Abstract
Objective
Copper (Cu) is essential micronutrient and its dysregulation is implicated in aortic aneurysm (AA) development. The Cu exporter ATP7A delivers Cu via the Cu chaperone Atox1 to secretory Cu enzymes, such as lysyl oxidase (LOX), and excludes excess Cu. LOX is shown to protect against AA formation. However, the role and mechanism of ATP7A in AA pathogenesis remains unknown.
Approach and Results
Here we show that Cu chelator markedly inhibited angiotensin II (Ang II)-induced abdominal AA (AAA) in which ATP7A expression was markedly downregulated. Transgenic ATP7A overexpression prevented AngII-induced AAA formation. Conversely, Cu transport dysfunctional ATP7Amut/+/ApoE−/− mice exhibited robust AAA formation and dissection, excess aortic Cu accumulation as assessed by X-ray fluorescence microscopy, and reduced LOX activity. In contrast, AAA formation was not observed in Atox1−/−/ApoE−/− mice, suggesting that decreased LOX activity, which depends on both ATP7A and Atox1, was not sufficient to develop AAA. Bone marrow (BM) transplantation suggested importance of ATP7A in vascular cells, not BM cells, in AAA development. MicroRNA (miR) array identified miR125b as a highly upregulated miR in AAA from ATP7Amut/+/ApoE−/− mice. Furthermore, miR125b target genes (histone methyltransferase Suv39h1 and the NFkB negative regulator TNFAIP3) were downregulated, which resulted in increased proinflammatory cytokine expression, aortic macrophage recruitment, MMP-2/9 activity, elastin fragmentation and vascular smooth muscle cell loss in ATP7Amut/+/ApoE−/− mice and reversed by LNA-anti-miR125b infusion.
Conclusion
ATP7A downregulation/dysfunction promotes AAA formation via upregulating miR125b, which augments pro-inflammatory signaling in a Cu-dependent manner. Thus, ATP7A is a potential therapeutic target for inflammatory vascular disease.
INTRODUCTION
Abdominal aortic aneurysms (AAA) are a life-threatening condition occurring in up to 9% of elderly individuals (above 65 years old)1, 2. Although morphologically similar, ascending thoracic aortic aneurysms (TAA) and AAA represent distinct disease processes. TAA frequently result from a non-inflammatory process of medial degeneration including smooth muscle cell (VSMC) loss and elastin fragmentation3. Conversely, AAAs involve chronic aortic wall inflammation, extracellular matrix (ECM) degradation and compromised smooth muscle function1, 2, 4 which causes weakening of the vessel wall and consequent progressive aortic dilation, rupture and death. Currently, the only form of treatment for AAA is endovascular or surgical repair, which is associated with significant procedural risks and complications1, 2. Therefore, understanding the cellular and molecular mechanisms underlying AAA progression is crucial to developing new, effective therapeutic strategies.
Copper (Cu), an essential micronutrient and catalytic cofactor, is involved in physiological processes such as wound repair, while excess Cu contributes to various inflammatory vascular diseases including atherosclerosis5–7. It has been shown that tissue Cu levels are significantly increased in pathological inflammatory conditions such as atherosclerosis and AA8, 9. Implanting a Cu cuff promotes neointima thickening in response to vascular injury10, while Cu chelators prevent this response11. Cu also plays an important role in inflammatory responses involved in innate and adaptive immunity7. Cu deficiency alters intravascular adhesion of leukocytes to activated endothelial cells (ECs) and expression of adhesion molecules, such as ICAM-1/VCAM-112, while Cu chelators inhibit atherosclerosis and inflammation13, 14. However, the role of Cu in AAA development is unknown.
Since excess Cu is toxic, intracellular Cu levels are tightly controlled by Cu transport proteins7, 15. These include Cu importer CTR1, which uptakes and transports Cu through the plasma membrane 16; Cu chaperone Antioxidant-1 (Atox1), which obtains Cu from CTR1 and delivers it to the Cu exporter ATP7A, which localizes at the trans-Golgi network (TGN) in the basal state. Then, ATP7A transports Cu to secretary Cu enzymes (e.g. lysyl oxidase (LOX))7, 17, 18. In pathological conditions in which excess Cu occurs, ATP7A translocates from the TGN to the plasma membrane to export the excess Cu to the extracellular space18. Thus, ATP7A is a key regulator of secretory Cu enzymes and intracellular Cu levels. X-linked loss-of-function mutation of ATP7A has been shown to cause Menkes disease in humans19, 20. Of note, global ATP7A knockout mice are embryonic lethal due to vascular defects 21. Mice carrying the X-linked blotchy ATP7A mutation (ATP7Amut/y mice) have a splice site mutation introducing a new stop codon at amino acid residue 794 with an associated loss of Cu transport function. They survive more than 6 months of age18, 22, and are a well characterized animal model to study the function of ATP7A in adult organisms. Using ATP7Amut/y mice, we previously reported that ATP7A plays an important role in VSMCs to regulate mesenteric arterial tone in hypertensive23 and diabetic mice24, 25. Interestingly, hemizygous male blotchy ATP7Amut/y mice, but not heterozygous female blotchy ATP7Amut/+ or control animals, had a progressive increase in the incidence of aneurysms with aging, primarily in the ascending aorta26, 27. However, the role and mechanisms for vascular ATP7A in AAA formation induced by Ang II infusion, which is an established AAA model 28, remains unclear.
MicroRNAs (miRs) are a family of ~22 nucleotide endogenous short noncoding RNAs which regulate gene expression either via translational inhibition or destabilization of target mRNAs. Environmental chemicals such as heavy metals can interfere with the biogenesis and expression of miRNAs, leading to toxicological consequences. Changes in Cu concentrations can alter the expression of a diverse number of miRNAs in the olfactory system of zebrafish that are involved in signal transduction and other critical neurological processes29. Recent studies have shown that miRNAs play an important role in in AAA formation30, 31by regulating pathways including proteinases, extracellular matrix production, vascular cell homeostasis, and inflammation. Thus, it is possible that microRNAs might be involved in ATP7A-mediated aortic aneurysm development.
In the present study, we examined the role of ATP7A in Ang II-induced AAA formation using heterozygous ATP7Amut/+/ApoE−/− mice, which have a normal life span, no spontaneous AAA formation, and reduced Cu transporter function, and the Cu chelator tetrathiomolybdate (TTM). We demonstrate that endogenous ATP7A protects against AAA formation by inhibiting vascular inflammation, MMP activity, elastin fragmentation and vascular apoptosis via limiting expression of pro-inflammatory miR-125b in a Cu-dependent manner. Thus, our results provide novel insights into the potential of ATP7A as a future therapeutic target for inflammatory vascular disease.
MATERIALS AND METHODS
The authors declare that all supporting data are available within the article and its online-only Data Supplement.
Animals
Heterozygous blotchy ATP7A mutant (ATP7Amut/+) mice backcrossed to the C57BL/6J background, Atox1−/− mice on C57BL/6J background and heterozygous transgenic mice overexpressing ATP7A on C57BL/6J background were weaned at 4 weeks of age and maintained on normal laboratory diet (Teklad diet 2918) for 3 months. ApoE−/− mice were purchased from Jackson Laboratory (Stock No: 002052; Bar Harbor, Maine). ATP7Amut mice carrying the X-linked blotchy ATP7A mutation have a splice site mutation introducing a new stop codon at amino acid residue 794 and show impaired copper transport function, but survive to more than 6 months of age18, 22, 23. ATP7A transgenic (ATP7A Tg) mice that overexpress the human ATP7A from the composite beta actin promoter (CAG) were generated24,32. ATP7Amut or Atox1−/− or ATP7A Tg mice previously backcrossed into C57BL/6J genetic background for at least ten generations were crossed with ApoE−/− mice on C57BL/6J background to generate ATP7Amut/ApoE−/−, Atox1−/−/ApoE−/− or ATP7A Tg/ ApoE−/− mice. ApoE−/− mice served as controls. The protocol for animal use was approved by Institutional Animal Care and Use Committee at Medical College of Georgia.
Experimental design and AAA Model
Mice were studied at 2–3 months old. Ang II (1000 ng/kg body weight/min; Cat# A2900, Sigma-Aldrich) was infused into the subcutaneous space in the interscapular area of female ApoE−/−, ATP7Amut/+/ApoE−/−, or Atox1−/−/ApoE−/− mice under parenteral anesthesia via Alzet osmatic mini pump (Alzet Model # 2004; Durect; Cupertino, CA, USA), as described previously33. For some experiments, male or female ApoE−/− or ATP7A Tg/ApoE−/− mice were continuously fed a high fat diet (HFD) (Diet # TD.88137; Harlan Teklad) containing 21% (wt/wt, which equals 42% kcal) saturated fat extracted from milk, 48.5% (wt/wt) carbohydrate, 17.3% (wt/wt) protein, and 0.2% (wt/wt) cholesterol (0.15% supplemented, and 0.05% from the fat source) during 4 weeks of Ang II infusion28, 34, 35. For TTM treatment, mice were randomly assigned and gavaged with water (control) or 0.7 mg/day/30g mice tetrathiomolybdate (TTM) daily for 4 weeks, as described previously13, 14, 36. Ceruloplasmin activity was measured in the serum of mice before and after TTM treatment using a colorimetric assay based on substrate oxidation (Sigma, Cat# MAK177).
Aortic tissue samples were harvested at several time points over the aneurysm induction period (28 days in the Ang II model). The chest and abdominal cavities were opened, and blood was drawn from the right ventricle at the time of euthanasia. Aortas were perfused with cold PBS through the left ventricle. Using a dissection microscope, the periadventitial tissue was carefully dissected from the wall of the aorta. Aortic measurements were determined with a stage micrometer and optical eyepiece reticle. An aortic aneurysm (AA) was defined as an increase in the baseline outer diameter of 50%. The aneurysmal areas were removed, fixed in paraformaldehyde (4% (wt/vol) Cat#15712-S, Electron Microscopy Sciences) for immunohistochemistry, or snap frozen in liquid nitrogen, and then stored at –80°C for biochemical assays. Aneurysm severity was graded according to the following criteria37, 38: grade 1, remodeled tissue in the suprarenal region frequently containing thrombus; grade 2, pronounced bulbous form of grade 1 containing thrombus; grade 3, multiple aneurysms containing thrombus; or ruptured, ruptured aortic aneurysm.
