Abstract
Background
Deep learning algorithms of cerebral blood flow (CBF) were employed to classify cognitive impairment and frailty in people living with HIV (PLWH). Feature extraction techniques identified brain regions that were the strongest predictors.
Setting
Virologically suppressed (<50copies/mL) PLWH (n=125) on combination antiretroviral therapy were enrolled. Participants averaged 51.4 (11.4) years of age and 13.7 (2.8) years of education. Participants were administered a neuropsychological battery, assessed for frailty, and completed structural neuroimaging.
Methods
Deep neural network (DNN) models were trained to classify PLWH as cognitively unimpaired or impaired based on neuropsychological tests (Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised, Trail making, Letter-Number Sequencing, Verbal Fluency, and Color Word Interference), as well as frail, pre-frail, or non-frail based on the Fried phenotype criteria (at least three of following five: weight loss, physical inactivity, exhaustion, grip strength, walking time).
Results
DNNs classified individuals with cognitive impairment in the learning, memory, and executive domains with 82%−86% accuracy (.81-.87 AUC). Our model classified non-frail, pre-frail, and frail PLWH with 75% accuracy. The strongest predictors of cognitive impairment were cortical (parietal, occipital, temporal) and subcortical (amygdala, caudate, hippocampus) regions, while the strongest predictors of frailty were subcortical (amygdala, caudate, hippocampus, thalamus, pallidum, cerebellum)
Conclusion
DNN models achieved high accuracy in classifying cognitive impairment and frailty status in PLWH. Feature selection algorithms identified predictive regions in each domain and identified overlapping regions between cognitive impairment and frailty. Our results suggest frailty in HIV is primarily subcortical while cognitive impairment in HIV involves subcortical and cortical regions.
Keywords: Machine learning, HIV, cerebral blood flow, cognitive impairment, frailty
Introduction
More than 37 million people worldwide are infected with HIV1. HIV affects the brain soon after seroconversion by reducing CD4 cells and thus compromising the immune system. Treatment with combination antiretroviral therapy (cART) slows the progression of the disease and can help prevent transmission2. cART has converted HIV into a chronic disease with the life expectancy of people living with HIV (PLWH) approaching the lifespan of HIV uninfected individuals3.
Neurocognitive disorders due to HIV remain prevalent in PLWH despite the introduction of cART4. Cognitive symptoms related to HIV often involve multiple cognitive domains5. Since the introduction of cART, the degree of cognitive deficits in PLWH are now less severe, but studies demonstrate that even mild symptoms significantly impact quality of life and overall health outcomes5. The persistence of cognitive deficits may reflect multiple etiologies including legacy effects of prior damage, persistent inflammation, and/or viral reservoirs within the brain4.
Due to increases in the life expectancy of PLWH, frailty has emerged as a significant age-related comorbidity. Frailty, as defined by the Fried Frailty Index involves five symptoms, including unintentional weight loss, physical inactivity, exhaustion, weak grip strength, and slowed walking time6. An individual is considered to be frail if (s)he has at least three of these symptoms. In the HIV-uninfected population, frailty is associated with functional decline, decreased resilience to physiologic stress, and mortality7–10. Persistent inflammation due to chronic immune dysfunction in PLWH can cause premature aging and has been associated with frailty8. PLWH exhibit a heightened burden of frailty compared to HIV-uninfected individuals9, possibly due to premature aging. Both frailty and cognitive impairment have been associated with changes in brain structure and function using neuroimaging.
Neuroimaging provides a non-invasive measure to assess brain function and integrity. Cerebral blood flow (CBF), as measured by arterial spin labeling (ASL), measures the amount of blood supplied to the brain within a given time interval. Alterations in CBF are observed in multiple neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, etc.)11–14. In HIV, a decrease in CBF has been reported soon after seroconversion in subcortical brain regions, including the putamen, globus pallidus, and caudate15,16. A decrease in CBF in the temporoparietal region has also been associated with cognitive impairment and motor dysfunction in chronically infected PLWH17. However, others have seen an increase in CBF in the posterior inferior parietal white matter18. This has been hypothesized to signify increased recruitment of brain systems to compensate for neural damage. As such, CBF could act as a predictive biomarker that exhibits specific temporal and spatial patterns. The identification of these patterns that are unique to cognitive impairment and/or frailty in PLWH would allow clinicians to develop treatments that are tailored to the individual.
