Abstract
Periodontitis (PD) is a common dysbiotic inflammatory disease leading to local bone deterioration and tooth loss. PD patients experience low grade bacteremias with oral microbes implicated in risk of heart disease, cancer and kidney failure. While Th17 effectors are vital to fighting infection, functional imbalance of Th17 effectors and regulatory T cells (Tregs) promote inflammatory diseases. Here we investigated, in a small pilot randomized clinical trial, whether expansion of inflammatory blood myeloid dendritic cells (mDCs) and conversion of Tregs to Th17 cells could be modulated with antibiotic (AB) as part of initial therapy in PD patients. PD subjects were randomly assigned to either 7 days of peroral metronidazole/amoxicillin AB treatment or no AB (non-AB), along with standard care debridement and chlorhexidine mouthrinse. 16s rRNA analysis of keystone pathogen Porphyromonas gingivalis (Pg) and its consortium members Fusobacterium nucleatum (Fn) and Streptococcus gordonii (Sg) confirmed presence of all three species in reservoirs (subgingival pockets and blood DCs) of PD patients before treatment. Of the three species, Pg was reduced in both reservoirs 4–6 weeks after therapy. Further, the frequency of CD1C+CCR6+ mDCs and IL-1R1 expression on IL17A+FOXP3+ CD4+ T cells in PD patients were reduced to healthy control levels. The latter led to decreased IL-1β-stimulated Treg-plasticity in PD patients and improvement in clinical measures of PD. Overall, we identified an important, albeit short-term beneficial role of AB therapy in reducing inflammatory DCs and Treg-Th17 plasticity in humans with periodontitis.
Introduction
PD is a common infectious disease, caused by a dysbiotic microflora (1, 2), that leads to disruption of immune homeostasis and destruction of bone supporting the dentition (3, 4). CD4+ T cell effector responses are reported to drive progression of PD (5, 6), while dendritic cells (DCs) are key arbiters of T cell effector responses. Circulating blood myeloid DCs (mDCs) clear bacterial pathogens, such as the keystone oral pathogen Pg (7) from the oral tissues and bloodstream of PD patients (8). Clearance of Pg stimulates non-canonical differentiation of pre-DCs into myeloid DCs in vitro (9) and in PD patients in vivo (8). Pg-induced myeloid DCs reportedly induce long lived FoxP3+ regulatory T cells (Treg) in vitro and in vivo (10). Induction of Tregs by Pg-infected DCs would appear paradoxical, as Tregs are thought to minimize tissue destruction in the late stage of PD (11, 12). However, when considering that double-positive, IL-17A+Foxp3+ CD4+T cells infiltrate PD sites in mice and humans, with Th17 cells essential for eradication of bacteria in oral mucosa (13), but responsible for bone degradation (14), it is feasible that induction of (unstable) Tregs by Pg infected DCs (10) may be a prelude to excessive Th17 responses (15). The pathogenic role of Th17 cells in humans with PD is supported by the histopathology of PD lesions (16–22). Recently an expansion of resident Th17 cells in human periodontitis was demonstrated and that genetic defect in Th17 cell differentiation conferred protection from immunopathology that lessened the periodontal inflammation and bone loss (23). Hence, conversion of Tregs to Th17 cells in PD ultimately may drive disease pathogenesis (14, 24, 25); however, presently unclear are the mechanisms of Treg-Th17 conversion and whether it can be prevented in PD.
The present study employed an AB regimen to temporarily eradicate local and hematogenous microbes in PD patients. This enabled us to determine how these microbes influence frequency of inflammatory blood mDCs and Treg to Th17 cell plasticity. We show that AB therapy reduced content of Pg and Fn in blood mDCs and reduced Pg at local reservoir, the subgingival pocket. Furthermore, AB therapy reduced Treg plasticity in the peripheral blood of PD patients and further prevented IL1β- and IL-6-boosted induction of Th17 cells through IL-1R1 and IL-6Rα modulation on Tregs. Thus, we identified a mechanism whereby including per-oral AB as part of initial therapy, combined with debridement and antimicrobial rinse, provides short term beneficial effects in modulating local and systemic inflammation in PD.
Material and Methods
Chronic periodontitis patients and healthy volunteers
This study was approved by the Augusta University Institutional Review Board and is listed in clinical trials.gov (NCT01568944). All subjects provided informed consent prior to participation in the study. PD subjects (n=18) with moderate to severe generalized disease (probing pocket depth (PPD) of >4 mm, attachment loss of >3 mm, bleeding on probing, and alveolar bone crest >3 mm from the cementoenamel junction), as described by Armitage 1999 (26), were randomly assigned to an antibiotic treatment (AB) consisting of 7 days of p.o. metronidazole (250mg)/amoxicillin (500mg) T.I.D. combined with local chlorhexidine rinses (n=8) or none (n=10). Exclusion criteria were pregnancy, allergy to antibiotics, current smoker, diabetes, autoimmune disease, cancer, liver or renal disease, taking statins, steroids or NSAIDS and inability to cease alcohol consumption during 7 days of AB therapy. At day 6 (24 hrs before SRP) all PD subjects were administered intensive single visit SRP. Peripheral blood was collected from healthy (n=9) and PD patients at baseline, 6 days, 7 days (24 hrs after SRP) and 4–6 weeks after AB (final visit). Gingival tissues and subgingival plaque samples were collected from PD patients at BL and final time points. Human peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient centrifugation using Lymphoprep™ (StemCell Technologies, Vancouver, BC, Canada).
Isolation and phenotypic analysis of blood DCs
Circulating blood DCs were enriched from PBMCs by negative selection using human panDC enrichment kit (StemCell Technologies, Vancouver, BC, Canada). Enriched DCs were analyzed by 8 color flow cytometry (MACS quant, Miltenyi Biotec). For blood DC subsets (mDCs and pDCs), the following conjugated antibodies were used: anti-human CD1C (clone AD5–8E7), CD141 (Clone AD5–14H12), CD303 (Clone AC-144) and CCR6 (clone REA190) (Miltenyi Biotec) and anti-human CCR7 (clone 3D12) (e-biosciences). Unstained cells and Isotype-matched antibodies (directly conjugated) were used as controls. Dead cells were excluded by using Live/Dead fixable aqua dead cell stain kit (Thermo Fisher Scientific).
Detection of dysbiotic microflora in blood DCs and gingival plaque
Total RNA was purified from blood DCs using RNAeasy Plus mini kit according to manufacturers’ instruction (Qiagen). Two rounds of blood DC isolation from PBMCs were performed to remove residual monocytes, with the purity of resultant blood DCs being consistently 80–90%. Genomic DNA was extracted from gingival plaque using QIAamp DNA Investigator kit according to manufacturer instructions (Qiagen). One-step quantitative real-time polymerase chain reaction (qRT-PCR) was performed using StepOnePlus real time RT-PCR system (Applied Biosystems, Life Technologies) using Express qPCR Super Mix according to manufacturers’ instruction (Invitrogen, Cat. no. A10312). Custom made primers (Applied biosystems, Thermo Fisher Scientific) specific for 16s rRNA of Porphyromonas gingivalis (Pg) (GenBank: AB035455.1; accession number: AIY9ZZ2) (https://www.ncbi.nlm.nih.gov/nuccore/AB035455.1) Fusobacterium nucleatum (Fn) (GenBank: AJ810280.1; accession number: APWCWT2) (https://www.ncbi.nlm.nih.gov/nuccore/AJ810280.1) and Streptococcus gordonii (Sg) (GenBank: D38483.1; accession number: AIT97D1) (https://www.ncbi.nlm.nih.gov/nuccore/D38483.1) were used to detect the presence of Pg, Fn and Sg in blood DCs and plaque samples. Universal bacterial 16s rRNA gene (total bacteria) (catalogue no. 4351372; Thermo Fisher Scientific) was used as an internal reference gene and relative abundance of Pg, Fn and Sg to the total bacteria were determined using 2-ΔΔCT method.
