Summary
The AHOD0831 study for paediatric patients with high risk Hodgkin lymphoma tested a response-based approach designed to limit cumulative alkylator exposure and reduce radiation volumes. Patients (Stage IIIB/IVB) received two cycles of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide). Rapid early responders [RER, no positron emission tomography (PET) activity above mediastinal blood pool] were consolidated with 2 cycles of ABVE-PC. Slow early responders (SER) received 2 cycles of ifosfamide/vinorelbine and 2 cycles of ABVE-PC. Radiotherapy was administered to sites of initial bulk and/or SER. By intent-to-treat analysis, 4-year second event-free survival (EFS; freedom from second relapse or malignancy) was 91.9% [95% confidence interval (CI):86.1%−95.3%], below the projected baseline of 95% (p=0.038). Five-year first EFS and overall survival (OS) rates are 79.1% (95% CI:71.5%−84.8%) and 95% (95% CI:88.8%−97.8%). Eight of 11 SER patients with persistent PET positive lesions at the end of chemotherapy had clinical evidence of active disease (3 biopsy-proven, 5 with progressive disease or later relapses). Although this response-directed approach did not reach the ambitiously high pre-specified target for second EFS, EFS and OS rates are comparable with results of recent trials despite the reduction in radiotherapy volumes from historical involved fields. Persistent PET at end of chemotherapy identifies a cohort at an especially high risk for relapse/early progression.
Keywords: Hodgkin lymphoma, paediatric, response adapted, PET, radiation
Introduction
Outcomes for paediatric patients with classical Hodgkin lymphoma (HL) are excellent using dose intensive, response-based therapy.(Schwartz et al. 2009, Mauz-Korholz et al. 2010, Kelly et al. 2011, Friedman et al. 2014) However, it has been challenging to achieve excellent cure rates among those with high-risk disease without routine use of radiotherapy (RT) or significant exposure to alkylating agents. (Hodgson et al. 2007). New strategies aimed at maximizing the therapeutic index are needed.
Tailoring therapy to early response potentially allows those patients with more chemosensitive tumours to have decreased cumulative treatment exposure and, consequently, reduced risk for long term toxicity. In contrast, those who demonstrate relative chemotherapy resistance with a slower response to therapy can be given augmented therapy with a novel regimen to 1) overcome resistance to the initial agents and 2) prevent long term complications associated with continued exposure to the initial agents, including doxorubicin and bleomycin. In P9425, tailoring therapy to response allowed a reduction in anthracycline and alkylator exposure for 63% of patients, with no statistical difference in 3-year event free survival (EFS) for 3 vs. 5 cycles of chemotherapy. However all patients received regional RT.(Schwartz et al. 2009) Similarly, there were no significant differences in outcome among the rapid and slow early responding patients on CCG 59704.(Kelly et al. 2011)
Among patients with high risk HL who develop a first relapse after dose-intensive regimens, high dose salvage therapies with stem cell rescue can result in long term cure in approximately 50%.(Harker-Murray et al. 2014; Josting et al. 2005) Given that toxicity is enhanced when secondary retrieval therapies are needed, the benefits of reduced dose therapies to the larger cohort who do not develop a recurrence must be considered. Quantifying the overall outcome of initial therapy and, if necessary, any salvage regimen, is essential.
Based on the experience of prior studies in the Children’s Oncology Group (COG), the AHOD0831 study tested a response-based treatment approach for paediatric patients with stages IIIB and IVB HL through the use of a treatment approach designed to limit cumulative alkylator exposure and reduce radiation volumes in rapid responding patients. Response measured by 18F-fluorodeoxyglucose (FDG)-positron-emission tomography (PET) following 2 cycles of dose intensive chemotherapy was used to assign consolidation chemotherapy and risk-adapted RT. Ifosfamide and vinorelbine were chosen for augmentation of therapy for slow early responding patients, as the combination was shown to have a high response rate in children with relapsed/refractory HL.(Trippett et al. 2015). This regimen should be associated with less toxicity than augmentation with escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone), a regimen often used for therapy intensification in slow responders.(Johnson et al. 2016, Press et al. 2016, Straus et al. 2018)
Methods
Patients
Children and adolescents aged ≤21 years, with classical or nodular lymphocyte predominant HL were enrolled on AHOD0831, a prospective nonrandomized phase 3 multicentre study. Stages, based on clinical evaluation by history and physical examination, imaging studies and bilateral bone marrow biopsies, were defined by the Ann Arbor staging system.(Lister et al. 1989) Patients were eligible for the study if they had HL stage III or IV, and B symptoms. Bulky disease was defined as any one of (1) Large mediastinal mass: tumour diameter > 1/3 the thoracic diameter on a posterior-anterior chest X-ray; (2) A continuous aggregate of nodal tissue that measures > 6 cm in the longest transverse diameter in the axial plane in any nodal area; or (3) Macroscopic splenic nodules: focal defects in the spleen seen on computed tomography (CT), PET or magnetic resonance imaging studies consistent with HL (deemed to be the functional equivalent of bulk disease in this study).
Treatment protocol
Details of the treatment regimens are listed in Table I. All patients received 2 cycles of induction chemotherapy with doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) administered every 21 days. Patients with a rapid early response to therapy, defined as a complete response (CR) after 2 cycles of therapy, received consolidation therapy with 2 more cycles of ABVE-PC followed by RT to sites of initial bulky involvement only. Patients with a slow early response to induction chemotherapy were administered 2 cycles of ifosfamide and vinorelbine every 21 days, followed by 2 more cycles of ABVE-PC. Involved field RT (21 Gy in 14 fractions) was delivered to sites of initial bulk disease and slow-responding sites. Radiotherapy plans were prospectively reviewed by the Quality Assurance Review Center (QARC). Radiotherapy boosts beyond 21 Gy were not allowed, even for sites of persistent FDG-PET uptake at the end of chemotherapy.