Machine learning (ML) algorithms have shown promise for supporting clinical decision making and predictive analytics using neuroimaging19–21. Compared with traditional statistics that target group-level results, ML algorithms can predict clinical outcomes at the level of the individual participant, and ultimately inform personalized treatments22,23. Deep learning is a branch of ML that has gained increased traction in the neuroimaging community. Deep learning models have outperformed independent component analyses in identifying key hidden features in neuroimaging data and have successfully identified early changes associated with “healthy” aging24,25 and neurodegenerative disease [e.g. Huntington’s disease26, schizophrenia27, and brain tumors28]. However, we are unaware of any studies that have utilized deep learning in conjunction with CBF to classify cognitive impairment or frailty in PLWH.
In this study, we investigated a large cohort of virologically suppressed PLWH (n=125) to: 1) utilize deep learning to classify individuals according to cognitive status and identify brain regions associated with specific changes in cognition, 2) discriminate between frail, pre-frail, and non-frail PLWH, and delineate neuroimaging features that best separate these three groups, and 3) identify predictors that are common and unique to cognitive impairment and frailty. Application of the data-driven approach in the current study represents an opportunity to discover novel mechanisms of frailty and cognition in PLWH.
Methods
Participants
PLWH were selected from ongoing studies conducted by the Infectious Disease clinic at Washington University in Saint Louis (WUSTL). A participant was excluded if (s) he was less than 18 years old, had a history of confounding neurological disorders, current or past opportunistic central nervous system (CNS) infection, traumatic brain injury (loss of consciousness >30 minutes), major psychiatric disorders, or met criteria for current substance use disorder according to the Diagnostic and Statistics Manual of Mental Disorders 5th edition. All PLWH were on stable cART for at least 6 months and had an undetectable viral load (<50 copies/ml). The WUSTL Institutional Review Board approved this study. Written informed consent was obtained from all participants.
Neuropsychological assessment
Neuropsychological testing targeted three neurocognitive domains that are frequently affected by HIV29–33. A total of 8 tests were administered that covered the following domains: 1) Learning: Total recall across the learning trials on the Hopkins Verbal Learning Test-Revised (HVLT-R34) and Brief Visuospatial Memory Test-Revised (BVMT-R35); 2) Memory: Delayed recall on the HVLT-R and BVMT-R; 3) Executive: Trail Making Test B36, Letter-Number Sequencing37, Verb fluency38, and Color Word Interference Test trial 339. Time to completion and total correct served as the dependent measures in accord with standard methods. Raw test scores were transformed into Z-scores using published norms corrected for age, education, sex, and race where applicable40–46. Individual Z-scores were then aggregated into domain scores. Individuals with at least one domain score < −1.5 SD were designated as cognitively impaired. We utilized a conservative definition of neurocognitive impairment to minimize false positives.
Frailty assessment
PLWH were classified using the Fried Frailty Index6. The Fried criteria include unintentional weight loss >10 pounds in the past 12 months, physical inactivity (health limiting an individual from participating in vigorous activities), exhaustion (present 5–7 days in the past week), weak grip strength (adjusted for sex and body mass index (BMI)), and slowed walking time (adjusted for sex and height). Consistent with prior studies47, PLWH were classified as frail if they met 3 or more symptoms (n= 12), pre-frail if they met 2 symptoms (n= 50) and non-frail if they met 0–1 symptom (n= 30).
