Abstract
Background:
There is little evidence to guide surgical management of biopsies yielding the histologic descriptor “atypical intraepidermal melanocytic proliferation” (AIMP).
Objective:
Determine frequency of and factors associated with melanoma and melanoma in-situ (MIS) diagnoses after excision of AIMP and evaluate margins used to completely excise AIMP.
Methods:
Retrospective, cross-sectional study of 1127 biopsies reported as AIMP and subsequently excised within one academic institution.
Results:
Melanoma (in-situ, stage 1A) was diagnosed after excision in 8.2% (92/1127) of AIMP samples. Characteristics associated with melanoma/MIS diagnosis included age 60–79 (OR 8.1, 95% CI 2.5–26.2), age ≥80 (OR 7.2, 95% CI 1.7–31.5), head/neck location (OR 4.9, 95% CI 3.1–7.7), clinical lesion partially biopsied (OR 11.0, 95% CI 6.7–18.1), and lesion extending to deep biopsy margin (OR 15.1, 95% CI 1.7–136.0). Average surgical margin used to excise AIMP lesions was 4.5mm (SD 1.8).
Limitations:
Single-site, retrospective, observational study; interobserver variability across dermatopathologists.
Conclusion:
Dermatologists and pathologists can endeavor to avoid ambiguous melanocytic designations whenever possible through excisional biopsy technique, interdisciplinary communication, and ancillary studies. In the event of AIMP biopsy, physicians should consider the term a histological description rather than a diagnosis, and, during surgical planning, use clinicopathologic correlation while bearing in mind factors that might predict true melanoma/MIS.
Keywords: Atypical intraepidermal melanocytic proliferation, atypical junctional melanocytic hyperplasia, atypical junctional melanocytic proliferation, lentiginous junctional melanocytic proliferation, Melanoma, melanoma in situ, excision, biopsy, atypical melanocytic proliferation, ambiguous melanocytic lesions
Melanocytic proliferations lie along a continuum of increasing histologic atypia from benign to malignant. Within this spectrum exists a subset of lesions that is intrinsically difficult to classify. Atypical intraepidermal melanocytic proliferation (AIMP) is a descriptive histopathologic term commonly used in these cases to denote morphology sharing some features with melanoma but failing to meet criteria of a definitive benign or malignant diagnosis.1 Given the ambiguous malignant potential of these lesions and the possibility of remaining subclinical melanoma or melanoma in-situ (MIS), a biopsy report describing AIMP warrants consideration for formal surgical excision with margins outside the biopsy site and any remaining pigmented lesion.
We aim to elucidate AIMP ontology and to add data to a growing body of dermatologic and dermatopathologic literature describing surgical outcomes of ambiguous melanocytic lesions.2–4 We report the percentage of AIMP biopsies with post-excision melanoma/MIS diagnoses, describe clinical and histopathologic biopsy characteristics associated with this outcome, and evaluate the margins used for complete AIMP excision. This data will aid in patient counseling and surgical planning for AIMP.
METHODS
Experimental design
The Memorial Sloan-Kettering Cancer Center institutional review board approved this analysis. A text search of surgical pathology reports (January 1992 – July 2016) using AIMP and synonyms included: “atypical junctional melanocytic proliferation / hyperplasia,” “atypical melanocytic proliferation,” “lentiginous junctional melanocytic proliferation,” and “proliferation of solitary units of melanocytes at the dermoepidermal junction.” Inclusion criteria were biopsied lesions: (1) with slides described using the above search terms by in-house pathologists and (2) subsequently excised at our institution.
Excluded biopsies were those reported to most likely represent melanoma/MIS, given the assumption that our dermatologists would interpret – and subsequently treat – this description as true malignancy. Conversely, AIMP reported “unlikely” to be melanoma/MIS were included, assuming that our dermatologists would not approach these as definitively benign entities.