Table I.
Details of the chemotherapy regimen.
| ABVE-PC: |
|---|
| Doxorubicin 25 mg/m2 i.v.days 1,2 |
| Bleomycin 5 u/m2 day 1, 10 u/m2 i.v.day 8 |
| Vincristine 1.4 mg/m2 i.v.days 1, 8 (max 2.8 mg) |
| Etoposide 125 mg/m2 i.v.days 1–3 |
| Prednisone 40 mg/m2 p.o.days 1–7 |
| Cyclophosphamide 600 mg/m2 i.v.days 1, 2 |
| G-CSF 5 μg/kg/day s.c days 9+ until ANC >1 × 109/l |
| IV: |
| Ifosfamide 3000 mg/m2 i.v.days 1–4 |
| Vinorelbine 25 mg/m2 i.v.days, 1, 5 |
| G-CSF 5 μg/kg/day s.c days 6+ until ANC >1 ×109/l |
ANC: absolute neutrophil count; G-CSF: granulocyte colony-stimulating factor; i.v.: intravenously; p.o.: orally; s.c.: subcutaneously.
The protocol was reviewed and approved by the National Cancer Institute and the Pediatric Central Institutional Review Board or the institutional review boards of the participating institutions. Written informed consent was obtained from patients, parents and/or guardians in accordance with the Declaration of Helsinki as required by government regulations.
Response assessment
Response to chemotherapy was defined on the basis of changes from diagnosis in anatomic radiological and FDG-PET evidence of disease adapted from the 2007 International Working Group lymphoma response criteria (Cheson et al. 2007, Juweid et al. 2007). A secondary objective was to investigate whether very early response by FDG-PET after 1 cycle of chemotherapy would identify a subject cohort that can be studied in future trials and that is distinguishable from currently defined rapid early responders (RER) after 2 cycles. Therefore, early response for determination of consolidation therapy was assessed by central review after either one or two cycles of induction chemotherapy (i.e., if post-Cycle 1 PET was negative it was not required to be repeated after Cycle 2). Patients meeting criteria for CR after one or two cycles were classified as RER. Patients classified as partial response (PR) or stable disease (SD) were considered slow early responders (SER). Patients with progressive disease (PD) came off protocol-directed therapy but were followed for second EFS (2nd EFS) and overall survival (OS).
Complete response was defined by response according to PET scan, as well as resolution of B symptoms. At sites where the PET scan was positive before therapy, a post-treatment residual mass of any size was permitted as long as it was PET-negative (equivalent to Deauville 1 or 2), with return to normal size and resolution of pre-therapy nodules in the spleen. For patients with at least one remaining PET-positive (equivalent to Deauville 3, 4, 5) site of the previously involved sites, less than complete disappearance but a ≥50% decrease in sum of the product of the perpendicular diameters (SPPD) of up to 6 of the largest dominant nodes or nodal masses was considered to be a PR. Stable disease was defined as < 50% decrease and < 50% increase in SPPD of any of the nodal masses, with no new lesions. Progressive disease (PD) or relapse following CR was classified as clinical or radiographic evidence of PET-positive increased tumour volume in a previously involved site with ≥50% increase in the SPPD to a size >1.5 cm in the long axis or as involvement of a new site.
Study design and statistical methods
Many patients with relapsed HL can be successfully cured with retrieval therapy. We therefore defined a novel endpoint, 2nd EFS, that might more accurately reflect long-term OS than primary EFS because many patients with relapsed HL can be successfully cured with retrieval therapy. For this reason, the primary aim of this non-randomized study was to achieve satisfactory survival outcome in the very high-risk patients treated in this response-based paradigm, as defined by a 4-year 2nd EFS at or above 95%. In contrast to studies in patients with relapsed disease, the 2nd EFS in this study is calculated from time of enrolment to the time of the second event. The first event can be relapse/progression of HL, second malignant neoplasm (SMN), biopsy-proven HL following completion of consolidation for SER patients, or death. Second event was defined as any subsequent relapse/progression (of HL or a previously reported SMN), a new SMN or death after a first event. As a severe event, death cannot happen twice. In this study it was important to capture death as an event in the survival analysis. Hence, if death occurred as the first event, it also counted as the second event. Patients who are alive without a 2nd event were censored at the date last seen. All evaluable patients were included in the survival analyses. A formal sample size was calculated based on a comparison of the observed 2nd EFS curve to a baseline 2nd EFS curve derived from prior paediatric high risk HL trials (Schwartz et al 2009; Kelly et al 2011). It was expected that 150 eligible and evaluable patients would result in 91% power to detect the departure from the baseline 2nd EFS in a one-sided one-sample log rank test at alpha level of 0.1. The 2nd EFS of the experimental approach was deemed unsatisfactory compared with the baseline if the p value was <0.1, which was calculated based on a comparison of the two survival curves across the entire follow-up time.
The secondary objectives included (1) maintaining 3-year EFS ≥93%; and (2) investigating whether very early response assessment measured by PET after 1 cycle of chemotherapy could identify a subject cohort distinguishable from the currently defined cohort of patients achieving an RER after 2 cycles. If so, this cohort could be studied in future trials for reduced therapy.