Magnetic resonance imaging (MRI) acquisition
All neuroimaging was performed on a 3T Siemens Tim Trio MR scanner (Siemens AG, Erlangen, Germany) with a 12-channel head coil. A high-resolution, 3-dimensional, sagittal, magnetization-prepared rapid gradient echo T1 scan was acquired (repetition time [TR] = 2400 ms; echo time (TE) = 3.16 ms; flip angle = 8°; inversion time = 1000 ms; voxel size = 1 × 1 × 1 mm3 voxels; 256 × 256 × 176 acquisition matrix; 162 slices). A 2-dimensional multislice oblique axial spin density/T2-weighted fast spin echo scan (TE = 450 milliseconds; TR = 3200 milliseconds; 256 × 256 acquisition matrix; 1 × 1 × 1 mm voxels) was acquired for registration. We utilized pseudo-continuous arterial spin labeling (pCASL), which is a non-invasive neuroimaging technique. pCASL uses water in the arterial blood as a contrast medium to measure CBF and determines the delivery rate of oxygen and nutrients to the capillary bed48. In addition to an improved signal-to-noise ratio, pCASL has lower inter-subject variability49. pCASL was obtained with 1.5 seconds labeling time, 1.2 seconds post labeling delay, TR = 3500 seconds, TE = 9.0 milliseconds, 64 × 64 acquisition matrix, 90° flip angle, 22 axial slices with a 1-mm gap, and voxel size of 3.4 × 3.4 × 5.0. Two pCASL scans were acquired with each containing 60 volumes. CBF values were computed for each control-label pair using a single compartment model50:
Here f is CBF, R1a (0.606 seconds−1 at 3T) is the longitudinal relaxation rate of the blood, M0 is the equilibrium magnetization, α is the tagging efficiency (0.85), τ (18.4 ms x number of radiofrequency blocks) is the duration of the labeling pulse, λ is the blood/tissue water partition co-efficient (0.9 g/mL), and w is the amount of post labeling delay.
MRI processing
Preprocessing methods were performed as previously described51. In short, motion correction was achieved in a two-step process. First, rigid registration to the mean volume was performed, and second, estimated motion parameters from the first step were used as weights in calculating the mean CBF. Each participant’s mean CBF was registered to a common atlas through a series of 3 linear registrations: (1) the mean control volumes were registered to the corresponding T2 images, (2) T2 images were registered to the T1 image, and (3) T1 images were registered to a common atlas. T1 and T2 images were used only for registration and segmentation purposes.
A total of 82 brain regions were generated using FreeSurfer version 5.3 (Laboratory for Computational Neuroimaging, Charlestown, MA, USA) based on a participant’s T1 image. Visual inspection of the automated segmentation results was performed for quality assurance purposes and corrections were made when necessary. CBF measurements were obtained from each of these 82 FreeSurfer defined regions. CBF values from these 82 regions were then grouped into 12 cortical and subcortical regions, including: cerebellum, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, frontal, parietal, temporal, cingulate, and occipital lobes. Within each group, CBF values were averaged to derive a mean CBF value for each region of interest. These regions were chosen as they have been previously been shown to be affected by HIV52. Each brain region was normalized to mean 0 and standard deviation 1 and rescaled to a [1, −1] interval.
Deep Learning Analysis
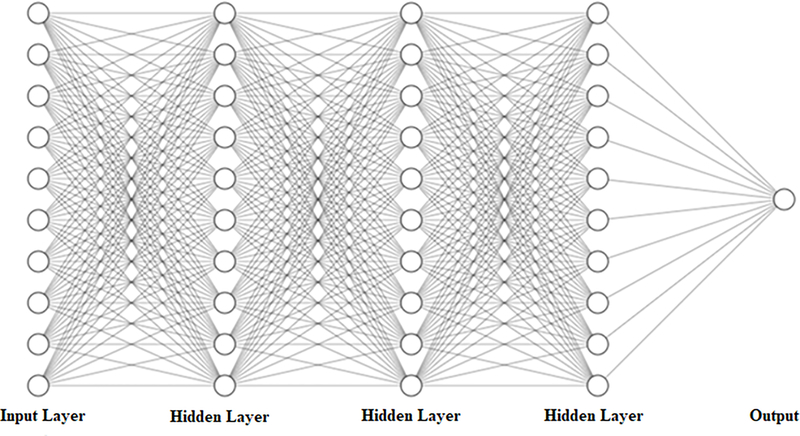
Feed forward deep neural network models (DNN) are a method of mapping an input to an output by composing it into sets of smaller functions at each layer of the network and feeding the results into the next layer of the network53. Layers between the input and output layer are called hidden layers, and each hidden layer offers another level of abstraction for feature identification and learning. A sample feed forward DNN with ten inputs, a single output, and three hidden layers is depicted in Figure 1. The feasibility of these networks is based on the Universal Approximation
Figure 1:
Deep neural network (DNN) architecture consisting of ten inputs, a single output, and three hidden layers.