Detection of inflammatory mediators in gingival tissues
Total RNA was extracted from gingival tissues using RNAeasy Plus mini kit according to manufacturers’ instruction (Qiagen) after homogenizing in TRIzol (Invitrogen), and cDNA generated using High capacity reverse transcriptase kit (Applied Biosystems, Life Technologies). Quantitative real-time PCR (qRT- PCR) was performed using Taqman fast universal PCR mix and Taqman primer/probe mixtures: CD1C (Hs00233509_m1), CCL20 (Hs00355476_m1), Foxp3 (Hs01085834_m1), TGF-β (Hs00998133_m1), IL-10 (Hs00961622_m1), RANKL (Hs00243522_m1), RORC (Hs01076112_m1), HIF-1α (Hs00153153_m1), IL-6 (Hs00174131_m1), IL-1β (Hs01555410_m1), IL-17A (Hs00174383_m1) and GAPDH (Hs0275899_m1) (Applied biosystems, Thermo Fisher Scientific). GAPDH was used as housekeeping gene and the relative mRNA expression was calculated using 2-ΔΔCT method.
Ex vivo T cell isolation, stimulation and culture
CD4+ T cells from PBMCs were purified by negative selection using Human CD4+ T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada). The purity of CD4+ T cells was always >95%. Treg-TH17 plasticity was analyzed by stimulating ex-vivo isolated CD4+ T cells with plate bound functional grade purified anti-CD3 (clone UCHT1) (1 μg/ml) and functional grade purified soluble anti-CD28 (CD28.2) (2 μg/ml) for 72 hrs in serum free XVIVO 15 (Lonza) medium in humidified incubators at 37° C with 5% CO2 and with or without IL-1β, IL-6 alone and both. At the end of 72 hrs the CD4+ T cells were restimulated for 6 hrs with PMA (50 ng/ml) (Sigma Aldrich) and Ionomycin (1μg/ml) (Sigma Aldrich) along with Brefeldin A (BFA) (eBiosciences) for the detection of IL17A+ Th17, Foxp3+ Tregs and IL17A+FOXP3+ T cells in total CD4+ and in Foxp3+ T cells.
Antibodies and FACS staining for T cells
The following fluorescence conjugated antibodies, from Miltenyi Biotec were used: CD4 (clone: M-T466), CD45RA (clone: REA562), CD196 (clone: REA190), CD127 (clone: REA614), IL-17A (clone: CZ8–23G1). Conjugated antibodies against the following were from eBiosciences: CD25 (clone BC96), CTLA4 (clone 14D3), CD161 (clone HP-3G10), FOXP3 (clone 236A/E7), IL-10 (clone JES3-9D7) and CD45RO (clone UCHL1). Conjugated antibodies against the following were from R&D systems: RORγT (clone AFKJS-9) and IL-1R1 (Catalog number FAB269F). Antibodies against TGF-β (clone TW4–9E7) and CD126 (clone M5) was from BD Biosciences. Intracellular antigens were detected using Foxp3 Fixation/Permeabilization buffer set (eBiosciences) according to the manufactures instruction after blocking with anti-human FcR (Miltenyi Biotec). Cells were acquired immediately after staining on MACSQuant 8-color flow cytometer and data were analyzed using MACS quant analyzer 10 (Miltenyi Biotec). Unstained cells and Isotype-matched antibodies (directly conjugated) were used as controls. Dead cells were excluded by using Live/Dead fixable aqua dead cell stain kit (Thermo Fisher Scientific).
Cytokine detection
The serum levels of IL-1β and MIP-3α/CCL20 was quantified by ELISA using Human IL-1β and CCL20/MIP-3α Quantikine ELISA kits (R and D Systems Inc., Minneapolis, Minnesota, USA).
Clinical data assessment
All PD patients were subjected to full mouth periodontal probing at six sites per tooth, using a Michigan “O”probe, to record probing depths, gingival recession, and clinical attachment loss, at baseline, day 6, and 4–6 weeks after SRP as previously reported (8).
Statistical analysis
Statistical analysis was carried out using Graphpad Prism and SAS 9.4 (SAS Institute, Inc., Cary, NC, 2012 software. Values were expressed as mean ± SEM or Least square mean (LSM) ± SE where appropriate. Two-group repeated measures analysis, Student’s t test, Wilcoxon signed-ranks test, Mann Whitney U test, Dunn’s multiple comparisons test were used where appropriate for comparison between the groups as indicated. Statistical significance was defined as P < 0.05. Spearman’s correlation analyses were used and r values stated where relevant.
Results
AB treatment ablates keystone pathogen Pg from both oral and blood DC reservoirs
Analysis of dysbiotic pathogen Pg, and its consortium members, Sg and Fn in PD patients confirms presence of all three microbial signatures in oral reservoir (Fig. 1A) and in blood reservoir (DCs) (8) (Fig. 1E, 1F, 1G). AB treatment as part of a standard anti-infective protocol (27) resulted in a decrease of Pg in both reservoirs at final time point (Fig. 1B, 1E). No changes were observed in the levels of Fn (Fig. 1C) and Sg (Fig. 1D) at final time point in oral reservoir. Fn (Fig. 1F) but not Sg (Fig. 1G) content of DCs was significantly reduced in AB group at final time point compared to non-AB patients, consistent with added efficacy of local debridement and mouth rinse.
Figure 1.
AB treatment reduced Pg from both oral and blood DC reservoirs. Genomic DNA isolated from the gingival plaque samples of non-AB (n = 7–9) and AB (n = 6–9) patients were analyzed for the presence of Pg (B), Fn (C) and Sg (D) by qRT- PCR. (A) Graph represents that all PD patients were colonized orally by Pg, Fn and Sg at base line (BL). (B) Significant reduction in Pg 16s rDNA in AB group compared to non-AB subjects at final time point. (Data are Mean ± SEM, *p<0.05). (E-G) Relative expressions of 16s rRNA specific for Pg (E), Fn (F) and Sg (G) were determined in ex-vivo isolated human blood DCs in both non-AB (n = 7–9) and AB (n = 6–9) patients at final time point. AB patients show significant reduction in the expression of 16s rRNA specific for Pg (E) and Fn (F) at final time point compared to non-AB group. (Data are LSM ± SE, **p<0.01, *p<0.05). Relative abundance of Pg, Fn and Sg to the total bacteria were determined in indicated plaque samples.
AB-mediated microbial ablation inhibits expansion of CD1C+CCR6+ inflammatory blood DCs and increase in serum CCL20 levels
Major blood DC subsets, including CD303+ plasmacytoid DCs (pDCs), CD1C+ and CD141+ myeloid DCs (mDCs) were enumerated by flow cytometry from PBMCs of healthy and all PD patients at baseline before any treatment (Supplemental Fig. 1A, 1B) and after antibiotic treatment (Supplemental Fig. 1C). There was a significantly higher frequency of CD1C+CCR6+ mDCs in PD patients at baseline compared to healthy control (HC) subjects (Fig. 2A, 2B), indicative of in vivo expansion as reported (8). Accordingly, we assessed the influence of AB treatment on expansion of CD1C+CCR6+ blood mDCs in PD subjects. In AB group, expansion of CD1C+CCR6+ blood mDCs from BL was significantly inhibited at day 6, day 7 and final time points (Fig. 2C). AB treatment also prevented the spike in blood mDCs after debridement-induced bacteremia in PD patients (8). As CCL20 attracts immature mDCs into inflamed tissue sites (28), we posited that serum levels of CCL20, chemokine of CCR6, present in inflamed periodontal tissues (29) might be involved in influencing mDC blood pool. While there was a non-significant trend for elevated CCL20 in sera of PD patients vs. healthy controls (HC) (Supplemental Fig. 1D), AB treatment decreased serum CCL20 relative to baseline levels at the final time point (Fig. 2D).
