Abstract
Background:
Early-onset or familial gastric cancer (GC) is known to have clinicopathologic profiles different from those of sporadic GC. We aimed to compare DNA damage response marker expression between early-onset or familial GC and sporadic GC.
Methods:
GC samples were obtained from patients who underwent gastrectomy for GC at Seoul National University Hospital. Immunohistochemical analyses of various DNA damage response markers, including BRCA1, BRCA2, MRE11, RAD51C, and γH2AX, were performed using 54 early-onset GC, 59 familial GC, and 337 sporadic GC tissue microarray samples. Correlations between marker expression and clinicopathologic features were evaluated by univariate and multivariate analyses, and overall survival was analyzed.
Results:
The rate of γH2AX positivity was significantly higher (p < 0.001) in early-onset or familial GC than in sporadic GC. In contrast, the rates of MRE11 negativity and RAD51C negativity were significantly higher in sporadic GC than in early-onset or familial GC. BRCA1 negativity was associated with decreased overall survival in sporadic GC (p = 0.002), and MRE11 negativity was associated with decreased overall survival in sporadic GC (p = 0.012).
Conclusion:
Our results show significant differences in DNA damage response marker expression between early-onset or familial GC and sporadic GC.
Keywords: DNA damage response, Early-onset gastric cancer, Immunohistochemistry
Introduction
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide (McLean and El-Omar, 2014). The incidence of GC in young patients declined from 6.34% of all patients with GC 20 years ago to 4.49% in the most recent decade (Kitamura et al., 1996). The overall incidence of early-onset GC in Korea is estimated to be 3.55% (Korea Central Cancer Registry, 2014). The incidence of advanced GC in young patients is higher than that in the general patient population (Lee et al., 2016). As GC is usually detected at an advanced stage, improved surveillance and chemotherapy strategies are needed (Lee et al., 2016).
It is postulated that genetic factors may be more important in young patients with early-onset GC than in older patients with GC as the patients with early-onset GC are less exposed to environmental carcinogens (Correa and Shiao, 1994). The different clinicopathological profiles of early-onset GC compared to those of conventional gastric carcinomas suggest that early-onset GC represents a separate entity within gastric carcinogenesis (Milne et al., 2007). It is known that microsatellite instability, which usually occurs at a frequency of 15% in older patients with gastric carcinomas, is consistently absent in young patients (Hayden et al., 1997; Lim et al., 2003; Carvalho and Kanaar, 2014). Approximately 10% of young GC patients have a positive family history (Kokkola and Sipponen, 2001).
Regarding familial GC, truly hereditary cases are thought to account for 1–3% of global GC cases and includes at least three main syndromes: hereditary diffuse GC (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach, and familial intestinal GC. A genetic basis has been found in only around 40% of families affected by HDGC (Oliveira et al., 2015). In early-onset GC, alterations in E-cadherin-mediated adhesion proteins are common whereas microsatellite instability is not (Hayden et al., 1997; Lim et al., 2003). Recently, germline mutations in PALB2, BRCA1, and RAD51C have been identified in patients with HDGC, indicating that defects in homologous recombination (HR) increase the GC risk (Sahasrabudhe et al., 2017). However, specific susceptibility genes in early-onset or familial GC have not been identified.
The DNA damage response (DDR) is a collective term for the plethora of intra- and intercellular signaling events and enzyme activities that result from the induction and cellular detection of DNA damage. These include events that lead to cell-cycle arrest, regulation of DNA replication, and repair or bypass of DNA damage (O’Connor, 2015).
Tumor cells harboring mutations in genes involved in HR, such as BRCA1, BRCA2, and MRE11, are particularly vulnerable to DNA damage (Vilar et al., 2011). A deficiency in DNA damage repair is a fundamental etiological factor in various human cancers. Germline mutations in a single copy of either BRCA1 or BRCA2 can cause hereditary breast and ovarian cancer syndrome, characterized by early-onset breast cancer, as well as increased risks of ovarian, pancreatic, stomach, laryngeal, fallopian tube, and prostate cancer. BRCA1 and BRCA2 expression patterns and their prognostic significance in digestive system cancers have been described to be associated with more advanced tumor stage (Wang et al., 2018).