Theorem, which states a neural network with a single hidden layer contains a finite set of neurons that can approximate continuous functions on compact subsets of Rn54. Our DNN contained 3 hidden layers with 10 neurons each. Every neuron utilized a sigmoid transfer function for activation:
which is a smooth differentiable function and mathematically equivalent to a hyperbolic tangent. The models were trained using scaled conjugate gradient backpropagation. Training was terminated at either 200 iterations or 10 successive validations. All analyses were performed in MATLAB R2018a. Models were evaluated using accuracy and the area under the curve. Each DNN was validated using 5-fold Monte Carlo cross-validation55. Cross-validation was used to estimate how well a model fit data independent of the information used to train the model, as well as insure the model was not overfitting due to small sample size. On each iteration, data were randomly partitioned into training and testing data, with 80% reserved for training and 20% used for testing. The results reported were the average accuracy across the five validations.
Features were ranked according to importance using a Relief algorithm56. Relief algorithms detect conditional dependencies between attributes using a nearest neighbor approach with features ranked by estimating how well their values distinguish between proximal comparisons.
In our analysis, separate DNN models were trained to discriminate cognitively impaired vs. unimpaired individuals in any one domain and separately by domain. A single DNN was also trained to classify participants as either frail, pre-frail, or not frail. In each of the above cases, the Relief algorithm was utilized to identify the strongest predictors of a given outcome.
Results
Demographics of the cohort
A majority of the cohort were African American (53%) males (66%), with an average age of 51.4 (11.4) years and 13.7 (2 .8) years of education. Detailed demographics are presented in Table 1. PLWH were further categorized as either frail (n= 12), pre-frail (n= 50), or non-frail (n= 30) using the Fried criteria, as well as cognitively normal or impaired.
Table 1.
Demographics of persons living with HIV (PLWH). SD=standard deviation, cART=combination antiretroviral therapy, IQR=interquartile range
| PLWH (n=125) | |
|---|---|
| Age (years, SD) | 51.4 (11.4) |
| Sex (% Male) | 66% |
| Education (years, SD) | 13.7 (2.8) |
| Race (% African American) | 53% |
| Duration of infection (years, SD) | 15.6 (8.8) |
| cART duration (years, SD) | 12.7 (7.5) |
| Current CD4 (cells/μl, IQR) | 585 (399, 858) |
| Nadir CD4 (cells/μl, IQR) | 180 (31–320) |
Cognitive impairment in PLWH
The DNN algorithm successfully identified individuals with neurocognitive impairment with an average accuracy of 82% and .81 AUC (True Positive Rate (TPR) = 84%, True Negative Rate (TNR) = 80%). The Relief algorithm identified CBF of both subcortical and cortical regions, including the amygdala (1), temporal lobe (2), hippocampus (3), occipital lobe (4), parietal lobe (5), and caudate (6) as the strongest predictors (numbers indicate rank of importance). In classifying cognitive impairment in the learning domain, the algorithm achieved 85% accuracy and 0.83 AUC (TPR = 93%, TNR = 68%). The best predictors within the learning domain were the amygdala (1), cingulate (2), hippocampus (3), frontal lobe (4), and parietal lobe CBF. In the memory domain, the algorithm achieved an average accuracy of 86% and 0.87 AUC (TPR = 94%, TNR = 79%), with the best predictive regions being CBF in the amygdala (1), temporal lobe (2), parietal lobe (3), caudate (4), and hippocampus (5). In classifying cognitive impairment in the executive domain, the algorithm achieved 86% accuracy and 0.85 AUC (TPR = 87%, TNR = 84%). CBF in the hippocampus (1), temporal lobe (2), cingulate (3), parietal lobe (4), and cerebellum (5) were the strongest predictors of executive dysfunction.
Frailty in PLWH
The DNN analysis distinguished between frail, pre-frail, or non-frail PLWH with 75% accuracy. The strongest predictors of frailty in PLWH were CBF in subcortical regions including the thalamus (1), pallidum (2), cerebellum (3), caudate (4), amygdala (5), and hippocampus (6).
Alternative Analysis Methods
To compare our algorithm to other machine learning methods, we tested our data with other commonly used machine learning algorithms including: decision trees, linear discriminant analysis, logistic regression, naïve Bayes, support vector machines, and K nearest neighbors. With the exception of decision trees in the executive domain, the DNN out performed all of the methods in classifying cognitive impairment. Our method also out-performed all other methods in classifying frailty status. The results of these analysis can be seen in Table 2, and the feature weights defined by the Relief algorithm for cognitive impairment and frailty are seen in figure 2.