Descriptive statistics were used to tabulate toxicity rates [National Cancer Institute Common Toxicity Criteria, v3.0 (CTCAE; http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf)] among patients treated with ABVE-PC and among those treated with ifosfamide/vinorelbine. Early response was tabulated; the rate of achieving RER and its corresponding confidence interval (CI) were calculated. Kaplan-Meier curves were used to estimate 2nd EFS, EFS and OS for the entire study population, for RER and SER separately, as well as those for Stage IVB patients only. Log rank test was used to explore whether there was a difference in 2nd EFS or EFS between the RER vs. SER on study. The survival analysis on the 2nd EFS was conducted on the intent to treat population. The proportion of patients that were PET-positive at Cycle 1 among those with positive baseline PET, as well as the proportion of patients whose PET became negative at Cycle 2 among those with positive PET at Cycle 1 was calculated; these evaluations examined whether PET at Cycle 1 identified a subgroup of patients achieving a very early CR that may be studied in future trials. As exploratory analyses, we performed log rank test to see if PET results after 1 cycle of ABVE-PC correlated with survival endpoints.
Results
Patients
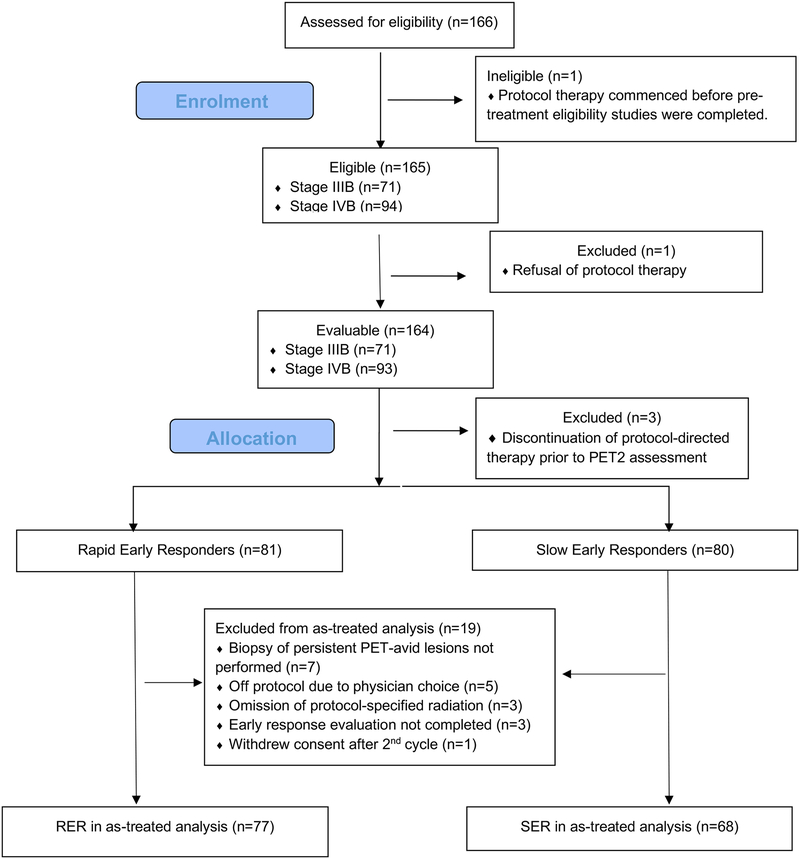
One hundred and sixty-six patients were enrolled in this study from December 2009 to January 2012, of whom 165 were eligible (Figure 1). Protocol therapy commenced in one patient before the pre-treatment eligibility studies (PET) were completed and thus the patient was classified as ineligible. The characteristics of the eligible patients are summarized in Table II. Eligible patients ranged in age from 5.2 to 21.4 years (median, 15.8 years) and 61% were male. The most frequent histology was nodular sclerosis (109 of 165; 66%).
Figure 1.
Flowchart diagram
PET: positron emission tomography; PET2: positron emission tomography after Cycle 2 of treatment; RER: rapid early responder; SER: slow early responder.
Table II.
Patient characteristics.
| N=165 | |
|---|---|
| Age (years) | |
| Median (range) | 15.8 (5.2–21.4) |
| Sex | |
| male | 101 (61%) |
| female | 64 (39%) |
| Race | |
| White | 111 (67%) |
| African American | 26 (16%) |
| Asian | 7 (4%) |
| American Indian/Alaskan Native | 2 (1%) |
| Unknown | 19 (12%) |
| Ethnicity | |
| Non-Hispanic | 128 (78%) |
| Hispanic | 33 (20%) |
| Unknown | 4 (2%) |
| Stage | |
| III | 71 (43%) |
| IV | 94 (57%) |
| Bulk | |
| Yes | 138 (84%) |
| No | 27 (16%) |
| Institutional Pathology | |
| Nodular Sclerosis | 109 (66%) |
| Mixed Cellularity | 13 (8%) |
| Lymphocyte Predominance | 2(1%) |
| Lymphocyte Rich | 10 (6%) |
| Not otherwise specified (NOS) | 28 (17%) |
| Lymphocyte depletion, NOS | 3 (2%) |
There were 71 stage III (43%) and 94 stage IV (57%) patients. By definition of study eligibility, all patients had B symptoms. Eighty-five (51.5%) patients presented with a large anterior mediastinal mass (LMA), 44 (26.7%) with extramediastinal bulk and 85 (51.5%) with macronodular splenic involvement. Overall, bulk disease was observed in 138 (84%) patients.
Among the 165 eligible patients, one patient refused any protocol-directed therapy after registration due to concerns related to a family history of cardiomyopathy. Thus 164 patients were evaluable for the primary aim of the study.
