Abstract
When a woman becomes pregnant, there is a vast series of physiological, vascular, and psychological changes. Among the most commonly reported changes are those involving sleep. Pregnant women report that their ability to maintain sleep and acquire continuous refreshing sleep is impaired during the perinatal period as compared to the non-pregnant period. A growing literature supports the hypothesis that disturbed sleep (which comes in many forms) during the perinatal period is associated with an increased risk of adverse maternal, delivery, and infant outcomes. Among the suggested biological pathways linking sleep and adverse outcomes are disturbances in the immune and hormonal systems. The following paper will discuss (1) the various sleep processes that are commonly disturbed during the perinatal period and the methods used to collect sleep data; (2) the evidence linking sleep to adverse outcomes; and (3) how one specific biological pathway, the immune system, likely mediates these associations. The goal of this paper is to clarify the role that sleep disturbance has during pregnancy.
Keywords: Sleep, Pregnancy, inflammation, Outcomes, Stress, Cytokines, Postpartum
Introduction
The state of pregnancy is accompanied by numerous physical and emotional changes. Among these are immunological and alterations in sleep patterns. These changes, either solo or in concert with one another, can impact the outcome of the pregnancy either positively or negatively.
A normal pregnancy is one in which the health of the mother and baby are secure; there are no complications associated with gestation, labor, or delivery. An aberrant pregnancy is one in which the health of the mother or child is compromised. This can take many forms but the most noteworthy and commonly experienced are preeclampsia, preterm labor/delivery, small-for-gestational-age babies, or maternal gestational diabetes. During pregnancy, it is critical that the maternal immune system does not reject the child. The literature suggests that for a normal pregnancy to occur, there must be a maternal shift to a Th2 or an anti-inflammatory cytokine (immune) profile which appears protective for the allograft fetus. Anomalous pregnancies may be characterized by either a failure of this shift to occur or by an over-secretion of Th1 or pro-inflammatory cytokines. Subsequently, it can have consequences for the mother and/or child if not attended to in the earliest of stages.
Numerous factors that influence a pregnancy and its subsequent outcome have been identified, including illness (Hartert et al., 2003; Lapinsky, 2010; Ellington et al., 2011; O’Brien et al., 2014; Guntupalli, Hall, Karnad, Bandi, & Belfort, 2015; Atta et al., 2016; Mouyis, Thornton, Williams, & Giles, 2017), stress(Sandman, Wadhwa, Chicz-Demet, Dunkel-Schetter, & Porto, 1997; Rini, Dunkel-Schetter, Wadhwa, & Sandman, 1999; Carmichael & Shaw, 2000; Livingston, Otado, & Warren, 2003; Coussons-Read, Okun, & Nettles, 2007; Davis, Glynn, Waffarn, & Sandman, 2011; Christian, 2014), emotional states such as depression and anxiety (Orr, James, & Blackmore, 2002; Diego et al., 2004; Abedian, Soltani, Mokhber, & Esmaily, 2015; Lewis, Austin, Knapp, Vaiano, & Galbally, 2015), and disturbed sleep (Lee, 2006; Okun, Hall, & Coussons-Read, 2007; Okun, Hanusa, Hall, & Wisner, 2009; Okun, Schetter, & Glynn, 2011; Tomfohr, Buliga, Letourneau, Campbell, & Giesbrecht, 2015). While there is an extensive literature about the immunological consequences of illness, stress, and psychopathology during gestation,(Christian, Franco, Iams, Sheridan, & Glaser, 2010; Christian, 2014; Corwin & Pajer, 2008; Coussons-Read et al., 2007; Madan et al., 2009; Maes et al., 2000; Spradley, Palei, & Granger, 2015), little is known regarding the effects of chronic or persistent sleep loss/disruption during pregnancy and its impact on immune dysregulation that may impact maternal and fetal health and pregnancy outcomes.
The goals of the current review are to provide the reader with a general understanding of the importance of sleep, how pregnancy affects sleep, the role that sleep plays in increased risk for adverse pregnancy outcomes, and the biological pathways that link sleep with outcomes. At the conclusion of the paper, the reader should have a basic understanding of the importance of sleep, the various methods used to ascertain sleep data, a broad overview of the how sleep is disrupted during various points in pregnancy, common sleep disorders, and how immune parameters are impacted by disrupted sleep.
1. Importance of Sleep
Overview of Sleep
Sleep is gaining recognition as an important factor to consider when studying or treating pregnant women. For example, many epidemiological reports have linked poor sleep or insomnia to poor health outcomes, including depression(Okun et al., 2011; Okun, Kiewra, Luther, Wisniewski, & Wisner, 2011; Volkovich, Tikotzky, & Manber, 2016; Wolynczyk-Gmaj et al., 2017; Yang et al., 2017; Hux, Roberts, & Okun, 2017), preterm birth (Okun et al., 2011; Okun et al., 2011; Blair, Porter, Leblebicioglu, & Christian, 2015; Li et al., 2016), and longer labor times (Evans, Dick, & Clark, 1995; Lee & Gay, 2004). The undisputed association between stress and sleep is particulary poignant in pregnancy, as stress is independently associated with poor pregnancy outcomes (Okun et al., 2013; Palagini et al., 2014; Hux et al., 2017).
Restorative Function of Sleep
Although sleep is essential for survival, it is only in the past three decades that the role of sleep in restoring and repairing the human body, including protein synthesis and mitotic division has been identified (Adam & Oswald, 1977; Adam & Oswald, 1984; Adam, Tomeny, & Oswald, 1986; Akerstedt & Nilsson, 2003; Born, Muth, & Fehm, 1988; Born, Lange, Hansen, Molle, & Fehm, 1997; Born & Fehm, 2000). The need for sleep is a basic physiological need and has similar homeostatic properties to hunger or thirst in that sleep reverses sleepiness (Saper, Cano, & Scammell, 2005). There is an intrinsic 24-hour sleep-wake cycle that parallels the circadian rhythm of the endocrine and immune systems. This circadian cycle governs many of an individual’s behaviors (Saper et al., 2005). Hence, the impact of sleep loss reaches beyond the immediate realm of just making an individual sleepy. It impacts the entire biological entity either directly or indirectly, due to the multiple brain regions, endocrine factors, and various neurotransmitters involved in sleep regulation (Benca & Quintas, 1997). We know that certain hormones, neuropeptides, and cytokines are secreted in particular slepe stages. For instance, bodily restitution is thought to occur during slow wave sleep (SWS) as Growth Hormone (GH) is secreted primarily during these Stages and cortisol secretion is suppressed during sleep (Akerstedt & Nilsson, 2003). GH stimulates growth, cell regeneration and cell reproduction. Hence, SWS is an important and necessary sleep stage. Additionally, during SWS memory is consolidated, the body tends to conserve energy, there is a cooling of the body and brain, and certain aspects of immune function are promoted (Bryant, Trinder, & Curtis, 2004).
To Provide a Stress Free Period
Born and Fehm (2000) discuss and describe how sleep, especially sleep occurring earlier in the night, can be regarded from a psychoneuroendocrinological perspective as a stress-free period. The release of adrenocorticotropic hormone (ACTH) and cortisol by pituitary-adrenal activity follows a circadian rhythm and is synchronized to the sleep-wake cycle. In typical situations, ACTH/cortisol concentrations are at a minimum in the early hours of sleep coinciding with SWS and are at their maximum during the second half of the night coinciding with REM sleep. The authors point out that this pattern of endocrine activity is unique to the state of sleep and can be modified by various forms of stress (Born & Fehm, 2000).
They acknowledge that various physical and psychological stressors can negatively influence the sensitivity of neuroendocrine regulation during sleep, thus shifting how much and when the nadirs of ACTH/cortisol occur. It has long been known that cortisol secretion is inhibited during sleep (Weitzman, Zimmerman, Czeisler, & Ronda, 1983). Furthermore, sleep restriction and poor sleep can alter secretion of cortisol (Born et al., 1986; Garde AH, Karlson B, Hansen AM, Persson R, & Akerstedt, 2012; Hori et al., 2011). Could pregnancy and the sleep disruption experienced during pregnancy be viewed as a chronic (albeit time constrained) stressor that can alter neuroendocrine activity? Or is altered endocrine activity during pregnancy a causal influence on the sleep disruption reported? There is vast support for the notion that sleep disruption is a stressor (McEwen, 2006; McEwen & Karatsoreos, 2015; Riemann et al., 2010). Events that simulate an immune challenge by stimulating the release of pro-inflammatory cytokines (including IL-1 and TNF-α) are considered stressors. Since total sleep deprivation showed increased pro-inflammatory cytokine levels, it can be argued that sleep deprivation is a stressor. However, the distinction between total sleep deprivation and partial sleep disruption and what that leads to immunological or endocrinological dysregulation during pregnancy has yet to be determined.