Human aortic tissues sections
We purchased deidentified formalin fixed paraffin embedded (FFPE) sections of human AAA (n=3 males) and normal aortas (n=3 males) from Origene (Supplement Table V). Samples were used for immunohistochemical staining. The specificity of primary antibody was confirmed by negative control procedures, which gave consistently negative results.
Cell culture and transfection
Bovine aortic endothelial cells (BAEC; VEC Technologies) were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% (vol/vol) fetal bovine serum and used for experiments until passage 10. Vascular smooth muscle cells (VSMC) were isolated from rat (RASM) and mouse (MASM) from thoracic aorta by enzymatic digestion as described previously25.
Immunoblotting
For protein expression in aortic tissue, mice were perfused with cold phosphate buffer saline. Aortae were harvested, frozen in liquid nitrogen and then crushed and cells lysed with RIPA buffer (5 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1%sodium deoxycholate, 0.1% SDS) containing protease inhibitor followed by brief sonication. Lysates were separated using SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, blocked in PBS containing 5% nonfat dry milk and 0.1% Tween 20, and incubated for overnight with primary antibodies. The following primary antibodies were used: anti-Atox1, anti-ATP7A, anti-Tubulin, anti-CCS, anti-Actin and anti-COX17. After incubation with secondary antibodies (Goat Anti-Rabbit IgG-HRP Conjugate, Bio-Rad, Goat Anti-Mouse IgG-HRP Conjugate, Bio-Rad), proteins were detected by ECL chemiluminescence.
Immunohistochemistry
Frozen sections were prepared by overnight 4% PFA incubation followed by sucrose dehydration and OCT embedding. Sections 7 μm in thickness were stained with antibodies against Mac3, CD45, MCP1, MMP2 or MMP9, incubated with biotin-conjugated anti-rat IgG antibody (Vector Laboratory) and visualized by VECTOR DAB following peroxidase labeling with VECTASTAIN Elite ABC Reagent (Vector Laboratories). Counterstaining with hematoxylin was performed. Images were captured by an Axio scope microscope and processed by AxioVision 4.8 software. All positive stained cells were counted in at least 3 microscopic fields (x40). The results were expressed as number of positive cells/mm2 area. Richard-Allan Scientific™ Elastic Stain (Thermo Scientific) was used for elastin staining. Elastin degradation was scored as described previously39. The grades were as follows: score 1, no degradation; score 2, mild elastin degradation; score 3, severe elastin degradation; and score 4, aortic rupture.
LOX activity assay
LOX activity in tissue lysates was measured by a high-sensitivity fluorescence assay, as previously described 40. Aortic tissues were homogenized in 1X LOX Urea buffer and protein concentration was determined. Equal amounts of protein samples were incubated in the presence and absence of 500 μmol/L BAPN at 37 °C for 30 min with final reaction mixture supplied by Amplite Fluorimetric Lysyl Oxidase Assay kit (AAT Bioquest) per the manufacturer’s instruction. The reaction was stopped on ice, and differences in fluorescence intensity (540-nm excitation wavelength and 590-nm emission wavelength) between samples with and without BAPN were determined.
Synchrotron X-ray Fluorescence Microscopy
Sections (5 μm thick) of formalin-fixed paraffin-embedded vascular tissues were prepared. For X-ray imaging, the sections were mounted intact on silicon nitride windows (area, 2 × 2 mm; thickness, 200 nm) manufactured by Silson (Cat# 11301147, Blisworth, U.K.) and attached by brief heating to 55°C, as previously described 40. Specimens were imaged with the scanning X-ray fluorescence microprobe at beamline 2-ID-E of the Advanced Photon Source (Argonne, IL). Undulator-generated x-rays of 10-keV incident energy were monochromatized with a single bounce Si <111> monochromator and focused to a measured spot size of 0.3 × 0.5 μm using Fresnel zone plate optics. Sections were raster-scanned in steps of 4.0 μm, and fluorescence spectra were collected for 1- to 2-sec dwell times by using a single-element silicon drift detector (Vortex-EX, SII Nanotechnology, CA). Quantitation and image-processing of the X-ray fluorescence (XRF) data sets was performed with MAPS software. Quantitation of elemental content was achieved by fitting XRF spectra at each pixel, and comparing against a calibration curve derived from measurements of thin-film standards NBS-1832 and NBS-1833 (National Bureau of Standards, Gaithersburg, MD).
Quantitative Real Time-polymerase chain reaction
Total RNA was isolated from aorta using TRI reagent (Molecular Research Center, Inc) according to the manufacturer’s instructions. 2 μg of total RNA were used to synthesize first stranded cDNA with a High-Capacity cDNA Reverse Transcription Kits. PCR was performed according to the manufacturer’s protocol using ABI PRISM® 7000 Sequence Detection System 26 (Applied Biosystems, CA) and QuantiFast SYBR Green PCR Kit (Qiagen, CA). Amplification conditions were performed with a 5 min preincubation at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 60°C. PCR products were subjected to melting curve analysis, using the ABI PRISM® 7000 Sequence Detection System, to exclude amplification of unspecific products. All real-time PCR primers were purchased from predesigned primers of QuantiTect primer assays (Qiagen). Results were normalized by 18S or HPRT expression levels.
Gelatin zymography for detection of MMP-2 and MMP-9
Protein was extracted from isolated abdominal aortas that had been snap frozen in liquid nitrogen and homogenized in a buffer containing 1 M NaCl, 2 M urea, 0.2 mM PMSF, 50 mM Tris (pH 7.4), 0.1% EDTA, 0.1% Brij-35, and protease inhibitors (10 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin), as previously described41. Samples were sonicated on ice and centrifuged, and supernatants were used to quantify protein content. Protein lysate was placed in a nonreducing zymogram buffer (Cat#161–0764, Bio-Rad, Hercules, CA) and applied without boiling to a 10% zymogram gel (#161–1167, Bio-Rad). Gels were incubated in 2% Triton X-100 at room temperature for 30 min and then rinsed in H2O for 5 min. Gels were incubated overnight at 37°C with gentle agitation in Zymogram developing buffer (Cat# 161–0766, Bio-Rad) containing 50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2, 0.02% Brij-35. Proteins were stained with Coomassie brilliant blue R-250 solution (Cat# 161–0436, Bio-Rad) and destained with a solution containing 40% methanol, 10% acetic acid, and 50% H2O.
Immunofluorescence analysis
Frozen sections were prepared by overnight 4% PFA incubation followed by sucrose dehydration and OCT embedding. The tissue sections (7 μm) were incubated with blocking buffer (3%BSA in PBS) for 1 h. Next, the sections were incubated with anti-ATP7A (Sigma Aldrich) or anti-actin α-smooth muscle Cy3 (Sigma Aldrich) for 18 h at 4°C, rinsed in PBS/BSA, and then incubated in Goat Anti-IgY Antibody, FITC Conjugate (Genway) for 1 h at room temperature and rinsed with PBS. Tissue sections were mounted onto cover glass using Vectashield (Cat#H-1200, Vector Laboratories) and visualized using confocal microscopy.
TUNEL assay
TUNEL staining was carried out to detect apoptotic cells in aortic tissue using In Situ Cell Death Detection Kit according to the manufacturer’s instructions (Cat#11684795910, Roche Diagnostics Co.)42. DAPI counterstaining was performed on aorta sections to label nuclei using VECTASHIELD mounting medium (Vector Laboratories). The number of TUNEL-positive nuclei per aortic section was normalized to total nuclei. Results are presented as percentage of apoptotic cells.
LNA-anti-miR-125b injection
Either locked nucleic acid (LNA)-anti-miR-125b or scrambled control-miR (miRCURY LNA miR inhibitor from Exiqon) was injected via retro-orbital approach at a concentration of 10 mg/kg. The mice were injected 1 day after AAA induction and were then given a weekly maintenance injection throughout the experiment period of 4 weeks. The sequenced of the LNA-anti-miRNA-125b was 5’-TCACAAGTTAGGGTCTCAGGGA-3’. The sequence of LNA scramble miR control was 5’-CATGTCATGTGTCACATCTCTT-3’ as reported previously43.
Quantitative PCR for miRNA and mouse inflammatory miRNA PCR array
Aorta were dissected, flash frozen using liquid nitrogen, and stored at −80C for processing. Total RNA was isolated using the Qiagen miRNeasy mini kit (cat #217004) according to the manufacturer’s protocol. RNA concentrations were determined using the Nanodrop Spectrophotometer. RNA was then transcribed into cDNA using Qiagen miScript RT kit (cat#218161, Qiagen). miR-125b specific primer (cat #MS00005992, Qiagen) was used and samples were run on the Applied Biosystems 7900HT Real-Time PCR System with RNU6 as the control.
For the PCR array, RNA was isolated as described above. Small RNAs were retro-transcribed from 200 ng of total RNA using the miScript II RT kit (Qiagen). Then, reaction mixtures were pooled according to their specific group and added into Immunopathology miScript PCR array (Cat# MIMM-104ZA, Qiagen). The reaction was run according to the manufacturer’s protocol. The expression of 84 mouse miRNAs predicted to regulate inflammatory genes was assayed in this array. Data were analyzed using software provided by Qiagen specific to these assays (miScript miRNA PCR Array Data Analysis software in GeneGlobe Data Analysis Center), normalized to the average Ct of six snoRNA/snRNAs housekeeping miRNAs controls (SNORD61, SNORD68, SNORD72, SNORD95, SNORD96A, RNU6–2).