Figure 2.
Increased expression of CD1C+CCR6+ Inflammatory blood DCs in periodontitis (PD) patients. Blood DCs isolated using human panDC isolation kit from PBMCs of HC, non-AB and AB patients were analyzed for CD1C+CCR6+ mDCs (A) Representative flow cytometry plots show the CCR6 expression in CD1C+ blood mDCs in healthy controls (HCs) and periodontitis (PD) patients at base line (BL) gated on total blood DCs. (B) Graph shows the frequency of CD1C+CCR6+ blood mDCs in periodontitis (PD) (n=16) and HC (n=14) subjects at base line (BL) (Data are Mean ± SEM, *p<0.05). (C) Mean change scores of CD1C+CCR6+ blood mDCs in AB (n = 6–8) and non-AB (n = 9–10) groups from base line (BL) to day 6 (24 hrs before SRP) day 7 (24 hrs after SRP) and final visits (4–6 weeks post-SRP). In AB patients, frequencies of CD1C+CCR6+ blood mDCs from base line (BL) was significantly decreased at day 6, day 7 and final time points (Data are LSM ± SE, *p<0.05, **p<0.01). (D) Only in AB group (n = 5–6), mean change scores of serum CCL20 differed significantly at final time compared to day7 (Data are LSM ± SE, * p<0.05).
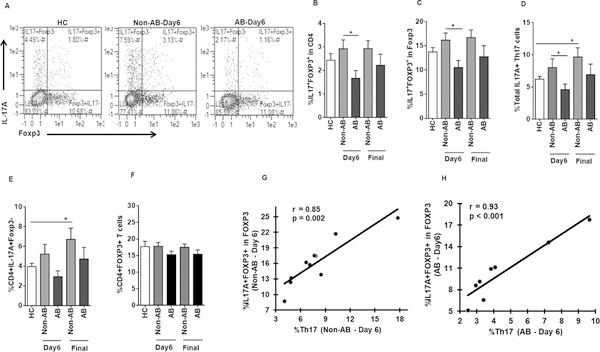
Frequency of IL-17A+FOXP3+ T cells in PD patients reduced by AB treatment
We have previously shown that Pg invades DCs and evades autophagy to stay viable and grow within (30); moreover, Pg infection induces IDO-competent immunoregulatory DCs (10). We therefore analyzed Th17 cell effectors and Tregs in the blood CD4+ T cells of AB and non-AB PD groups, as well as healthy controls, specifically focusing on double-positive IL-17A+Foxp3+ T cells (Fig. 3A, gated on total CD4+ T cells). One of the limitations of our study was the limited number of PBMCs available from PD patients for the isolation of homogeneous population of Tregs, hence we analyzed the double-positive IL-17A+Foxp3+ T cells in total CD4+Foxp3+ T cells rather from isolated Tregs population. AB treatment significantly reduced IL-17A+FOXP3+ T cells at day 6 (Fig. 3B and 3C, gated on total CD4+ and on Foxp3+ T cells, respectively). This was further reflected by a reduction of total CD4+IL17A+Foxp3+/− Th17 cells (Figure 3D) compared to non-AB patients. Further no reduction in the frequency of IL17A+Fopx3- Th17 cells was detected in AB patients compared to non-AB group at day 6 (Fig. 3E), suggesting that the observed decrease in the total Th17 cells in AB group might be due to the reduction in IL17A+FOXP3+ T cells. Further the reduction in IL17A+FOXP3+ T cells in AB group was not due to the difference in the starting population of CD4+Foxp3+ T cell population, as the frequency of CD4+Foxp3+ T cells did not change due to AB treatment in PD patients, although we observed a significant increase in CD4+Foxp3+ T cells in HC-BL compared to non-AB PD patients at day6 (data not shown). Further, no change in total CD4+FOXP3+ Tregs was observed in any of the groups after stimulation at day 6 and final visits (Fig. 3F). Further no difference was observed in the frequencies of IL17A+FOXP3+ (Fig. 3B and 3C), IL17A+FOXP3+/− (Fig. 3D) and IL17A+Foxp3- (Fig. 3E) T cells between healthy controls and PD patients at baseline (day 6 of non-AB). Only in the non-AB group at final time point was there a significant increase in the Th17 cell response, compared to healthy subjects (Fig. 3D and 3E). Notably, IL17A+FOXP3+ T cells showed significant positive correlation with IL17A+Foxp3- Th17 cells in both non-AB and AB groups at day 6 (Fig. 3G and 3H, respectively), while no significant correlation was found between 17A+FOXP3+ T cells and CD4+Foxp3+ Tregs. Together these data might suggest a role for AB therapy in short-term reduction of bone damaging Th17 cells (14) through modulation of Tregs to Th17 conversion.
Figure 3.
AB treatment reduced the frequencies of IL17A+FOXP3+ T cells in PD patients. CD4+ T cells from PBMCs of healthy controls (HCs) and periodontitis (PD) patients were isolated and stimulated in vitro with plate bound anti-CD3 and soluble anti-CD28 for 3 days followed by 6 hrs re-stimulation with PMA/Ionomycin along with BFA. (A) Representative flow cytometry profile show IL17A+FOXP3-, IL17A+FOXP3+ and IL17A-FOXP3+ populations in CD4+ T cells in healthy controls (HC) (n = 9) and day 6 of non-AB (non-AB day 6 is PD at baseline since no treatment was rendered) and AB patients. Graph shows the increased induction of IL17A+FOXP3+ T cells in CD4+ (B) and CD4+FOXP3+ populations (C) in non-AB (n = 10) compared to AB (n = 7–8) subjects at day 6 (Data are mean ± SEM, * p<0.05). (D) Reduced frequencies of total Th17 cells in AB (n = 7–8) subjects at day 6 compared to non-AB group (Data are mean ± SEM, * p<0.05). No change was observed in the frequencies of IL17A+FOXP3+ T cells (B and C) and total Th17 cells (D) between healthy controls (HC) and periodontitis (PD) patients at base line (non-AB day 6 is PD at baseline since no treatment was rendered). (E) No change in the frequencies of CD4+IL17A+Foxp3- Th17 cells in AB (n = 7–8) subjects at day 6 compared to non-AB group (n = 10) (Data are mean ± SEM, * p<0.05). (F) Frequencies of CD4+FOXP3+ T cells in healthy controls (HC) (n = 9), non-AB (n = 10) and AB (n = 7–8) patients at day 6 and final time points after stimulation. (G-H) Graphs depicts the Spearman’s positive correlation between %IL17A+Foxp3+ T cells and %IL17A+Foxp3- Th17 cells at day 6 in non-AB (n = 10) (G) and AB (H) groups (n = 8).