Restorative Function of Sleep during Pregnancy
Data suggest that sleep during pregnancy supports restoration and facilitation of the developing fetus similar to what was previously discussed (Adam & Oswald, 1977; Adam, 1980). Recognition of the myriad alterations in metabolism and arousal that occur as a result of pregnancy is important to better understand how sleep may biologically influence outcomes. Pregnancy is a high metabolic effort situation and much of the maternal energy is directed toward the fetal-placental component in a time-dependent manner (Richardson, 1996). There is a shift in the favoring of anabolic activity. This physiological shift is timed to facilitate the appropriate growth and maturation of the fetus, as well as to be protective (Richardson, 1996). This occurrence may explain why expectant mothers describe their activity levels as more passive. Their bodies may be in a mode of energy conservation during high metabolic expenditure. An interesting phenonmonon that supports the energy conservation hypothesis is that the sleep architecture (percent time spent in SWS or REM) of the non-pregnant state is maintained during pregnancy. While some some studies show some alterations in SWS and in Stage 1 at various points of gestation (Driver & Shapiro, 1992; Hertz et al., 1992; Schorr et al., 1998; Wilson et al., 2011), the patterns are quite similar to non-pregnant women. This is observed even in the incidence of an increase of arousals and number of minutes spent awake at night. This suggests that there may be an evolutionary and/or adaptive process to the increased time spent awake at night observed during pregnancy. The mother learns, prior to having the child, to be more adept at acquiring consolidated sleep in shorter time periods. This may be conducive to allow the mother to attend to the newborn’s requirements during the postpartum. This hypothesis remains speculative as there are no data to support or deny the possibility that there is an evolutionary benefit associated with disrupted sleep.
2. Pregnancy and Alterations in Sleep
From conception through delivery, sleep is distinctly altered in the pregnant woman. Sleep disturbances are typically classified as: disturbed sleep quality, poor sleep continuity, insomnia, short/long sleep duration, restless legs syndrome, and sleep disordered breathing. A methodological concern with many studies is the definition used to define poor sleep. For example, sleep quality can be defined by a single question or a series of validated questions. Likewise, sleep apnea is defined as having > 5 breathing events per hour determined by in-lab or in-home PSG. The broader term, sleep disordered breathing refers to a group of disorders characterized by abnormal respiratory patterns or insufficient ventilation during sleep. These can include sleep apnea, snoring, or hypoxia. The terms are often used interchangeably. Equally, insomnia can be determined with a single question or clinically which rely on the report of significant sleep complaints accompanied by significant daytime impairment.
Sparse data are available that inform women what to expect with regards to the timing, degree, frequency, and/or severity of a specific pregnancy-related sleep disturbance. Although up to 75% of pregnant women experience some form of sleep disruption during pregnancy, the number of women who report significant sleep complaints, of any kind, in the first trimester is about 25% (Okun & Coussons-Read, 2007; Okun, 2013). It is clear that sleep quality and quantity fluctuations vary by trimester. The changes in sleep have been previously described (Lee, Zaffke, & McEnany, 2000; Lee, 2006; Okun, 2013). So the focus here will be on the various methods used to ascertain or record sleep, their pros and cons, and any significant findings.
Reports that utilize validated measures indicate that sleep disorders are quite common during pregnancy. Insomnia, affects ~12% in early pregnancy with rates as high as 75% in late pregnancy (Okun et al., 2013; Okun, Buysse, & Hall, 2015), whereas SDB affects about 4% in early pregnancy and 8.3% in mid pregnancy (Louis et al., 2018). Moreover, according to the National Sleep Foundation, up to 26% report restless legs syndrome and 30–50% have gastroesophageal reflux disease (GERD). Taken together, it is clear that sleep problems and complaints are significantly greater in the pregnant state compared to the non-pregnant state.
Measurement of Sleep
Polysomnography (PSG)
Polysomnography (PSG) is the current gold standard for measuring sleep. It is a technique that collects multiple physiologic parameters of sleep via surface electrodes, including brain dynamics of electroencephalography (EEG), eye movements, muscle activity, heart physiology, and respiratory function (Marino et al., 2013). Sleep is composed of two distinct states, REM sleep and NREM sleep. REM sleep is a unique phase of sleep in that it has physiological similarities to wake, characterized by rapid, high-frequency, low amplitutde EEG waves. The distinguishing feature of REM sleep is the random/rapid movement of the eyes, low/diminished muscle tone, and the propensity to experience vivid, hallucinogenic-like dreams. Its function is postulated to involve immune function stability, memory preservation (e.g., procedural, spatial and emotional memory), and brain development during infancy (Hall, Okun, Atwood, Buysse, & Strollo, 2008; Patel & Araujo, 2018). Within NREM sleep three distinct stages can be identified by characteristic EEG waveforms. Lighter stages of sleep N1 and N2 are characterized by low-amplitude, fast frequency EEG activity, whereas the deepest stage of sleep, N3, is characterized by high amplitude, slow frequency EEG(Hall et al., 2008) Figure 1. N1 is referred to as transition sleep and lasts only 5–10 minutes. This is where physiological changes occur, including heart and breathing rate slowing, muscles relax, and body temperature decreases. N2 is typified by additional physiological markers such as sleep spindles and K-complexes which are thought to play a role in transitioning to a deeper sleep (Patel & Araujo, 2018). N3 is where deep sleep occurs. It is thought that this sleep is necessary for an individual to feel refreshed and restored the next day. REM and NREM alternate within one sleep cycle (~80–110 minutes).
Figure 1.
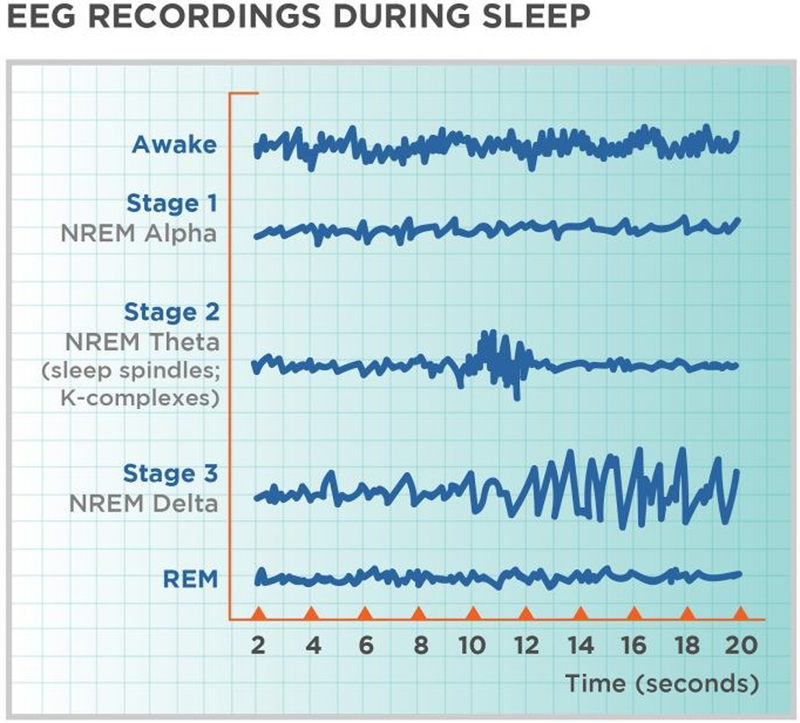
Visual depiction of the sleep stages assessed by PSG.
Actigraphy
Actigraphy is a method commonly used in perinatal sleep assessment. Actigraphy is a non-invasive method of monitoring human rest/activity cylces. The actigraph is a wrist-watch like device that measures gross motor activity constantly. The unit has a piezoelectric accelerometer that uses a low-pass filter to remove everything except the 2–3 Hz band (Ancoli-Israel et al., 2015; Marino et al., 2013; Martin & Hakim, 2011). By measuring wrist movements with an accelerometer in a wrist worn device, the presence or absence of sleep or wake can be determined (Marino et al., 2013). This approach supports evaluation of larger sample sizes and longitudinal assessments as it is inexpensive, unobtrusive, and enables measurement across a wide range of circumstances and locations. The majority of studies that use actigraphy also use sleep diaries (see below) as they help corroborate the data.