Statistical Analysis
Data are presented as mean ± SEM. Normality of the data (using Shapiro-Wilk test) and the equality of group variance (using Brown-Forsythe test) were performed on all data using SigmaPlot 14. Data were compared between groups of cells and animals by Student t-test when one comparison was performed or by ANOVA for multiple comparisons. When significance was indicated by ANOVA, the Tukey post-hoc test was used to specify between group differences. Values of *p<0.05, **p<0.01 and ***p<0.001 were considered statistically significant. Statistical tests were performed using Prism v4 (GraphPad Software, San Diego, CA).
RESULTS
Cu chelator TTM prevents AAA formation
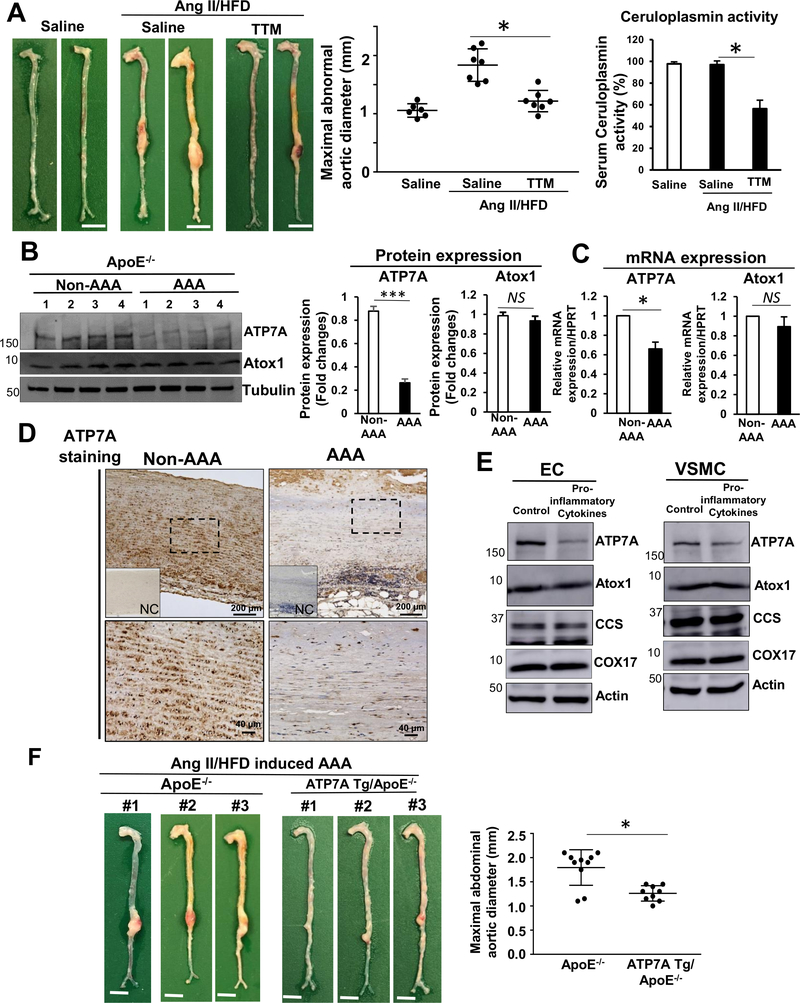
To determine the role of Cu in AAA formation, we examined the effect of the Cu chelator tetrathiomolybdate (TTM) on AAA formation in Apo E−/− mice following Ang II infusion and a high fat diet (HFD), which recapitulates some of the pathological characteristics of AAA in humans28, 34, 35. Figure 1A shows that TTM significantly reduced AAA formation as assessed by maximal aortic diameter. The efficacy of TTM treatment to lower Cu status was confirmed by a reduction in activity of the serum Cu-dependent enzyme ceruloplasmin of 42% as assessed by its ferroxidase activity, which is an established surrogate maker of bioavailable Cu13, 14, 36. This finding suggests that excess Cu contributes to AAA formation induced by Ang II/HFD.
Figure 1. ATP7A expression is reduced in Ang II-induced AAA, while ATP7A overexpression or administration of a Cu chelator prevents Ang II-induced AAA formation in ApoE−/− mice.
(A, B, C) ApoE−/− mice were infused with Ang II on a HFD or saline for 4 weeks with or without Cu chelator TTM. A, Representative images of whole aorta (left). Scale bars: 3 mm. Maximal abdominal aortic diameter (middle panel) and serum ceruloplasmin activity in aorta (right). (n=4). B, Western blot of ATP7A and Atox1 proteins in mouse abdominal aorta tissues (n=8). C, Quantitative real-time PCR analysis of ATP7A or Atox1 mRNA expression in abdominal aorta (n=4). D, Immunohistochemical staining of ATP7A in human AAA and non-AAA tissue (n=3 for AA or N=3 for non-AA). Inset, absence of staining when mouse IgG was used as antibody isotype negative controls (NC). High magnification of region within rectangle are shown at lower panel. E, ECs from bovine aorta (BAEC) and VSMC from rat aorta (RASM) were incubated with pro-inflammatory cytokine cocktail (TNFα (10 ng/ml), IL-1β (10 ng/ml) and IL-6 (10 ng/ml) for 24 hrs and then used to measure protein expression. (n=4). Quantifications were showed in Supplementary figure II. F, Representative images of whole aorta from ApoE−/− or ATP7A Tg/ApoE−/− mice following 4 weeks Ang II infusion with HFD. Scale bars: 3 mm. Maximal abdominal aortic diameter (right panel, n=9). *p<0.05.
Reduction of ATP7A expression in human and mouse AAA tissues and inflamed vascular cells
Since ATP7A plays an important role in exporting excess Cu to maintain intracellular Cu homeostasis7, 17, 18, we next examined ATP7A expression levels in the abdominal aorta from Ang II/HFD-induced AAA mice. Figure 1B and 1C show that ATP7A protein and mRNA expression were markedly reduced (P<0.05) in AAA aorta. Furthermore, immunofluorescence analysis showed a dramatic decrease of ATP7A expression in αSMA positive smooth muscle cell (SMC) layer and endothelial cell (EC) layer in AAA tissue compared to non-AAA tissue (Figure I in the Online-only Data Supplement). Reduction of ATP7A expression was also shown in aorta of AAA patients compared to non-AAA controls using immunostaining (Figure 1D). To address whether inflammation associated with AAA may be mechanistically linked to ATP7A downregulation, we examined the effects of inflammatory cytokines on ATP7A expression. We found that pro-inflammatory cytokines (TNFα, IL-1β and IL-6), which are involved in AAA pathology44, significantly decreased ATP7A mRNA and protein expression in cultured ECs and vascular SMCs (VSMCs), without affecting expression of other pathways regulating copper metabolism (Figure 1E and Figure II in the Online-only Data Supplement). These data suggest that ATP7A expression is reduced in AAA in conjunction with inflammation.
Overexpression of ATP7A suppresses Ang II-induced AAA formation
To determine the functional significance of ATP7A in the formation of AAA, we examined the effects of overexpression of ATP7A on the development of Ang II/HFD-induced AAA using ATP7A overexpressing transgenic (Tg) mice32, as we have previously described24. ATP7A overexpression significantly blunted the progression of AAA and related pathological changes (as discussed below) induced by Ang II infusion (Figure 1F and Figure XI in the Online-only Data Supplement). These data suggest that the reduced ATP7A expression observed in AAA tissues may be linked to AAA pathogenesis.
ATP7A dysfunction accelerates AAA formation
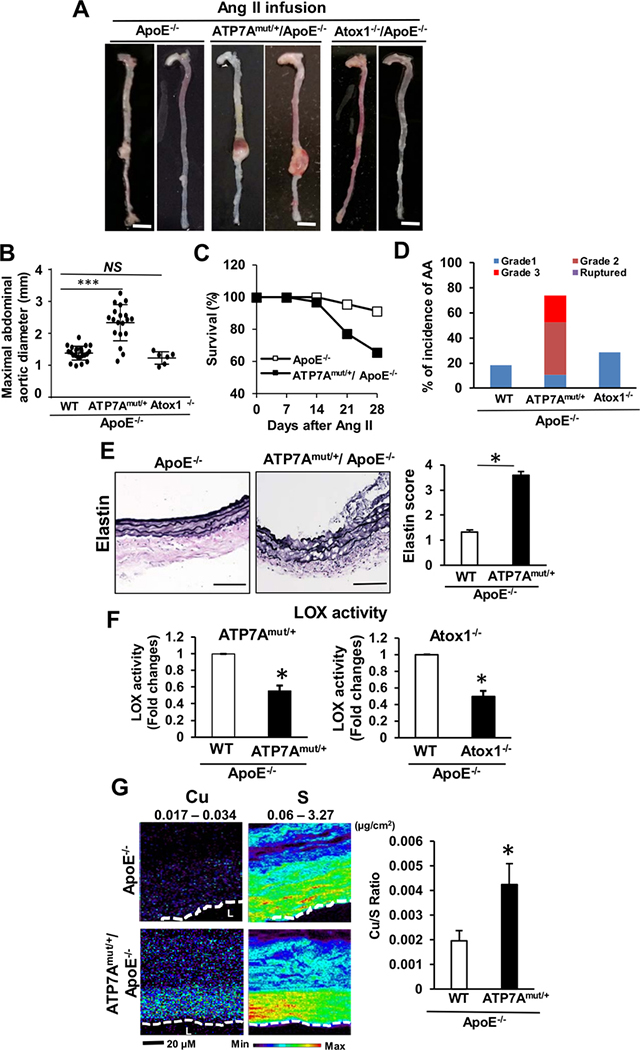
To examine the protective role of endogenous ATP7A in AAA formation, we used X-linked blotchy ATP7A mutant mice18, 22–24, which have a splice site mutation introducing a new stop codon at amino acid residue 794 with reduced Cu transport function18, 22 and typically survive to more than 6 months of age18, 22, 23. Using the Ang II-induced AAA model without HFD, which produces a less severe AAA phenotype28, 34, 35, we found that hemizygous male blotchy ATP7Amut/y/ApoE−/− mice died within a week after Ang II infusion. Thus, we employed heterozygous female ATP7Amut/+/ApoE−/− mice which had reduced Cu transport function without spontaneous AAA formation and age-matched female ApoE−/− (i.e. 2–3 month old) mice as a control. Of note, female control ApoE−/− mice exhibit much less incidences of AngII-induced AAA formation and inflammation than males, consistent with previous reports45, 46. There was no significant difference in blood pressure or serum cholesterol between ATP7Amut/+/ApoE−/− mice and ApoE−/− mice following Ang II infusion (Figure III and IV in the Online-only Data Supplement). With saline infusion, AAA were not observed in either ATP7Amut/+/ApoE−/− or ApoE−/−mice (Figure V in the Online-only Data Supplement). However, Ang II infusion for 28 days markedly increased abdominal aortic dilation in ATP7Amut/+/ApoE−/−, as compared to ApoE−/− mice (Figures 2A and 2B). Of note, 35% (11/32) of Ang II-infused ATP7Amut/+/ApoE−/− mice died versus 9% (2/23) of the ApoE−/− mice (Figure 2C). Moreover, ATP7Amut/+/ApoE−/− mice exhibited a higher complexity of aneurysm pathology and dissection with thrombus as compared to control mice (Figure 2D and Figure VIA in the Online-only Data Supplement). Furthermore, ATP7Amut/+/ApoE−/− mice exhibited significantly higher Cu levels in the AAA tissues as compared to ApoE−/− mice, as assessed by X-ray fluorescence microscopy (XFM)(Figure 2G).
