Abstract
Radiation-induced pulmonary fibrosis (RIPF) is a serious treatment complication that affects about 9-30% cancer patients receiving radiotherapy for thoracic tumors. RIPF is characterized by progressive and irreversible destruction of lung tissues and deterioration of lung function, which can compromise quality of life and eventually lead to respiratory failure and death. Unfortunately, the mechanisms by which radiation causes RIPF have not been well established nor has an effective treatment for RIPF been developed. Recently, an increasing body of evidence suggests that induction of senescence by radiation may play an important role in RIPF and clearance of senescent cells (SnCs) with a senolytic agent, small molecule that can selectively kill SnCs, has the potential to be developed as a novel therapeutic strategy for RIPF. This review discusses some of these new findings to promote further study on the role of cellular senescence in RIPF and the development of senolytic therapeutics for RIPF.
Introduction
Thoracic radiotherapy (RT) is an essential treatment modality for lung, breast and esophageal cancers, and various mediastinal tumors. Despite the increasing use of highly conformal RT techniques, many patients treated with thoracic RT remain at risk of developing radiation-induced pulmonary fibrosis (RIPF).1–3 The necessity of avoiding pneumonitis and RIPF limits the dose and intensity of irradiation and thus reduces the efficacy of RT. In addition, RIPF is a latent disease that may occur several years after exposure to ionizing radiation (IR). Therefore, long-term cancer survivors who were previously treated with conventional RT or preconditioned with total body irradiation (TBI) for bone marrow transplantation are still at risk to develop RIPF. However, RIPF is not a universal outcome of RT and there is no reliable prognosticator to predict which patients are at risk of developing RIPF after thoracic RT. Treating all patients after thoracic RT with a radiation protectant/mitigator to prevent and mitigate RIPF would unnecessarily expose a tremendous number of patients to the risk of drug toxicity and incur great cost. Furthermore, RIPF is insidious and most patients with RIPF have a significant fibrotic burden at the time of clinical presentation, limiting treatment to mitigants that would likely only slow the progression of RIPF. Currently, pirfenidone and nintedanib are the only drugs that have been approved to treat idiopathic pulmonary fibrosis (IPF) but their effectiveness against RIPF has yet to be determined.4,5 In addition, there is no therapeutics that can halt or reverse the disease progression of IPF and RIPF. Without an effective treatment, RIPF can continue progressing to chronic pulmonary insufficiency that not only affects the quality of life but may eventually lead to cor pulmonale or death. Therefore, new strategies that can stop and even reverse the course of the disease are urgently needed. An accumulating body of evidence suggests that induction of senescence by radiation may play an important role in RIPF and clearance of senescent cells (SnCs) with senolytic agents, a class of small molecules that can selectively kill SnCs has the potential to be developed as a novel therapeutic strategy for RIPF.6,7 Therefore, in this review we discuss some of these new findings to promote further study on the role of cellular senescence in RIPF and the development of senolytic therapeutics for RIPF.
RIPF
RIPF is a late effect of radiation on the lungs.8 It develops in the third phase of RT-induced lung tissue damage. The first phase is asymptomatic. The second phase, called the radiation-induced pneumonitis, occurs within a few weeks up to several months after radiation, resulting in a noninfectious inflammation of the lungs. Although post-radiation pneumonitis is often radiographically evident and asymptomatic, in some patients, it may progress to oxygen dependence, requirement for mechanical ventilation, respiratory failure, and, in some cases, death. With or without anti-inflammatory treatment, most patients can recover from radiation-induced pneumonitis but some may proceed to the third phase, i.e. fibrotic phase, which is characterized by progressive and irreversible pulmonary fibrosis that causes destruction of lung tissues and deterioration of lung function and eventually results in cor pumonale, respiratory failure and death.2 Extensive efforts have been devoted to elucidate the mechanisms by which IR induces RIPF. The suggested mechanisms include increased production of reactive oxygen species (ROS), induction of chronic inflammation, activation of the TGF-β signaling pathway, and dysregulation of extracellular matrix degradation.6
However, therapeutics targeting each of these individual causes of RIPF have some effects on preventing and mitigating the disease but have limited effectiveness in retarding and reversing the progression of the disease if they cannot be given to patients before or shortly after RT. The lack of an effective treatment for RIPF stimulates further studies to elucidate the fundamental causes of the disease. An increasing body of evidence suggests that induction of cellular senescence by IR may be a driver of the pathogenesis of RIPF, which might be targetable to develop more effective treatments for RIPF.