Subjective Reports
Subjective data can be acquired via many methods. The most common is self-report questionnaires, such as the Pittsburgh Sleep Quality Index(Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), the Insomnia Symptom Questionnaire (Okun et al., 2009), or the Epworth Sleepiness Scale (Johns, 1991). These are retrospective accounts of one’s habitual sleep. They can provide information on sleep quality, insomnia, or daytime sleepiness. Visual analogue scales are also a simple tool to determine the previous night’s sleep quality, quantity or other dimension. The reliance on questionnaire-derived sleep duration (or other variable) may lead to spurious associations among studies that evaluate sleep and health outcomes in pregnancy(Herring et al., 2013). Although not considered methodologically ideal, purely restrospective and subjective studies often provide a beginning point to further inquiry. It is often a cost-effective means to ascertain pilot data for future study. A frequently used method that provides prospective, daily sleep data, is the sleep diary. It is used in conjunction with actigraphy to corroborate validity. The sleep diary allows for prospective daily accounting of specific aspects of sleep including sleep onset latency, number of awakenings, wake after sleep onset and sleep duration. This method is extememely useful for ascertaining long term sleep and variablity in sleep patterns.
What is the best choice to study sleep in pregnant women?
The appropriate method relies heavily on the hypotheses, resources, sample size and parameters of interest of the investigators. If a health psychologist wants to address sleep as a covariate, then the easiest method would be questionanires. If temporal questions regarding a health condition are put forth, for instance, then actigraphy and sleep diaires should suffice. The focus in the last decade has been on collecting sleep data from as many women as possible or for long periods of time. This has resulted in actigraphy, questionnaires, and sleep diaries to be the predominant methods used. These methods are ideal for gathering sleep duration, sleep quality, sleep continuity, and insomnia data. For investigations of SDB, in-home sleep testing devices have become so small and portable, there is little need to bring pregnant women to the laboratory for an over-night sleep study.
Findings on sleep disturbance during pregnancy
Polysomnography
Older data collected via PSG in perinatal women suggested an increase in REM sleep (paradoxical sleep) during late pregnancy(Branchey & Petre-Quadens, 1968). Others show an increase in awake times, differences in total sleep time (compared to non-pregnant and across pregnancy), an increase in slow wave sleep (SWS), and a decrease in REM sleep across pregnancy(Brunner et al., 1994; Driver & Shapiro, 1992; Hertz et al., 1992; Schorr et al., 1998). Recent data from Wilson and colleagues who conducted PSG in first and third trimester women and compared them to non-pregnant controls, found that their results confer with the older studies. Sleep duration is longer in the first trimester compared to the third, and pregnant women have shorter sleep duration than non-pregnant women(Wilson, Fung, Walker, & Barnes, 2013; Wilson et al., 2011). Taken together the sparse data and restricted sample sizes suggest that sleep architecture is not significantly modified during pregnancy. The modest change is sleep duration and awakenings, for instance, are likely a reflection of the changing habitus and hormone fluctuations, rather than an adaptive and important mechanism. The primary utility of using PSG to study pregnant women presently appears to be to identify pathological conditions such as sleep apnea or periodic leg movements. This segment of the literature is riddled by a small number of studies and vast methodological differences among the studies described. It has been argued that 1–2 nights of PSG-collected sleep data will not facilitate a complete understanding of sleep patterns and how they impact health outcomes in perinatal women. Other methodologies appear to be better suited to address this question.
Actigraphy
As mentioned, actigraphy is a method that determines sleep based on the absence or presence of movement, It is less intrusive and able to collect longitudinal sleep data. A consistent finding, similar to PSG, is that sleep is quite disturbed in the 1st trimester, modestly improves in the 2nd trimester, and progressively worsens by the end of gestation (Lee & Gay, 2004; Bei, Milgrom, Ericksen, & Trinder, 2010; Elek, Hudson, & Fleck, 1997; Okun et al., 2013; Volkovich et al., 2016; Tsai, Lee, Lin, & Lee, 2016; Reid et al., 2017; Shinkoda, Matsumoto, & Park, 1999). The nocturnal sleep period is peppered by an increase in awakenings, often to urinate or result of physical discomfort. It is further characterized by restless sleep and a decline in total sleep time that increases daytime fatigue and sleepiness (Bourjeily, Raker, Chalhoub, & Miller, 2013; Herring et al., 2013). Unfortunately, since actigraphy defines sleep by lack of movement, many movements are identified as wake. Likewise, non-movement while awake is identified as sleep. Hence, there is often a large discrepancy between questionnaire-derived data and actigraphy-meausred sleep time(Herring et al., 2013). Thus, a major caveat of actigraphy is that it often overestimates awakenings and underestimates sleep duration (Herring et al., 2013). Nonetheless, it is an excellent method to use in order to collect longitudinal sleep in pregnant women.
Subjective Reports
There are dozens of studies utilizing various subjective reports. Two early studies assessed the primary reasons for sleep disturbance during pregnancy. The need to urinate was the main complaint found in a secondary analysis by Baratte-Beebe & Lee (Baratte-Beebe & Lee, 1999) of women in childbearing years before and during pregnancy. This increase is primarily a result of bladder compression by the uterus, during the 1st trimester, and the descending fetus pressing on the bladder during the 3rd trimester (Baratte-Beebe & Lee, 1999). The second most common complaint was nighttime awakenings. Mindell & Jacobson (Mindell & Jacobson, 2000) questioned 127 women at one of four points during pregnancy about their sleep habits and sleep disturbances. Consistent with other studies, nighttime awakening was the most common sleep disturbance. In support of an insomnia-like sleep disturbance, a significant number of women in this sample reported difficulty staying and/or falling asleep as well as waking too early. Also, over 40% reported “thoughts” as the cause for these problems, another indication of an insomnia-like disorder. It is known that pregnancy-related anxiety is indeed associated with adverse pregnancy outcomes (Abedian et al., 2015; Lewis et al., 2015; Misri & Swift, 2015; Dolatian et al., 2016; Tham et al., 2016). While it is unclear as to whether there is an adaptive advantage to the increase in nocturnal awakenings and disrupted sleep (i.e., preparation for caring for infant), the evidence supports the hypothesis that abnormal sleep patterns, (e.g., insomnia, short sleep duration or poor sleep continuity) are associated with an increase risk in medical conditions, such as preterm birth or growth restricted babies(Micheli et al., 2011).
3. Sleep and Adverse Pregnancy Outcomes
A rapidly growing literature supports an independent and substantive role for antenatal sleep disturbance to negatively affect maternal and infant outcomes. Sleep disorders such as sleep disordered breathing and insomnia, as well as poor sleep quality, circadian misalignment, and short sleep duration are implicated in increased risk for adverse outcomes. Poor sleep quality, short sleep duration, and insomnia, for instance, are associated with new and recurrent depressive episodes (Bei et al., 2010; Dorheim, Bjorvatn, & Eberhard-Gran, 2012; Goyal, Gay, & Lee, 2007; Kamysheva, Skouteris, Wertheim, Paxton, & Milgrom, 2009; Okun et al., 2009; Okun et al., 2011; Okun et al., 2013; Skouteris, Wertheim, Germano, Paxton, & Milgrom, 2009), preterm birth(Guendelman et al., 2013; Micheli et al., 2011; Okun et al., 2011; Strange, Parker, Moore, Strickland, & Bliwise, 2009), gestational diabetes(Balserak BI, Jackson N, Ratcliffe SA, Pack AI, & Pien GW, 2013; Bourjeily, Raker, Chalhoub, & Miller, 2010; Facco, Grobman, Kramer, Ho, & Zee, 2010; O’Brien et al., 2012; O’Keeffe & St-Onge, 2012; Qiu, Enquobahrie, Frederick, Abetew, & Williams, 2010; Reutrakul et al., 2011), gestational hypertension/preeclampsia(Ayrim et al., 2011; Bourjeily et al., 2010; Champagne et al., 2009; O’Brien et al., 2012; Williams et al., 2010), labor and delivery outcomes (Beebe & Lee, 2007; Evans et al., 1995; Lee & Gay, 2004), immune/endocrine dysregulation(Okun & Coussons-Read, 2007; Okun et al., 2007; Okun et al., 2011; Okun, Luther, Wisniewski, & Wisner, 2013), and a few examining infant outcomes(Ayrim et al., 2011; Bourjeily et al., 2010; Franklin et al., 2000; Goyal, Gay, & Lee, 2009; Nishihara & Horiuchi, 1998). Sleep disordered breathing, which involves hypoxia and sleep disruption, is similary associated with the adverse outcomes, and predominantly associated with preeclampsia, gestational diabetes, gestational hypertension, intrauterine growth restriction, and low birth weight (Carnelio, Morton, & McIntyre, 2017). Despite the increased interest, our knowledge remains limited by predominantly retrospective studies or small sample sizes. With regards to infant outcomes, our knowledge is even more limited. Historically, the emphasis has been on sleep disordered breathing (SDB) or sleep apnea and infant outcomes(Bourjeily et al., 2010; Chen et al., 2012; Franklin et al., 2000; Koken et al., 2007; Tauman et al., 2011), however recent data has emphasized sleep quality and insomnia and how they impact the neonate(Dorheim et al., 2012; Okun et al., 2011; Okun et al., 2015; Rognmo, Sivertsen, & Eberhard-Gran, 2016; Sivertsen, Hysing, Dorheim, & Eberhard-Gran, 2015; Wolynczyk-Gmaj et al., 2017).