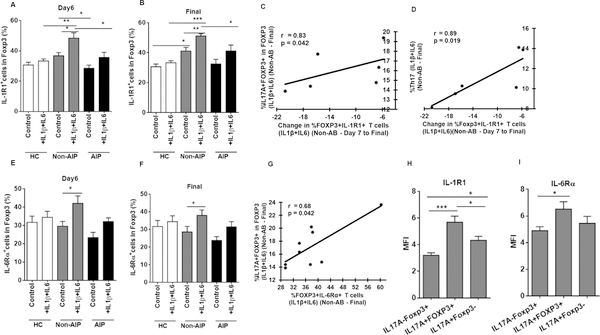
AB treatment decreases cytokine-mediated boosting of IL-17+FOXP3+ T cells
Human monocyte derived DCs secrete high levels of IL1-β and IL-6 when infected with Pg (59), cytokines important in differentiation of IL17+FOXP3+ T cells (31–33). Moreover, IL1-β and IL-6 are elevated in lesions (18, 19, 21, 34) and serum of PD patients (35). We hypothesized a role for IL1-β and IL-6 in conversion of Tregs to IL17+FOXP3+ T cells in PD patients not treated with AB. We show that indeed, CD4+ T cells of non-AB group responded robustly to either IL-1β, IL-6 or both together, differentiating into IL17A+FOXP3+ T cells at all-time points (Fig. 4A and 4B), compared to HC. Conversely, CD4+ T cells of AB group were resistant to IL1-β and IL-6 mediated differentiation of CD4+Foxp3+ T cell into IL17+FOXP3+ T cells, equivalent to non-cytokine treated control at day 6 (Fig. 4A) and final (Fig. 4B) time points and remained non-significant compared to HC, which was further reflected in the frequencies of total Th17 cells at day6 (Fig. 4C) and final (Fig. 4D) time points. Likewise, as observed in the absence of cytokines in Fig. 3D and 3E, the observed significant reduction in total Th17 cells (Fig. 4C, 4D) in the presence of both IL1-β and IL-6 in AB group might be due to the reduction in the double positive IL17+FOXP3+ T cells, as no change was detected in IL17A+Foxp3- Th17 cells between non-AB and AB groups at day 6 (Fig. 4E, 4F). Further, the increase in total Th17 cells in non-AB group (Fig. 4C) in the presence of both IL1-β and IL-6 when compared to its respective no cytokine control group at day 6 might be due to the increase in the double positive IL17A+Foxp3+ T cells (Fig. 4A), as no change was detected in the frequency of IL17A+Foxp3- Th17 cells in non-AB in the presence of both IL1-β and IL-6 (Fig. 4E). While we could not detect significant differences in relative IL1-β and IL-6 serum levels in the groups, there was a significant inverse correlation between serum IL-1 β and frequency of Tregs (Fig. 4G) in non-AB at final time point. These findings suggest that elevated levels of inflammatory cytokines IL1-β and IL-6 might provide a polarizing environment for peripheral expansion of Th17 cells by the induction of double positive IL17A+Foxp3+ T cells in PD and PD associated systemic disorders.
Figure 4.
Inflammatory cytokines in periodontitis (PD) patients enhanced the induction of IL17A+FOXP3+ T cells. CD4+ T cells were stimulated as in figure 3 in the presence and absence of either IL-1β or IL-6 or both. (A and B) Presence of IL-1β and IL-6 increased the induction of IL-17A+FOXP3+ T cells in non-AB (n = 10) patients at day 6 (A) and final (B) time points compared to healthy controls (HC) (n = 9) and AB (n = 7–8) patients (Data are mean ± SEM, * p<0.05, **p<0.01). (C and D) Increased induction of total Th17 cells in non-AB (n = 10) patients at day 6 (C) and final (D) time points in the presence of IL-1β, IL-6 or both when compared to healthy controls (HC) (n = 9) and AB (n = 7–8) groups (Data are mean ± SEM, * p<0.05, **p<0.01). (E and F) No change in CD4+IL17A+Foxp3- Th17 cells between AB (n = 7–8) and non-AB groups (n = 10) in the presence of both IL-1β or IL-6 at day 6 (E) and final time point (F) (Data are mean ± SEM, * p<0.05). (G) Graph depicts the Spearman’s inverse correlation of serum IL-1β and %Tregs at final time point in non-AB group (n = 8).
AB treatment reduces IL-1R1 expression on Tregs, involved in induction of IL17+Foxp3+ T cells
In vitro expanded human (36–40) and murine (41, 42) Tregs that express IL-1R1 and IL-6Rα are early intermediates of Th17 effectors under inflammatory conditions (14, 36, 39, 41–43). We therefore analyzed IL-1R1 and IL-6Rα expression on CD4+Foxp3+ T cells of all groups. CD4+Foxp3+ T cells expressing IL1R1 and IL6Rα in HC, non-AB and AB groups both in the presence and absence of IL1β and IL6 were depicted in supplemental Figure 2A. We show that FoxP3+ T cells from non-AB group expressed higher IL-1R1 expression relative to HC and AB group, and were highly responsive to cytokine stimulation by further upregulation of IL-1R1 (Fig. 5A, 5B); moreover, change in IL-1R1 expression on CD4+FoxP3+ T cells in the presence of cytokines correlated positively with IL17A+FOXP3+ T cells (Fig. 5C) and Th17 cells (Fig. 5D) at final time point in non-AB group. On the contrary, AB treatment comparatively decreased the expression of IL-1R1 both in the presence and absence of IL1-β at day 6 (Fig. 5A) and final (Fig. 5B) time points compared to non-AB group. IL-6R1 expression on CD4+Foxp3+ T cells of non-AB group was more dependent than IL-1R1, on cytokine stimulation (Fig. 5E and 5F). Nevertheless, a significant positive correlation between Foxp3+IL-6Rα+ population and IL17A+FOXP3+ T cells was detected in response to IL1-β and IL-6 in non-AB group at final time point (Fig. 5G). Moreover, there was a significant increase detected in IL-1R1 and IL-6Rα expressions in IL17A+FOXP3+ T cells of PD patients, compared to conventional IL17A-Foxp3+ Tregs (Fig. 5H, 5I, respectively) and IL17A+Foxp3- Th17 cells (Fig. 5H). Additionally, we found that IL-17A+Foxp3+ T cells were enriched in IL-1R1+Foxp3+CD45RO+ MTregs (data not shown), which is in consistent with the previous reports (36) (39). Overall, these data suggest an important role for oral microbial burden in driving expression of IL-1R1 on CD4+FOXP3+ Tregs, leading to induction of IL17+Foxp3+ T cells through IL-1β signaling. This, in combination with IL-6/IL-6Rα, enhances the differentiation of Tregs to IL-17A producing T cells. The above data also suggest an essential role of AB treatment in reducing the expression of IL1R1 on Tregs via regulating the constant low-grade bacteremia in PD patients.
Figure 5.
Increased expression of IL-1R1 and IL-6Rα in CD4+Foxp3+ T cells in PD patients. CD4+ T cells were stimulated as in figure 3 in the presence and absence of IL-1β+IL-6. (A and B) Frequencies of C4+Foxp3+ T cells expressing IL-1R1 was increased in non-AB (n = 6–9) patients compared to healthy controls (HC) (n = 8) and AB (n = 5–6) subjects at day6 (A) and final (B) time points (Data are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001). (C and D) Graphs depicts the Spearman’s positive correlation of Foxp3+IL-1R1+ population with %IL17A+Foxp3+ T cells (C) and Th17 cells (D) at final time point in response to IL1-β and IL-6 in non-AB group (n=6). (E and F) Graph shows the percentage of C4+Foxp3+ T cells expressing IL6Rα in healthy controls (HC) (n = 8), non-AB (n = 6–9), and AB (n = 5–6) subjects at day6 (E) and final (F) time points (Data are mean ± SEM, *p<0.05, **p<0.01). (G) Graph depicts the Spearman’s positive correlation between the Foxp3+IL-6Rα+ population and %IL17A+Foxp3+ T cells at final time point in response to IL1-β and IL-6 in non-AB group (n=9). (H) Increased expression of IL-1R1 in IL-17A+Foxp3+ Treg compared to conventional Treg and Th17 populations in periodontitis (PD) patients (Data are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001). (I) Increased expression of IL-6Rα in IL-17A+Foxp3+ Treg compared to conventional IL17A-Foxp3+ Treg (Data are mean ± SEM, *p<0.05).