Table 2:
Accuracy of classifying each of the cognitive domains, cognitive impairment (CI), and frailty status using a deep neural network (DNN) model compared to other commonly used machine learning algorithms. LDA = Linear Discriminant Analysis, SVM = Support Vector Machine, KNN = K Nearest Neighbor, CI = cognitively impaired (in any domain).
| Learning | Memory | Executive | CI | Frailty | |
|---|---|---|---|---|---|
| Decision Tree | 70% | 75% | 87% | 68% | 60% |
| LDA | 68% | 71% | 75% | 64% | 55% |
| Logistic Regression | 68% | 73% | 76% | 61% | - |
| Naïve Bayes | 72% | 71% | 67% | 61% | 51% |
| SVM | 76% | 82% | 85% | 69% | 60% |
| KNN | 76% | 82% | 85% | 68% | 66% |
| Deep Net | 85% | 86% | 86% | 82% | 75% |
Figure 2:
Distribution of feature weights generated from the Relieff algorithm for each of the brain regions of interest for cognitive impairment (CI) and frailty.
Discussion
Our models yielded high accuracy in classifying both cognitive impairment and domain specific impairment (82%−86%). In each cognitive impairment model, the primary predictors contained both cortical and subcortical regions. DNN models discriminated between frail, pre-frail, and non-frail PLWH with 75% accuracy, with the strongest predictors being primarily subcortical regions (thalamus, caudate, amygdala, hippocampus, cerebellum cortex, pallidum, and putamen). Importantly, the data-driven approach accurately classified individuals as frail and/or cognitively impaired despite otherwise effective use of cART.
Our research defines robust predictive models of cognitive impairment in PLWH. In particular, we showed 82%−86% accuracy in classifying cognitive impairment and domain specific impairment (learning, memory, and executive domains). We observed that changes in the amygdala, temporal lobe, parietal lobe, and hippocampus occurred in the majority of our analysis. Others have also observed that PLWH had decreased CBF bilaterally in the inferior lateral frontal cortices and in the inferior medial parietal brain region18. They also found an increase in CBF in the posterior inferior parietal white matter and noted these abnormalities correlated with disease severity.
In the frailty analysis, our model achieved 75% accuracy classifying three groups. The majority of those labeled incorrectly belonged to the pre-frail group. The strongest predictors were primarily subcortical structures (thalamus, caudate, amygdala, hippocampus, cerebellum cortex, pallidum, and putamen). Previous research has shown that HIV and aging can lead to a decrease in CBF and functional connectivity in these regions57,58. It is possible that there is an interaction between the neuropathogenic mechanisms of HIV and the neural substrates of frailty that exacerbates changes in CBF in PLWH and may result in the recruitment of additional regions in chronically infected PLWH.
Figure 3 shows the overlap between the strongest predictors of cognitive impairment and frailty. These results suggest frailty in HIV is primarily a subcortical disease while cognitive impairment in PLWH reflects changes in subcortical and cortical areas. While certain regions (amygdala, caudate and hippocampus) are susceptible to both frailty and HIV, there are also differences. This indicates alterations in CBF in cortical regions are more related to cognition while changes in subcortical areas are due to frailty.
Figure 3:
Venn diagram of cerebral blood flow (CBF) showing overlapping regions that were strong predictors in cognitive impairment and frailty. Italics indicate subcortical regions.
These findings have clinical importance for the management and care of PLWH. The interaction between HIV, cognitive impairment, and frailty has not been well studied. Accurately identifying PLWH with defined CBF patterns could allow for personalized medicine. The implementation of decision support models can be useful in evaluating the effects of specialized therapy for PLWH.
A number of limitations exist in this study. The data used for training our models was cross sectional in nature. Longitudinal data would give information about the rate of change over time, which may increase the accuracy of the models. Another issue is the ambiguous nature of a “pre-frail” classification. The majority of DNN model errors for frailty occurred due to misclassification of the pre-frail group. Multiple frailty assessments should be conducted to ensure those in the pre-frail group maintain that status, or the pre-frail group should be removed, and considered as one of the other two classes. There was also some overlap in impairment in the cognitive domains, primarily because most individuals that had cognitive impairment had changes in multiple domains. Future studies with larger sample sizes should evaluate individuals with impairment in only one cognitive domain. Lastly, our analysis evaluated the average CBF within lobes and subcortical regions bilaterally. Future studies should evaluate the individual regions in the brain where changes may be present, but were “washed out” due to averaging regions.