Cellular senescence – a double-edged sword in the fight against cancer with RT
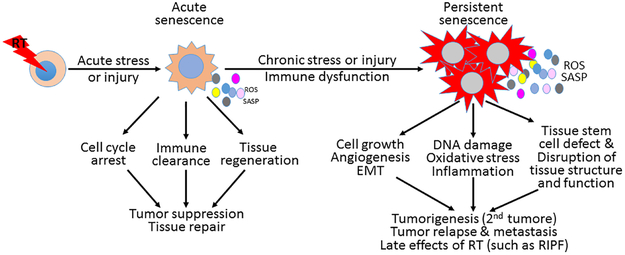
IR is a potent inducer of cellular senescence. Induction of cellular senescence is considered an important cellular mechanism for cancer prevention and treatment by IR, because it causes a permanent growth arrest of DNA damaged cells to prevent their propagation and stimulates the immune system to rapidly eliminate these genetically unstable cells. However, if the increase in SnC production persists to exceed the immune clearance capacity or the immune system is compromised and cannot efficiently remove SnCs, SnCs accumulate.9–12 Under such circumstances, SnCs can potentially promote tumorigenesis, increase tumor resistance to RT, and facilitate tumor relapse and metastasis in a cell non-autonomous manner by producing increased levels of ROS and secreting a plethora of inflammatory mediators (e.g. cytokines and chemokines), growth factors, and extracellular proteases – termed the senescence-associated secretory phenotype (SASP). SASP creates a tumor-promoting environment by causing chronic oxidative stress and inflammation, stimulating cell proliferation and angiogenesis, inducing epithelial-to-mesenchymal transition, and disrupting tissue structure and function.13–15 In addition, SnCs can also contribute to IR-induced late effects, such as lung, kidney, intestinal, and skin fibrosis; cataract; cognitive dysfunction; and cardiovascular diseases.6,16–21 Therefore, cellular senescence is a double-edged sword in the fight against cancer with RT. Inhibiting the induction of senescence is detrimental, but promoting SnC clearance after IR is beneficial (Fig.1).
Figure 1.
Cellular senescence – a double-edged sword in the fight against cancer with thoracic radiotherapy (RT). ROS, reactive oxygen species; SASP, senescence-associated secretary phenotype; RIPF, radiation-induced pulmonary fibrosis; and EMT, epithelial-mesenchymal transition.
Cellular senescence and RIPF
An increasing body of evidence demonstrates that senescence plays an important role in tissue remodeling, injury, and repair both in normal development and physiology and in various pathological conditions and diseases.9–12 Induction of senescence during the acute phase of tissue injury is presumably beneficial, because SnCs can promote tissue repair by recruiting immune cells to clear damaged cells including SnCs and stimulating the proliferation and differentiation of neighboring cells including tissue stem and progenitor cells to repopulate the damaged tissue through SASP. This presumption is supported by the finding that mice that are deficient in induction of senescence due to lacking of Trp53 and/or Cdkn2a developed severe liver fibrosis after suffering from carbon tetrachloride-induced liver injury.22 In addition, SnCs also play an essential role in cutaneous wound healing through secretion of PDGF-AA and production of the matricellular protein CCN1.23,24 However, SnCs may regulate tissue repair and fibrosis in a tissue-specific manner, because inhibition of senescence induction does not promote, but inhibits, bleomycin- or IR-induced pulmonary fibrosis.25–27 In addition, induction of senescence during tissue injury has to be tightly regulated to avoid aberrant wound healing responses, because persistent induction of senescence or inability to clear SnCs after tissue injury results in SnC accumulation, which can lead to various age-related diseases and tissue fibrosis including those induced by IR and thus is detrimental.6,10,11 Thus, the extent of senescence within an injured tissue may impact the capacity for beneficial (wound healing) versus deleterious (chronic inflammation) effects, with accumulation of SnCs eventually reaching a harmful threshold.