Response
PET responses following 1 and 2 cycles of ABVE-PC are summarized in Table III for 164 evaluable patients. Overall, 27 (16%) patients were PET-negative after 1 cycle, and 81 (49%) were PET-negative after 2 cycles. One hundred and sixty-one patients were included in the comparison of RER and SER groups; three patients were not evaluable for early response after discontinuing protocol-directed therapy prior to the response evaluation time point. As resolution of splenic macronodules by CT after 2 cycles was required for classification as RER, 81 (50%) were RER (i.e., CR) and 80 (50%) were SER (78 PR and 2 SD). The RER percentage was 58% (40/69) for Stage III patients and 45% (41/92) for Stage IV patients. Among 138 patients with bulk disease, 62 (45%) were RER, 72 (52%) had PR, and 4 (3%) SD or PD.
Table III.
PET response among 164 evaluable patients following cycle 1 and 2 by International Working Group criteria.(Cheson et al. 2007)
| PET-0 (On-Study, Site review) | PET-1 (Cycle 1, Central review) | PET2 (Cycle 2, Central review) |
|---|---|---|
| 163 positive | 133 positive | |
| 1 missing | ||
| 26 negative | ||
| 2 negative | ||
| 2 not done | 2 negative | |
| 2 missing | 2 missing | |
| 1 negative | 1 negative | 1 negative |
PET: positron emission tomography
Radiotherapy
Of the 164 patients, 125 (76%) patients received RT. Sixty of the 125 were RER and 65 were SER. No patients received a boost beyond the prescribed 21 Gy. Two patients stopped RT early at a dose of 9 Gy, in one instance due to an acute fatality.
Toxicity
Grade 3 and 4 acute non-haematological toxicities occurred in <5% of patients by phase of treatment, while febrile neutropenia was reported in 39 (24%) and mucositis in 23 (14%), rates similar to those observed in other studies utilizing the ABVE-PC regimen, (Schwartz et al. 2009, Friedman et al. 2014) even with the higher cyclophosphamide dose in this protocol. There was one toxic death in a RER patient during the RT phase, attributable to disseminated fungal infection with a brain abscess and multiple hepatosplenic lesions, disseminated intravascular coagulation and multi-organ dysfunction.