Data collection
Data abstracted from biopsy reports included date, patient age, biopsy type, and gross specimen dimensions. Pathology report text was assessed for term used (AIMP or synonyms listed above), presence of pagetoid spread, description of a second surrounding or colliding histopathologic component (i.e. nevus, carcinoma), positive margin presence/location, and special studies (immunohistochemical stains, molecular studies). The pathologist’s description was ranked by degree of diagnostic uncertainty: (1) unlikely melanoma/MIS or melanoma/MIS not mentioned, (2) “cannot exclude melanoma/MIS,” and (3) melanoma /MIS listed in the differential diagnosis.
Pre-biopsy clinical lesion size, personal melanoma/MIS history, and any local treatment prior to biopsy were recorded from the medical record. Clinical, operative, and pathology reports corresponding to each biopsy’s subsequent excision were assessed for: residual pigment presence/absence, treating specialty, surgical treatment type, treatment date, days between biopsy and treatment, margins of clinically normal-appearing tissue removed around the biopsied lesion, and final histopathologic description/diagnosis. When serial excisions (multiple standard excisions or staged-excision technique with delayed reconstruction) were required to attain negative histopathologic margins, total number of excisions was recorded with margins summed to yield a “total margin used to clear.”
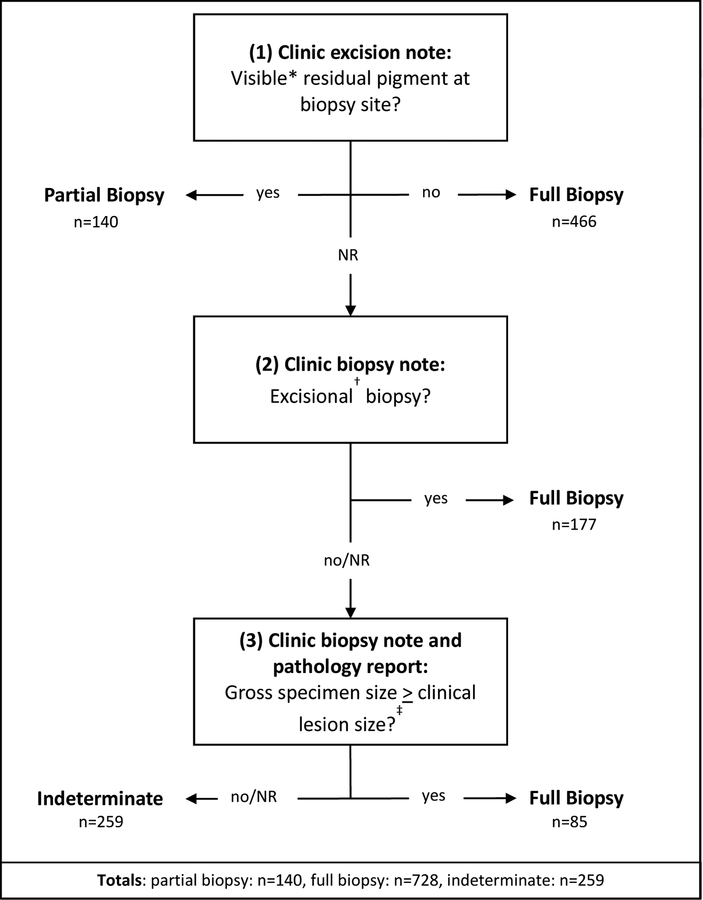
Figure 1 details the algorithm used to determine whether biopsies represented full or partial samples of the clinically-apparent pigment of their original lesions. The excision note was first examined for commentary about residual pigment at the previously-biopsied site. If no such notation was found, the original biopsy note from clinic was assessed for biopsy type. If there was no record of an intentional excisional biopsy, gross specimen size was compared to clinical lesion size as a tertiary assessment.
Figure 1. Algorithm to Determine Full Versus Partial Biopsy for 1127 AIMP Lesions.
NR, not recorded
*Including Woods Lamp examination
†Including shave removal, shave excision, punch removal, punch excision, saucerization
‡Assumes shrinkage of gross tissue
Data analysis
Descriptive statistics and graphical methods were used to describe patient characteristics, lesions, surgical procedures, and histologic lesion evaluations. Descriptive and relative frequencies and 95% exact binomial confidence intervals were used to present melanoma/MIS diagnosis burden. Logistic regression was used to present association between post-excision melanoma/MIS diagnosis and patient/lesion characteristics. All analyses were performed with Stata v14.2, Stata Corporation, College Station, TX.
