatment. Thirty-three percent of patients specifically receiving anthracycline and trastuzumab combination chemotherapy regimens transitioned to Stage B HF at three months.
Table 3.
Distribution of HF Stages by Treatment-Related Variables
| 3 Months | 6 Months | 12–24 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage A (n = 116) | Stage B (n = 27) | p-value | Stage A (n=51) | Stage B (n=22) | p-value | Stage A (n= 51) | Stage B (n=16) | p-value | |
| Cancer | |||||||||
| Acute myelogenous leukemia | 4 (3.4) | 5 (18.5) | 0.012§ | 4 (7.8) | 3 (13.6) | 0.424 | -- | -- | |
| Breast | 61 (52.6) | 16 (59.3) | 0.531 | 35 (68.6) | 14 (63.6) | 0.677 | 31 (60.8) | 7 (43.8) | 0.230 |
| Lymphoma | 42 (36.2) | 5 (18.5) | 0.078 | 11 (21.6) | 4 (18.2) | 0.742 | 18 (35.3) | 7 (43.8) | 0.542 |
| Renal cell carcinoma | 3 (2.6) | 0 (0.0) | 1.000 | 1 (2.0) | 1 (4.5) | 0.515 | -- | -- | |
| Sarcoma | 6 (5.2) | 1 (3.7) | 1.000 | -- | -- | 2 (3.9) | 2 (12.5) | 0.239 | |
| Prior cancer treatment | 17 (14.7) | 7 (25.9) | 0.158 | 10 (19.6) | 6 (27.3) | 0.468 | 9 (17.6) | 6 (37.5) | 0.096 |
| Prior anthracycline treatment | 10 (8.6) | 7 (25.9) | 0.012 | 5 (9.8) | 6 (27.3) | 0.056 | 7 (13.7) | 5 (31.3) | 0.111 |
| Anthracycline before trastuzumab | 11 (9.7) | 7 (25.9) | 0.024 | 6 (11.8) | 8 (36.4) | 0.014§ | 7 (14.3) | 5 (31.3) | 0.129 |
| Chemotherapy drugs | 0.053 | 0.075 | 0.329 | ||||||
| Anthracycline (w/o trastuzumab) | 63 (54.3) | 13 (48.2) | 26 (51.0) | 9 (40.9) | 23 (45.1) | 9 (56.3) | |||
| Anthracycline + trastuzumab | 15 (12.9) | 9 (33.3) | 7 (13.7) | 9 (40.9) | 10 (19.6) | 5 (31.3) | |||
| Trastuzumab (w/o anthracycline) | 19 (16.4) | 4 (14.8) | 12 (23.5) | 2 (9.1) | 11 (21.6) | 2 (12.5) | |||
| Neither anthracycline nor trastuzumab | 19 (16.4) | 1 (3.7) | 6 (11.8) | 2 (9.1) | 7 (13.7) | 0 (0.0) | |||
| Cumulative doxorubicin dose (mg/m2)** | 202.4±7.4 | 161.9±25.1 | 0.141 | 216.4±14.1 | 212.5±27.0 | 0.898 | 272.9±11.0 | 295.5±36.1 | 0.563 |
| Cumulative trastuzumab dose (mg) | 2608.3±317.7 | 2189.8±380.4 | 0.383 | 3083.9±449.7 | 1555.2±380.1 | 0.022§ | 4289.0±859.3 | 11324.7*** | |
| Cumulative paclitaxel dose (mg) | 1598.6±423.5 | 1093.4±162.5 | 0.273 | 1974.0±651.0 | 850.3±168.6 | 0.110 | 1519.5±326.3 | ||
| Cumulative docetaxel dose (mg) | 598.3±47.3 | 602.3±56.9 | 0.958 | 604.3±102.7 | 490.5*** | 656.8±50.0 | 918.0±42.0 | 0.111 | |
| Cumulative cyclophosphamide dose (mg) | 5240.4 ±226.2 | 4438.0±456.2 | 0.128 | 4634.6±242.5 | 4335.8±300.1 | 0.444 | 7109.0±514.1 | 7710.6±1443.2 | 0.704 |
Values are listed as the number n(%) or mean ± standard error.
Other includes lapatinib, sunitunib, bortezomib, bevacizumab, pertuzumab, gemtuzumab, and mitomycin
All anthracyclines were recorded in doxorubicin equivalents (34) and indexed to body surface area (BSA)
Only one value
p-values ≤ 0.05 were considered significant.