It is not entirely clear as to how sleep increases risk for adverse outcomes, however there are various mechanistic pathways proposed to mediate the association between sleep and adverse pregnancy outcomes. Sleep is not merely the absence of waking. On the contrary, it is a state during which specialized physiological activities occur in the brain and throughout the body. It is an active process in which metabolism, tissue restoration, memory consolidation, and general homeostatic balance is maintained (Fuller, Gooley, & Saper, 2006; Markov & Goldman, 2006). Homeostatic and circadian influences dictate that we spend approximately one-third of our lives asleep(Saper et al., 2005). Yet, to date, the primary focus in the pathogenesis of disease has been on the role of waking factors with little attention to sleep. Okun et al (Okun, Roberts, Marsland, & Hall, 2009) proposed a conceptual model as to how sleep may increase risk of hypertensive disorders of pregnancy. They indicate that disturbed sleep affects several biological pathways associated with cardiovascular morbidity, including neuroendocrine, metabolic, and inflammatory systems. One way that disturbed sleep may increase cardiovascular disease is by exacerbating well recognized cardiovascular risk factors, such as obesity and insulin resistance. Importantly, many of these are also risk factors for adverse pregnancy outcomes. While increased oxidative stress, endothelial dysfunction and inflammation are implicated in the pathogenesis of cardiovascular disease and adverse pregnancy outcomes, this paper will focus on dysregulation of the immune system.
4. Pregnancy and Alterations in Immune Function
The clinical data showing an association between poor pregnancy outcomes and high pro-inflammatory cytokine levels strongly suggest that an appropriate shift to a response favoring anti-inflammatory cytokine secretion appears to be crucial in maintaining normal gestation and delivery. Th1 cytokines relevant to pregnancy include interleukin 1 β (IL-1β), interleukin 8 (IL-8), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6). Elevated levels of IL-6, IL-8, and TNF-α are associated with premature labor and delivery (Arck et al., 2001; Coulam, 2000). Failure to effectively switch off aspects of the inflammatory response during pregnancy also has been associated with the development of preeclampsia (Hennessy, Pilmore, Simmons, & Painter, 1999; Schiessl, 2007; Dekker & Sibai, 1999). Significantly more TNF-α, IL-6, and IL-1β are produced by lymphocytes from preeclamptic women than those produced by lymphocytes from women with normal pregnancies (Hayashi, Ueda, Ohkura, & Inaba, 2005; Saito et al., 1999; Munno et al., 1999). These data show that the balance of the maternal immune system in pregnancy is critical for successful pregnancy, and that increased levels of Th1-type or pro-inflammatory cytokines may be related to the development of serious complications.
Pregnancy is characterized by a plethora of immunologic change and these changes are often referred to as the “immunological paradox of pregnancy” because even with the paternal antigens present, the fetus is immunologically tolerated by the mother and often brought to term (Clark et al., 1996; Coussons-Read et al., 2002; Hegde, Ranpura, D’Souza, & Raghavan, 2001; Piccinni, Scaletti, Maggi, & Romagnani, 2000; Saito et al., 1999; Wadhwa, 2005). Some researchers purport that in order to maintain the viability of the fetus, a chronic immunosuppressive state must take place (Hegde et al., 2001; Piccinni et al., 2000). Some have even stated the maternal-fetal relationship is unique and represents a more step-by-step, programmed interactive symbiosis, rather than a simple host/tumor or host/allograft relationship (Chaouat et al., 2002). Data indicate that immediately following insemination, the female reproductive tract undergoes inflammatory changes in response to semen (Hegde et al., 2001). These inflammatory changes are due to the high levels of immunoregulatory molecules called prostaglandins, which are a potent immunosuppressor contained in seminal fluid (Hegde et al., 2001). Similarly, the uterus is exposed to antigens from the fetus once conception has occurred and the maternal-fetal compartment is able to distinguish between self and non-self. Because of this remarkable ability, other scientists contend that the preponderance of the changes occur at the maternal-fetal interface, not throughout the entire body (Luppi, Haluszczak, Trucco, & DeLoia, 2002; Piccinni et al., 2000). Regardless of the degree of immunosuppression, including a shift from a pro-inflammatory to an anti-inflammatory cytokine profile along with secretion of appropriate anti-abortive chemicals such as TGF-β and IL-10, it is accepted that various immune modifications must take place in order to achieve a successful pregnancy.
5. Consequences of the Inflammatory Response During Pregnancy
Despite advances in medical technology adverse pregnancy outcomes continue to plague pregnant women all over the world. For instance, preterm birth complicates almost 12% of all pregnancies (CDC, 2014; Peltier, 2003). Preterm labor and delivery appear to be a consequence of an early or premature inflammatory response. Normal parturition involves three physiologically interdependent processes: (1) restructuring of the cervix to allow it to stretch; (2) weakening and rupture of membranes; and (3) initiation of rhythmic contractions that will force the fetus and placenta out (Peltier, 2003). It appears that certain pro-inflammatory cytokines play a significant role in initiating this normal process. With a decrease of progesterone, IL-8, IL-1β, IL-6, and TNF-α production is increased, which commences the cascade of labor events (Peltier, 2003). It is infection or an inflammatory response that results in a premature rise in pro-inflammatory cytokine levels that ultimately begins an early labor process (Peltier, 2003). If infection accounts for 30% of all preterm labor and delivery (Peltier, 2003), other factors, such as stress and sleep loss, may account for a significant portion of the remaining variance. These associations are actively being evaluated.
Similar to preterm birth, preeclampsia affects the newborn and its health. However, preeclampsia is especially dangerous to the health of the mother and contributes to mortality in childbirth (Tjoa, Oudejans, van Vugt, Blankenstein, & van Wijk, 2004). Recent studies have attempted to identify markers that may indicate susceptibility to preterm birth or preeclampsia prior to the development of the disease (Bertran et al., 2005; Fialova et al., 2004; Freeman et al., 2004; Tjoa et al., 2004). One example is C-reactive protein (CRP) as a potential marker. CRP is a sensitive indication of tissue damage and inflammation. It has been shown that women with preeclampsia (Fialova et al., 2004; Tjoa et al., 2004) and gestational diabetes mellitus (Qiu, Sorensen, Luthy, & Williams, 2004) have elevated levels of CRP. By measuring CRP levels early in pregnancy, data suggest that an early warning of compromised placental development may be indicated (Bertran et al., 2005; Tjoa et al., 2004). To date, no single biomarker has proven to be reliably indicative of an adverse pregnancy outcome.
A growing concern is the development of gestational diabetes mellitus (GDM) and its subsequent impact on the health and lifestyle of the mother and infant. Gestational diabetes mellitus afflicts approximately 4% of all pregnancies annually and may be as high as 14% annually (Qiu et al., 2004). In order to maintain a proper provision of maternal nutrients to the feto-placental unit, some insulin resistance is required (Radaelli, Varastehpour, Catalano, & Hauguel-De, 2003). When women develop GDM, insulin resistance is more severe with accelerated fetal development as one result. GDM increases the risk of fetal macrosomia and other neonatal morbidities including hypoglycemia, hypocalcemia, polycythemia, and jaundice. GDM is associated with an increased frequency of maternal hypertensive disorders and the need for cesarean delivery (Qiu et al., 2004). Comparable to the use of CRP in early detection of preeclampsia, increased leukocyte counts are used as a marker of inflammation that is associated with development of type 2 diabetes. There is growing evidence that insufficient sleep may contribute to GDM.
Women who have several recurrent spontaneous abortions (RSA) have higher levels of Th1-type cytokines (IL-2, IFNγ and TNFα) than women who had normal pregnancies(Arck et al., 2001; Liu et al., 2011; Nepomnaschy et al., 2006; Raghupathy, 1997). The same studies also showed that normal pregnancies were associated with higher levels of IL-4, IL-5, IL-6 and IL-10 than those women with RSA (Raghupathy, 2001). Some investigators purport that the absence of a type 2 bias may be associated with, and possibly a predictor of, pathologic events(Marzi et al., 1996). Thus, from an immunological point of view, successful pregnancy is characterized by altered ratios of Th1-type and Th2-type cytokines in a fashion that is consistent with at least partial immunosuppression and downregulation of a cell-mediated immune response. The data clarify the need to understand and better interpret how inflammation can influence the progression and success of pregnancy, and how the behavior of sleep influences these processes.