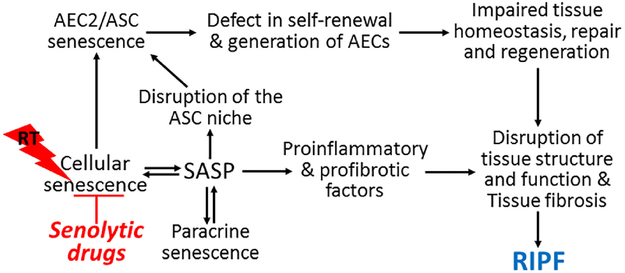
Indeed, accumulating evidence suggests that induction of cellular senescence may play an important role in the pathogenesis of RIPF 6,27 and various other fibrotic lung diseases including IPF and pulmonary fibrosis induced by bleomycin.28 Senescent alveolar epithelial cells (AECs) have been detected in fibrotic foci in the lungs of IPF patients .29,30 Similarly, mice treated with bleomycin31 or thoracic irradiation27 demonstrate increases in senescent type II alveolar epithelial cells (AEC2), the putative alveolar stem cells (ASCs). Inhibition of cellular senescence by targeted inhibition of NADPH oxidases (NOXs) can prevent RIPF and delay the progression of bleomycin-and radiation-induced pulmonary fibrosis in mice.27,31 Similar findings, e.g. inhibition of senescence induction to protect against lung fibrosis, were also observed when mice were treated with a recombinant truncated plasminogen activator inhibitor-1 protein (rPAI-123)32 or rapamycin33 prior to their exposure to thoracic irradiation or with rupatadine shortly after intra-tracheal administration of bleomycin.34 Furthermore, genetic clearance of SnCs through expression of a suicide transgene delayed age-associated decline in lung function and bleomycin-induced pulmonary fibrosis in mice.25,35 These findings suggest that lungs may be different from other tissues and organs, such as liver, in response to induction of cellular senescence. Accordingly, it has been hypothesized that SnCs function as the perpetuators that are primarily responsible for both initiating the pathogenesis and driving the progression of RIPF. SnCs, particularly senescent AEC2, can potentially promote RIPF via multiple mechanisms as shown in Fig.2. First, AEC2 function as ASCs. When AEC2/ASCs become senescent, they cannot self-renew and generate AEC to maintain the homeostasis of alveolar epithelium and to repair the epithelium after tissue injury; however, they continue to occupy the stem cell niche. In addition, senescent AEC2 can set in motion a self-perpetuating vicious cycle of abnormal tissue repair process and secondary senescence by initiating oxidative stress and inflammation. This in turn leads to disruption of normal tissue structure and function in part via ROS and SASP, which eventually leads to pulmonary fibrosis. This hypothesis is supported by the findings that induction of AEC2 senescence by AEC2-specific deletion of telomeric repeat-binding factor 2 (Trf2) in adult mice causes ASC failure, provokes pulmonary inflammation and fibrotic responses, and sensitizes mice to bleomycin-induced pulmonary fibrosis.36 In contrast, inhibition of cellular senescence can prevent RIPF and delay the progression of bleomycin-induced pulmonary fibrosis.27,31 In addition, senescent fibroblasts were identified in the lungs from IPF patients and fibrotic lung tissues from mice after bleomycin treatment.31,37 These senescent fibroblasts may also contribute to the pathogenesis of pulmonary fibrosis under various pathological conditions but their roles in RIPF have yet to be determined. However, IR also induces pneumonitis priori to RIPF. Whether SnCs plays a role in IR-induced pneumonitis has yet to be determined.
Figure 2.