Figure 5 shows the ORs for various factors that could influence an individual’s transition from Stage A to Stage B HF at three, six, and 12–24 months after initiating chemotherapy. At three months (Figure 5A), participants who received combination anthracycline and trastuzumab chemotherapy were more likely to transition to Stage B HF (OR 3.37, 95% CI [1.28–8.85]). This remained significant at six months (OR 4.35, 95% CI [1.36–13.96]) (Figure 5B). At both six and 12–24 months (Figure 5B and C), initiating a potentially cardiotoxic chemotherapeutic regimen with an LVEF in the bottom quartile of the study group was associated with progression to a more advanced HF Stage (OR 5.12, 95% CI [1.75–14.96]) at six months and (OR 3.77, 95% CI [1.04–13.68]) at 12–24 months.
Figure 5 – Forest Plots of ORs for Transition to Stage B HF at 3, 6, and 12–24 Months.
The Forest plots graph the ORs for various factors possibly contributing to transition to Stage B HF at three (A), six (B), and 12–24 months (C). Error bars represent 95% confidence interval.
Discussion
There are several important findings related to this study. First, 18.9% of individuals receiving potentially cardiotoxic chemotherapy transition from Stage A to Stage B HF three months after initiating their treatment (Figure 1). Second, those who remain classified as Stage A HF three months after initiation of chemotherapy are more likely to remain classified as Stage A HF six and 12–24 months after initiating potentially cardiotoxic chemotherapy (Figure 4). In contrast, for participants who transition to Stage B HF three months after starting chemotherapy, the majority remain in Stage B HF at six and 12–24 months after initiating their therapy (Figure 4). This suggests that an early assignment of HF Stage, as early as three months after starting chemotherapy, may be predictive of future HF Stages. Finally, and somewhat surprisingly, change in LVEF and HF symptom scores from MLHFQ subset of 5 questions did not correlate with one another (Figure 3).
Currently, LVEF represents one of the key components of the current American Society of Echocardiography Consensus Statements and American Society of Clinical Oncology Statements on monitoring patients for cardiac injury after receipt of potentially cardiotoxic chemotherapy (1,2,11,15,18). Cardinale et al. found that 82% of those started on guideline-based HF therapy for an LVEF decline of more than ten absolute percentage points or a LVEF decline to <50% three to four months into treatment experienced a future improvement in LVEF (19). Results from our study indicate that early monitoring of patients receiving a regimen that contains an anthracycline identifies 21.0% of those that transition to Stage B HF three months into treatment. Based on Cardinale’s data, it is plausible that these patients may be candidates for guideline-based HF treatment. Furthermore, as shown in Figure 4, participants who remain with Stage A HF three months after initiating chemotherapy are more likely to remain in Stage A HF at both six and 12–24 months after initiating their treatment. At the same time, those who progress to Stage B HF at three months are more likely to remain with Stage B HF at six and 12–24 months rather than return to Stage A HF.
With regard to cancer type, there was a high percentage (55.6%) of individuals with AML who transitioned from Stage A to Stage B HF three months after initiating chemotherapy. This could be related to the fact that these individuals receive relatively large doses of chemotherapy (usually anthracycline-based) over three days during their induction. We did not observe a relationship between the number of risk factors for cardiovascular disease and transition from Stage A to Stage B HF (Table 2) (6,16,20–22). These findings may be related to our relatively small sample size.
In this study, the OR for transitioning from Stage A to Stage B HF after receipt of anthracycline and trastuzumab chemotherapy regimen during this study was 3.37 (95% CI [1.28–8.85]) at three months and 4.35 (95% CI [1.36–13.96]) at six months (Figure 5). This is mostly consistent with previously published results which have established the incidence of LV systolic dysfunction with trastuzumab ranging from 21–34% at some point during receipt of trastuzumab (3,4,9,12,23). Also interestingly, those participants who entered the study with their LVEF in the bottom quartile of participants were more likely to exhibit Stage B HF at later time points. This finding was particularly noticeable at six and 12–24 months; OR 5.12 (95% CI 1.75–14.96) at six months and OR 3.77 (95% CI 1.04–13.68) at 12–24 months (Figure 5). Further testing and data collection is required to determine the cause of this phenomenon.
In an additional analysis, as shown in Figure 3A–C, our observed increase in MLHFQ subset scores did not correlate with LVEF decline even though roughly one-fifth of participants showed declines in LVEF to qualify their classification of Stage B HF at each time point. This finding could be due to: a) many of the symptoms included in the MLHFQ are similar to chemotherapy-related side effects but not necessarily indicative of “true HF;” b) HF is occurring but not necessarily related to a perceptible LVEF decline – similar to the syndrome of HF with “preserved” LVEF; c) some of the decline in LVEF may have been related to isolated decrements in LV end diastolic volume as a result of decreased oral intake as opposed to impaired LV systolic function; or d) a combination of the above (24,25). While our data do not allow us to determine the reason for the lack of a correlation between LVEF and MLHFQ scores, they underscore the need for additional research to clarify the relationship between LVEF and HF symptomatology among patients receiving treatment for cancer with potentially cardiotoxic agents.