Relapse and Survival Outcomes
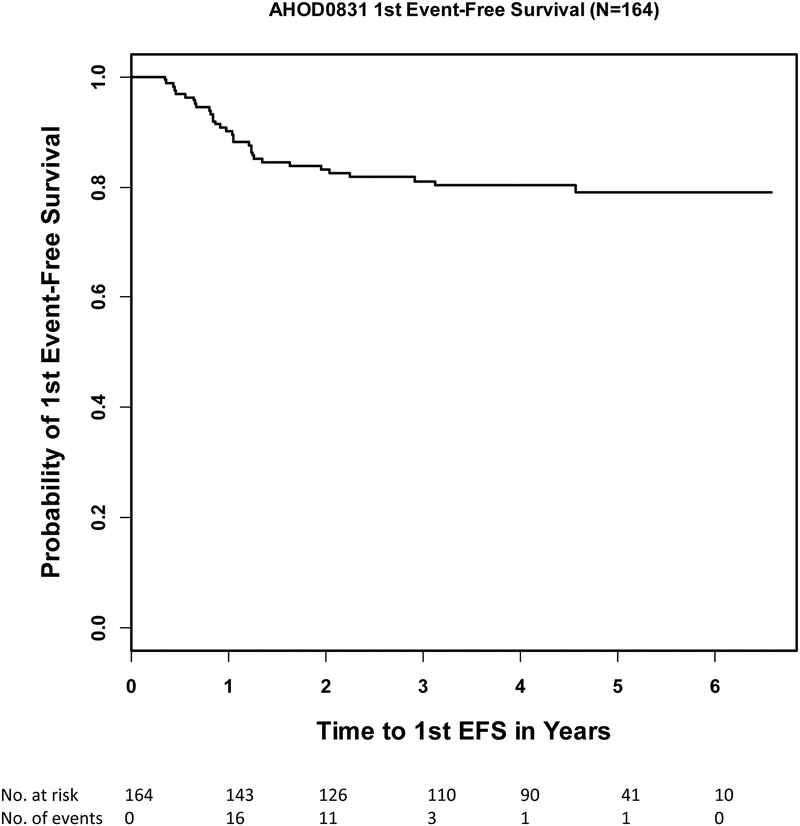
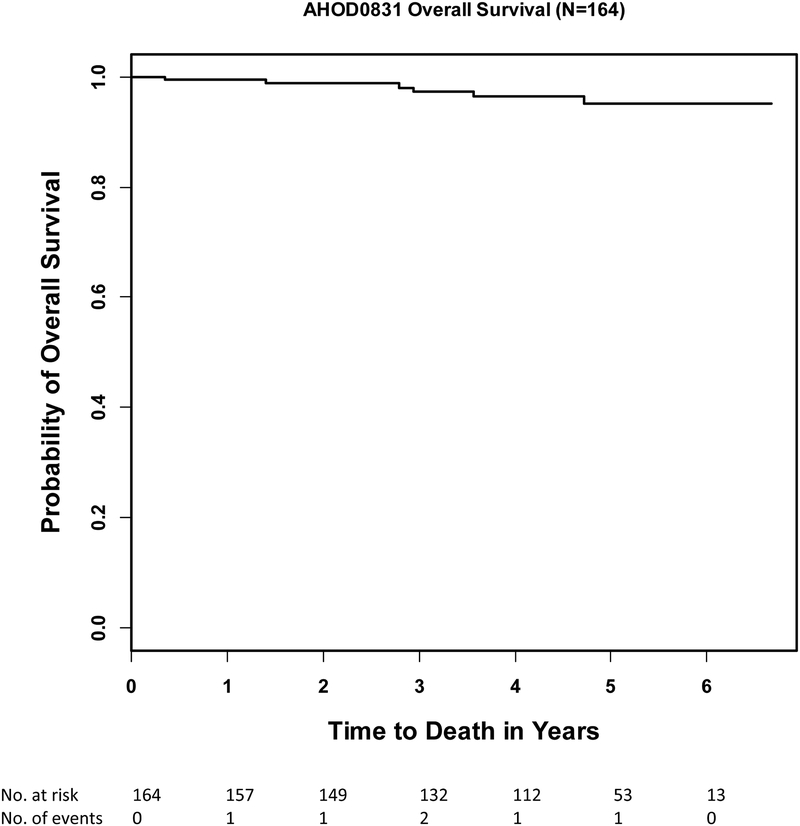
Standard 3-year 1st EFS and OS rates of the evaluable patients (n=164) were 81% (95% CI:74%−86.3%) and 97.3% (95% CI:92.9%−99%) and 5-year 1st EFS and OS rates were 79.1% (95% CI:71.5%−84.8%) and 95% (95% CI:88.8%−97.8%)(Figure 2a, 2b). Five-year 1st EFS rates for RER and SER patients were 83.5% (73.2%−90.1%) and 73.2% (60.6%−82.3%), respectively. The 1st EFS events reported include 30 relapse/progressions, 1 SMN (large cell lymphoma) and 1 death secondary to disseminated fungal infection during RT. Ten of the 32 first events occurred during or within 3 months after the last treatment and 22 events happened beyond 3 months after the last treatment. During the follow-up, 29 of the 32 patients received anti-cancer treatment: 4 patients received chemotherapy only, 23 patients were treated with multiple therapeutic modalities including stem cell transplant, and 2 patients received multiple therapeutic modalities without stem cell transplant.
Figure 2a.
First event free survival (1st EFS) of all evaluable patients (n=164)
Figure 2b.
Overall survival of all evaluable patients (n=164)
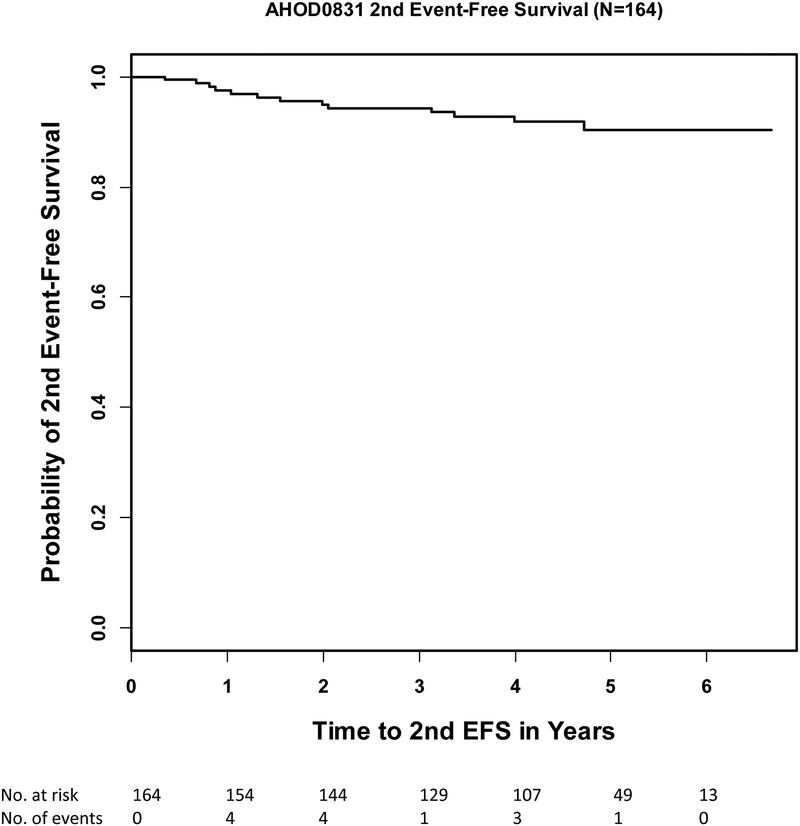
The primary endpoint of the study was 2nd EFS at 4 years. By “intent to treat” analysis of 164 evaluable patients with the median follow-up time of 4.5 years the 4-year 2nd EFS was 91.9% (95% CI: 86.1%−95.3%, Figure 2c). This survival rate is below the projected baseline 4-year rate of 95% by one-sided one-sample log-rank test (p=0.038). Subgroup analyses showed that 4-year 2nd EFS for RER (n=81) was 94.6% (95% CI:86.3%−98.0%), SER (n=80) was 88.6% (95% CI:78.4%−94.2%), stage IIIB (n=70) was 92.5% (95% CI:82.8%−96.8%) and stage IVB (n=94) 91.5% (95% CI:82.8%−95.9%). To date, six deaths have been reported, among two RER and four SER, three stage III and three stage IV. Time to death ranged from 4.1 to 56.6 months.
Figure 2c.