Disordered Sleep and Immune Function in Pregnancy
Pregnancy is typically associated with changes in immune function, changes that may be associated with beneficial or harmful outcomes (i.e., uncomplicated birth or preeclampsia). Changes in immune function during pregnancy may also exert negative effects on sleep. For example, the shift toward a pronounced Th2 or anti-inflammatory cytokine profile seen in pregnancy would intimate that Th1 or pro-inflammatory cytokines, such as interleukin 1 (IL-1) and tumor necrosis factor alpha (TNF-α), would be reduced. IL-1 and TNF-α not only defend against antigens, but they also are important mediators of the sleep process (Krueger et al., 1998). Hence, one would anticipate a reduction in sleep during normal pregnancy as a consequence of this decrease in IL-1 and TNF-α. When considering the complex interactions among sleep, the physiological state of pregnancy, and the altered immune functions occurring during pregnancy, it is possible that exacerbated sleep disruption may occur in pregnancy. This could ultimately skew the pro-inflammatory cytokine profile leading to an inflammatory state rather than the desired anti-inflammatory state. For this reason, excessive sleep disruption and/or deprivation may be a potential contributor to poor maternal health during pregnancy as well as negative pregnancy outcomes.
The concept that sleep disruption alters cytokine profiles and immune function in nonpregnant populations has previously been addressed (Krueger & Fang, 1999; Opp & Krueger, 2015). What is of interest here is how disturbed sleep experienced during pregnancy can dysregulate inflammation during pregnancy which thereby increases risk for adverse outcomes. To date, only a handful of studies have assessed disrupted sleep and immune function in pregnant women, and even fewer have considered sleep disorders and their impact on immune function in pregnany.
Summarizing the literature affords a plausible pathway in which sleep disturbance throughout pregnancy may negatively affect immune functioning and impact outcomes. It is known that secretions of IL-1β and TNF-α occur primarily during SWS, while increases in secretion of IL-6 are linked to REM sleep (Krueger et al., 1998; Redwine, Hauger, Gillin, & Irwin, 2000; Opp & Krueger, 2015). Abberations in these cytokines have been implicated in the development of pregnancy complications(Piccinni et al., 2000; Saito et al., 1999). Some evidence suggests that dramatically reduced REM sleep is commonly seen in preeclampsia (Edwards, Blyton, Kesby, Wilcox, & Sullivan, 2000) which could affect the nocturnal secretion of IL-6. However, data suggest that high levels of IL-6 are in fact associated with preeclampsia. Others suggest that a dramatic increase in Stage 2 sleep may be the cause of elevated IL-6(Brunner et al., 1994). Reductions in SWS are correlated with increases in IL-1β and TNF-α (Krueger et al., 1998). Importantly, TNF-α has been shown to inhibit trophoblast growth and subsequent recurrent miscarriages and preeclampsia (Dekker & Sibai, 1999). The paucity of research, even to this day, indicates significant gaps in the knowledge regarding disturbed sleep and immunity during pregnancy.
To date, only a few studies have considered assaying immune parameters associated with both sleep and pregnancy simultaneously. Okun and colleagues have published the majority of the available literature. They found that poor sleep quality was associated with higher circulating and stimulated levels of IL-6(Okun & Coussons-Read, 2007; Okun et al., 2007; Okun, Coussons-Read, & Hall, 2009), and that symptoms of insomnia and poor sleep quality dysregulate several cytokines(Okun, Roberts JM, Begley, Catov, & Patrick, 2013; Okun et al., 2013). One other study corroborated these findings(Blair et al., 2015). All of the aforementioned cytokines play a role in both sleep and pregnancy. How the interleukins, the interferons and other immune substances interact with one another during these two physiological events is currently under investigation, but the evidence strongly suggests a synergistic relationship.
Conclusion
Clearly pregnant women experience more sleep disturbance than their non-pregnant counterparts. The degree to which this occurs appears to be quite variable, not only by trimester but within an individual as well. Disturbed sleep is emerging as a prominent factor contributing to risk for adverse pregnancy outcomes. Inflammation is a key biological pathway linking myriad behaviors with health outcomes. The evaluation of sleep and inflammation as a contributing factor to adverse pregnancy outcomes is still in its nascency. Hopefully this will change. In a previous publication, Okun and colleagues proposed a model linking sleep disturbances in early gestation to adverse pregnancy outcomes via increased inflammation(Okun et al., 2009). Specifically, they proposed a feed forward loop between sleep disruption and inflammation during a critical period of early pregnancy when inflammation can act, for instance, to inhibit the trophoblast invasion and associated remodeling of maternal blood vessels that perfuse the placenta. This author purports that in order to truly understand the implications of disturbed sleep on health, both the immediate and long-term effects of disturbed sleep on immunocompetence need to be ascertained through longitudinal study designs. Assessing sleep disturbances as a risk factor for adverse outcomes could provide a target for intervention especially since sleep problems are amenable to treatment.
Acknowledgements:
Funding sources NIH NR010813 for financial support
Footnotes
Conflict of Interest: The author has no conflicts to report
Reference List
- Abedian Z, Soltani N, Mokhber N, & Esmaily H (2015). Depression and anxiety in pregnancy and postpartum in women with mild and severe preeclampsia. Iran J.Nurs.Midwifery Res, 20, 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam K (1980). Sleep as a restorative process and a theory to explain why. Progress in Brain Research, 53, 289–305. [DOI] [PubMed] [Google Scholar]
- Adam K & Oswald I (1977). Sleep is for tissue restoration. J R.Coll.Physicians Lond, 11, 376–388. [PMC free article] [PubMed] [Google Scholar]
- Adam K & Oswald I (1984). Sleep helps healing. Br.Med.J.(Clin.Res.Ed), 289, 1400–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam K, Tomeny M, & Oswald I (1986). Physiological and psychological differences between good and poor sleepers. Journal of Psychiatric Research, 20, 301–316. [DOI] [PubMed] [Google Scholar]
- Akerstedt T & Nilsson PM (2003). Sleep as restitution: an introduction. Journal of Internal Medicine, 254, 6–12. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ et al. (2015). The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behavioral Sleep Medicine, 13 Suppl 1, S4–S38. [DOI] [PubMed] [Google Scholar]
- Arck PC, Rose M, Hertwig K, Hagen E, Hildebrandt M, & Klapp BF (2001). Stress and immune mediators in miscarriage. Human Reproduction, 16, 1505–1511. [DOI] [PubMed] [Google Scholar]
- Atta DS, Girbash EF, Abdelwahab SM, Abdeldayem HM, Tharwat I, & Ghonaim R (2016). Maternal cytokines and disease severity influence pregnancy outcomes in women with rheumatoid arthritis. J.Matern.Fetal Neonatal Med., 29, 3358–3363. [DOI] [PubMed] [Google Scholar]
- Ayrim A, Keskin EA, Ozol D, Onaran Y, Yiidirim Z, & Kafali H (2011). Influence of self-reported snoring and witnessed sleep apnea on gestational hypertension and fetal outcome in pregnancy. Archives of Gynecology and Obstetrics, 283, 195–199. [DOI] [PubMed] [Google Scholar]
- Balserak BI, Jackson N, Ratcliffe SA, Pack AI, & Pien GW (2013). Sleep-Disordered Breathing and Daytime Napping are Associated with Maternal Hyperglycemia. Sleep Breath, 17, 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratte-Beebe KR & Lee K (1999). Sources of midsleep awakenings in childbearing women. Clinical Nursing Research, 8, 386–397. [DOI] [PubMed] [Google Scholar]
- Beebe KR & Lee KA (2007). Sleep disturbance in late pregnancy and early labor. Journal of Perinatal and Neonatal Nursing, 21, 103–108. [DOI] [PubMed] [Google Scholar]
- Bei B, Milgrom J, Ericksen J, & Trinder J (2010). Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep, 33, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca R & Quintas J (1997). Sleep and host defenses: A review. Sleep, 20, 1027–1037. [PubMed] [Google Scholar]
- Bertran N, Camps J, Fernandez-Ballart J, Murphy MM, Arija V, Ferre N et al. (2005). Evaluation of a high-sensitivity turbidimetric immunoassay for serum C-reactive protein: Application to the study of longitudinal changes throughout normal pregnancy. Clin Chem.Lab Med, 43, 308–313. [DOI] [PubMed] [Google Scholar]