IL-17+FOXP3+ T cells in PD patients demonstrate phenotype characteristics of both conventional Treg and Th17 cells
As we used total CD4+ T cells, the phenotype of IL-17A producing CD4+FOXP3+ T cells was further examined for markers of conventional CD4+IL17A-FOXP3+ Treg or IL17A+Foxp3- Th17 cells. Compared to CD4+IL17A-Foxp3+ Tregs, IL17A+Foxp3+ T cells had significantly higher expression of Treg markers FOXP3, TGF- β, IL-10, CTLA4 and CD127 (Fig. 6A - 6E). CD127 was also significantly higher in Th17 cells (44) (Fig. 6E). Moreover, human Th17 markers RORγt, IL17A, CD161 and CCR6 (45, 46) were significantly higher in IL17A+Foxp3+ T cells compared to CD4+IL17A-FOXP3+ Tregs (Fig. 6F - 6I). Overall, IL-17-producing CD4+FOXP3+ T cells possess phenotypes of both conventional Th17 and Treg cell populations.
Figure 6.
IL17A+FOXP3+ T cells display phenotype characteristics of Th17 and Treg cells in PD patients. Expressions of Foxp3, TGF-β, IL-10, CTLA4, CD127, RORγt, IL17A, CD161 and CCR6 were measured in CD4+FOXP3+ conventional Treg, IL17A+FOXP3+ T cells and IL-17A+ Th17 cells in periodontitis (PD) patients. (A - I) Graphs show the difference in the mean fluorescence intensity (MFI) of the above mentioned markers between the subsets in periodontitis (PD) patients (Data are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001).
Influence of AB treatment on gingival tissue markers of inflammation
To determine the local gingival response to AB therapy, gingival tissue biopsies from AB and non-AB groups were analyzed for mRNA of immature DCs (CD1C), tissue recruitment chemokine (CCL20), Th17 and Treg cells specific cytokines and transcription factors (RORC, RANKL, IL-1β, IL-6, Foxp3, IL-10, TGF-β and HIF-1α) (Supplemental Fig. 3A - 3K). When comparing AB group to non-AB group, we observed a significant decrease in the local mRNA expression of IL-1β, RANKL and IL-10 in gingiva of AB group compared to non-AB group at final time point (Supplementary Fig. 3F, 3G and 3J, respectively), consistent with improvements in local clinical response (Table 1) (supplementary Fig. 4A and 4B). Further we observed a trend for decreased CD1C, CCR6, CCL20, HIF-1α, RORC, IL-6, Foxp3, and TGF-β mRNA in AB group at final time point, compared to non-AB (Supplementary Fig. 3A, 3B – 3E, 3H, 3I and 3K, respectively). Additionally, in non-AB group at final time point the expression of CCR6 and CCL20 (Supplementary Fig. 3B and 3C, respectively) were significantly associated with clinical symptoms of PD (Supplementary Fig. 4C and 4D).
Table 1.
Antibiotic treatment improved the PPD and CAL in PD patients.
| Clinical Indices | Treatment Group | Exam | n | Mean ± SD (Each Exam) | Mean ± SD (Improvement) |
|---|---|---|---|---|---|
| PPD | Non-AB | BL | 10 | 3.6 ± 0.5 | 0.3 ± 0.2** |
| Final | 10 | 3.3 ± 0.4 | |||
| AB | BL | 7 | 3.5 ± 0.9 | 0.6 ± 0.5* | |
| Final | 7 | 2.9 ± 0.4 | |||
| CAL | Non-AB | BL | 10 | 4.1 ± 0.9 | 0.2 ± 0.4 |
| Final | 10 | 3.9 ± 0.9 | |||
| AB | BL | 7 | 3.4 ± 0.9 | 0.5 ± 0.7* | |
| Final | 7 | 2.8 ± 0.3 |
PPD; Probing Depth, CAL; Clinical attachment loss.
p<0.05
p<0.01.
Association between the frequencies of Tregs, Th17, IL17A+Foxp3+ T cells and clinical improvement of PD
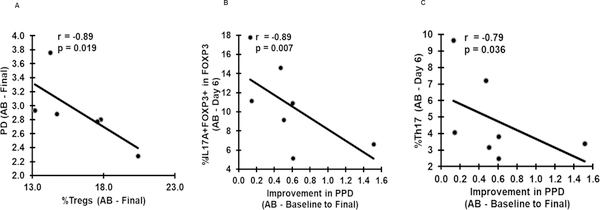
Initial AB therapy, as part of standard debridement and mouthrinse significantly decreased the signs of periodontitis, probing pocket depths (PPD) and clinical attachment loss (CAL) (Table 1). There was also improvement in PPD but not CAL in non-AB patients, attributed to the scaling (Table 1). In AB group the frequencies of Tregs was inversely correlated with PPD (r=−0.89, p=0.019, Fig. 7A) at final time point; moreover, decreases in the frequencies of IL17A+Foxp3+ T cells (Fig. 3C) and Th17 cells (Fig. 3D) at day 6 were strongly correlated with the improvement in PPD (Fig. 7B, 7C, respectively). This in turn supports the role of AB treatment and Treg stability in the improvement of clinical measures of PD. Overall, these results emphasize the important role that systemic and local clearance of dysbiotic microflora plays in efforts to improve clinical outcomes and in modulation of Treg to Th17 balance.
Figure 7.
Association of Pg, Tregs, Th17 and IL17A+FOXP3+ T cells with clinical improvement of periodontitis (PD). (A) Graph depicts the spearman’s inverse correlation between the %Tregs and periodontitis (PD) at final time point in AB group (n=7). (B and C) Graphs depicts the spearman’s negative correlations of % IL17A+FOXP3+ T cells (C) and %Th17 cells with improvement in periodontitis (PD) in AB group (n=7) at final time point. PPD, probing depth.