RESULTS
Our database search yielded 1898 potential AIMP biopsies subsequently excised in-house. After excluding samples deemed “most likely” melanoma/MIS, 1127 biopsies remained for inclusion. Clinical and histopathologic characteristics are recorded in Table I and Table II, respectively.
Table I.
Clinical characteristics of sample
| Clinical variables | N (%) |
|---|---|
| Mean age at biopsy (SD)[range], years | 56.4 (15.2) [7–93] |
| Sex | |
| Female | 658 (58.3) |
| Male | 469 (41.6) |
| Anatomic location | |
| Head / neck | 192 (17.0) |
| Trunk / extremity | 827 (73.4) |
| Hand / foot | 86 (7.6) |
| Nail | 13 (1.2) |
| Genitalia | 9 (0.8) |
| Mean lesion diameter (SD)[range], mm* | 6.6 (5.6)[1 −50] |
| Melanoma/MIS history | |
| Yes | 440 (39.0) |
| No | 676 (60.0) |
| Not recorded | 11 (1.0) |
| Prior treatment for lesion | |
| Yes (laser/biopsy/excision/not specified) | 60 (5.3) |
| No | 1056 (93.7) |
| Not recorded | 11 (1.0) |
| Treating specialty | |
| Dermatology | 909 (80.7) |
| Surgical oncology | 188 (16.7) |
| Other (head/neck surgery, plastic surgery, hand surgery, gynecologic surgery) | 30 (2.7) |
| Follow-up treatment | |
| Excision | 1062 (94.2) |
| Shave excision | 47 (4.2) |
| Punch excision | 16 (1.4) |
| Amputation | 2 (0.2) |
| Average time between biopsy and treatment (SD) [range], days† | 69 (45) [3–2214] |
| AIMP re-excision diagnosis | |
| Melanoma | 6 (0.5) |
| Melanoma in-situ | 86 (7.6) |
| Atypical melanocytic lesion§ | 266 (23.6) |
| Atypical/dysplastic nevus | 37 (3.3) |
| Benign nevus | 23 (2.0) |
| Other, benign | 24 (2.1) |
| Scar | 685 (60.8) |
| Average margin used to clear (SD) [range], mm‡ | 4.5 (1.8) [1–19] |
| Serial excisions needed | |
| No | 1070 (94.9) |
| Yes, 2 | 53 (4.7) |
| Yes, 3 | 4 (0.4) |
SD, standard deviation; mm, millimeters; AIMP, atypical intraepidermal melanocytic proliferation
Data available for 505 samples
Data available for 1121 samples
Data available for 945 excisions
Composite category of atypical melanocytic diagnoses and descriptors, including AIMP, atypical junctional melanocytic hyperplasia, melanocytic hyperplasia, etc.
Table II.
Histopathologic characteristics of sample biopsies
| Clinical variables | N (%) |
|---|---|
| Biopsy type | |
| Shave | 702 (62.3) |
| Punch | 66 (5.9) |
| Excisional biopsy | 247 (21.9) |
| Nail biopsy | 12 (1.1) |
| Not recorded | 100 (8.9) |
| Full vs. partial biopsy | |
| Full | 728 (64.6) |
| Partial | 140 (12.4) |
| Indeterminate | 259 (23.0) |
| Term used to describe pathology | |
| Atypical intraepidermal melanocytic proliferation | 728 (64.6) |
| Lentiginous / junctional melanocytic proliferation | 328 (29.1) |
| Atypical melanocytic proliferation | 71 (6.3) |
| Degree of melanoma/MIS uncertainty | |
| Least likely melanoma/MIS / does not mention melanoma | 501 (44.5) |
| Cannot exclude melanoma/MIS | 248 (22.0) |
| Melanoma/MIS in differential | 378 (33.5) |
| Pagetoid spread | |
| Yes | 266 (23.6) |
| No | 861 (76.4) |
| Collision lesion | |
| With a nevus / other melanocytic lesion | 289 (25.6) |
| With a non-melanocytic lesion | 85 (7.5) |
| No collision reported | 753 (66.8) |
| Margins involved in biopsy specimen? | |
| Lateral | 334 (29.6) |
| Deep / deep and lateral | 31 (2.8) |
| Unspecified positive margin | 274 (24.3) |
| Negative margin explicitly noted | 80 (7.1) |
| Not recorded | 408 (36.2) |
| Special stains performed in-house?* | |
| Yes | 296 (48.3) |
| Not recorded | 316 (51.6) |
612 biopsies performed in-house, 515 biopsies performed at outside institutions
Of 1127 AIMP biopsies, 515 were performed at outside institutions with slides subsequently reviewed and labeled AIMP by our pathologists. Of the remaining 612 biopsies performed in-house, 296 (48%) received adjunctive dermatopathologic studies in addition to hematoxylin/eosin staining prior to biopsy reporting. The institution’s three dermatopathologists at time of data collection read 85.4% of biopsies and excisions.