RAD51C is a paralog of RAD51, with an important role in the DDR (Min et al., 2013). RAD51C-deficient cancer cells are highly sensitive to the PARP inhibitor olaparib (Min et al., 2013). In non-small cell lung cancer, increased expression of RAD51C has been suggested to confer resistance to chemotherapy and/or radiotherapy (Chen et al., 2016).
Meiotic recombination 11 (MRE11), DNA repair protein Rad50 (RAD50), and Nijmegen breakage syndrome 1 (NBS1) form the MRN complex, which is required for the maintenance of genomic instability (Carvalho and Kanaar, 2014). This complex, in combination with ataxia telangiectasia mutated (ATM), coordinates the cellular detection and repair of DNA double-strand breaks (DSBs) (Altan et al., 2016). High expression of MRE11–RAD50–NBS1 is associated with poor prognosis and chemoresistance in GC (Altan et al., 2016) as well as poor response to neoadjuvant radiotherapy and prognosis in rectal cancer (Ho et al., 2018). The MRN complex is frequently not detected in low-grade epithelial ovarian cancer, suggesting high sensitivity to the PARP inhibitor (Brandt et al., 2017).
The formation of γH2AX foci is commonly used to quantitatively analyze DSB induction and repair. Analyses of γH2AX revealed deficiencies in nonhomologous end-joining, the dominant DSB repair pathway in mammalian cells (Rogakou, 1998; Rothkamm, 2003). Phosphorylated H2AX, designated as γH2AX, is visualized by immunofluorescence microscopy as discrete nuclear foci, reflecting sites of DSBs. γH2AX is significantly associated with a shorter survival in patients with gastric carcinoma (Hussein et al., 2018).
We compared the immunohistochemical profiles of four DDR markers and one pharmacodynamic biomarker, γH2AX, between early-onset or familial GC and sporadic GC tissues.
Materials and Methods
Patients
Early-onset GC is defined as GC diagnosed before age 40 (Lai et al., 2008; Takatsu et al., 2016). Patients were selected from January 1993 to December 1999 at Seoul National University Hospital. Of 4123 patients who underwent gastrectomy for GC, 54 patients were 40 years old or younger. To determine clinical outcomes, each patient was followed up from the date of surgery. The mean follow-up time was 42 months (range 1–60 months). The 59 familial cases included in this study were individuals who had GC with at least two first-degree relatives with GC, including at least one patient diagnosed before the age of 50 years. As a sporadic GC cohort, the files of 337 patients with surgically resected primary gastric carcinoma of 40 years or older examined at the Department of Pathology, Seoul National University Hospital in 2004 were analyzed. This study was approved by the Institutional Review Board/Independent Ethics Committee of Seoul National University Hospital (H-1010-065-336).
Tissue arrays and immunohistochemistry (IHC)
Four-micrometer tissue microarray sections were subjected to immunohistochemical staining by the standard streptavidin-biotin complex method. Sectioning was performed after the specimens were de-paraffinized and rinsed in phosphate-buffered saline. Antigen retrieval was performed using rehydrated sections. The primary antibodies used were as follows: anti-BRCA1 (MS110; mouse monoclonal; 1:200; Abcam, Cambridge, UK), anti-BRCA2 (MAB2476; mouse monoclonal; 1:500; R&D Systems, Inc. Minneapolis, MN, USA), anti-Mre11 (ab214; mouse monoclonal; 1:100; Abcam, Cambridge, UK), anti-RAD51C (NBP1-19647; rabbit polyclonal; 1 : 300; Novus biologicals, Littleton CO, USA), and anti-phospho-Histone H2A.X (Ser139) (clone JBW300; mouse monoclonal; 1 : 300; Upstate, NY, USA).