Figure 2. ATP7A dysfunction, but not lack of Atox1, augments AAA formation.
(A-F) ApoE−/−, ATP7Amut/+/ApoE−/− or Atox1−/−/ApoE−/− mice following 4 weeks of Ang II infusion without HFD were examined. A, Representative images of whole aorta. Scale bars: 3 mm. B and C, Maximal abdominal aortic diameter (B), Kaplan–Meier curves of survival (C) in ApoE−/− (n=23), ATP7Amut/+/ApoE−/− (n=19) and Atox1−/−/ApoE−/− (n=6) mice. D, Incidence and severity of AA. See the Online Methods for grading aneurysm severity. E, Representative images of elastin integrity of abdominal aortic sections from ApoE−/− (n=23) and ATP7Amut/+/ApoE−/− (n=21) mice and quantitative analysis of grading for elastin degradation. Scale bars: 20 μm. F, LOX activity in aortic tissue. n=4. G, Aortic Cu content in ApoE−/− or ATP7Amut/+/ApoE−/− mice following 4 weeks of Ang II infusion as measured by synchrotron-based x-ray fluorescence (XFM). XFM scans (1–2 seconds per pixel) were performed in paraffin-embedded tissues. The maximum and minimum threshold values in microgram per square centimeter are given above each frame. Map of Cu shows areas of the lowest to the highest content scaled to a rainbow color (bottom). Total sulfur is used as a surrogate for total cellular protein and to visualize the morphology of tissue sections. n=5. *p<0.05, ***p<0.001.
ATP7A dysfunction in vascular cells, not bone marrow (BM) cells, contributes to AAA development
Since ATP7A is expressed in both BM cells and vascular cells23, 47, we performed reciprocal BM transplantation (BMT) experiments to interrogate the functional importance of ATP7A expression in BM cells. For these BMT studies, heterozygous blotchy ATP7Amut/+/ApoE−/−, or WT ApoE−/− mice, were irradiated, followed by intravenous injection of BM (Figure VIIB in the Online-only Data Supplement). BMT studies confirmed the development of severe AngII-induced AAA formation in ATP7Amut/+/ApoE−/− mice reconstituted with BM from WT ApoE−/− mice, while reconstitution of WT ApoE−/− mice with BM from ATP7Amut/+/ApoE−/− mice did not alter AAA development. The mean maximal abdominal aortic diameter was 2.18 ± 0.15 mm in the ATP7Amut/+/ApoE−/− mice reconstituted with ATP7Amut/+/ApoE−/− BM, which was significantly greater than that of the WT ApoE−/− mice transplanted with ATP7Amut/+/ApoE−/− BM (1.31 ± 0.02 mm) (Figure VIIA in the Online-only Data Supplement). Thus, these BMT studies suggest that ATP7A expression in vascular cells, but not BM cells, plays a critical role in transducing the AAA phenotype.
Impaired Atox1-ATP7A-LOX pathway is insufficient to induce AAA formation
ATP7A transports Cu to the secretory Cu enzyme pro-LOX for activity, which is essential for the crosslinking of collagen and elastin48. We thus examined if enhanced AAA formation in Cu transporter dysfunctional ATP7A mutant mice might be due to a decrease in LOX activity. Previous studies have demonstrated that LOX−/− mice or mutant mice exhibit perinatal death from AA formation and spontaneous dissection49, and that LOX mutant mice exhibit AAA characterized by fragmented elastin fibers and aberrant SMC layers50. Elastin van Gieson (EVG) staining of the aortas demonstrated enhanced degradation and disruption of aortic elastin in Ang II-induced AAA from ATP7Amut/+/ApoE−/− mice compared with ApoE−/− mice (Figure 2E). Unexpectedly, deletion of Atox1 (Cu chaperone for ATP7A) in ApoE−/− mice, which reduced LOX activity to a similar extent as observed in ATP7Amut/+/ApoE−/− mice (45–50% reduction) (Figure 2F), failed to enhance AngII-induced AAA (Figure 2A). These findings indicate that reduced LOX activity, induced by impaired Atox1-ATP7A pathway, alone is insufficient to promote AAA.
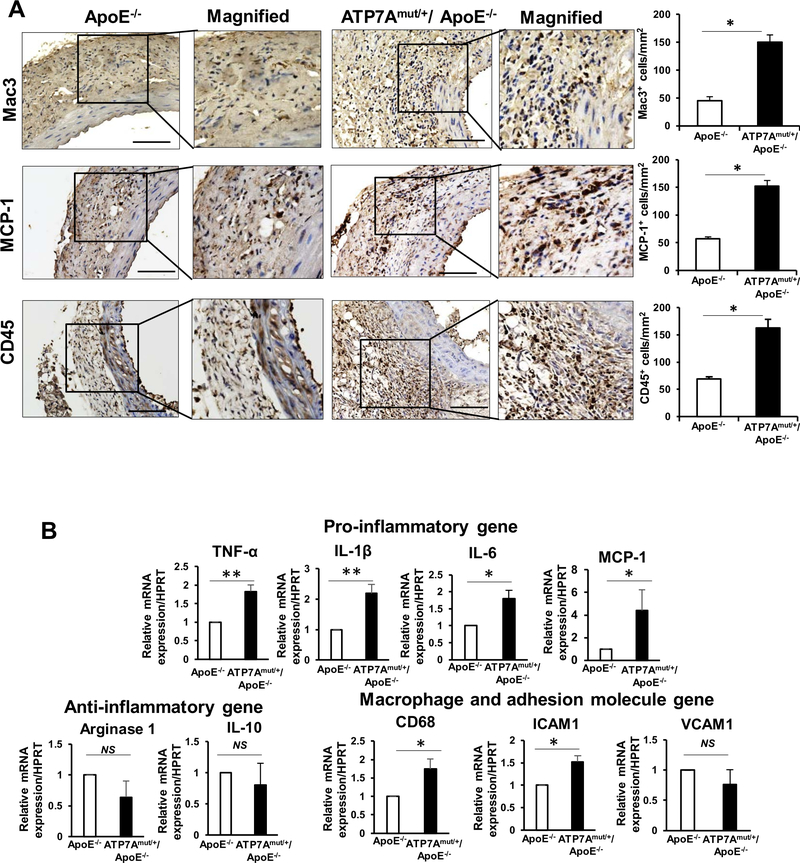
ATP7A dysfunction exacerbates vascular inflammation and matrix metalloproteinase (MMP) activity in Ang II-induced AAA mice
To investigate the mechanisms by which ATP7A dysfunction promotes Ang II-induced AAA formation, independent of LOX, we first examined the accumulation of inflammatory cells4. We found that accumulation of inflammatory cells including Mac3+, MCP-1+ and CD45+ cells (Figure 3A) as well as mRNA expression of pro-inflammatory genes (TNFα, IL-1β, MCP-1 and IL-6) and macrophage and adhesion molecules (CD68 and ICAM1), but not anti-inflammatory genes (such as arginase-1, IL-10) were significantly increased in the AAA region of ATP7Amut/+/ApoE−/− mice compared to ApoE−/− mice (Figure 3B). By contrast, ATP7A-Tg/ApoE−/− mice exhibited a significant reduction in the expression of proinflammatory genes (Figure XI in the Online-only Data Supplement).
Figure 3. ATP7A dysfunction promotes proinflammatory responses in AAA.
(A and B) Abdominal aorta tissues from ApoE−/− and ATP7Amut/+/ApoE−/− mice following 4 weeks of Ang II infusion were examined. A, Representative images of immunohistochemical staining for macrophages (Mac3) and Monocytes chemoattractant protein-1 (MCP-1) and quantification. B, Dynamic expression profiles of inflammatory genes expression measured by qPCR (n=4). *p<0.05.