The two cases ultimately treated with amputation were both AIMP reported in nail units, one of which yielded a post-amputation diagnosis of MMIS.
Melanoma/MIS diagnosis after excision and surgical margins
Of 1127 biopsies interpreted as AIMP, 92 (8.2%, 95% CI: 6.6–9.9%) were diagnosed as MIS (86/92) or invasive melanoma (6/92, ranging 0.2–0.6mm) following excision. Excluding samples yielding scar on excision, 21% (92/442) were diagnosed as melanoma/MIS. Factors associated with melanoma/MIS diagnosis are outlined in Table III. Characteristics with greatest odds of melanoma/MIS diagnosis were age 60–79 (OR 8.1, 95% CI 2.5–26.2), age ≥80 (OR 7.2, 95% CI 1.7–31.5), head/neck location (OR 4.9, 95% CI 3.1–7.7), partial biopsy of visible clinical lesion (OR 11.0, 95% CI 6.7–18.1), and deep biopsy margin involvement (OR 15.1, 95% CI 1.7–136.0). Male sex, prior local treatment, and melanoma/MIS noted in differential diagnosis yielded statistically significant but smaller odds ratios.
Table III.
Clinical and histopathologic variables associated with melanoma/melanoma in-situ diagnosis after excision of AIMP
| Characteristics | Total n (%) | Melanoma/MIS diagnosis after excision | Odds ratio, melanoma/MIS diagnosis after excision (95% CI) | ||
|---|---|---|---|---|---|
| No n (%) | Yes n (%) | ||||
| Sex | |||||
| Female | 658 (58.4) | 619 (59.8) | 39 (42.4) | 1.0 (referent) | |
| Male | 469 (41.6) | 416 (40.2) | 53 (57.6) | 2.0 (1.3 – 3.1) | |
| Age | |||||
| <40 | 172 (15.3) | 169 (16.4) | 3 (3.3) | 1.0 (referent) | |
| 40–59 | 428 (38.2) | 404 (39.3) | 24 (26.1) | 3.3 (1.0 – 11.3) | |
| 60–79 | 477 (42.6) | 417 (40.5) | 60 (65.2) | 8.1 (2.5 – 26.2) | |
| ≥80 | 44 (3.9) | 39 (3.8) | 5 (5.4) | 7.2 (1.7 – 31.5) | |
| Anatomic location | |||||
| Trunk/extremity | 827 (73.4) | 782 (75.6) | 45 (48.9) | 1.0 (referent) | |
| Head/neck | 192 (17) | 150 (14.5) | 42 (45.7) | 4.9 (3.1 −7.7) | |
| Hand/foot | 86 (7.6) | 85 (8.2) | 1 (1.1) | 0.2 (0.0 – 1.5) | |
| Other (nail & genital) | 22 (2.0) | 18 (1.7) | 3 (4.3) | 2.9 (0.8 – 10.2) | |
| Melanoma/MIS history | |||||
| No | 676 (60.6) | 629 (61.4) | 47 (51.1) | 1.0 (referent) | |
| Yes | 440 (39.4) | 395 (38.6) | 45 (48.9) | 1.5 (1.0– 2.3) | |
| Prior treatment for lesion | |||||
| No | 1056 (94.6) | 974 (95.1) | 82 (89.1) | 1.0 (referent) | |
| Yes | 60 (5.4) | 50 (4.9) | 10 (10.9) | 2.4 (1.2 – 4.9) | |
| Biopsy type | |||||
| Shave | 702 (68.3) | 638 (67.4) | 64 (79.0) | 1.0 (referent) | |
| Punch | 66 (6.4) | 56 (5.9) | 10 (12.4) | 1.8 (0.9 – 3.7) | |
| Excision | 247 (24.1) | 242 (25.6) | 5 (6.2) | 0.2 (0.1 – 0.5) | |