Immunohistochemical assessment
Immunohistochemical results for BRCA1, BRCA2, MRE11, and RAD51C were scored according to nuclear staining. If fewer than 10% of nuclei were positive, the result was considered negative. Otherwise, the result was considered positive (Kim et al., 2013). Immunohistochemical staining was performed to detect nuclear γH2AX. The staining intensity was scored on a scale of zero to three (0, no staining; 1, weak staining; 2, intermediate staining; 3, strong staining). The staining area was scored on a scale of zero to five (0, no stained cells; 1, 1% of the cells were positive; 2, 2–10% of the cells were positive; 3, 11–33% of the cells were positive; 4, 34–66% of the cells were positive; 5, 67–100% of the cells were positive). The sum of the scores ranged from zero to eight. Subsequently, the summed scores for the nuclear expression of γH2AX were grouped as positive or negative in the receiver operating characteristic curve analysis using the highest positive likelihood ratio for the prediction of GC-related death (Park et al., 2015; Hussein et al., 2018).
Statistical analysis
Statistical analyses were performed using SPSS 17.0. Univariate and multivariate analyses were performed by chi-squared and Log-rank tests. Kaplan and Meier (1958) plots were used to visualize the survival distribution of patient subgroups, with differences in survival estimated using Log-rank tests. A value of p < 0.05 (two-tailed) was considered significant.
Results
DNA damage response (DDR) -related protein expression
The expression levels of BRCA1, BRCA2, MRE11, RAD51C, and γH2AX were determined by IHC. Representative results of IHC staining are shown in Figure 1. For comparative analysis of the IHC results, we grouped the samples into early-onset, familial, early-onset or familial, and sporadic GC according to previously established criteria (Lai et al., 2008; Oliveira et al., 2015; Takatsu et al., 2016).
Figure 1.
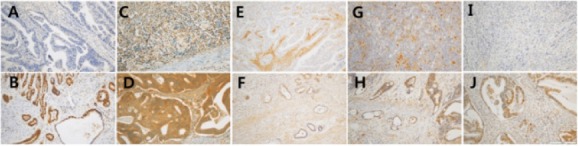
Immunohistochemical Analysis of DNA Damage Response Proteins in Gastric Cancer Tissues. A, BRCA1 negative; B, BRCA1 positive; C, BRCA2 negative; D, BRCA2 positive; E, MRE11 negative; F, MRE11 positive; G, RAD51C negative; H, RAD51C positive; I, γH2AX negative; D, γH2AX positive (x200).
Comparison of clinicopathological and IHC parameters in early-onset or familial and sporadic GCs
Histological parameters were compared among sporadic, early-onset, and familial GC, as summarized in Table 1. The ratio of males to females was significantly higher for sporadic GC than for early-onset or familial GC (p<0.001). Significantly more early-onset and familial tumors were poorly differentiated than sporadic cancers (p < 0.001); 85.4% of early-onset and familial tumors were poorly differentiated compared with 49.9% of the sporadic cancers. Similarly, diffuse type early-onset and familial tumors were significantly more frequent than diffuse type sporadic cancers (p < 0.001); 80.9% of early-onset cancers were poorly differentiated compared with 37.1% of the sporadic cancers. Higher T stage, presence of lymph node metastasis, and higher TNM stage were significantly associated with early-onset or familial cancers (p<0.001, p=0.002, and p<0.001, respectively).
Table 1.