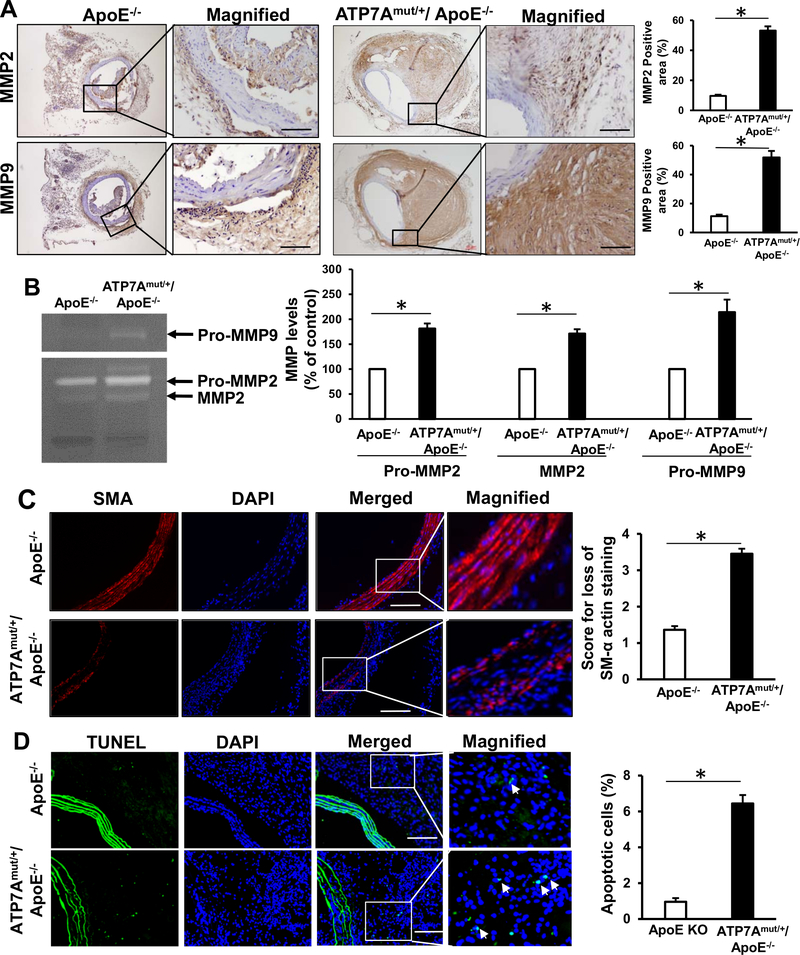
MMPs play a key role in the initiation and progression of AAA by promoting matrix degradation, thereby weakening the arterial wall and favoring aneurysm formation4, 51, 52. Since infiltrating inflammatory cells are major sources of MMPs4, 51, 52, we measured MMP activity and expression in the aortas of Ang-II infused mice. Immunohistochemical analysis revealed that MMP2 and MMP9 protein expression (Figure 4A) and proteolytic activity (Figure 4B), which are known to contribute to ECM remodeling and aneurysm formation4, 51, 52, were significantly increased in the arteries of ATP7Amut/+/ApoE−/− mice compared to control ApoE−/− mice. These results indicate that ATP7 dysfunction exacerbates AAA formation by augmenting inflammatory cell accumulation and associated MMP activation.
Figure 4. ATP7A dysfunction promotes MMP expression, apoptosis and loss of VSMCs in aortas following 4 weeks of Ang II infusion.
(A-D) Abdominal aorta tissues from ApoE−/− and ATP7Amut/+/ApoE−/− mice following 4 weeks of Ang II infusion were examined. A, Immunohistochemical staining for MMP2 and MMP9 and quantification (n=7–11). B, Representative zymogram (top) and quantified data (bottom) of MMP levels in aorta homogenates (n=4). C, Immunofluorescence staining for SMC loss assessed by SMA staining and quantification (n=11). Score 1, intact media, no SMC loss; score 2, modest loss of SMC; score 3, severe loss of SMC; score 4, rupture of media, almost no SMC left. D, Immunofluorescence staining of apoptotic cells using TUNEL staining and quantification (n=10). Scale bars: 20 μm. *p<0.05.
ATP7A dysfunction increases VSMC loss in Ang II-induced AAA mice
Since loss of VSMC is a characteristic feature of AA formation42, 53, we next examined VSMC density and apoptosis in the aorta of Ang II-infused mice. Immunofluorescence analysis of αSMA staining demonstrated that the VSMC density in the aortic media was significantly lower in ATP7Amut/+/ApoE−/− mice as compared to ApoE−/− mice (Figure 4C). Furthermore, a significant increase in the number of apoptotic cells in the aortic wall, as measured by TUNEL, was seen in ATP7Amut/+/ApoE−/− mice as compared to control ApoE−/− mice, indicating the enhanced apoptosis of vascular cells (Figure 4D). These results indicate that ATP7A dysfunction increases vascular apoptosis and decreases cell number of VSMCs, which may contribute to AAA formation. To examine if ATP7A knockdown in vascular cells directly induce apoptosis, we performed Annexin V-FITC and propidium iodide (PI) double staining using flow cytometry in cultured human VSMCs and ECs (Figure VIII in the Online-only Data Supplement). ATP7A knockdown in both cells did not induce early or late apoptotic cells and necrotic cells, compare to control cells (Figure VIII in the Online-only Data Supplement). Thus, vascular apoptosis increase in aorta of ATP7Amut/+/ApoE−/− mice may be secondary due to the inflammatory cell accumulation.
ATP7A dysfunction augments miRNA-125b expression in aorta of Ang II-induced AAA mice
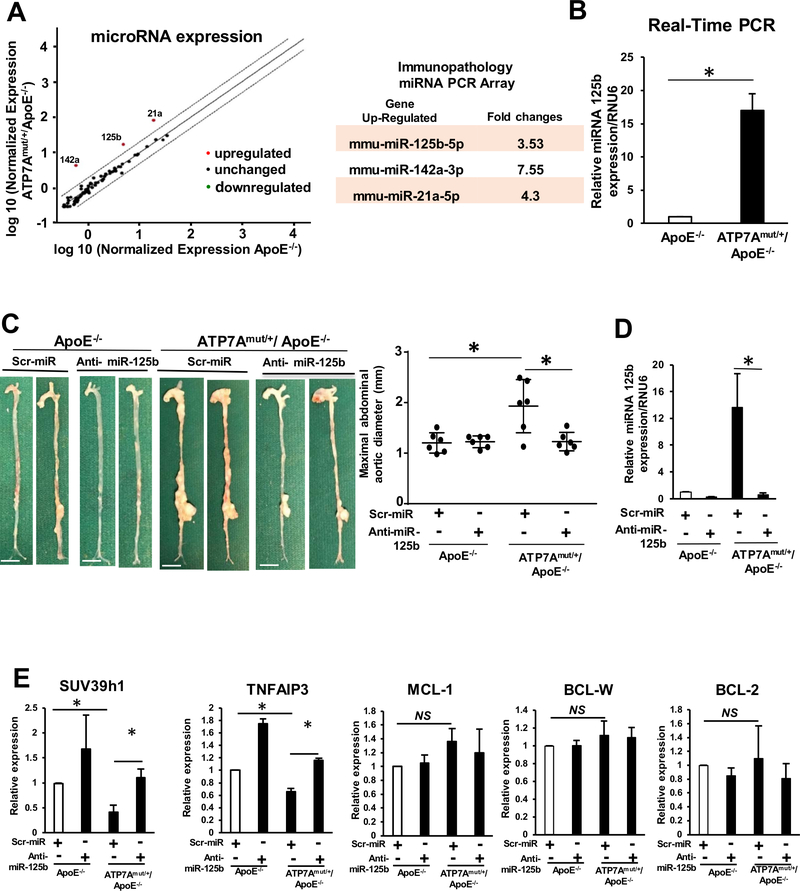
Given the involvement of miRNAs in AAA pathogenesis30, 31, we examined whether miRNAs regulate the inflammatory response and AAA development induced by ATP7A dysfunction. We performed the miScript miRNA qPCR Array (Immunopathogenesis kit; Qiagen) in aortas from Ang II-infused ATP7Amut/+/ApoE−/− and control ApoE−/− mice. We found that 3 of 88 miRNAs tested were upregulated at least 3- fold in aorta of Ang II-infused ATP7Amut/+/ApoE−/− mice, as compared to control ApoE−/− mice, including the following miRNAs: miR-125b, miR-142a, miR-21a, (Figure 5A). Since miR-125b is a highly expressed miRNA and was reported to regulate proinflammatory gene expression in VSMC54, we validated miR-125b expression using qPCR and confirmed that miRNA-125b was significantly upregulated in aortas of Ang II-infused ATP7Amut/+/ApoE−/− mice (Figure 5B) as well as in VSMCs isolated from those aortas (Figure IX in the Online-only Data Supplement). These results suggest that ATP7A dysfunction increases miR-125b expression, which may in turn promote inflammation and AAA formation.
Figure 5. Anti-miRNA 125b treatment abolishes the development of Ang II-induced AA formation in ATP7Amut/+/ApoE−/− mice.
Abdominal aorta tissues from ApoE−/− and ATP7Amut/+/ApoE−/− mice following 4 weeks of saline or Ang II infusion were examined. (A) Identification of aortic miRNAs in ApoE−/− or ATP7Amut/+/ApoE−/− mice infused with Ang II for 4 weeks using the Qiagen miScript miRNA. Immunopathology array (n=3/group) and miScript PCR Array Data Analysis Tool. (B) miRNA 125b was validated using Real time-qPCR (n=4). (C,D,E) Representative images and maximum diameter of aorta (C), quantification of miRNA 125b expression (n=4) (D), andmiR-125b target gene expression analysis (n=4) using real-time qPCR (E) of mice with or without intravenous injection of LNA modified Anti-miRNA 125b or scrambled control. In C, scale bars: 3 mm. Maximal abdominal aortic diameter (Middle) with or without anti-miRNA 125b treatment (n=4). *p<0.05.