Discussion
The purpose of the current small pilot randomized clinical trial was not to examine the therapeutic efficacy of AB for PD, which has been previously reviewed (27). The purpose here was to determine whether ablating the dysbiotic oral microbes could reverse local and systemic inflammatory responses in PD patients. It is important to express caution in interpretation of this study, as it was only a 4–6 week study and patients were not followed for cellular responses afterward. However, by temporarily ablating the oral microbiome, i.e. the “mobile microbiome” (47) with AB, we were able to analyze its role in induction of inflammatory mDCs and CD4+IL-17A+Foxp3+ T cells in circulation. This AB combination is highly effective against the species analyzed here, including obligate anaerobes Pg and Fn (i.e. metronidazole) (48) and facultative anaerobes such as Sg (i.e. amoxicillin) (49). Of the three periodontal pathogens, the keystone pathogen Pg (50) was significantly reduced by AB treatment in both oral and systemic reservoirs. The increased trend in Sg load in oral reservoir in the AB group might be due to poor antibiotic exposure to the subgingival pockets, as Sg precedes the colonization of Fn and Pg in the biofilm (51, 52). However, we cannot rule out direct immunomodulatory effects of AB, the classic example being the tetracyclines, which inhibit matrix metalloproteinases at sub-anti-microbial doses (53). Beta-lactam antibiotics such as amoxicillin can be biotransformed by human microbiota into products that can activate NF-κB signaling in human monocytes (54). Metronidazole reportedly inhibits the production of IL-1β, IL-6, IL-8, IL-12 and TNFα by human periodontal ligament cells in vitro in response to Pg LPS (55) and modulates the mitogenic response of human lymphocytes to phytohemagglutinin (PHA) (56). Oral metronidazole modulates the severity of experimental autoimmune uveitis (EAU) in B10.RIII mice by increasing Tregs in the gut and extra intestinal tissues, as well as decreasing effector T cells and cytokines, but the role of the microflora was not examined in this previous study (57). When part of a broad spectrum AB combination, metronidazole also dramatically decreased cecal content of Firmicutes and Bacteroidetes, as well as the bacterial class, Alphaproteobacteria. Metronidazole reportedly decreases circulating neutrophils and monocytes in mice (58), a finding particularly relevant to the present study, as monocytes are a major precursor to myeloid DCs reduced by AB in our study. We have previously shown that expansion of mDCs in circulation in PD patients was not mediated by DC poietins and that the expanded mDCs contained Pg, as detected by 16s rRNA and immunofluorescence microscopy, using monoclonal antibody against the Mfa1 fimbriae (8). The Mfa1 fimbriae is a DC invasin (59, 60), which promotes non-canonical mDC differentiation in vitro (9) and evasion of autophagy by Pg (30). Inflammatory mDCs infiltrate diseased tissues of patients with PD and form immune conjugates with CD4+ T cell in situ (61–63). The double positive (IL-17A+Foxp3+) CD4+ T cells examined here infiltrate periodontitis tissues of both mice and humans. The conversion of Tregs to Th17 effectors is thought to contribute to disease pathogenesis (14, 24, 25). Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction (64). Furthermore, it has been demonstrated that dysbiotic microbiome triggers the expansion of Th17 cells in human periodontitis (23). Hence, these findings of AB mediated reduction in Th17 conversion are not only relevant to bone destruction, but to management of patients with comorbid conditions such as cardiovascular disease (65), chronic kidney disease requiring dialysis (66) and rejection of a transplanted kidney (67, 68).
In the current study we cannot exclude the possibility that the observed IL-17A-producing Foxp3+ T cells in our ex-vivo stimulated cultures might have arisen from non-regulatory CD4+ T cells. CD4+CD25- conventional T cells transiently express Foxp3 following in vitro stimulation (69), the detected IL17A-producing Foxp3+ CD4+ T cells in healthy controls and PD patients might have differentiated mostly from memory Tregs (MTregs) (70) (31), as the percentage of MTregs was significantly higher in PD patients compared to their naïve counterparts (supplemental Fig. 4E). Moreover, the Treg population is heterogeneous with respect to the expression of cell surface markers (CD25 and CD45RA) and transcription factor Foxp3 (71). Although it has been reported that both FOXP3low- and Foxp3high- expressing Tregs are capable of producing IL17A, in the current study most of the IL17A-producing Foxp3+ CD4 T cells were within the CD45RA-FOXP3low and CD45RA-Foxp3high populations, whereas relatively fewer IL17A-producing Foxp3+ CD4 T cells were distributed within the CD45RA+Foxp3low resting Tregs (supplemental Fig. 4F). These findings clearly demonstrate that IL17A-producing Foxp3+ CD4 T cells were derived from diverse Treg lineages, and Treg plasticity toward Th17 occurred in all these subpopulations. Additionally, the IL17A-producing Foxp3+ CD4 T cells in the current study were characterized by the elevated expressions of both Th17 and Treg cell markers. These results were in consistent with the previous reports demonstrating that CD4+FOXP3+ Tregs expressing CCR6 (72) (31), RORγt (73) (74) (70), CD161 (45) (75) and CTLA4 (76) were capable of differentiating into IL17A-producing Tregs (77) (78) and could secrete moderate levels of TGF-β (79).
Based on our current findings, the lower frequency of IL17A+ Foxp3+ CD4+ T cells in healthy controls and in AB treated group can be attributed to the decreased expression of IL-1R1 on CD4+Foxp3+ Tregs in these groups. IL1β, an essential cytokine for the development of IL-17A-producing Th17 cells, signals through engagement of the receptor complex formed by IL-1R1 and IL-1 receptor accessory protein (IL-1RAcP), which is recruited to the complex after the binding of IL-1β to IL-1R1 (80). Human Th17 cell differentiation is predominantly regulated via differential expression of IL-1RI (81). Further additional studies have documented the selective expression of IL-1R1 by both ex vivo and in in vitro expanded human Tregs (36–40), and were considered to be an early intermediate in the differentiation of Tregs to Th17 effectors under inflammatory conditions, through IL-1β/IL-1R1 mediated signals (36, 39, 43). IL-6 exerts its effect via binding to IL-6Rα and their interaction with gp130 (41, 42). Although IL-6 alone has been reported to be sufficient in the induction of IL17-producing Tregs in mice (82, 83), later studies revealed that IL-1β/IL-1R mediated signaling was essential for the early differentiation of murine Th17 cells (84). While we could not detect significant differences in IL1-β and IL-6 serum levels in the groups, we detected significant correlations of serum IL-1 β with %Tregs in non-AB group (Figure 4G).
In conclusion our findings support a novel anti-inflammatory function of AB therapy, through its immunomodulatory properties and disruption of dysbiosis. We showed reduced expansion of tissue homing myeloid DC subset in blood and tissues associated with reduction in IL-1β mediated conversion of Tregs to bone damaging Th17 cells. Increased IL17A-producing Foxp3+ CD4+ T cells in the peripheral blood of PD patients could have detrimental properties in other comorbid diseases such as kidney disease and cardiovascular disease. This however, is a short-term study, and the residual longer term (i.e. 6 mo-1 yr) effects of 7 days of AB therapy will need to be further examined to determine if we can reduce conversion of Tregs to Th17 in periodontitis patients in the long term.
Supplementary Material
Key Points.
AB therapy in PD subjects ablated P.gingivalis from oral and hematogenous reservoirs.
This reduced inflammatory blood mDCs and IL-1R expression on Tregs in PD subjects.
A favorable clinical response was related with Pg ablation and decrease in Treg→Th17.
Acknowledgements
We are grateful to David Munn (Augusta University, Augusta, GA) for the expertise and feedback. We thank Zoya B. Kurago (Augusta University, Augusta, GA) for helpful scientific discussion.
Footnotes:
This study was funded in part by NIH-NIDCR (R01 DE014328) and the Carlos and Marguerite Mason trust to improve kidney transplant outcomes in Georgia (to CWC).
Abbreviation used in this article:
- PD
periodontitis
- Tregs
regulatory T cells
- AB
antibiotics
- DCs
dendritic cells
- mDCs
myeloid dendritic cells
- non-AB
no antibiotics
- Pg
Porphyromonas gingivalis
- Fn
Fusobacterium nucleatum
- Sg
Streptococcus gordonii
- IL1R1
interleukin receptor 1
- IL6Rα
interleukin receptor alpha
- HC
healthy controls
- PPD
probing depth
- CAL
clinical attachment loss
- MTregs
memory Tregs
- NTregs
naïve Tregs.
Footnotes
Disclosures
The authors have no conflicts of interest.