- Blair LM, Porter K, Leblebicioglu B, & Christian LM (2015). Poor Sleep Quality and Associated Inflammation Predict Preterm Birth: Heightened Risk among African Americans. Sleep, 38, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J & Fehm HL (2000). The neuroendocrine recovery function of sleep. Noise.Health, 2, 25–38. [PubMed] [Google Scholar]
- Born J, Kern W, Bieber K, Fehm-Wolfsdorf G, Schiebe M, & Fehm HL (1986). Night-time plasma cortisol secretion is associated with specific sleep stages. Biological Psychiatry, 21, 1415–1424. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, & Fehm HL (1997). Effects of sleep and circadian rhythm on human circulating immune cells. Journal of Immunology, 158, 4454–4464. [PubMed] [Google Scholar]
- Born J, Muth S, & Fehm HL (1988). The significance of sleep onset and slow wave sleep for nocturnal release of growth hormone and cortisol. Psychoneuroendocrinology, 13, 233–243. [DOI] [PubMed] [Google Scholar]
- Bourjeily G, Raker C, Chalhoub M, & Miller M (2013). Excessive daytime sleepiness in late pregnancy may not always be normal: results from a cross-sectional study. Sleep Breath, 17, 735–740. [DOI] [PubMed] [Google Scholar]
- Bourjeily G, Raker CA, Chalhoub M, & Miller MA (2010). Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. European Respiratory Journal, 36, 849–855. [DOI] [PubMed] [Google Scholar]
- Branchey M & Petre-Quadens O (1968). A comparative study of sleep parameters during pregnancy. Acta Neurologica et Psychiatrica Belgica, 68, 453–459. [PubMed] [Google Scholar]
- Brunner DP, Munch M, Biedermann K, Huch R, Huch A, & Borbély AA (1994). Changes in sleep and sleep electroencephalogram during pregnancy. Sleep, 17, 576–582. [DOI] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, & Curtis N (2004). Sick and tired: Does sleep have a vital role in the immune system? Nat.Rev.Immunol, 4, 457–467. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carmichael SL & Shaw GM (2000). Maternal life event stress and congenital anomalies. Epidemiology, 11, 30–35. [DOI] [PubMed] [Google Scholar]
- Carnelio S, Morton A, & McIntyre HD (2017). Sleep disordered breathing in pregnancy: the maternal and fetal implications. Journal of Obstetrics and Gynaecology, 37, 170–178. [DOI] [PubMed] [Google Scholar]
- CDC. (2014). Preterm Birth. Ref Type: Online Source
- Champagne K, Schwartzman K, Opatrny L, Barriga P, Morin L, Mallozzi A et al. (2009). Obstructive sleep apnoea and its association with gestational hypertension. European Respiratory Journal, 33, 559–565. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N et al. (2002). A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod.Immunol, 53, 241–256. [DOI] [PubMed] [Google Scholar]
- Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, & Lin HC (2012). Obstructive sleep apnea and the risk of adverse pregnancy outcomes. American Journal of Obstetrics and Gynecology, 206, 136–5. [DOI] [PubMed] [Google Scholar]
- Christian LM (2014). Effects of stress and depression on inflammatory immune parameters in pregnancy. American Journal of Obstetrics and Gynecology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Iams JD, Sheridan J, & Glaser R (2010). Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain, Behavior, and Immunity, 24, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Arck PC, Jalali R, Merali FS, Manuel J, Chaouat G et al. (1996). Psycho-neuro-cytokine/endocrine pathways in immunoregulation during pregnancy. American Journal of Reproductive Immunology, 35, 330–337. [DOI] [PubMed] [Google Scholar]
- Corwin EJ & Pajer K (2008). The psychoneuroimmunology of postpartum depression. Journal of Women’s Health, 17, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulam CB (2000). Understanding the immunobiology of pregnancy and applying it to treatment of recurrent pregnancy loss. Early Pregnancy, 4, 19–29. [PubMed] [Google Scholar]
- Coussons-Read ME, Mazzeo RS, Whitford MH, Schmitt M, Moore LG, & Zamudio S (2002). High altitude residence during pregnancy alters cytokine and catecholamine levels. American Journal of Reproductive Immunology, 48, 344–354. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, & Nettles CD (2007). Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity, 21, 343–350. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker GA & Sibai BM (1999). The immunology of preeclampsia. Seminars in Perinatology, 23, 24–33. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, & Kuhn C (2004). Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry, 67, 63–80. [DOI] [PubMed] [Google Scholar]
- Dolatian M, Mahmoodi Z, Alavi-Majd H, Moafi F, Ghorbani M, & Mirabzadeh A (2016). Psychosocial factors in pregnancy and birthweight: Path analysis. Journal of Obstetrics and Gynaecology Research, 42, 822–830. [DOI] [PubMed] [Google Scholar]
- Dorheim SK, Bjorvatn B, & Eberhard-Gran M (2012). Insomnia and depressive symptoms in late pregnancy: a population-based study. Behavioral Sleep Medicine, 10, 152–166. [DOI] [PubMed] [Google Scholar]
- Driver HS & Shapiro CM (1992). A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep, 15, 449–453. [DOI] [PubMed] [Google Scholar]
- Edwards N, Blyton CM, Kesby GJ, Wilcox I, & Sullivan CE (2000). Pre-eclampsia is associated with marked alterations in sleep architecture. Sleep, 23, 619–625. [PubMed] [Google Scholar]
- Elek SM, Hudson DB, & Fleck MO (1997). Expectant parents’ experience with fatigue and sleep during pregnancy. Birth, 24, 49–54. [DOI] [PubMed] [Google Scholar]
- Ellington SR, Hartman LK, Acosta M, Martinez-Romo M, Rubinson L, Jamieson DJ et al. (2011). Pandemic 2009 influenza A (H1N1) in 71 critically ill pregnant women in California11. American Journal of Obstetrics and Gynecology, 204, S21–S30. [DOI] [PubMed] [Google Scholar]
- Evans ML, Dick MJ, & Clark AS (1995). Sleep during the week before labor: relationships to labor outcomes. Clinical Nursing Research, 4, 238–249. [DOI] [PubMed] [Google Scholar]
- Facco FL, Grobman WA, Kramer J, Ho KH, & Zee PC (2010). Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. American Journal of Obstetrics and Gynecology, 203, 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialova L, Kalousova M, Soukupova J, Malbohan I, Madar J, Frisova V et al. (2004). Markers of inflammation in preeclampsia. Prague Med Rep, 105, 301–310. [PubMed] [Google Scholar]
- Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, & Svanborg E (2000). Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest, 117, 137–141. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE et al. (2004). Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension, 44, 708–714. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, & Saper CB (2006). Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. Journal of Biological Rhythms, 21, 482–493. [DOI] [PubMed] [Google Scholar]
- Garde AH, Karlson B, Hansen AM, Persson R, & Akerstedt, T. (2012). Sleep and Salivary Cortsiol In Kristenson M, Garvin P, & Lundberg U (Eds.), The Role of Saliva Cortisol Measurement in Health and Disease (pp. 116–128). Bentham Science Publishers. [Google Scholar]
- Goyal D, Gay C, & Lee K (2009). Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Archives of Womens Mental Health, 12, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal D, Gay CL, & Lee KA (2007). Patterns of sleep disruption and depressive symptoms in new mothers. Journal of Perinatal and Neonatal Nursing, 21, 123–129. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Pearl M, Kosa JL, Graham S, Abrams B, & Kharrazi M (2013). Association between preterm delivery and pre-pregnancy body mass (BMI), exercise and sleep during pregnancy among working women in Southern California. Maternal and Child Health Journal, 17, 723–731. [DOI] [PubMed] [Google Scholar]
- Guntupalli KK, Hall N, Karnad DR, Bandi V, & Belfort M (2015). Critical illness in pregnancy: part I: an approach to a pregnant patient in the ICU and common obstetric disorders. Chest, 148, 1093–1104. [DOI] [PubMed] [Google Scholar]
- Hall M, Okun ML, Atwood CW, Buysse DJ, & Strollo PJ (2008). Measurement of sleep by polysomnography In Luecken LL & Gallo LC (Eds.), Handbook of Physiological Research Methods in Health Psychology (pp. 341–367). Sage Publications. [Google Scholar]
- Hartert TV, Neuzil KM, Shintani AK, Mitchel EF Jr., Snowden MS, Wood LB et al. (2003). Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season4. American Journal of Obstetrics and Gynecology, 189, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ueda Y, Ohkura T, & Inaba N (2005). Interleukin-6 concentrations in the placenta and blood in normal pregnancies and preeclampsia. Horm.Metab Res, 37, 419–424. [DOI] [PubMed] [Google Scholar]
- Hegde UC, Ranpura S, D’Souza S, & Raghavan VP (2001). Immunoregulatory pathways in pregnancy. Indian J Biochem.Biophys, 38, 207–219. [PubMed] [Google Scholar]