Second event free survival (2nd EFS) of all evaluable patients (n=164)
A secondary “as-treated” analysis was conducted, excluding 19 of the 164 patients who had premature termination or deviation from protocol-directed therapy. Specifically, patients were excluded from this analysis if they did not undergo biopsy of PET-avid lesions at the end of consolidation (n=7), were taken off protocol therapy by physician choice (n=5), did not receive protocol-directed RT by physician choice (n=3), did not complete early response evaluation (n=3) or withdrew consent for further treatment after the second cycle (n=1). Median follow-up among the remaining 145 evaluable patients was 4.6 years, with 81% (118/145) of patients followed for a minimum of 3 years. In this “as-treated” analysis, the 4-year 2nd EFS for these 145 patients was 91.7% (95% CI: 85.5%−95.3%), which is also significantly below the projected baseline 4-year rate of 95% by one-sided one-sample log-rank test (p=0.017). Among 24 RER and 24 SER patients no longer in follow-up, the median time from enrolment to last follow-up end date for this group of patients was 2.6 years for the RER patients and 2.9 years for the SER patients, a time period in which the majority of events would be expected to occur.
A total of 161 patients were evaluable for early response after one cycle of therapy (Table III). Twenty-seven were PET1-negative, 78 were PET2-negative- and 56 were PET2-positive. The corresponding 3-year 1st EFS rates were 92.6% (73.5%−98.1%), 81.3% (70.5%−88.5%) and 73.6% (59.4%−83.5%), respectively (p=0.07). One hundred and five patients achieved a negative PET by Cycle 2, whereas 56 remained positive. The corresponding 3-year 1st EFS were 84.3% (75.7%−90.1%) and 73.6% (59.4%−83.5%), respectively (p=0.06).
Eleven SER patients had persistent PET-positive lesions at the end of chemotherapy (and an additional RER patient had progressive disease as identified by PET). Eight of these 11 had clinical evidence of active disease (3 biopsy-proven HL, 5 with progressive disease or later relapses). In retrospective analysis, no specific Deauville score could be identified to predict which patients were at highest risk for progression (Table SI).
Discussion
AHOD0831 did not meet its primary objectives; both 1st and 2nd EFS were below the prespecified targets. Nevertheless, EFS and OS rates were comparable to those seen in a predecessor COG trial (Table IV; Schwartz et al., 2009), despite a short total duration of chemotherapy and limited radiation fields. In addition, augmenting treatment intensity with ifosfamide/vinorelbine appears to be a successful strategy for reducing relapse risk among those patients with a slow early response to ABVE-PC.
Table IV.
Outcomes for patients enrolled on AHOD0831 compared with stage IIIB/IVB patients enrolled on P9425 (Schwartz et al 2009).
| AHOD0831 Stage IIIB/IVB, n=164 |
P9425 (Schwartz et al 2009) Stage IIIB/IVB, n=88 |
|
|---|---|---|
| 4-year EFS (95% CI) |
80.3% (73.1–85.7%) |
81.7% (71.8%−88.3%) |
| 4-year OS (95% CI) |
96.5% (91.7–98.5%) |
92.9% (84.9%−96.8%) |
CI: confidence interval; EFS: event-free survival; OS: overall survival.
In retrospect, the prespecified 1st and 2nd EFS targets may have been ambitiously set too high. AHOD0831 included only paediatric patients with very high-risk disease (stages IIIB and IVB), of whom 84% had bulky disease at presentation. The EFS targets were derived from prior studies (Schwartz et al. 2009, Kelly et al. 2011) that included a wider range of high risk patients, of which over 30% would have not been eligible for the present study (e.g., stages IB, IIB, IIIA, IVA). Despite this, EFS and OS rates are in a comparable range to these prior studies.
Our study achieved these outcomes while reducing RT volumes compared to the predecessor trials (Schwartz et al. 2009, Kelly et al. 2011), which treated all initially involved sites of disease. For patients with advanced stage disease, involved-field RT historically encompassed significant volumes of normal tissue, whereas the approach utilized in this study of limiting RT to sites of bulk or SER allowed the omission of RT in approximately 25% of RER patients and a reduction in normal tissue exposure for all others. SER on this study also received less doxorubicin than on P9425.(Schwartz et al. 2009)
The role of RT in advanced stage HL has evolved since the initiation of this trial. In the pre-PET era, the UK LY09 (Johnson et al. 2010) and the German Hodgkin Study Group. HD12(Borchmann et al. 2011) trials found a significant benefit to the use of RT as part of initial therapy, and the indication for RT in this trial was similar to that in the Stanford V arm of the Eastern Cooperative Oncology Group (E2496)(Gordon et al. 2013) trial. More recently, the Gruppo Italiano Terapie innovative nel Linfomi/Italian Lymphoma Foundation HD 0607 trial found no significant benefit to RT among patients with nodal masses ≥5 cm who had a negative PET scan after two and four cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) (Casasnovas et al. 2019) and adult HL protocols that incorporate escalated dose BEACOPP chemotherapy have reported 5-year progression-free survival (PFS) rates >85% with limited or no routine use of RT.(Borchmann et al. 2018) These findings suggest that PET may be utilized to avoid RT for the majority of advanced stage patients who achieve a rapid metabolic response, provided chemotherapy is sufficiently intensive.