References
- 1.Darveau RP 2009. The oral microbial consortium’s interaction with the periodontal innate defense system. DNA and cell biology 28: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socransky SS, and Haffajee AD. 2005. Periodontal microbial ecology. Periodontology 2000 38: 135–187. [DOI] [PubMed] [Google Scholar]
- 3.Teles R, Teles F, Frias-Lopez J, Paster B, and Haffajee A. 2013. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000 62: 95–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajishengallis G 2010. Complement and periodontitis. Biochemical pharmacology 80: 1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gemmell E, Yamazaki K, and Seymour GJ. 2007. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontology 2000 43: 14–40. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki K, Yoshie H, and Seymour GJ. 2003. T cell regulation of the immune response to infection in periodontal diseases. Histology and histopathology 18: 889–896. [DOI] [PubMed] [Google Scholar]
- 7.Hajishengallis G, Darveau RP, and Curtis MA. 2012. The keystone-pathogen hypothesis. Nature reviews. Microbiology 10: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, Bear A, Genco CA, Brown DL, and Cutler CW. 2012. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. Journal of immunology (Baltimore, Md. : 1950) 189: 3178–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miles B, Scisci E, Carrion J, Sabino GJ, Genco CA, and Cutler CW. 2013. Noncanonical dendritic cell differentiation and survival driven by a bacteremic pathogen. Journal of leukocyte biology 94: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyagi RK, Miles B, Parmar R, Garg NK, Dalai SK, Baban B, and Cutler CW. 2017. Human IDO-competent, long-lived immunoregulatory dendritic cells induced by intracellular pathogen, and their fate in humanized mice. Scientific reports 7: 41083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garlet GP, Cardoso CR, Mariano FS, Claudino M, de Assis GF, Campanelli AP, Avila-Campos MJ, and Silva JS. 2010. Regulatory T cells attenuate experimental periodontitis progression in mice. Journal of clinical periodontology 37: 591–600. [DOI] [PubMed] [Google Scholar]
- 12.Ernst CW, Lee JE, Nakanishi T, Karimbux NY, Rezende TM, Stashenko P, Seki M, Taubman MA, and Kawai T. 2007. Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease. Clinical and experimental immunology 148: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffen SL, and Hajishengallis G. 2008. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. Journal of dental research 87: 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K, and Takayanagi H. 2018. Host defense against oral microbiota by bone-damaging T cells. Nature communications 9: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, Oukka M, and Kuchroo VK. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nature immunology 8: 345–350. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Honda T, Domon H, Oda T, Okui T, Amanuma R, Nakajima T, and Yamazaki K. 2005. Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral microbiology and immunology 20: 382–386. [DOI] [PubMed] [Google Scholar]
- 17.Honda T, Aoki Y, Takahashi N, Maekawa T, Nakajima T, Ito H, Tabeta K, Okui T, Kajita K, Domon H, and Yamazaki K. 2008. Elevated expression of IL-17 and IL-12 genes in chronic inflammatory periodontal disease. Clinica chimica acta; international journal of clinical chemistry 395: 137–141. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, and Silva JS. 2009. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral microbiology and immunology 24: 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, Yamada N, Hata M, Yamane J, and Terada N. 2009. The involvement of IL-23 and the Th17 pathway in periodontitis. Journal of dental research 88: 633–638. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RB, Wood N, and Serio FG. 2004. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. Journal of periodontology 75: 37–43. [DOI] [PubMed] [Google Scholar]
- 21.Lester SR, Bain JL, Johnson RB, and Serio FG. 2007. Gingival concentrations of interleukin-23 and −17 at healthy sites and at sites of clinical attachment loss. Journal of periodontology 78: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 22.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, and Gamonal J. 2005. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. Journal of clinical periodontology 32: 383–389. [DOI] [PubMed] [Google Scholar]
- 23.Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, Morell RJ, Freeman AF, Lazarevic V, Trinchieri G, Diaz PI, Holland SM, Belkaid Y, Hajishengallis G, and Moutsopoulos NM. 2018. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Science translational medicine 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okui T, Aoki Y, Ito H, Honda T, and Yamazaki K. 2012. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. Journal of dental research 91: 574–579. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Guan N, Jin Y, Lin X, and Gao H. 2015. Subcutaneous vaccination with Porphyromonas gingivalis ameliorates periodontitis by modulating Th17/Treg imbalance in a murine model. International immunopharmacology 25: 65–73. [DOI] [PubMed] [Google Scholar]
- 26.Armitage GC 1999. Development of a classification system for periodontal diseases and conditions. Annals of periodontology 4: 1–6. [DOI] [PubMed] [Google Scholar]
- 27.Keestra JA, Grosjean I, Coucke W, Quirynen M, and Teughels W. 2015. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated chronic periodontitis: a systematic review and meta-analysis. Journal of periodontal research 50: 294–314. [DOI] [PubMed] [Google Scholar]
- 28.Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M, Nishimura A, Endo H, Kitasato H, Kawai S, Takagishi K, and Kondo H. 2001. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clinical and experimental immunology 125: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosokawa Y, Nakanishi T, Yamaguchi D, Takahashi K, Yumoto H, Ozaki K, and Matsuo T. 2002. Macrophage inflammatory protein 3alpha-CC chemokine receptor 6 interactions play an important role in CD4+ T-cell accumulation in periodontal diseased tissue. Clinical and experimental immunology 128: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, Waller JL, Genco CA, Slocum C, Manning M, Schoenlein PV, and Cutler CW. 2015. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS pathogens 10: e1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, and Liu YJ. 2009. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proceedings of the National Academy of Sciences of the United States of America 106: 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, and Joosten I. 2008. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112: 2340–2352. [DOI] [PubMed] [Google Scholar]
- 33.Deknuydt F, Bioley G, Valmori D, and Ayyoub M. 2009. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clinical immunology (Orlando, Fla.) 131: 298–307. [DOI] [PubMed] [Google Scholar]
- 34.Honda T, Domon H, Okui T, Kajita K, Amanuma R, and Yamazaki K. 2006. Balance of inflammatory response in stable gingivitis and progressive periodontitis lesions. Clinical and experimental immunology 144: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, and Madalinski K. 2003. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. Journal of clinical periodontology 30: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 36.Mercer F, Kozhaya L, and Unutmaz D. 2010. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PloS one 5: e8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, and Shevach EM. 2009. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood 113: 5125–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valmori D, Raffin C, Raimbaud I, and Ayyoub M. 2010. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proceedings of the National Academy of Sciences of the United States of America 107: 19402–19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raffin C, Raimbaud I, Valmori D, and Ayyoub M. 2011. Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells. Journal of immunology (Baltimore, Md. : 1950) 187: 5196–5202. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, and Zheng SG. 2014. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proceedings of the National Academy of Sciences of the United States of America 111: E3432–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, and Zheng SG. 2010. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. Journal of immunology (Baltimore, Md. : 1950) 185: 2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor RA, Floess S, Huehn J, Jones SA, and Anderton SM. 2012. Foxp3(+) Treg cells in the inflamed CNS are insensitive to IL-6-driven IL-17 production. European journal of immunology 42: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 43.Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, Pear WS, Hatton RD, and Weaver CT. 2015. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nature immunology 16: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulahad WH, Boots AM, and Kallenberg CG. 2011. FoxP3+ CD4+ T cells in systemic autoimmune diseases: the delicate balance between true regulatory T cells and effector Th-17 cells. Rheumatology (Oxford, England) 50: 646–656. [DOI] [PubMed] [Google Scholar]