Anti-miRNA-125b Inhibits ATP7A dysfunction-induced acceleration of AAA formation
To determine the functional significance of miR-125b upregulation, we tested whether the anti-miR-125b can prevent ATP7A dysfunction-induced AAA formation in ATP7Amut/+/ApoE−/− mice. We designed locked nucleic acid (LNA)-modified anti-miR-125b (LNA-Anti-miR-125b) to silence the expression of miR-125b in vivo and a control LNA-modified scrambled miR (scr-miR). Intravenous injection of LNA-anti miR-125b significantly inhibited the increase in aortic diameter (Figure 5C) and reduced miR-125b expression (Figure 5D) in Ang II-infused ATP7Amut/+/ApoE−/− mice. Taken together, these results suggest that ATP7A dysfunction promotes AAA at least in part due to upregulation of miR-125b expression. Because inflammation and apoptosis are involved in AAA formation, we examined expression of miR-125b targeted inflammatory genes and anti-apoptotic genes in Ang II-infused ATP7Amut/+/ApoE−/− mice treated with LNA-anti-miR-125b. Figure 5E shows that miR-125b targeted inflammatory genes (SUV39h154 or TNFAIP355), but not anti-apoptotic genes (MCL-1, BCL-W, BCL-256) were significantly decreased in Ang II-infused ATP7Amut/+/ApoE−/− mice and rescued by anti-miR-125b treatment. Consistently, anti-miR-125b treatment significantly decreased inflammatory cell (Mac3) infiltration as well as expression of pro-inflammatory genes and adhesion molecules such as (IL-1β, IL-6, TNFa, MCP1, ICAM1) in aorta of ATP7Amut/+/ApoE−/− mice (Figure 6A and 6B). Furthermore, anti-miR-125b also decreased MMP activity, elastin degradation, loss of VSMC density and vascular cell apoptosis in aortae of ATP7Amut/+/ApoE−/− mice (Figure 6C, 6D, and Figure X in the Online-only Data Supplement). Collectively, these findings indicate that the hallmark features of the AAA phenotype seen in the dysfunctional aortas from ATP7A-ApoE−/− mice are due to miR-125b upregulation.
Figure 6. Anti-miRNA 125b decreased Mac3 expression, MMP expression, apoptosis and loss of SMCs in aorta from Ang II-infused ATP7Amut/+/ApoE−/− mice.
Abdominal aorta tissues from ApoE−/− or ATP7Amut/+/ApoE−/− mice following 4 weeks of Ang II infusion with or without anti-miRNA 125b treatment were examined. A, Representative images of immunohistochemical staining for macrophages (Mac3) and quantification (n=4). Scale bars: 20 μm. B, Expression profiles of inflammatory genes by real-time qPCR (n=4). C, Immunohistochemical staining for MMP2 (top) and MMP9 (middle) and representative images of elastin integrity (bottom) and quantification (right) (n=4). D, Representative zymogram (top) of pro-MMP-2 and pro-MMP-9 levels and quantification (right). Scale bars: 20 μm. *p<0.05. E, Proposed model for the protective role of ATP7A against inflammation and smooth muscle cell apoptosis in experimental aortic aneurysm. ATP7A downregulation promotes AA formation by increasing vascular inflammation, in part via inducing miR-125b, thereby increasing MMP activity, elastin fragmentation, vascular apoptosis, and VSMC loss,. Thus, ATP7A is a potential therapeutic target for inflammatory vascular disease.
Role of ATP7A-miR125b pathway in AngII-induced AAA formation
We next examined the expression of miR-125b in Ang II/HFD-induced AAA in which ATP7A expression was markedly downregulated (Figure 1B, 1C and Figure I in the Online-only Data Supplement) and found that miR-125b expression was increased in AAA aorta compare to control mice (Figure XIA and XIIA in the Online-only Data Supplement). Furthermore, ATP7A overexpression, which inhibited AAA formation (Figure 1F), reduced miR-125b upregulation induced by Ang II infusion (Figure XIA in the Online-only Data Supplement), suggesting the causal role of ATP7A downregulation in upregulation of miR-125b and AAA formation. Moreover, these mice showed increase of miR-125b target anti-inflammatory genes (SUV39h154 or TNFAIP355), but not anti-apoptotic genes (MCL-1, BCL-W, BCL-256), thereby reducing the expression of pro-inflammatory genes and adhesion molecules (Figure XIB, XIC in the Online-only Data Supplement). Mechanistically, Cu chelator TTM treatment in vivo which inhibited AAA formation (Figure 1A) prevented upregulation of miR-125b induced by AngII infusion (Figure XIIA in the Online-only Data Supplement). Furthermore, ATP7A knockdown in cultured VSMCs increased miR125b expression, which was inhibited by TTM (Figure XIIB in the Online-only Data Supplement). Given that Cu exporter ATP7A dysfunction increased intracellular Cu level in vascular cells19, 20, 57, these results suggest that ATP7A dysfunction-induced Cu elevation might contribute to miR-125b upregulation. Functional significance of miR-125b elevation was demonstrated by the observation that LNA-modified anti-miR-125b infusion significantly inhibited AAA formation induced by AngII/HFD. Furthermore, there was no additional preventive effect of ATP7A overexpression on anti-miR-125b-induced inhibitory effects on AAA formation, suggesting ATP7A-miR125b pathway is involved in AAA formation (Figure XIII in the Online-only Data Supplement). Taken together, these results suggest that decreased ATP7A expression in Ang II/HFD mice or ATP7A dysfunctional mutant mice may contribute to AAA formation mainly through Cu-dependent miR125b upregulation.
DISCUSSION
This study provides novel evidence that the Cu exporter ATP7A protects against AAA formation by suppressing inflammation and protecting vascular wall integrity (Figure 6E). Major findings in this study are as follows: 1) ATP7A expression is significantly reduced during AAA formation in AngII-infused mice and human AAA tissue; 2) the Cu chelator TTM, or ATP7A overexpression, prevented Ang II-induced AAA; 2) LOX activity was reduced to a similar extent in Cu transport dysfunctional ATP7Amut/+/ApoE−/− mice and Cu chaperone deficient Atox1−/−/ApoE−/− mice, but only the ATP7Amut/+/ApoE−/− mice exhibited enhanced AAA formation and dissection, in association with Cu accumulation as assessed by XFM analysis; 3) ATP7A is a negative regulator of vascular inflammation and vascular apoptosis, which are involved in aneurysm formation; 4) BM transplantation experiments suggest that ATP7A expression in vascular cells, but not BM cells, plays an important role in AAA development; 5) augmented vascular miRNA-125b expression provides a novel mechanism that is responsible for increased vascular inflammation and subsequent apoptosis in the pathogenesis of AAA in ATP7Amut/+/ApoE−/− mice and AngII/HFD ApoE−/− mice.
Cu chelator TTM which forms a tripartite TTM-copper-protein complex to chelates bioavailable Cu, has been used for the treatment of patients with Wilson disease who exhibit Cu toxicity 5, 6 and is shown to prevent atherosclerotic lesion development in ApoE−/− mice14, acute inflammation and pulmonary fibrosis,5–7, 13. However, the effects of Cu chelation on AAA development have not been reported. Thus, to our knowledge, this is the first study to demonstrate that Cu chelator significantly inhibits Ang II/HFD-induced AAA formation. The efficacy of TTM in chelating Cu was confirmed by the reduced activity of serum secretory Cu enzyme ceruloplasmin, an established surrogate marker of bioavailable Cu13, 36. These findings suggest that excess Cu may contribute to AAA formation induced by Ang II/HFD. In the present study, we found that expression of Cu exporter ATP7A, but not Atox1, was significantly reduced in AAA tissues, which may result in excess accumulation of Cu in AAA. Of note, ATP7A protein was also downregulated in human AAA tissues compared to that of non-AAA, supporting the clinical significance of ATP7A in AAA formation. Although the mechanism of downregulation of ATP7A in AAA tissues remains unclear, we found that inflammatory cytokines, which are involved in AAA pathology44, induced decrease in ATP7A mRNA and protein expression in cultured VSMCs. Addressing underlying molecular mechanism in detail is the subject of future study. Moreover, functional significance of ATP7A downregulation was demonstrated by our observation that ATP7A overexpression in ApoE−/− mice mitigates Ang II/HFD-induced AAA formation. Together with Cu transport dysfunctional ATP7Amut mice18, 22, these findings indicate that endogenous ATP7A functions to protect against AAA formation. Thus, Cu chelation or restoring ATP7A expression may be potentially effective therapeutic option for the treatment of AAA in patients.
Using Ang II-induced AAA model without HFD, which produces a less severe AAA phenotype, we showed that hemizygous male blotchy ATP7Amut/y/ApoE−/− mice died within seven days, while heterozygous female blotchy ATP7Amut/+/ApoE−/− mice showed enhanced AAA formation and dissection with increased Cu accumulation, as compared to WT female ApoE−/− mice which showed the low incidence of AAA 45, 46. It is shown that hemizygous blotchy ATP7Amut/y mice, but not heterozygous blotchy ATP7Amut/+mice, spontaneously develop aneurysms, mostly in the ascending aorta, with an increased incidence with aging26, 27. Notably, Menkes disease patients, as well as hemizygous blotchy ATP7Amut/y/ApoE−/− mice, also exhibit aneurysm formation in systemic arteries such as lumbar, iliac, splenic and hepatic arteries58, 59. Whether ATP7A limits aneurysm formation in arteries other than the aorta under various conditions such as hypertension, obesity or aging warrants future investigation. Furthermore, BMT experiments revealed that ATP7A in tissue-resident cells such as vascular cells such as VSMC and ECs plays an important role for preventing AAA development. VSMC loss is another key feature of human AAA that contributes to the degeneration of vascular wall integrity42, 53. The present study found that α-SMA staining as an index of VSMC density was markedly reduced, while apoptosis was highly increased, in the aortas of ATP7A dysfunctional mice. Furthermore, loss of EC integrity also contributes to AAA development in response to Ang II infusion37. Further studies are required to investigate the tissue specific role of ATP7A in VSMCs and ECs in limiting AAA formation.