Our data did not indicate that concurrent receipt of ACE inhibitors, statins, or beta-blockers for treatment of other cardiovascular risk factors such as hypertension influence the transition from Stage A to more advanced HF Stages. While several research studies suggest beneficial effects with renin-angiotensin inhibition and beta-blockade, other studies of those without underlying heart disease, elderly patients, or large numbers of comorbidities are less certain (16,22,26–29). Gulati et al. showed that candesartan, when used in healthy breast cancer patients receiving adjuvant anthracycline- containing chemotherapy, showed a small protective effect on LVEF decline as compared to placebo (30). Similarly, Cardinale et al. showed that enalapril helped to prevent LV dysfunction after anthracycline chemotherapy (31). Another study showed that breast cancer patients who were already taking statins had a smaller decrease in LVEF after initiation of anthracycline-based chemotherapy as compared to those not taking statins, though this was an observational study (32). Perhaps our small sample size, and the fact that our study is observational (i.e., we did not randomize patients to ACE inhibitors or other therapy) explains our findings.
The LVEF change used to identify Stage B-D HF included two criteria: drop in LVEF to <50% or a 10% decline in LVEF to a value <53%. Based on our prior assessments, in this study, we are reliably able to detect LVEF changes >3% (33). Of the 27 individuals who transitioned from Stage A to Stage B, fifteen exhibited >10% declines in LVEF to values <53%. The remaining patients who transitioned to Stage B experienced drops to <50%. Among these twelve patients, one patient transitioned from Stage A to Stage B based on a change in LVEF of 3% (the person dropped from a baseline of 52% to a follow-up of 49% LVEF). Thus, it is possible that one patient out of 27 (3.7% of those transitioning) may have been identified as more advanced HF due to chance. In addition, there were four patients who remained Stage A whose final LVEF values were 51% with their change in LVEF from baseline to follow-up <3%. This represents 3.4% (4/117) of the patients who were identified as not transitioning. These patients (five total) comprised nearly equal proportions of patients who did versus did not transition from Stage A HF to Stage B HF. Based on these analyses, we conclude that there is a possibility that, at most, 3.5% (5/144) of patients may have been misclassified at the follow-up visit due to the variability of our CMR measurement of LVEF; however, four patients remained Stage A and one patient transitioned from Stage A to Stage B HF, and we believe our findings are not due to chance or measurement error.
Our study has the following limitations. First, to detect Stage B HF, we required a marked drop in LVEF. Our LVEF metric was adopted from the consensus statement provided by the American Society of Echocardiography. To date, there have been no cardio-oncology guidelines for LVEF assessments using CMR imaging (2). Second, a small number of patients underwent LV myocardial strain measurements (see Online Supplement A). According to these data, there was a small difference in strain measurements, however, our sample size was too small to make definite conclusions. We also did not assess LV diastolic dysfunction. As such, we may have underestimated the incidence of Stage B HF in our study population. Measuring strain in a larger sample size could be an important future research study. Further studies incorporating more objective measurements with metrics like cardiopulmonary exercise or six-minute walk testing may provide greater insight into development of HF symptoms. Finally, due to sample size limitations and our study design (cohort as opposed to randomized clinical trial), we were unable to assess whether potentially cardioprotective medications, such as beta-blockers influenced the transition to more advanced HF stage.
In conclusion, almost one-fifth of participants who receive potentially cardiotoxic chemotherapy including anthracyclines and trastuzumab may transition from Stage A to Stage B HF three months after initiation of their treatment regimens. Baseline to three month changes in Stage of HF are associated with longer term HF stage assessments including baseline to six or 12–24 months after initiating potentially cardiotoxic chemotherapy.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
This study shows that there is evidence of transition from Stage A to Stage B HF early after receipt of anthracycline- or trastuzumab-based chemotherapy that correlates with HF Stage among survivors of these treatments. Additionally, the HF Stage assigned three months after starting chemotherapy is associated with HF Stage at six and 12–24 months post-treatment.
Transitional Outlook
Further prospective research is needed to determine the factors that contribute to and strategies that may prevent the development of HF in patients receiving potentially cardiotoxic chemotherapy.