Hypothetic model of mechanisms by which cellular senescence contributes to radiation-induced pulmonary fibrosis (RIPF). AEC2, type II alveolar epithelial cells; ASC, alveolar stem cells; ROS, reactive oxygen species SASP, senescence-associated secretary phenotype; RT, thoracic radiotherapy
Mechanisms of IR-induced cellular senescence
Cell and tissue injury as a result of exposure to IR occurs by direct ionization of cellular macromolecules or by the reaction of macromolecules with free radicals generated by the radiolysis of water. Since water constitutes about 75% of the mass of cells and tissues, the majority of energy of a low linear energy transfer radiation such as X-rays and γ-irradiation is mainly deposited in water to produce free radicals that, in turn, significantly contribute to IR-induced injury. Moreover, free radical-mediated cell injury can be enhanced in the presence of oxygen due to the formation of various reactive oxygen species (ROS). Ionization and/or reaction with free radicals/ROS disrupt the structure and function of DNA, lipids, and proteins, which lead to metabolic and functional alterations and ultimately to senescence or cell death. Among various macromolecules, DNA is considered to be the most critical molecular target for IR-induced senescence and cell death.38
IR induces several different types of damage to DNA, which include base damages and changes, cross linking, single-strand breaks, and double-strand breaks (DSBs). Among them, DSBs are the most detrimental DNA damage to a cell because they can ultimately lead to chromosome breaks and translocations that are associated with many human diseases including cancer if they are left unrepaired or misrepaired. DSBs trigger a series of cellular reactions termed DNA damage response (DDR) to ensure the rapid detection and repair of DSBs or to remove the damaged cells via induction of senescence and apoptosis in order to maintain genome integrity. Specifically, DSBs are recognized by ataxia telangiectasia mutated (ATM). Once activated, ATM phosphorylates various downstream substrates to initiate a signaling cascade that regulates DNA repair, cell cycle checkpoints, and cell survival. For example, phosphorylation of checkpoint kinase 2 at Thr68 and/or that of p53 at Ser15 by ATM activate the G1–S cell cycle checkpoint. Phosphorylation of p53 by ATM can upregulate the expression of p21, proapoptotic proteins such as Puma, and p16 to induce cell cycle arrest, apoptosis and senescence, respectively, in a cell type- and a cell context-dependent manner, because many intrinsic and extrinsic factors can affect cellular responses to IR-induced DNA damage.39 In addition, IR-induced oxidative stress can also activate the mitogen-activated protein kinase (MAPK) p38, which in turn causes cellular senescence by induction of p16 expression and/or interacting with p53.40 Although IR may induce senescence via different upstream signal transduction cascades (i.e. activation of the p53-p21 or/and p38 pathway), signals from these pathways eventually converge on the p16-Rb pathway, whose activation inescapably prevents senescent cells from reentering the cell cycle. This hypothesis is supported by the finding that activation of p53 and induction of p21 are transient events during the onset of senescence that subside when expression of p16 starts rising.41–43 Induction of senescence can be prevented by inactivation of p53 prior to upregulation of p16; however, once p16 is highly expressed, downregulation of p53 cannot reverse cell cycle arrest.43,44 This indicates that activation of the p53-p21 pathway is important role in initiation of senescence and that upregulation of p16 is required for maintenance of senescence.
Senolytics
The seminal finding that AP20187 can selectively eliminate SnCs in BubR1 hypomorphic progeroid mice through an INK-ATTAC transgene, prolonging the healthspan of the mice by delaying the onset of several age-related pathologies, has prompted a gold rush in the discovery of small molecules that can selectively kill SnCs without depending on a transgene.45–49 These small molecules, called senolytic drugs, have the potential to be used not only as novel antiaging drugs but also as new treatment for IR-induced late effects such as RIPF. However, resistance to apoptosis is a hallmark of SnCs,50 because SnCs are protected from induction of apoptosis using different SnC anti-apoptotic pathways (SCAPs).46–49 These SCAPs function as the Achilles’ heel of SnCs. Identification of SCAPs and molecularly targeted inhibition of SCAPs with a small molecule can selectively kill SnCs, which forms the basis for the development of senolytics. Several SCAPs have been identified, including the anti-apoptotic BCL-2 family proteins, HIF-1α, HSP90, and the MDM2/FOXO4-p53-p21 and receptor tyrosine kinase-PI3K-AKT pathways.46–49 Accordingly, several senolytic agents have been identified to target these SCAPs and other unknown targets, including natural compounds such as quercetin,51 fisetin,52 and piperlongumine,53,54 and targeted therapeutics such as dasatinib, a non-specific tyrosine kinase inhibitor;51 inhibitors of the anti-apoptotic BCL-2 family proteins,55,56 HSP90 and histone deacetylase;57 UBX101, an inhibitor of the MDM2/p53 protein interaction;58 and a modified FOXO4-p53 interfering peptide (IP).59 Some of these senolytics can effectively clear SnCs in mice. It has yet to be determined whether they can also effectively clear SnCs in humans. One of the most recent study showed that treatment with dasatinib plus quercetin was well tolerated by IPF patients.60 However, their effectiveness in clearing SnCs in patients has yet to be demonstrated in future studies.