Conclusion
In the post cART era, cognitive impairment and frailty remain prevalent. In order to provide targeted treatment to PLWH, novel biomarkers are needed to detect cognitive impairment and frailty. In our analysis, we have shown DNN models can classify cognitive impairment in PLWH. We also showed a DNN can discriminate between frail, pre-frail, and non-frail PLWH with high accuracy. Lastly, using feature extraction methods, we have identified the strongest predictors of impairment and frailty across 12 brain regions. Our results showed cognitive impairment is both cortical and subcortical and nature, while frailty is primarily subcortical. Our results also show an overlap in strong predictors in subcortical brain regions between frailty and cognitive impairment.
Acknowledgements
The study was supported by grants from the National Institutes of Health (R01NR012657 and R01NR014449). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Funding: National Institutes of Health R01NR012657 and R01NR014449
References
- 1.HIV.gov. Global Statistics HIV. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. Published 2018.
- 2.Cohen MS, Smith MK, Muessig KE, et al. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: Where do we go from here? Lancet. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Sighem A, Gras L, Reiss P, et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter Study. Neurology. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–986. http://linkinghub.elsevier.com/retrieve/pii/S147330991370269X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. Journals Gerontol Ser A Biol Sci Med Sci. 2001. [DOI] [PubMed] [Google Scholar]
- 7.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. Journals Gerontol - Ser A Biol Sci Med Sci. 2007. [DOI] [PubMed] [Google Scholar]
- 8.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: State of knowledge and areas of critical need for research. a report to the NIH office of AIDS research by the HIV and aging working group. J Acquir Immune Defic Syndr. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Curr HIV/AIDS Rep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terzian AS, Holman S, Nathwani N, et al. Factors Associated with Preclinical Disability and Frailty among HIV-Infected and HIV-Uninfected Women in the Era of cART. J Women’s Heal. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firbank MJ, He J, Blamire AM, et al. Cerebral blood flow by arterial spin labeling in poststroke dementia. Neurology. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colloby SJ, Firbank MJ, He J, et al. Regional cerebral blood flow in late-life depression: arterial spin labelling magnetic resonance study. Br J Psychiatry. 2012. [DOI] [PubMed] [Google Scholar]
- 14.Chen JJ, Salat DH, Rosas HD. Complex relationships between cerebral blood flow and brain atrophy in early Huntington’s disease. Neuroimage. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ances BM, Roc AC, Wang J, et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006. [DOI] [PubMed] [Google Scholar]
- 16.Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: A potential biomarker of the effects of HIV in the brain. Neurology. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst, Itti E, Itti L, et al. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered 1H MRS and SPECT study. J Magn Reson Imaging. 2000. [DOI] [PubMed] [Google Scholar]
- 18.Chang L, Ernst T, Leonido-Yee M, et al. Perfusion MRI detects rCBF abnormalities in early stages of HIV-cognitive motor complex. Neurology. 2000. [DOI] [PubMed] [Google Scholar]
- 19.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemm S, Blankertz B, Dickhaus T, et al. Introduction to machine learning for brain imaging. Neuroimage. 2011. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini E, Ballerini L, Hernandez M del CV, et al. Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: A systematic review. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passos IC, Mwangi B, Kapczinski F. Big data analytics and machine learning: 2015 and beyond. The Lancet Psychiatry. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Adeli E, Zahr NM, Pfefferbaum A, et al. Novel Machine Learning Identifies Brain Patterns Distinguishing Diagnostic Membership of HIV, Alcoholism, and Their Comorbidity of Individuals. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019. https://linkinghub.elsevier.com/retrieve/pii/S2451902219300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole JH, Poudel RPK, Tsagkrasoulis D, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017. [DOI] [PubMed] [Google Scholar]