| Nail | 12 (1.2) | 10 (1.1) | 2 (2.5) | 2.0 (0.4 – 9.3) | |
| Biopsy | |||||
| Full | 728 (64.6) | 696 (67.3) | 32 (34.8) | 1.0 (referent) | |
| Partial | 140 (12.4) | 93 (9) | 47 (51.1) | 11.0 (6.7 – 18.1) | |
| Indeterminate | 259 (23) | 246 (23.8) | 13 (14.1) | 1.1 (0.6 – 2.2) | |
| Term used to describe pathology | |||||
| AIMP | 728 (64.6) | 666 (64.4) | 62 (67.4) | 1.0 (referent) | |
| L/JMP | 328 (29.1) | 303 (29.3) | 25 (27.2) | 0.9 (0.5 – 1.4) | |
| AMP | 71 (6.3) | 66 (6.4) | 5 (5.4) | 0.8 (0.3 – 2.1) | |
| Degree of melanoma/MIS uncertainty | |||||
| Least likely melanoma/MIS / does not mention melanoma/MIS | 501 (44.5) | 481 (46.5) | 20 (21.7) | 1.0 (referent) | |
| Cannot exclude melanoma/MIS | 248 (22) | 230 (22.2) | 18 (19.6) | 1.9 (1.0 – 3.6) | |
| Melanoma/MIS in differential | 378 (33.5) | 324 (31.3) | 54 (58.7) | 2.0 (1.5 – 2.6) | |
| Pagetoid spread | |||||
| No | 861 (76.4) | 786 (75.9) | 75 (81.5) | 1.0 (referent) | |
| Yes | 266 (23.6) | 249 (24.1) | 17 (18.5) | 0.7 (0.4 – 1.2) | |
| Collision lesion | |||||
| No collision reported | 753 (66.8) | 690 (66.7) | 63 (68.5) | 1.0 (referent) | |
| With a nevus / other melanocytic lesion | 289 (25.6) | 264 (25.5) | 25 (27.2) | 1.0 (0.6 −1.7) | |
| With a non-melanocytic lesion | 85 (7.5) | 81 (7.8) | 4 (4.4) | 0.5 (0.2 – 1.5) | |
| Margins involved in biopsy specimen | |||||
| Negative margin explicitly noted | 80 (7.1) | 79 (10.1) | 1 (1.1) | 1.0 (referent) | |
| Lateral | 334 (29.6) | 306 (39.0) | 28 (30.4) | 7.2 (1.0 – 53.9) | |
| Deep / deep and lateral | 31 (2.8) | 26 (3.3) | 5 (5.4) | 15.1 (1.7 −136.0) | |
| Unspecified positive margin | 274 (24.3) | 250 (31.8) | 24 (26.1) | 7.6 (1.0 – 57.0) | |
| Not recorded | 408 (36.2) | 374 (47.6) | 34 (37.0) | 7.2 (1.0 – 53.2) | |
| Special stains performed in-house* | |||||
| No | 316 (51.6) | 288 (51.1) | 28 (58.3) | 1.0 (referent) | |
| Yes | 296 (48.3) | 276 (48.9) | 20 (41.7) | 0.7 (0.4 – 1.4) | |
AIMP, atypical intraepidermal melanocytic proliferation; CI, confidence interval; L/JMP, lentiginous/junctional melanocytic proliferation; AMP, atypical melanocytic proliferation
515 biopsies performed at outside institution; 612 biopsies performed in-house
After controlling for partial biopsy, all melanoma/MIS-associated characteristics remained significant (male sex, OR 2.0, 95% CI 1.3–3.2; age 60–79, OR 9.0, 95% CI 2.7–30.0; age ≥ 80, 95% CI 1.8–38.3; head/neck location, OR 3.4; 95% CI 2.1–5.6; melanoma/MIS in differential diagnosis, OR 4.0, 95% CI 2.3–7.0; positive deep margin, OR 18.3, 95% CI 1.9–172.6), except for history of prior treatment, which lost significance (OR 1.4; 95% CI 0.6–3.2).