While natural senolytic compounds have the advantages of low toxicity, they are usually less potent than targeted senolytics and thus have to be combined with other senolytic agents to be effective in clearing SnCs.51 The mechanisms of action of most natural senolytics have not been well defined nor have their molecular targets been identified and characterized, making it very difficult to rationally modify the compounds to improve their senolytic activity. In contrast, almost all of the targeted senolytics discovered are repurposed anticancer agents with the exception of the FOXO4-p53-IP.46–49 These repurposed senolytics usually possess various on-target and/or off-target toxicities, which could preclude their clinical use as anti-aging agents because older people are more susceptible to adverse drug effects than young individuals and less tolerant of cancer drug toxicity. Therefore, strategies to reduce on-target and/or off-target toxicity of known targeted senolytics are urgently needed. For example, we and others have found that ABT263 and ABT737, which are potent BCL-2 and BCL-xL dual inhibitors, can potently kill a variety of SnCs in cell culture with a few exceptions (such as senescent chondrocytes and synovial fibroblasts in the OA joint), whereas they have minimal effect on their non-senescent counterparts.51,55,56 These findings suggest that ABT263 and ABT737 are potent and broad-spectrum senolytic agents. This suggestion is supported by the finding that treatment of mice with ABT263 or ABT737 can effectively clear SnCs in various murine tissues. More importantly, clearance of SnCs with ABT263 can rejuvenate aged hematopoietic stem cells (HSCs) and the senescent hematopoietic system in aged mice55and ameliorate several pathological conditions associated with aging such as atherosclerosis, dementia and pulmonary fibrosis.7,61,62 Interestingly, BCL-xL specific inhibitors such as A-1331852 and A-1155463 can also selectively kill a variety of SnCs,52 whereas the BCL-2 specific inhibitor ABT199 and MCL-1 specific inhibitor A-1210477 have no such effect. These findings suggest that among these anti-apoptotic BCL-2 family proteins, BCL-xL is one of the most important survival factors for these SnCs.