- Hennessy A, Pilmore HL, Simmons LA, & Painter DM (1999). A deficiency of placental IL-10 in preeclampsia. Journal of Immunology, 163, 3491–3495. [PubMed] [Google Scholar]
- Herring SJ, Foster GD, Pien GW, Massa K, Nelson DB, Gehrman PR et al. (2013). Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep Breath, 17, 1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, & Fein AM (1992). Sleep in normal late pregnancy. Sleep, 15, 246–251. [DOI] [PubMed] [Google Scholar]
- Hori H, Teraishi T, Sasayama D, Ozeki Y, Matsuo J, Kawamoto Y et al. (2011). Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. Journal of Psychiatric Research, 45, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Hux VJ, Roberts JM, & Okun ML (2017). Allostatic load in early pregnancy is associated with poor sleep quality. Sleep Medicine, 33, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep, 14, 540–545. [DOI] [PubMed] [Google Scholar]
- Kamysheva E, Skouteris H, Wertheim EH, Paxton SJ, & Milgrom J (2009). A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. Journal of Affective Disorders. [DOI] [PubMed] [Google Scholar]
- Koken G, Sahin FK, Cosar E, Saylan F, Yilmaz N, Altuntas I et al. (2007). Oxidative stress markers in pregnant women who snore and fetal outcome: a case control study. Acta Obstetricia et Gynecologica Scandinavica, 86, 1317–1321. [DOI] [PubMed] [Google Scholar]
- Krueger JM & Fang J (1999). Cytokines and sleep regulation In Lydic R & Baghdoyan HA (Eds.), Handbook of Behavioral State Control: Cellular and Molecular Mechanisms (pp. 609–622). Boca Raton: CRC Press. [Google Scholar]
- Krueger JM, Fang J, Taishi P, Chen Z, Kushikata T, & Gardi J (1998). Sleep. A physiologic role for IL-1 beta and TNF-alpha. Annals of the New York Academy of Sciences, 856, 148–159. [DOI] [PubMed] [Google Scholar]
- Lapinsky SE (2010). Critical illness as a result of influenza A/H1N1 infection in pregnancy4. BMJ, 340, c1235. [DOI] [PubMed] [Google Scholar]
- Lee KA (2006). Sleep during pregnancy and postpartum In Lee-Chiong T (Ed.), Encyclopedia of Sleep Medicine (pp. 629–635). John Wiley & Sons, Inc. [Google Scholar]
- Lee KA & Gay CL (2004). Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics and Gynecology, 191, 2041–2046. [DOI] [PubMed] [Google Scholar]
- Lee KA, Zaffke ME, & McEnany G (2000). Parity and sleep patterns during and after pregnancy. Obstetrics and Gynecology, 95, 14–18. [DOI] [PubMed] [Google Scholar]
- Lewis AJ, Austin E, Knapp R, Vaiano T, & Galbally M (2015). Perinatal Maternal Mental Health, Fetal Programming and Child Development. Healthcare.(Basel), 3, 1212–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhang J, Zhou R, Liu J, Dai Z, Liu D et al. (2016). Sleep disturbances during pregnancy are associated with cesarean delivery and preterm birth. J.Matern.Fetal Neonatal Med, 1–6. [DOI] [PubMed] [Google Scholar]
- Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX et al. (2011). Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. American Journal of Reproductive Immunology, 65, 503–511. [DOI] [PubMed] [Google Scholar]
- Livingston IL, Otado JA, & Warren C (2003). Stress, adverse pregnancy outcomes, and African-American females. Journal of the National Medical Association, 95, 1103–1109. [PMC free article] [PubMed] [Google Scholar]
- Louis JM, Koch MA, Reddy UM, Silver RM, Parker CB, Facco FL et al. (2018). Predictors of sleep-disordered breathing in pregnancy. American Journal of Obstetrics and Gynecology, 218, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi P, Haluszczak C, Trucco M, & DeLoia JA (2002). Normal pregnancy is associated with peripheral leukocyte activation. American Journal of Reproductive Immunology, 47, 72–81. [DOI] [PubMed] [Google Scholar]
- Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R et al. (2009). Maternal obesity and markers of inflammation in pregnancy. Cytokine, 47, 61–64. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, De Jongh R et al. (2000). Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology, 25, 121–137. [DOI] [PubMed] [Google Scholar]
- Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM et al. (2013). Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep, 36, 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov D & Goldman M (2006). Normal sleep and circadian rhythms: neurobiologic mechanisms underlying sleep and wakefulness. Psychiatric Clinics of North America, 29, 841–853. [DOI] [PubMed] [Google Scholar]
- Martin JL & Hakim AD (2011). Wrist actigraphy. Chest, 139, 1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E et al. (1996). Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clinical and Experimental Immunology, 106, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2006). Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism: Clinical and Experimental, 55, S20–S23. [DOI] [PubMed] [Google Scholar]
- McEwen BS & Karatsoreos IN (2015). Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med.Clin, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M et al. (2011). Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology, 22, 738–744. [DOI] [PubMed] [Google Scholar]
- Mindell JA & Jacobson BJ (2000). Sleep disturbances during pregnancy. J Obstet.Gynecol Neonatal Nurs, 29, 590–597. [DOI] [PubMed] [Google Scholar]
- Misri S & Swift E (2015). Generalized Anxiety Disorder and Major Depressive Disorder in Pregnant and Postpartum Women: Maternal Quality of Life and Treatment Outcomes. J.Obstet.Gynaecol.Can, 37, 798–803. [DOI] [PubMed] [Google Scholar]
- Mouyis MA, Thornton CC, Williams D, & Giles IP (2017). Pregnancy Outcomes in Patients with Psoriatic Arthritis. Journal of Rheumatology, 44, 128–129. [DOI] [PubMed] [Google Scholar]
- Munno I, Chiechi LM, Lacedra G, Putignano G, Patimo C, Lobascio A et al. (1999). Spontaneous and induced release of prostaglandins, interleukin (IL)-1beta, IL-6, and tumor necrosis factor-alpha by placental tissue from normal and preeclamptic pregnancies. American Journal of Reproductive Immunology, 42, 369–374. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, & England BG (2006). Cortisol levels and very early pregnancy loss in humans. Proc.Natl.Acad.Sci.U.S.A, 103, 3938–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara K & Horiuchi S (1998). Changes in sleep patterns of young women from late pregnancy to postpartum: relationships to their infants’ movements. Perceptual and Motor Skills, 87, 1043–1056. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Bullough AS, Chames MC, Shelgikar AV, Armitage R, Guilleminualt C et al. (2014). Hypertension, snoring, and obstructive sleep apnoea during pregnancy: a cohort study. BJOG., 121, 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC et al. (2012). Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. American Journal of Obstetrics and Gynecology, 207, 487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe M & St-Onge MP (2012). Sleep duration and disorders in pregnancy: implications for glucose metabolism and pregnancy outcomes. Int.J.Obes.(Lond), 37, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Roberts JM, Begley A, Catov JM, & Patrick TE (2013). 24-hour Sleep Duration in Early Gestation is Associated with Increased Markers of Inflammation Among Women with a History of Preeclampsia. The Open Sleep Journal, 6, 14–22. [Google Scholar]
- Okun ML (2013). Sleep in Pregnancy and the Postpartum In Kushida CA (Ed.), Encyclopedia of Sleep (pp. 674–679). Waltham: Academic Press. [Google Scholar]
- Okun ML, Buysse DJ, & Hall MH (2015). Identifying Insomnia in Early Pregnancy: Validation of the Insomnia Symptoms Questionnaire (ISQ) in Pregnant Women. J.Clin.Sleep Med, 11, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read M, & Hall M (2009). Disturbed sleep is associated with increased C-reactive protein in young women. Brain, Behavior, and Immunity, 23, 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML & Coussons-Read ME (2007). Sleep disruption during pregnancy: how does it influence serum cytokines? Journal of Reproductive Immunology, 73, 158–165. [DOI] [PubMed] [Google Scholar]
- Okun ML, Hall M, & Coussons-Read ME (2007). Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod.Sci., 14, 560–567. [DOI] [PubMed] [Google Scholar]