Average surgical margin used to excise AIMP lesions was 4.5mm (SD 1.8), with 94.9% (1070/1127) requiring one excision. Positive or equivocal margins led to serial excisions in 5.1% (57/1127) of cases. For the 86 samples ultimately diagnosed as MIS, average surgical margin was 6.5mm (SD 2.4); for the six invasive melanomas, 10.4mm (SD 5.6).
DISCUSSION
Central to this study is identification of partial biopsies, here defined as those failing to capture all visible pigment from their source lesions. These showed odds of melanoma/MIS 11 times those of full biopsies: intentional excisional biopsies and punch/shave biopsies of all visible pigment. Although the American Academy of Dermatology and National Comprehensive Cancer Network recommend narrow excisional biopsies encompassing the clinically-apparent breadth of suspicious melanocytic lesions,5,6 a desire to spare tissue in cosmetically- and/or functionally- sensitive areas may prompt a decision to partially sample a lesion, inevitably increasing risk of sampling error. Ultimately, complete sampling of suspicious pigmented lesions – providing dermatopathologists with maximum pathologic diagnostic information – is likely to be in the patient’s best interest. Cases potentially inappropriate for complete excisional biopsy, such as broad lesions with suspected horizontal spread near vital anatomy, may benefit from multiple scouting biopsies to optimize diagnostic accuracy.
An average 4.5mm margin was used to clear AIMP lesions, aligning with the lower bound of NCCN’s 5–10mm recommendation for MIS wide excision.7 Those AIMP ultimately diagnosed MIS, however, yielded an average 6.5mm surgical margin for histologic clearance, possibly due to re-excisions carried out after MIS diagnosis. This further underscores the utility of full pigmented lesion biopsies, which may reduce likelihood of ambiguous biopsy descriptions leading to re-excisions.
Our study revealed nearly all characteristics associated with melanoma/MIS diagnosis to remain significant after controlling for partial biopsy, diverging from a recent analysis of head/neck, hand, and foot AIMP biopsies in which partial biopsy was the only clinicopathologic factor associated with post-excision melanoma/MIS.4 These other clinical characteristics may thus remain useful to prognosticate greater or lower melanoma/MIS odds even for full lesion biopsies.
Our study’s total melanoma/MIS rate is higher than that of a prior study – which reported 4.2% melanoma/MIS amongst 306 AIMP biopsies2 – likely secondary to our cancer center-derived study population. The analyses reveal similar melanoma/MIS-associated characteristic profiles, notably head/neck location, positive deep margin, and melanoma/MIS in initial biopsy differential.2 Diverging from the prior study, we report neither hand/foot location nor punch biopsy technique to associate with melanoma/MIS diagnosis.2 The significance of this is unclear and may reflect differing institutional sampling practices and pathology reporting.
There is interobserver variability inherently associated with a subjective histopathologic descriptor such as AIMP. That we do not explicitly account for this variability is a limitation of our study. Other limitations include the study’s retrospective nature and restriction to one academic cancer center subject to referral bias.
CONCLUSION
The intrinsic diagnostic uncertainty of biopsies reported as AIMP presents a therapeutic dilemma. Factors associated with post-excision melanoma/MIS diagnosis, particularly incomplete clinical pigment sampling, deep biopsy margin involvement, and age ≥60, should prompt consideration to excise with surgical margins in line with MIS guidelines. In carefully selected clinical settings, complete biopsies with few risk factors may lead the clinician not to intervene further.