The mechanism by which BCL-xL inhibition selectively induces apoptosis in SnCs may be attributable to the persistent stress endured by SnCs, which can upregulate the expression of some of proapoptotic proteins such as BCL-2 antagonist/killer (BAK).55 To counteract the effect of these proapoptotic proteins for survival, SnCs also express increased levels of antiapoptotic proteins such as BCL-xL.55 Therefore, inhibition of BCL-xL with an inhibitor can release BCL-2-interacting mediator of cell death (BIM) and other BH3 proteins, which in turn activates BAK and/or BCL-2-associated X protein (BAX). The activation of BAX and/or BAK at the mitochondrial membrane induces their oligomerization and formation of the macropores that causes mitochondrial outer membrane permeabilization (MOMP). MOMP results in the release of cytochrome C from mitochondria to the cytoplasm, which binds to the apoptotic proteaseactivating factor 1 (APAF1) to form the apoptosome. The apoptosome then induces a cascade activation of the initiator caspase (caspase 9) and executioner caspases (caspases 3, 6 and 7) to dismantle the cells.63
However, the on-target toxicity of thrombocytopenia induced by BCL-xL inhibition prevents the use of ABT-263 and other BCL-xL specific inhibitors in clinic even for cancer patients, because platelets also depend on BCL-xL for survival.64–67 We hypothesize that we can overcome this on-target toxicity to generate a safer and more effective senolytic agent by converting ABT-263 and BCL-xL specific inhibitors into platelet-sparing BCL-xL Proteolysis Targeting Chimera (BCL-xL PROTACs). PROTACs are bivalent small-molecules containing a ligand that recognizes the target protein linked to another ligand that binds to a specific E3 ubiquitin ligase.68 Such molecules can recruit the target protein to the E3 ligase, promote proximity-induced ubiquitination of the target protein, and lead to its degradation by proteasomes. Therefore, we have been working on developing a novel ABT-263-based BCL-xL PROTAC designed to target BCL-xL to an E3 ubiquitin ligase such as cereblon (CRNB) and von Hippel-Lindau (VHL) that are minimally expressed in platelets for ubiquitination and proteasomal degradation to reduce ABT-263 platelet toxicity.69 In addition, PROTACs act catalytically to induce protein degradation in a super-stoichiometric manner and their effect is not limited by equilibrium occupancy. Therefore, BCL-xL PROTACs should have a better and longer-acting senolytic activity than ABT-263. This will significantly improve the safety and effectiveness of BCL-xL targeted senolytic drug for RIPF treatment. We have already generated some proof-of-concept preliminary data demonstrating that an ABT-263-based BCL-xL PROTAC targeting BCL-xL to the CRL4CRBN E3 ligase is significantly less toxic to human platelets than ABT-263 while retaining their selectivity and potency against SnCs.69 We expect that the safety and effectiveness of BCL-xL PROTACs can be further improved after we optimize the linker between ABT-263 and the E3 ligase ligand in the future.
Clearance of SnCs with senolytics to treat RIPF
Most of the studies reported previously have focused on inhibiting pathways associated with induction of cellular senescence in an effort to prevent and mitigate IR- or chemotherapy-induced pulmonary fibrosis, but not on halting or reversing pulmonary fibrosis by clearing existing SnCs. 27,32,33 We hypothesize that clearance of SnCs with a senolytic drug that can selectively kill SnCs has the potential not only to prevent but also to stop and reverse RIPF. This hypothesis is supported by our recent studies, which showed that treatment of the thoracically irradiated mice with ABT-263 almost completely reversed RIPF, even when the initiation of ABT-263 treatment was delayed until RIPF was established.7 This finding suggests that unlike other known radiation protectants and mitigators which have to be used either before or shortly after IR to be effective in preventing and delaying the progression of RIPF, senolytic drugs such as ABT-263 has the potential to be used as an effective treatment for RIPF even after RIPF becomes a progressive disease. Besides ABT-263, it will be of a great interest to determine whether other senolytic agents such as the combination of dasatinib and quercetin can also be used to effectively clear senescent AEC2 cells in the lungs induced by radiation to stop or reverse RIPF. This is because senolytic treatment with dasatinib and quercetin could effectively induce apoptosis in senescent AEC2 cells and fibroblasts, reduce the expression of senescence and SASP markers, and increased the expression of alveolar epithelial markers in isolated AEC2s from bleomycin-treated mice ex vivo and improved the lung function in mice after bleomycin treatment.25,26 The reversal of RIPF by clearance of SnCs with a senolytic drug is likely not only dependent on the suppression of IR-induced pulmonary oxidative stress and inflammation but also rely on the restoration of the function of ASCs. Our recent studies showed that genetic or pharmacological clearance of SnCs rejuvenated various tissue stem cells in normally aged mice and prematurely aged mice induced by IR.55 Therefore, it will be of great interest to determine whether clearance of SnCs can not only inhibit IR-induced chronic pulmonary oxidative stress and inflammation, but also restore the function of ASCs by rejuvenating AEC2 and/or by improving the ASC niche.