- Okun ML, Hanusa BH, Hall M, & Wisner KL (2009). Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behavioral Sleep Medicine, 7, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Kiewra K, Luther JF, Wisniewski SR, & Wisner KL (2011). Sleep disturbances in depressed and nondepressed pregnant women. Depress.Anxiety., 28, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Kline CE, Roberts JM, Wettlaufer B, Glover K, & Hall M (2013). Prevalence of sleep deficiency in early gestation and its associations with stress and depressive symptoms. J.Womens Health (Larchmt.), 22, 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, & Hall M (2009). Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J.Clin.Sleep Med., 5, 41–51. [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, & Wisner KL (2011). Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. Journal of Affective Disorders, 130, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Luther JF, Wisniewski SR, Sit D, Prairie BA, & Wisner KL (2011). Disturbed Sleep, a Novel Risk Factor for Preterm Birth? Journal of Women’s Health, 21, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Luther JF, Wisniewski SR, & Wisner KL (2013). Disturbed sleep and inflammatory cytokines in depressed and nondepressed pregnant women: an exploratory analysis of pregnancy outcomes. Psychosomatic Medicine, 75, 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Roberts JM, Marsland AL, & Hall M (2009). How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstetrical & Gynecological Survey, 64, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Schetter CD, & Glynn LM (2011). Poor sleep quality is associated with preterm birth. Sleep, 34, 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR & Krueger JM (2015). Sleep and immunity: A growing field with clinical impact. Brain, Behavior, and Immunity, 47, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr ST, James SA, & Blackmore PC (2002). Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology, 156, 797–802. [DOI] [PubMed] [Google Scholar]
- Palagini L, Gemignani A, Banti S, Manconi M, Mauri M, & Riemann D (2014). Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Medicine, 15, 853–859. [DOI] [PubMed] [Google Scholar]
- Patel AK & Araujo JF (2018). Physiology, Sleep Stages. [PubMed] [Google Scholar]
- Peltier MR (2003). Immunology of term and preterm labor. Reprod.Biol.Endocrinol, 1, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni MP, Scaletti C, Maggi E, & Romagnani S (2000). Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol, 109, 30–33. [DOI] [PubMed] [Google Scholar]
- Qiu C, Enquobahrie D, Frederick IO, Abetew D, & Williams MA (2010). Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC.Womens Health, 10, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Sorensen TK, Luthy DA, & Williams MA (2004). A prospective study of maternal serum C-reactive protein (CRP) concentrations and risk of gestational diabetes mellitus. Paediatric and Perinatal Epidemiology, 18, 377–384. [DOI] [PubMed] [Google Scholar]
- Radaelli T, Varastehpour A, Catalano P, & Hauguel-De MS (2003). Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes, 52, 2951–2958. [DOI] [PubMed] [Google Scholar]
- Raghupathy R (1997). Th1-type immunity is incompatible with successful pregnancy. Immunology Today, 18, 478–482. [DOI] [PubMed] [Google Scholar]
- Raghupathy R (2001). Pregnancy: Success and failure within the Th1/Th2/Th3 paradigm. Seminars in Immunology, 13, 219–227. [DOI] [PubMed] [Google Scholar]
- Redwine L, Hauger RL, Gillin JC, & Irwin M (2000). Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. Journal of Clinical Endocrinology and Metabolism, 85, 3597–3603. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Facco FL, Grobman WA, Parker CB, Herbas M, Hunter S et al. (2017). Sleep During Pregnancy: The nuMoM2b Pregnancy and Sleep Duration and Continuity Study. Sleep, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA et al. (2011). Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P (1996). Sleep in pregnancy. Holistic Nursing Practice, 10, 20–26. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M et al. (2010). The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Medicine Reviews, 14, 19–31. [DOI] [PubMed] [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, & Sandman CA (1999). Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychology, 18, 333–345. [DOI] [PubMed] [Google Scholar]
- Rognmo K, Sivertsen B, & Eberhard-Gran M (2016). Self-reported short sleep duration and insomnia symptoms as predictors of post-pregnancy weight change: Results from a cohort study. Womens Health (Lond), 12, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, & Michimata T (1999). Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin.Exp.Immunol, 117, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-Demet A, Dunkel-Schetter C, & Porto M (1997). Maternal stress, HPA activity, and fetal/infant outcome. Annals of the New York Academy of Sciences, 814, 266–275. [DOI] [PubMed] [Google Scholar]
- Saper CB, Cano G, & Scammell TE (2005). Homeostatic, circadian, and emotional regulation of sleep. J.Comp Neurol, 493, 92–98. [DOI] [PubMed] [Google Scholar]
- Schiessl B (2007). Inflammatory response in preeclampsia. Molecular Aspects of Medicine, 28, 210–219. [DOI] [PubMed] [Google Scholar]
- Schorr SJ, Chawla A, Devidas M, Sullivan CA, Naef RW, & Morrison JC (1998). Sleep Patterns in Pregnancy: A Longitudinal Study of Polysomnography Recordings During Pregnancy. Journal of Perinatology, 18, 427–430. [PubMed] [Google Scholar]
- Shinkoda H, Matsumoto K, & Park YM (1999). Changes in sleep-wake cycle during the period from late pregnancy to puerperium identified through the wrist actigraph and sleep logs. Psychiatry and Clinical Neurosciences, 53, 133–135. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Hysing M, Dorheim SK, & Eberhard-Gran M (2015). Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC.Pregnancy.Childbirth., 15, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, & Milgrom J (2009). Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues, 19, 45–51. [DOI] [PubMed] [Google Scholar]
- Spradley FT, Palei AC, & Granger JP (2015). Immune Mechanisms Linking Obesity and Preeclampsia. Biomolecules., 5, 3142–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange LB, Parker KP, Moore ML, Strickland OL, & Bliwise DL (2009). Disturbed sleep and preterm birth: a potential relationship? Clinical and Experimental Obstetrics and Gynecology, 36, 166–168. [PubMed] [Google Scholar]
- Tauman R, Many A, Deutsch V, Arvas S, scher-Landsberg J, Greenfeld M et al. (2011). Maternal snoring during pregnancy is associated with enhanced fetal erythropoiesis--a preliminary study. Sleep Medicine, 12, 518–522. [DOI] [PubMed] [Google Scholar]
- Tham EK, Tan J, Chong YS, Kwek K, Saw SM, Teoh OH et al. (2016). Associations between poor subjective prenatal sleep quality and postnatal depression and anxiety symptoms. Journal of Affective Disorders, 202, 91–94. [DOI] [PubMed] [Google Scholar]
- Tjoa ML, Oudejans CB, van Vugt JM, Blankenstein MA, & van Wijk IJ (2004). Markers for presymptomatic prediction of preeclampsia and intrauterine growth restriction. Hypertens.Pregnancy., 23, 171–189. [DOI] [PubMed] [Google Scholar]
- Tomfohr LM, Buliga E, Letourneau NL, Campbell TS, & Giesbrecht GF (2015). Trajectories of Sleep Quality and Associations with Mood during the Perinatal Period. Sleep, 38, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Lee PL, Lin JW, & Lee CN (2016). Cross-sectional and longitudinal associations between sleep and health-related quality of life in pregnant women: A prospective observational study. International Journal of Nursing Studies, 56, 45–53. [DOI] [PubMed] [Google Scholar]
- Volkovich E, Tikotzky L, & Manber R (2016). Objective and subjective sleep during pregnancy: links with depressive and anxiety symptoms. Archives of Womens Mental Health, 19, 173–181. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD (2005). Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology, 30, 724–743. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA, & Ronda J (1983). Cortisol secretion is inhibited during sleep in normal man. J.Clin.Endocrinol.Metab, 56, 352–358. [DOI] [PubMed] [Google Scholar]
- Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, & Enquobahrie D (2010). Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep, 33, 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DL, Barnes M, Ellett L, Permezel M, Jackson M, & Crowe SF (2011). Decreased sleep efficiency, increased wake after sleep onset and increased cortical arousals in late pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology, 51, 38–46. [DOI] [PubMed] [Google Scholar]
- Wilson DL, Fung A, Walker SP, & Barnes M (2013). Subjective reports versus objective measurement of sleep latency and sleep duration in pregnancy. Behavioral Sleep Medicine, 11, 207–221. [DOI] [PubMed] [Google Scholar]
- Wolynczyk-Gmaj D, Rozanska-Waledziak A, Ziemka S, Ufnal M, Brzezicka A, Gmaj B et al. (2017). Insomnia in Pregnancy Is Associated With Depressive Symptoms and Eating at Night. J.Clin.Sleep Med, 13, 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mao J, Ye Z, Zeng X, Zhao H, Liu Y et al. (2017). Determinants of sleep quality among pregnant women in China: a cross-sectional survey. J.Matern.Fetal Neonatal Med, 1–6. [DOI] [PubMed] [Google Scholar]