While AIMP and other similar descriptors may periodically be unavoidable, further steps can and should be taken to minimize ambiguity in melanocytic biopsies. Complete excisional biopsies of suspicious pigmented lesions and clinical information-sharing with pathologists (lesion size, appearance, and history; full vs. partial biopsy) enhance clinicopathologic correlation. Noninvasive imaging such as dermoscopy and reflectance confocal microscopy aid in diagnosis and mapping before or after biopsy, particularly in cosmetically- or functionally-sensitive areas unfavorable for excisional biopsy.8 Pathologists might also curtail ambiguous biopsy descriptions with ancillary diagnostic techniques. Immunohistochemistry, cytogenetics, and gene expression assays have shown potential in improving diagnostic precision, particularly in combination with clinical imaging approaches.1 The conversation about AIMP must underscore effective interdisciplinary communication between dermatologist and pathologist to minimize ambiguity and optimize management of atypical melanocytic lesions.
Capsule Summary.
Factors associated with malignancy diagnosis after excision of atypical intraepidermal melanocytic proliferation include incomplete sampling (lateral/deep margin), head/neck location, and age ≥60.
Ambiguous melanocytic biopsy designations should be avoided, but, when present, warrant clinicopathologic correlation, with malignancy risk factors prompting consideration to excise as melanoma in-situ.
Acknowledgements
We would like to acknowledge the entire dermatopathology department of Memorial Sloan-Kettering Cancer Center, including Drs. Cecilia Lezcano, Travis Hollmann, and Melissa Pulitzer.
This study was performed under the Memorial Sloan Kettering Cancer Center Retrospective Research IRB Protocol 16–450
Funding Sources: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Abbreviations and acronyms
- AIMP
- AMP
- L/JMP
- MIS
- NR
- OR
- SD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
Non Relevant Disclosures for Anthony M. Rossi, MD: Mavig: Travel; Merz: Consultant; Dynamed: Consultant; Canfield Scientific: Consultant; Allergan Inc: Advisory Board; Evolus: Consultant; Biofrontera: Consulatant; Quantia MD: Consultant; Lam Therapeutics; Consultant
ASLMS: A Ward Memorial Research Grant
Skin Cancer Foundation: Research Grant
References
- 1.Ensslin CJ, Hibler BP, Lee EH, Nehal KS, Busam KJ, Rossi AM. Atypical Melanocytic Proliferations: A Review of the Literature. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al. ]. 2018;44(2):159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Miller CJ, Sobanko JF, Shin TM, Etzkorn JR. Diagnostic Change From Atypical Intraepidermal Melanocytic Proliferation to Melanoma After Conventional Excision-A Single Academic Institution Cross-Sectional Study. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al. ]. 2016;42(10):1147–1154. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Miller CJ, Sobanko JF, Shin TM, Etzkorn JR. Frequency of and factors associated with positive or equivocal margins in conventional excision of atypical intraepidermal melanocytic proliferations (AIMP): A single academic institution cross-sectional study. Journal of the American Academy of Dermatology. 2016;75(4):688–695. [DOI] [PubMed] [Google Scholar]
- 4.Jew O, Miller CJ, Shin TM, Sobanko JF, Etzkorn JR. Diagnostic Change From Atypical Intraepidermal Melanocytic Proliferation to Melanoma is More Likely When Clinically Visible Residual Pigment Remains After Biopsy. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al. ]. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. Journal of the National Comprehensive Cancer Network : JNCCN. 2009;7(3):250–275. [DOI] [PubMed] [Google Scholar]
- 6.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. Journal of the American Academy of Dermatology. 2011;65(5):1032–1047. [DOI] [PubMed] [Google Scholar]
- 7.Melanoma. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2018; Version 2. 2018:https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed 16 February 2018. [Google Scholar]
- 8.Yelamos O, Cordova M, Blank N, et al. Correlation of Handheld Reflectance Confocal Microscopy With Radial Video Mosaicing for Margin Mapping of Lentigo Maligna and Lentigo Maligna Melanoma. JAMA dermatology. 2017;153(12):1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]