Conclusion
Here we provide a brief review to discuss some of the recent findings suggesting that SnCs may play a causative role in RIPF. However, all these findings were observed in mice after they were exposed to a high dose thoracic irradiation and thus have yet to be confirmed in patients who undergo RT for cancer. The development of senolytics could potentially provide the opportunity to validate the role of SnCs in RIPF in humans if an effective senolytic agent can be developed and used safely to treat patients. This remains a daunting task considering that most of the known senolytics are either natural compounds that probably lack sufficient potency to effectively clear SnCs as a single therapeutic agent or repurposed targeted anticancer agents that have many side effects. Therefore, discovery and development of more potent and safer senolytics will be important to translate the findings from mice to humans. We hope that this review will stimulate more studies on the role of SnCs in RIPF and promote further development of senolytic therapeutics for RIPF as well as other human diseases.
ACHKNOWLEDGEMENTS
This work was supported in part by grants from NIH (R01CA211963 and R01CA219836) and the Intramural Program of the NIH (CCR, NCI). All authors have read the journal's policy on conflicts of interest and the journal's authorship agreement.
Abbreviations:
- AEC2
type II alveolar epithelial cells
- AECs
alveolar epithelial cells
- APAF1
apoptotic protease-activating factor 1
- ASCs
alveolar stem cells
- CRNB
cereblon
- ATM
ataxia telangiectasia mutated
- BAK
BCL-2 antagonist/killer
- BAX
BCL-2-associated X protein
- DDR
DNA damage response
- DSB
double-strand break
- HSCs
hematopoietic stem cells
- IPF
idiopathic pulmonary fibrosis
- IP
interfering peptide
- IR
ionizing radiation
- MAPK
mitogen-activated protein kinase
- MOMP
mitochondrial outer membrane permeabilization
- NOXs
NADPH oxidases
- RIPF
radiation-induced pulmonary fibrosis
- ROS
reactive oxygen species
- rPAI-1
recombinant truncated plasminogen activator inhibitor-1 protein
- RT
thoracic radiotherapy
- SASP
senescence-associated secretory phenotype
- SCAPs
SnC anti-apoptotic pathways
- SnCs
senescent cells
- TBI
total body irradiation
- Trf2
telomeric repeat-binding factor 2
- VHL
von Hippel-Lindau
Footnotes
CONFLICTS OF INTEREST
G.Z. and D.Z. are inventors of a pending patent application for the development of BCL-xL targeted senolytic agents. D.Z. is a co–founder and an advisor of Unity that develops senolytic agents to treat various age-related diseases in humans.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13(4):242–248. [DOI] [PubMed] [Google Scholar]
- 2.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. [DOI] [PubMed] [Google Scholar]
- 3.Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20(3):201–207. [DOI] [PubMed] [Google Scholar]
- 4.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. [DOI] [PubMed] [Google Scholar]
- 6.Citrin DE, Prasanna PGS, Walker AJ, et al. Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate. Report of an NCI Workshop, September 19, 2016. Radiat Res. 2017;188(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan J, Li D, Xu Y, et al. Inhibition of Bcl-2/xl With ABT-263 Selectively Kills Senescent Type II Pneumocytes and Reverses Persistent Pulmonary Fibrosis Induced by Ionizing Radiation in Mice. Int J Radiat Oncol Biol Phys. 2017;99(2):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Wu Z, Ning W. Advances in Molecular Mechanisms and Treatment of Radiation-Induced Pulmonary Fibrosis. Transl Oncol. 2019;12(1):162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017;169(6):1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. [DOI] [PubMed] [Google Scholar]
- 12.Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E, Lowe SW. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol. 2008;73:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. Trends Cell Biol. 2012;22(4):211–219. [DOI] [PubMed] [Google Scholar]
- 14.Campisi J, Aging cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. [DOI] [PubMed] [Google Scholar]
- 16.Douple EB, Mabuchi K, Cullings HM, et al. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep. 2011;5 Suppl 1:S122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys. 2013;52(4):435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. [DOI] [PubMed] [Google Scholar]
- 19.Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS). Int J Radiat Biol. 2011;87(8):851–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yablokov AV, Nesterenko VB, Nesterenko AV. 15. Consequences of the Chernobyl catastrophe for public health and the environment 23 years later. Ann N Y Acad Sci. 2009;1181:318–326. [DOI] [PubMed] [Google Scholar]
- 21.Yablokov AV. 4. Accelerated aging as a consequence of the Chernobyl catastrophe. Ann N Y Acad Sci. 2009;1181:55–57. [DOI] [PubMed] [Google Scholar]