LOX activity is essential for crossing of collagen and elastin, and loss of LOX activity is shown to lead to vascular dilation and rupture49. We thus examined whether AAA formation in ATP7A dysfunctional mutant mice could be due to either abnormal Cu absorption or reduced Cu transport to the secretory Cu-dependent enzyme pro-LOX (the precursor of LOX) 60. Unexpectedly, we found that dysfunction of ATP7A, but not deficiency of Atox1 (Cu chaperone for ATP7A), augmented Ang II-induced AAA. Since both ATP7A dysfunction and Atox1 deficiency reduced LOX activity at the similar extent, these results indicate that the decreased LOX activity alone is insufficient to promote AAA. These findings also suggest that Atox1 and ATP7A may have distinct functions such that Atox1 functions as a Cu-dependent transcription factor 40, 61, 62 in addition to Cu chaperone for the ATP7A-LOX pathway7. Menkes disease is characterized by the extensive decrease in Cu levels in most tissues except for the kidney and small intestine due to abnormal Cu absorption in the intestine19, 20. Importantly, ATP7A dysfunctional mice exhibited increased Cu levels, as assessed by XFM analysis, in the AAA tissues. These results suggest that the reduced LOX activity in both strains of mice is not due to decreased tissue Cu levels. Consistent with this, cells isolated from Menkes patients show increased cellular Cu level due to a Cu efflux defect19, 20. Collectively, these results suggest that enhanced AAA formation in ATP7Amut mice is not simply due to a decrease in LOX activity, but to other (Cu-dependent) factors such as Cu-dependent transcription factor function of Atox1 as discussed below.
Inflammatory cell infiltration and proteolytic enzymes (MMPs) also contribute to AAA development in humans and animals. We found that inflammatory cell infiltration and expression/activity of MMP2 and 9 were significantly increased in the aorta of ATP7Amut/+/ApoE−/− mice compared to control mice. As reported51, infiltration of macrophages into the vessel wall presents a major source of MMPs and VSMC is also a major source of MMP252. The increased MMPs promote matrix degradation, thus impairing the integrity of the arterial wall and promoting AAA development. We also found that transcription of pro-inflammatory genes, but not anti-inflammatory genes, was significantly increased in aorta with AAA from ATP7Amut/+/ApoE−/− mice. There are two possible mechanisms underlying this response. First, Cu accumulation in ATP7A dysfunctional mice itself may promote vascular inflammation. For example, Cu was reported to induce IL-6 secretion in cultured cells,63 while implantation of Cu coated discs in rats induced IL-6 production and recruitment of IL-1α-secreting cells64. Moreover, Cu chelation by TTM inhibited vascular inflammation and atherosclerosis in ApoE−/− mice14. Second, epigenetic regulation of inflammatory genes induced by ATP7A dysfunction may also contribute to vascular inflammation during AAA. Indeed, ATP7Amut/+/ApoE−/− mice exhibit reduced expression of anti-inflammatory genes such as SUV39h1 and TNFAIP3, a negative regulator of NFkB55. Previous study reported dysregulation of Suv39h1 and associated chromatin H3K9me3 (which increases inflammatory gene expression) in VSMC of diabetic mice54. We recently reported that decreased ATP7A protein expression in diabetic mice contributed to endothelial dysfunction25. Thus, it is conceivable that ATP7A dysfunction may cause vascular inflammation via reducing the SUV39h1 and TNFAIP3 gene expression.
Inflammatory miRNAs also play an important role in AAA formation 30, 31. The microRNA array and qPCR analysis in AAA tissues indicate that at least three inflammatory miRNAs (miR-125b, −142a and −21a) were significantly upregulated (more than 3-fold) in ATP7A dysfunctional mice as compared with control. Among them, we focused on miRNA-125b, since it is one of the highly expressed miRNAs involved in inflammation54, 55 and also upregulated in the abdominal aorta from Ang II/HFD-induced AAA ApoE−/− mice. Previous studies also reported the upregulation of miR-125b in VSMCs isolated from human AAA samples compared to those from normal aortas65. Functional significance of miR-125b elevation was demonstrated by the evidence that LNA-modified anti-miR-125b treatment significantly prevented AngII/HFD-induced AAA formation. ATP7A overexpression had no additional inhibitory effects on anti-miR-125b-treated AngII/HFD mice, suggesting the ATP7A-miR125b pathway (Figure XIII in the Online-only Data Supplement). Of note, miR-125b is shown to repress SUV39h154 and TNFAIP355 as well as induces apoptosis in cancer cells by targeting anti-apoptotic Bcl-2, Bcl-W, and Mcl-1proteins56. In the present study, we found that LNA-anti-miR-125b treatment also rescued the decreased SUV39h1 and TNFAIP3 expression in ATP7Amut/+ mice, which was associated with reduced inflammatory cytokine expression, aortic dilation, apoptosis and MMP activity without affecting anti-apoptotic genes. We also found that ATP7A dysfunction-induced Cu elevation was not sufficient to induce apoptosis in vascular cells. Given that vascular apoptosis is induced by pro-inflammatory cytokines 42, 53, it is tempting to speculate that the enhanced apoptosis in ATP7Amut mice via upregulating miR-125b may be due to the increased vascular inflammation during AAA formation.
Experiments using Cu chelator TTM suggest that decreased ATP7A expression in Ang II/HFD-induced or ATP7A dysfunction-induced AAA formation is mainly through the Cu-dependent miR125b-mediated inflammatory response. We previously reported that the Cu chaperone Atox1 functions as a Cu-dependent transcription factor to increase expression of the NADPH oxidase organizer p47phox in inflamed ECs, cyclin D1 and SOD3 in VSMCs or fibroblasts7. We found that the human, mouse or rat miR-125b promoter contains Atox1-binding sites (5’-GAAAGA-3’). Thus, it is likely that increased miR-125b expression in ATP7Amut mice could be mediated through Cu-dependent transcription factor function of Atox1. Addressing this point in detail and role of other microRNAs in AAA formation in ATP7Amut mice is the subject of future investigation.
In summary, we demonstrate that the Cu transporter ATP7A protects against AAA formation by limiting vascular inflammation and MMP activity, at least in part via suppressing pro-inflammatory miR-125b in a Cu-dependent manner (Figure 6E). Our studies provide novel insights into the Cu transporter ATP7A as a potential therapeutic target for treatment of vascular inflammatory diseases and reveal new complexities in the role of Cu-dependent processes in AAA development.
Supplementary Material
Highlights.
Cu chelator markedly inhibited angiotensin II (Ang II)-induced abdominal aortic aneurysm (AAA).
Human and mouse AAA tissues showed decreased Cu exporter ATP7A expression compared to non-AAA tissues.
ATP7A dysfunctional mutant mice exhibited robust AAA formation and increased aortic Cu accumulation, vascular inflammation, and vascular smooth muscle cell loss.
Mechanistically, miR125b, which targets anti-inflammatory Suv39h1 and TNFAIP3, was highly upregulated in AAA from ATP7A dysfunctional mutant mice in a Cu-dependent manner.
LNA-modified anti-miR125b prevented vascular inflammation and AAA development in Ang II-infused or ATP7A dysfunctional mice.
Cu chelation therapy or enhancing Cu transporting function of ATP7A that limits miR-125b may be potential therapeutic approach for treating inflammatory vascular disease.
Acknowledgements
V.S. designed the study, performed experiments, analyzed data, and wrote the manuscript. A.D. D.A. T.H, S.L H. K. helped for preparing XFM sample and performed experiment, O.A, L.F. S.V. performed XFM experiment, B.S, G.C, J. W. provided non-AAA sample, J.H.K, D.F and N.W. gave suggestions to advance this project and edited manuscript. M.U.-F. and T.F. designed the overall study, analyzed data, and wrote, reviewed, and edited the manuscript. T.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
The US Department of Energy, Office of Science, Office of Basic Energy Sciences supported use of the Advanced Photon Source at Argonne National Laboratory, under contract No. DE-AC02–06CH11357. This research was supported by NIHR01HL070187 (to T.F.), Department of Veterans Affairs Merit Review grant 2I01BX001232 (to T.F.), NIHR01HL133613, NIHR01HL116976 (to T.F., M.U.-F.), NIHR01 HL135584 (to M.U.-F.), 15SDG25700406 (to V.S.), and HL124097, HL126949, HL134354 and AR070029 (to N.L.W), 17POST33660754 (to D.A.).
Non-standard Abbreviations and Acronyms
- AAA
Abdominal aortic aneurysm
- Ang II
Angiotensin II
- Atox1
Antioxidant 1
- Apo E
Apolipoprotein E
- BMT
Bone marrow transplantation
- BAEC
Bovine aortic endothelial cells
- Cu
Copper
- ATP7A
Copper-transporting P-type ATPase/Menkes ATPase
- EVG
Elastin van Gieson
- EC
Endothelial cells
- HFD
High fat diet
- IB
Immunoblot
- LNA
Locked nucleic acid
- LOX
Lysyl oxidase
- MMPs
Matrix Metalloproteinases
- MASMs
Mouse aortic smooth muscle cells
- RASMs
Rat aortic smooth muscle cells
- RT-PCR
Reverse transcription-polymerase chain reaction
- SUV39h1
Suppressor of Variegation 3–9 Homolog 1
- TTM
Tetrathiomolybdate
- TNFAIP3
TNF Alpha Induced Protein 3
- TGN
Trans-Golgi network
- VSMC
Vascular smooth muscle cell
- XFM
X-ray fluorescence microscopy
Footnotes
Disclosure
None.