Tailoring consolidation therapy to early interim PET resulted in comparable outcomes across RER and SER patients. Recent trials of PET response-adapted therapy in adults are associated with lower PFS rates among patients with interim PET-positive scans, even with the use of considerably more consolidation chemotherapy. In the phase II S0816 US intergroup study, a benefit of escalating therapy was observed in the 60 (18%) patients who were PET-positive after two cycles of ABVD and were subsequently changed to six cycles of BEACOPP.(Press et al. 2016) The 2-year PFS was 64% (95% CI, 50% to 75%), in comparison to the 3-year first EFS rate of 73.6% (59.4–83.5%) observed among interim PET-positive patients in this trial. Cumulative anthracycline, alkylator and etoposide were higher with the former. The HD0801 study also suggests a potential benefit with escalation of therapy for advanced-stage HL patients who remain PET-positive after two cycles of ABVD; 519 advanced-stage HL patients were treated with ABVD and 103 were PET-positive after two cycles (Zinzani et al. 2016). Among them, 81 received intensified therapy with ifosfamide and vinorelbine plus gemcitabine, followed by carmustine, cytarabine, etoposide and melphalan (BEAM) and autologous stem cell transplantation (Zinzani et al. 2016). With two years of follow-up, by intention to treat analysis, the 2-year PFS for the PET-positive group was 76% (95% CI, 66% to 84%), also comparable to the SER outcomes in our study, albeit with significantly more treatment burden. In our study, RER patients received only 12 weeks of chemotherapy, in contrast to the 24 weeks of therapy administered in other paediatric and adult regimens for high risk patients.(Mauz-Korholz et al. 2010, Press et al. 2016) Cumulative anthracycline doses were capped at 200 mg/m2, bleomycin at 60 units/m2 and cyclophosphamide at 4800 mg/m2, ranges which should be associated with a reduction in late toxicities, especially with the reduction in radiation volumes. Among SER patients, the current design allowed for further reduction of cumulative doxorubicin, bleomycin and etoposide through the addition of the cross-resistant regimen, ifosfamide and vinorelbine as compared with prior studies utilizing the ABVE-PC regimen.(Schwartz et al. 2009) In addition, RT was reduced to bulk and slow responding involved fields only. Ifosfamide/vinorelbine was well tolerated.
When additionally considering salvage treatment, this regimen was associated with an ability to cure approximately 90% with half receiving minimal therapy for very high risk disease. The ability to adjust treatment exposures with concurrent assessment of outcome for those requiring the augmentation due to either early response or to salvage therapy results in giving each patient optimal therapeutic intensity. Very early response by PET assessment after 1 cycle did not define a group with a statistically significant lower risk for relapse. Further evaluation utilizing better imaging assessments beyond solely visual assessment of PET response or predictive biomarkers are thus needed to further refine this approach.
Persistent PET positivity at end of chemotherapy identified a cohort at an especially high risk for relapse/early progression (8 of 11 patients with persistent PET positivity). Our results suggest that these patients need close surveillance, and probably alternative approaches to therapy beyond conventional chemotherapy. The incorporation of the agents specifically targeting the Hodgkin Reed-Sternberg cell or aberrant immune response may benefit this highest risk group of patients with newly diagnosed paediatric HL. The antibody-drug conjugate, brentuximab vedotin and the checkpoint inhibitors, nivolumab and pembrolizumab are being evaluated in clinical trials for initial therapy and relapses, including paediatric studies.(Kelly 2015) We did not identify a predictive Deauville score in post hoc analyses. Further analyses on FDG PET/CT are forthcoming, including evaluation of semi-quantitative PET measures, such as quantitative PET(Hasenclever et al. 2014) and total metabolic tumour burden.
Although the ambitiously high benchmarks for surrogates of long-term survival were not met with the prescribed treatment approach, early PET-directed treatment stratification in children and adolescents with very high risk HL is associated with comparable EFS and OS rates to other paediatric and adult high risk HL trials, even with reductions in radiation volumes and titration of cumulative chemotherapy doses. Further refinements in risk stratification and treatment augmentation, potentially through the introduction of novel agents, will facilitate improvements in outcome for the 20% of patients not achieving long term remission of disease.
Supplementary Material
Acknowledgements:
This study was supported in part by grants from the National Institutes of Health to the Children’s Oncology Group (U10CA098543), Statistics & Data Center Grant (U10CA098413), NCTN Operations Center Grant (U10CA180886), NCTN Statistics & Data Center Grant (U10CA180899), QARC (CA29511) and IROC RI (U24CA180803), and St. Baldrick’s Foundation.
Footnotes
Conflicts of Interest:
The authors declare no competing financial interests.
References:
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, Eich HT, Mueller-Hermelink HK, Kanz L, Greil R, Rank A, Paulus U, Smardova L, Huber C, Dorken B, Nerl C, Krause SW, Mueller RP, Fuchs M and Engert A (2011). “Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group.” J Clin Oncol 29(32): 4234–4242. [DOI] [PubMed] [Google Scholar]
- Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, Zijlstra JM, Markova J, Meissner J, Feuring-Buske M, Huttmann A, Dierlamm J, Soekler M, Beck HJ, Willenbacher W, Ludwig WD, Pabst T, Topp MS, Hitz F, Bentz M, Keller UB, Kuhnhardt D, Ostermann H, Schmitz N, Hertenstein B, Aulitzky W, Maschmeyer G, Vieler T, Eich H, Baues C, Stein H, Fuchs M, Kuhnert G, Diehl V, Dietlein M and Engert A (2018). “PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group.” Lancet 390(10114): 2790–2802. [DOI] [PubMed] [Google Scholar]