- 22.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun J-I, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12(7):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann M, Korfei M, Mutze K, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. European Respiratory Journal. 2017;50(2) :1602367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Citrin DE, Shankavaram U, Horton JA, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105(19):1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailleux AA, Crestani B. Licence to kill senescent cells in idiopathic pulmonary fibrosis? Eur Respir J. 2017;50(2). [DOI] [PubMed] [Google Scholar]
- 29.Disayabutr S, Kim EK, Cha S-I, et al. miR-34 miRNAs Regulate Cellular Senescence in Type II Alveolar Epithelial Cells of Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE. 2016;11(6):e0158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minagawa S, Araya J, Numata T, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011. ;300(3):L391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecker L, Logsdon NJ, Kurundkar D, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6(231):231ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung EJ, McKay-Corkum G, Chung S, et al. Truncated Plasminogen Activator Inhibitor-1 Protein Protects From Pulmonary Fibrosis Mediated by Irradiation in a Murine Model. Int J Radiat Oncol Biol Phys. 2016;94(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung EJ, Sowers A, Thetford A, et al. Mammalian Target of Rapamycin Inhibition With Rapamycin Mitigates Radiation-Induced Pulmonary Fibrosis in a Murine Model. Int J Radiat Oncol Biol Phys. 2016;96(4): 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv X, Wang X, Li K, et al. Rupatadine protects against pulmonary fibrosis by attenuating PAF-mediated senescence in rodents. PLoS ONE. 2013;8(7):e68631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto M, Asai A, Kawagishi H, et al. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight. 2016;1(12):e87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alder JK, Barkauskas CE, Limjunyawong N, et al. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA. 2015;112(16):5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waters DW, Blokland KEC, Pathinayake PS, et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315(2):L162–L172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000;301(1):133–142. [DOI] [PubMed] [Google Scholar]
- 39.Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367–401. [DOI] [PubMed] [Google Scholar]
- 40.Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8(2): 131–144. [DOI] [PubMed] [Google Scholar]
- 41.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998; 16(9): 1113–1123. [DOI] [PubMed] [Google Scholar]
- 42.te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62(6): 1876–1883. [PubMed] [Google Scholar]
- 43.Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. [DOI] [PubMed] [Google Scholar]
- 44.Beauséjour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBOJ. 2003;22(16):4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niedernhofer LJ, Robbins PD. Senotherapeutics for healthy ageing. Nat Rev Drug Discov. 2018;17(5):377. [DOI] [PubMed] [Google Scholar]
- 49.Ovadya Y, Krizhanovsky V. Strategies targeting cellular senescence. J Clin Invest. 2018;128(4):1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014; 15(11):1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY). 2017;9(3):955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Chang J, Liu X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY). 2016;8(ll):2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Zhang S, Liu X, et al. Oxidation resistance 1 is a novel senolytic target. Aging Cell. May 2018:el2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yosef R, Pilpel N, Tokarsky-Amiel R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeon OH, Kim C, Laberge R-M, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baar MP, Brandt RMC, Putavet DA, et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169(1):132–147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. [DOI] [PubMed] [Google Scholar]
- 64.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. [DOI] [PubMed] [Google Scholar]
- 65.Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29(7):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci TranslMed. 2015;7(279):279ra40. [DOI] [PubMed] [Google Scholar]
- 67.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. [DOI] [PubMed] [Google Scholar]
- 68.Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16(2):101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng G, Zhou D, Zhang X, Wang Y, Chang J. Compounds That Induce Degradation of Anti-Apoptotic Bcl-2 Family Proteins and the Uses Thereof. October 2017. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017184995. Accessed December 29, 2018. [Google Scholar]