Acknowledgments
Financial Support: This research was supported in part by National Institutes of Health grants R01CA167821, R33CA12196, and the Susan G. Komen Foundation, BCTR07007769.
Abbreviations
- AHA/ACCF
American Heart Association/American College of Cardiology Foundation
- HF
heart failure
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- CMR
cardiac magnetic resonance
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- BMI
body mass index
- ACE
angiotensin converting enzyme
- OR
odds ratio
- AML
acute myelogenous leukemia
Footnotes
Industry Relationships: The authors have no relationships with industry to disclose regarding this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: An overview of current uses and future directions of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2014;27:911–39. [DOI] [PubMed] [Google Scholar]
- 3.Bowles EJA, Wellman R, Feigelson HS et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst 2012;104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin M, Esteva FJ, Alba E et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: Review and expert recommendations. Oncologist 2009;14:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Long HD, Lin YE, Zhang JJ, Zhong WZ, Zheng RN. Risk of congestive heart failure in early breast cancer patients undergoing adjuvant treatment with trastuzumab: A meta-analysis. Oncologist 2016;21:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidman A, Hudis C, Pierri MK et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215–21. [DOI] [PubMed] [Google Scholar]
- 7.Eschenhagen T, Force T, Ewer MS et al. Cardiovascular side effects of cancer therapies: A position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail 2011;13:1–10. [DOI] [PubMed] [Google Scholar]
- 8.Steingart RM, Bakris GL, Chen HX et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J 2012;163:156–63. [DOI] [PubMed] [Google Scholar]
- 9.Tan-Chiu E, Yothers G, Romond E et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 2005;23:7811–9. [DOI] [PubMed] [Google Scholar]
- 10.Ho E, Brown A, Barrett P et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: A speckle tracking echocardiographic study. Heart 2010;96:701–7. [DOI] [PubMed] [Google Scholar]
- 11.Smith LA, Cornelius VR, Plummer CJ et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawaya H, Sebag IA, Plana JC et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt SA, Abraham WT, Chin MH et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in collaboration with the international society for heart and lung transplantation. J Am Coll Cardiol 2009;53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 14.Khouri MG, Douglas PS, Mackey JR et al. Cancer therapy-induced cardiac toxicity in early breast cancer: Addressing the unresolved issues. Circulation 2012;126:2749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure:Aa report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol 2016;68:1476–88. [DOI] [PubMed] [Google Scholar]
- 16.Heckbert SR, Post W, Pearson GD et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: The Multiethnic Study of Atherosclerosis. J Am Coll Cardiol 2006;48:2285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rector TS. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire. Am Heart J 1992; 124(4):1017–25. [DOI] [PubMed] [Google Scholar]
- 18.Armenian SH, Lacchetti C, Barac A et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 19.Cardinale D, Colombo A, Bacchiani G et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–8. [DOI] [PubMed] [Google Scholar]
- 20.Guenancia C, Lefebvre A, Cardinale D et al. Obesity As a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: A systematic review and meta-analysis. J Clin Oncol 2016;34:3157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J Am Coll Cardiol 2012;60:2384–90. [DOI] [PubMed] [Google Scholar]
- 22.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. J Clin Oncol 2005;23:8597–605. [DOI] [PubMed] [Google Scholar]
- 23.Slamon D, Eiermann W, Robert N et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melendez GC, Sukpraphrute B, D’Agostino RB Jr. et al. Frequency of left ventricular end-diastolic volume-mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am J Cardiol 2017;119:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhakal BP, Malhotra R, Murphy RM et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drafts BC, Twomley KM, D’Agostino R, Jr. et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swiger KJ, Singh J, Lenihan DJ. Cardiomyopathic toxicity from chemotherapy: Is there an opportunity for preemptive intervention? Curr Treat Options Cardiovasc Med 2017;19:20. [DOI] [PubMed] [Google Scholar]
- 28.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25:3808–15. [DOI] [PubMed] [Google Scholar]
- 29.Georgakopoulos P, Roussou P, Matsakas E et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: A prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol 2010;85:894–6. [DOI] [PubMed] [Google Scholar]
- 30.Gulati G, Heck SL, Ree AH et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;37:1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardinale D, Colombo A, Sandri MT et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006;114:2474–81. [DOI] [PubMed] [Google Scholar]
- 32.Chotenimitkhun R, D’Agostino R Jr., Lawrence JA et al. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. The Can J Cardiol 2015;31:302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natori S, Lai S, Finn JP et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–65. [DOI] [PubMed] [Google Scholar]
- 34.Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol 2001;28:2–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.