- 25.Cole JH, Underwood J, Caan MWA, et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizk-Jackson A, Stoffers D, Sheldon S, et al. Evaluating imaging biomarkers for neurodegeneration in pre-symptomatic Huntington’s disease using machine learning techniques. Neuroimage. 2011. [DOI] [PubMed] [Google Scholar]
- 27.Plis SM, Hjelm DR, Slakhutdinov R, et al. Deep learning for neuroimaging: A validation study. Front Neurosci. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akkus Z, Galimzianova A, Hoogi A, et al. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J Digit Imaging. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ances BM, Ortega M, Vaida F, et al. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004. [DOI] [PubMed] [Google Scholar]
- 31.Paul R, Rhee G, Baker LM, et al. Effort and neuropsychological performance in HIV-infected individuals on stable combination antiretroviral therapy. J Neurovirol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson K, Jiang H, Evans SR, et al. International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J Neurovirol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods SP, Moore DJ, Weber E, et al. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test-revised: normative data and analysis of inter-form and test re-test reliability. Clin Neuropsychol. 1998. [Google Scholar]
- 35.Benedict. The Brief Visuospatial Memory Test - Revised. Psychol Assess. 1997. [Google Scholar]
- 36.Reitan RM, Davison LA. Clinical neuropsychology: current status and applications. Winst Sons. 1974. [Google Scholar]
- 37.Wechsler D Wechsler Adult Intelligence Scale (WAIS-3R); 1997.
- 38.Piatt AL, Fields JA, Paolo AM, et al. Action (verb naming) fluency as an executive function measure: Convergent and divergent evidence of validity. Neuropsychologia. 1999. [DOI] [PubMed] [Google Scholar]
- 39.Delis D, Kaplan E, Kramer J. Delis-Kaplan executive function system (D-KEFS). Can J Sch Psychol. 2001. [Google Scholar]
- 40.Woods SP, Scott JC, Sires DA, et al. Action (verb) fluency: Test-retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 2005. [PubMed] [Google Scholar]
- 41.Gladsjo JA, Schuman CC, Evans JD, et al. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999. [DOI] [PubMed] [Google Scholar]
- 42.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s older African Americans normative studies: Norms for Boston naming test, controlled oral word association, category fluency, animal naming, token test, WRAT-3 reading, trail making test, stroop test, and judgment of line orientation. Clin Neuropsychol. 2005. [DOI] [PubMed] [Google Scholar]
- 43.Heaton RK, Miller S, Taylor M, et al. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Programs; 2004.
- 44.Duff K Demographically corrected normative data for the Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised in an elderly sample. Appl Neuropsychol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and caucasians on the hopkins verbal learning test-revised, Brief visuospatial memory test-revised, stroop color and word test, and wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman MA, Schinka JA, Mortimer JA, et al. Hopkins Verbal Learning Test – Revised: Norms for Elderly African Americans. Clin Neuropsychol. 2003. [DOI] [PubMed] [Google Scholar]
- 47.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. In: The Lancet.; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grade M, Hernandez Tamames JA, Pizzini FB, et al. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gevers S, Van Osch MJ, Bokkers RPH, et al. Intra-and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Zhang Y, Wolf RL, et al. Amplitude-modulated Continuous Arterial Spin-labeling 3.0-T Perfusion MR Imaging with a Single Coil: Feasibility Study. Radiology. 2005. [DOI] [PubMed] [Google Scholar]
- 51.Tanenbaum AB, Snyder AZ, Brier MR, et al. A method for reducing the effects of motion contamination in arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guha A, Brier MR, Ortega M, et al. Topographies of cortical and Subcortical volume loss in HIV and aging in the cART Era. J Acquir Immune Defic Syndr. 2016;73(4):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodfellow I, Bengio Y, Courville A. Deep Learning. Nature. 2016. [Google Scholar]
- 54.Hornik K Approximation capabilities of multilayer feedforward networks. Neural Networks. 1991. [Google Scholar]
- 55.Dubitzky W, Granzow M, Berrar D. Fundamentals of Data Mining in Genomics and Proteomics; 2007.
- 56.Kira K, Rendell LA. A Practical Approach to Feature Selection.; 1992.
- 57.Ances BM, Vaida F, Yeh MJ, et al. HIV Infection and Aging Independently Affect Brain Function as Measured by Functional Magnetic Resonance Imaging. J Infect Dis. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega M, Brier MR, Ances BM. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]