- Casasnovas R-O, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, Dupuis J, Gac A-C, Gastinne T, Joly B, Bouabdallah K, Nicolas-Virelizier E, Feugier P, Morschhauser F, Delarue R, Farhat H, Quittet P, Berriolo-Riedinger A, Tempescul A, Edeline V, Maisonneuve H, Fornecker L-M, Lamy T, Delmer A, Dartigues P, Martin L, André M, Mounier N, Traverse-Glehen A and Meignan M (2019). “PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study.” The Lancet Oncology 20(2): 202–215. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M & Diehl V (2007). “Revised response criteria for malignant lymphoma.” J Clin Oncol 25(5): 579–586. [DOI] [PubMed] [Google Scholar]
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, Kessel S, De Alarcon PA, Chen AR, Kobrinsky N, Ehrlich P, Hutchison RE, Constine LS and Schwartz CL (2014). “Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031.” J Clin Oncol 32(32): 3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD, Gospodarowicz M, Advani R, Kahl BS, Friedberg JW, Blum KA, Habermann TM, Tuscano JM, Hoppe RT and Horning SJ (2013). “Randomized Phase III Trial of ABVD Versus Stanford V With or Without Radiation Therapy in Locally Extensive and Advanced-Stage Hodgkin Lymphoma: An Intergroup Study Coordinated by the Eastern Cooperative Oncology Group (E2496).” Journal of Clinical Oncology 31(6): 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker-Murray PD, Drachtman RA, Hodgson DC, Chauvenet AR, Kelly KM and Cole PD (2014). “Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma.” Pediatr Blood Cancer 61(4): 579–586. [DOI] [PubMed] [Google Scholar]
- Hasenclever D, Kurch L, Mauz-Korholz C, Elsner A, Georgi T, Wallace H, Landman-Parker J, Moryl-Bujakowska A, Cepelova M, Karlen J, Alvarez Fernandez-Teijeiro A, Attarbaschi A, Fossa A, Pears J, Hraskova A, Bergstrasser E, Beishuizen A, Uyttebroeck A, Schomerus E, Sabri O, Korholz D and Kluge R (2014). “qPET - a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma.” Eur J Nucl Med Mol Imaging 41(7): 1301–1308. [DOI] [PubMed] [Google Scholar]
- Hodgson DC, Hudson MM and Constine LS (2007). “Pediatric hodgkin lymphoma: maximizing efficacy and minimizing toxicity.” Semin Radiat Oncol 17(3): 230–242. [DOI] [PubMed] [Google Scholar]
- Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, d’Amore F, Enblad G, Franceschetto A and Fulham M (2016). “Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma.” New England Journal of Medicine 374(25): 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PW, Sydes MR, Hancock BW, Cullen M, Radford JA and Stenning SP (2010). “Consolidation radiotherapy in patients with advanced Hodgkin’s lymphoma: survival data from the UKLG LY09 randomized controlled trial (ISRCTN97144519).” J Clin Oncol 28(20): 3352–3359. [DOI] [PubMed] [Google Scholar]
- Josting A, Rudolph C, Mapara M, Glossmann JP, Sieniawski M, Sieber M, Kirchner HH, Dorken B, Hossfeld DK, Kisro J, Metzner B, Berdel WE, Diehl V and Engert A (2005). “Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG).” Ann Oncol 16(1): 116–123. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD and Imaging L Subcommittee of International Harmonization Project in (2007). “Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma.” J Clin Oncol 25(5): 571–578. [DOI] [PubMed] [Google Scholar]
- Kelly KM (2015). “Hodgkin lymphoma in children and adolescents: improving the therapeutic index.” Blood 126(22): 2452–2458. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Sposto R, Hutchinson R, Massey V, McCarten K, Perkins S, Lones M, Villaluna D and Weiner M (2011). “BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group.” Blood 117(9): 2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA and Tubiana M (1989). “Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting.” J Clin Oncol 7(11): 1630–1636. [DOI] [PubMed] [Google Scholar]
- Mauz-Korholz C, Hasenclever D, Dorffel W, Ruschke K, Pelz T, Voigt A, Stiefel M, Winkler M, Vilser C, Dieckmann K, Karlen J, Bergstrasser E, Fossa A, Mann G, Hummel M, Klapper W, Stein H, Vordermark D, Kluge R and Korholz D (2010). “Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study.” J Clin Oncol 28(23): 3680–3686. [DOI] [PubMed] [Google Scholar]
- Press OW, Li H, Schoder H, Straus DJ, Moskowitz CH, LeBlanc M, Rimsza LM, Bartlett NL, Evens AM, Mittra ES, LaCasce AS, Sweetenham JW, Barr PM, Fanale MA, Knopp MV, Noy A, Hsi ED, Cook JR, Lechowicz MJ, Gascoyne RD, Leonard JP, Kahl BS, Cheson BD, Fisher RI and Friedberg JW (2016). “US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816.” J Clin Oncol 34(17): 2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, Lipshultz SE, Turner CS, deAlarcon PA and Chauvenet A (2009). “A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425.” Blood 114(10): 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DJ, Jung S-H, Pitcher B, Kostakoglu L, Grecula JC, Hsi ED, Schöder H, Popplewell LL, Chang JE and Moskowitz CH (2018). “CALBG 50604: risk-adapted treatment of non-bulky early stage Hodgkin lymphoma based on interim PET.” Blood: blood-2018–2001-827246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippett TM, Schwartz CL, Guillerman RP, Gamis AS, Gardner S, Hogan S, London WB, Chen L and de Alarcon P (2015). “Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: a children’s oncology group report.” Pediatr Blood Cancer 62(1): 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani PL, Broccoli A, Gioia DM, Castagnoli A, Ciccone G, Evangelista A, Santoro A, Ricardi U, Bonfichi M, Brusamolino E, Rossi G, Anastasia A, Zaja F, Vitolo U, Pavone V, Pulsoni A, Rigacci L, Gaidano G, Stelitano C, Salvi F, Rusconi C, Tani M, Freilone R, Pregno P, Borsatti E, Sacchetti GM, Argnani L and Levis A (2016). “Interim Positron Emission Tomography Response-Adapted Therapy in Advanced-Stage Hodgkin Lymphoma: Final Results of the Phase II Part of the HD0801 Study.” J Clin Oncol 34(12): 1376–1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.