REFERENCES
- 1.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. [DOI] [PubMed] [Google Scholar]
- 4.Dale MA, Ruhlman MK, Baxter BT. Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol. 2015;35:1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer GJ. Tetrathiomolybdate anticopper therapy for Wilson’s disease inhibits angiogenesis, fibrosis and inflammation. J Cell Mol Med. 2003;7:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discov Today. 2005;10:1103–1109. [DOI] [PubMed] [Google Scholar]
- 7.Fukai T, Ushio-Fukai M, Kaplan JH. Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol. 2018;315:C186–C201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koksal C, Ercan M, Bozkurt AK, Cortelekoglu T, Konukoglu D. Abdominal aortic aneurysm or aortic occlusive disease: role of trace element imbalance. Angiology. 2007;58:191–195. [DOI] [PubMed] [Google Scholar]
- 9.Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol. 2004;24:949–954. [DOI] [PubMed] [Google Scholar]
- 10.Volker W, Dorszewski A, Unruh V, Robenek H, Breithardt G, Buddecke E. Copper-induced inflammatory reactions of rat carotid arteries mimic restenosis/arteriosclerosis-like neointima formation. Atherosclerosis. 1997;130:29–36. [DOI] [PubMed] [Google Scholar]
- 11.Mandinov L, Mandinova A, Kyurkchiev S, Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, de Muinck ED, Lindner V, Post MJ, Simons M, Bellum S, Prudovsky I, Maciag T. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci U S A. 2003;100:6700–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuschke DA, Saari JT, Miller FN. Leukocyte-endothelial adhesion is impaired in the cremaster muscle microcirculation of the copper-deficient rat. Immunol Lett. 2001;76:139–144. [DOI] [PubMed] [Google Scholar]
- 13.Wei H, Frei B, Beckman JS, Zhang WJ. Copper chelation by tetrathiomolybdate inhibits lipopolysaccharide-induced inflammatory responses in vivo. Am J Physiol Heart Circ Physiol. 2011;301:H712–H720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei H, Zhang WJ, McMillen TS, Leboeuf RC, Frei B. Copper chelation by tetrathiomolybdate inhibits vascular inflammation and atherosclerotic lesion development in apolipoprotein E-deficient mice. Atherosclerosis. 2012;223:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan JH, Maryon EB. How Mammalian Cells Acquire Copper: An Essential but Potentially Toxic Metal. Biophys J. 2016;110:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. [DOI] [PubMed] [Google Scholar]
- 19.Mercer JF. The molecular basis of copper-transport diseases. Trends Mol Med. 2001;7:64–69. [DOI] [PubMed] [Google Scholar]
- 20.Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhu S, Weisman GA, Gitlin JD, Petris MJ. Conditional knockout of the Menkes disease copper transporter demonstrates its critical role in embryogenesis. PLoS One. 2012;7:e43039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Camakaris J, Mercer JF. Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein, in cells from blotchy (Mo blo) and brindled (Mo br) mouse mutants. Hum Mol Genet. 1999;8:1069–1075. [DOI] [PubMed] [Google Scholar]
- 23.Qin Z, Gongora MC, Ozumi K, Itoh S, Akram K, Ushio-Fukai M, Harrison DG, Fukai T. Role of Menkes ATPase in angiotensin II-induced hypertension: a key modulator for extracellular superoxide dismutase function. Hypertension. 2008;52:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudhahar V, Urao N, Oshikawa J, McKinney RD, Llanos RM, Mercer JF, Ushio-Fukai M, Fukai T. Copper transporter ATP7A protects against endothelial dysfunction in type 1 diabetic mice by regulating extracellular superoxide dismutase. Diabetes. 2013;62:3839–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudhahar V, Okur MN, Bagi Z, O’Bryan JP, Hay N, Makino A, Patel VS, Phillips SA, Stepp D, Ushio-Fukai M, Fukai T. Akt2 Stabilizes ATP7A, a Copper Transporter for SOD3 (Extracellular Superoxide Dismutase), in Vascular Smooth Muscles: Novel Mechanism to Limit Endothelial Dysfunction in Type 2 Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2018;38(3):529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brophy CM, Tilson JE, Braverman IM, Tilson MD. Age of onset, pattern of distribution, and histology of aneurysm development in a genetically predisposed mouse model. J Vasc Surg. 1988;8:45–48. [PubMed] [Google Scholar]
- 27.Andrews EJ, White WJ, Bullock LP. Spontaneous aortic aneurysms in blotchy mice. Am J Pathol. 1975;78:199–210. [PMC free article] [PubMed] [Google Scholar]
- 28.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Bammler TK, Beyer RP, Gallagher EP. Copper-induced deregulation of microRNA expression in the zebrafish olfactory system. Environ Sci Technol. 2013;47:7466–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maegdefessel L, Dalman RL, Tsao PS. Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu Rev Med. 2014;65:49–62. [DOI] [PubMed] [Google Scholar]
- 31.Raffort J, Lareyre F, Clement M, Mallat Z. Micro-RNAs in abdominal aortic aneurysms: insights from animal models and relevance to human disease. Cardiovasc Res. 2016;110:165–177. [DOI] [PubMed] [Google Scholar]
- 32.Ke BX, Llanos RM, Wright M, Deal Y, Mercer JF. Alteration of copper physiology in mice overexpressing the human Menkes protein ATP7A. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1460–R1467. [DOI] [PubMed] [Google Scholar]
- 33.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remus EW, O’Donnell RE Jr., Rafferty K, Weiss D, Joseph G, Csiszar K, Fong SF, Taylor WR. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2012;303:H1067–H1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 37.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, Aki S, Miyazawa H, Biswas K, Nagakura C, Ueno M, Iseki S, Schwartz RJ, Okamoto H, Sasaki T, Matsui O, Asano M, Adams RH, Takakura N, Takuwa Y. Endothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med. 2012;18:1560–1569. [DOI] [PubMed] [Google Scholar]
- 38.Manning MW, Cassi LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med. 2002;7:45–54. [DOI] [PubMed] [Google Scholar]
- 39.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das A, Sudhahar V, Chen GF, Kim HW, Youn SW, Finney L, Vogt S, Yang J, Kweon J, Surenkhuu B, Ushio-Fukai M, Fukai T. Endothelial Antioxidant-1: a Key Mediator of Copper-dependent Wound Healing in vivo. Sci Rep. 2016;6:33783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HW, Blomkalns AL, Ogbi M, Thomas M, Gavrila D, Neltner BS, Cassis LA, Thompson RW, Weiss RM, Lindower PD, Blanco VM, McCormick ML, Daugherty A, Fu X, Hazen SL, Stansfield BK, Huo Y, Fulton DJ, Chatterjee T, Weintraub NL. Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am J Physiol Heart Circ Physiol. 2017;313:H1168–H1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamanouchi D, Morgan S, Kato K, Lengfeld J, Zhang F, Liu B. Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:702–707. [DOI] [PubMed] [Google Scholar]
- 43.Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011;192:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. [DOI] [PubMed] [Google Scholar]
- 45.Alsiraj Y, Thatcher SE, Charnigo R, Chen K, Blalock E, Daugherty A, Cassis LA. Female Mice With an XY Sex Chromosome Complement Develop Severe Angiotensin II-Induced Abdominal Aortic Aneurysms. Circulation. 2017;135:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henriques TA, Huang J, D’Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. [DOI] [PubMed] [Google Scholar]
- 47.Qin Z, Konaniah ES, Neltner B, Nemenoff RA, Hui DY, Weintraub NL. Participation of ATP7A in macrophage mediated oxidation of LDL. J Lipid Res. 2010;51:1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. [DOI] [PubMed] [Google Scholar]
- 50.Lee VS, Halabi CM, Hoffman EP, Carmichael N, Leshchiner I, Lian CG, Bierhals AJ, Vuzman D, Brigham Genomic M, Mecham RP, Frank NY, Stitziel NO. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113:8759–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keeling WB, Armstrong PA, Stone PA, Bandyk DF, Shames ML. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg. 2005;39:457–464. [DOI] [PubMed] [Google Scholar]
- 52.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 54.Villeneuve LM, Kato M, Reddy MA, Wang M, Lanting L, Natarajan R. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes. 2010;59:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci U S A. 2012;109:7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong J, Zhang JP, Li B, Zeng C, You K, Chen MX, Yuan Y, Zhuang SM. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32:3071–3079. [DOI] [PubMed] [Google Scholar]
- 57.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A. 2003;100:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adaletli I, Omeroglu A, Kurugoglu S, Elicevik M, Cantasdemir M, Numan F. Lumbar and iliac artery aneurysms in Menkes’ disease: endovascular cover stent treatment of the lumbar artery aneurysm. Pediatr Radiol. 2005;35:1006–1009. [DOI] [PubMed] [Google Scholar]
- 59.Mentzel HJ, Seidel J, Vogt S, Vogt L, Kaiser WA. Vascular complications (splenic and hepatic artery aneurysms) in the occipital horn syndrome: report of a patient and review of the literature. Pediatr Radiol. 1999;29:19–22. [DOI] [PubMed] [Google Scholar]
- 60.Rowe DW, McGoodwin EB, Martin GR, Grahn D. Decreased lysyl oxidase activity in the aneurysm-prone, mottled mouse. J Biol Chem. 1977;252:939–942. [PubMed] [Google Scholar]
- 61.Thomas M, Gavrila D, McCormick ML, Miller FJ Jr., Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen GF, Sudhahar V, Youn SW, Das A, Cho J, Kamiya T, Urao N, McKinney RD, Surenkhuu B, Hamakubo T, Iwanari H, Li S, Christman JW, Shantikumar S, Angelini GD, Emanueli C, Ushio-Fukai M, Fukai T. Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function. Sci Rep. 2015;5:14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmalz G, Schuster U, Schweikl H. Influence of metals on IL-6 release in vitro. Biomaterials. 1998;19:1689–1694. [DOI] [PubMed] [Google Scholar]
- 64.Suska F, Esposito M, Gretzer C, Kalltorp M, Tengvall P, Thomsen P. IL-1alpha, IL-1beta and TNF-alpha secretion during in vivo/ex vivo cellular interactions with titanium and copper. Biomaterials. 2003;24:461–468. [DOI] [PubMed] [Google Scholar]
- 65.Cheuk BL, Cheng SW. Identification and characterization of microRNAs in vascular smooth muscle cells from patients with abdominal aortic aneurysms. J Vasc Surg. 2014;59:202–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.