- 45.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, and Annunziato F. 2008. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. The Journal of experimental medicine 205: 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, and Sallusto F. 2007. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nature immunology 8: 942–949. [DOI] [PubMed] [Google Scholar]
- 47.Han YW, and Wang X. 2013. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. Journal of dental research 92: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dabija-Wolter G, Al-Zubaydi SS, Mohammed MMA, Bakken V, and Bolstad AI. 2018. The effect of metronidazole plus amoxicillin or metronidazole plus penicillin V on periodontal pathogens in an in vitro biofilm model. Clinical and experimental dental research 4: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SY 2000. Postantibiotic effects and postantibiotic sub-MIC effects of amoxicillin on Streptococcus gordonii and Streptococcus sanguis. Journal of chemotherapy (Florence, Italy) 12: 379–384. [DOI] [PubMed] [Google Scholar]
- 50.Hajishengallis G, and Lamont RJ. 2012. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Molecular oral microbiology 27: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA Jr., and Progulske-Fox A. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arteriosclerosis, thrombosis, and vascular biology 25: e17–18. [DOI] [PubMed] [Google Scholar]
- 52.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, and Palmer RJ Jr. 2002. Communication among oral bacteria. Microbiology and molecular biology reviews: MMBR 66: 486–505, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan ME, Ramamurthy S, and Golub LM. 1996. Matrix metalloproteinases and their inhibition in periodontal treatment. Current opinion in periodontology 3: 85–96. [PubMed] [Google Scholar]
- 54.Oh J, Patel J, Park HB, and Crawford JM. 2018. beta-Lactam Biotransformations Activate Innate Immunity. The Journal of organic chemistry 83: 7173–7179. [DOI] [PubMed] [Google Scholar]
- 55.Rizzo A, Paolillo R, Guida L, Annunziata M, Bevilacqua N, and Tufano MA. 2010. Effect of metronidazole and modulation of cytokine production on human periodontal ligament cells. International immunopharmacology 10: 744–750. [DOI] [PubMed] [Google Scholar]
- 56.Elizondo G, and Ostrosky-Wegman P. 1996. Effects of metronidazole and its metabolites on histamine immunosuppression activity. Life sciences 59: 285–297. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura YK, Metea C, Karstens L, Asquith M, Gruner H, Moscibrocki C, Lee I, Brislawn CJ, Jansson JK, Rosenbaum JT, and Lin P. 2016. Gut Microbial Alterations Associated With Protection From Autoimmune Uveitis. Investigative ophthalmology & visual science 57: 3747–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fararjeh M, Mohammad MK, Bustanji Y, Alkhatib H, and Abdalla S. 2008. Evaluation of immunosuppression induced by metronidazole in Balb/c mice and human peripheral blood lymphocytes. International immunopharmacology 8: 341–350. [DOI] [PubMed] [Google Scholar]
- 59.Zeituni AE, Jotwani R, Carrion J, and Cutler CW. 2009. Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. Journal of immunology (Baltimore, Md. : 1950) 183: 5694–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeituni AE, McCaig W, Scisci E, Thanassi DG, and Cutler CW. 2010. The native 67-kilodalton minor fimbria of Porphyromonas gingivalis is a novel glycoprotein with DC-SIGN-targeting motifs. Journal of bacteriology 192: 4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jotwani R, and Cutler CW. 2003. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. Journal of dental research 82: 736–741. [DOI] [PubMed] [Google Scholar]
- 62.Jotwani R, Muthukuru M, and Cutler CW. 2004. Increase in HIV receptors/co-receptors/alpha-defensins in inflamed human gingiva. Journal of dental research 83: 371–377. [DOI] [PubMed] [Google Scholar]
- 63.Jotwani R, Palucka AK, Al-Quotub M, Nouri-Shirazi M, Kim J, Bell D, Banchereau J, and Cutler CW. 2001. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. Journal of immunology (Baltimore, Md. : 1950) 167: 4693–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, and Takayanagi H. 2006. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of experimental medicine 203: 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez NJ, Quintero A, Casanova PA, Ibieta CI, Baelum V, and Lopez R. 2012. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. Journal of periodontology 83: 267–278. [DOI] [PubMed] [Google Scholar]
- 66.Zhao D, Khawaja AT, Jin L, Li KY, Tonetti M, and Pelekos G. 2018. The directional and non-directional associations of periodontitis with chronic kidney disease: A systematic review and meta-analysis of observational studies. Journal of periodontal research. [DOI] [PubMed] [Google Scholar]
- 67.Segelnick SL, and Weinberg MA. 2009. The periodontist’s role in obtaining clearance prior to patients undergoing a kidney transplant. Journal of periodontology 80: 874–877. [DOI] [PubMed] [Google Scholar]
- 68.Zwiech R, and Bruzda-Zwiech A. 2013. Does oral health contribute to post-transplant complications in kidney allograft recipients? Acta odontologica Scandinavica 71: 756–763. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, and Toes RE. 2007. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. European journal of immunology 37: 129–138. [DOI] [PubMed] [Google Scholar]
- 70.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, and Valmori D. 2009. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proceedings of the National Academy of Sciences of the United States of America 106: 8635–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, and Sakaguchi S. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899–911. [DOI] [PubMed] [Google Scholar]
- 72.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, and Falk K. 2005. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood 105: 2877–2886. [DOI] [PubMed] [Google Scholar]
- 73.Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW, Panaccione R, and Ghosh S. 2013. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflammatory bowel diseases 19: 2522–2534. [DOI] [PubMed] [Google Scholar]
- 74.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, and Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. The Journal of experimental medicine 205: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basdeo SA, Moran B, Cluxton D, Canavan M, McCormick J, Connolly M, Orr C, Mills KH, Veale DJ, Fearon U, and Fletcher JM. 2015. Polyfunctional, Pathogenic CD161+ Th17 Lineage Cells Are Resistant to Regulatory T Cell-Mediated Suppression in the Context of Autoimmunity. Journal of immunology (Baltimore, Md. : 1950) 195: 528–540. [DOI] [PubMed] [Google Scholar]
- 76.Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C, Gao N, Jia Y, Xu D, Huang FP, Li N, Lu L, and Li ZG. 2015. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Annals of the rheumatic diseases 74: 1293–1301. [DOI] [PubMed] [Google Scholar]
- 77.Esposito M, Ruffini F, Bergami A, Garzetti L, Borsellino G, Battistini L, Martino G, and Furlan R. 2010. IL-17- and IFN-gamma-secreting Foxp3+ T cells infiltrate the target tissue in experimental autoimmunity. Journal of immunology (Baltimore, Md. : 1950) 185: 7467–7473. [DOI] [PubMed] [Google Scholar]
- 78.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, and Zou W. 2011. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. Journal of immunology (Baltimore, Md. : 1950) 186: 4388–4395. [DOI] [PubMed] [Google Scholar]
- 79.Ye ZJ, Zhou Q, Du RH, Li X, Huang B, and Shi HZ. 2011. Imbalance of Th17 cells and regulatory T cells in tuberculous pleural effusion. Clinical and vaccine immunology : CVI 18: 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Neill LA, and Dinarello CA. 2000. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunology today 21: 206–209. [DOI] [PubMed] [Google Scholar]
- 81.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, and Kang I. 2010. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood 115: 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu L, Kitani A, Fuss I, and Strober W. 2007. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. Journal of immunology (Baltimore, Md. : 1950) 178: 6725–6729. [DOI] [PubMed] [Google Scholar]
- 83.Zheng SG, Wang J, and Horwitz DA. 2008. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. Journal of immunology (Baltimore, Md. : 1950) 180: 7112–7116. [DOI] [PubMed] [Google Scholar]
- 84.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, and Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30: 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.