Patient Profiles and DNA Damage Response Protein Expression Results for Early-Onset or Familial Gastric Cancers and Sporadic Gastric Cancers
| Early-onset | Familial | Early-onset or familial | Sporadic | Early-onset vs. sporadic | Familial vs. sporadic | Early-onset vs. familial | Early-onset or familial vs. sporadic | ||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 23 (42.6) | 38 (64.4) | 61 (54) | 251 (74.5) | < 0.001 | 0.108 | 0.02 | < 0.001 |
| Female | 31 (57.4) | 21 (35.6) | 52 (46) | 86 (25.5) | |||||
| Differentiation | Well-differentiated | 0 (0) | 2 (4.3) | 2 (2.2) | 32 (9.5) | < 0.001 | 0.001 | not available | < 0.001 |
| Moderately differentiated | 3 (7) | 8 (17.4) | 11 (12.4) | 137 (40.7) | |||||
| Poorly differentiated | 40 (93) | 36 (78.3) | 76 (85.4) | 168 (49.9) | |||||
| Lauren classification | Intestinal | 3 (7) | 10 (21.7) | 13 (14.6) | 156 (46.3) | < 0.001 | < 0.001 | not available | < 0.001 |
| Diffuse | 37 (86) | 35 (76.1) | 72 (80.9) | 125 (37.1) | |||||
| Mixed | 3 (7) | 1 (2.2) | 4 (4.5) | 56 (16.6) | |||||
| T stage | T1 | 6 (14.3) | 17 (35.4) | 23 (25.6) | 115 (34.1) | < 0.001 | < 0.001 | not available | < 0.001 |
| T2 | 3 (7.1) | 5 (10.4) | 8 (8.9) | 179 (53.1) | |||||
| T3 | 18 (42.9) | 14 (29.2) | 32 (35.6) | 41 (12.2) | |||||
| T4 | 15 (35.7) | 12 (25) | 27 (30) | 2 (0.6) | |||||
| LN metastasis | Absent | 17 (39.5) | 14 (28.6) | 31 (33.7) | 176 (52.2) | 0.117 | 0.002 | 0.267 | 0.002 |
| Present | 26 (60.5) | 35 (71.4) | 61 (66.3) | 161 (47.8) | |||||
| TNM stage | I | 9 (16.7) | 17 (35.4) | 26 (25.5) | 185 (54.9) | < 0.001 | 0.009 | 0.03 | < 0.001 |
| II | 21 (38.9) | 20 (41.7) | 41 (40.2) | 75 (22.3) | |||||
| III | 24 (44.4) | 11 (22.9) | 35 (34.3) | 77 (22.8) | |||||
| BRCA1 | Negative | 24 (49) | 22 (38.6) | 46 (43.4) | 103 (36.5) | 0.098 | 0.767 | 0.282 | 0.215 |
| Positive | 25 (51) | 35 (61.4) | 60 (56.6) | 179 (63.5) | |||||
| BRCA2 | Negative | 21 (47.7) | 15 (26.3) | 36 (35.6) | 128 (39.4) | 0.29 | 0.06 | 0.026 | 0.5 |
| Positive | 23 (52.3) | 42 (73.7) | 65 (64.4) | 197 (60.6) | |||||
| MRE11 | Negative | 16 (33.3) | 2 (3.5) | 18 (17.1) | 117 (35.2) | 0.796 | < 0.001 | < 0.001 | < 0.001 |
| Positive | 32 (66.7) | 55 (96.5) | 87 (82.9) | 215 (64.8) | |||||
| Rad51C | Negative | 7 (14.3) | 9 (15.8) | 16 (15.1) | 148 (46) | < 0.001 | < 0.001 | 0.829 | < 0.001 |
| Positive | 42 (85.7) | 48 (84.2) | 90 (84.9) | 174 (54) | |||||
| H2AX | Negative | 16 (32) | 12 (21.1) | 28 (26.2) | 182 (64.1) | < 0.001 | < 0.001 | 0.199 | < 0.001 |
| Positive | 34 (68) | 45 (78.9) | 79 (73.8) | 102 (35.9) |
There were no significant differences in BRCA1 and BRCA2 protein expression between early-onset or familial GC and sporadic GC cases. In a comparison of DDR-related protein expression frequencies among the GC cohorts, RAD51C positivity was significantly higher in early-onset or familial GC (84.9%) than in sporadic GC (54.0%) (p < 0.001). γH2AX was more frequently positive in early-onset or familial GC (73.8%) than in sporadic GC (35.9%) (p<0.001) (Table 1).
Expression of DDR markers in early-onset or familial GC and sporadic GC
Clinicopathological features were not correlated with BRCA1, BRCA2, MRE11, and γH2AX expression. RAD51C expression was significantly associated with the presence of lymph node metastasis (p = 0.032) and higher TNM stage (p = 0.044) in early-onset or familial GC (Table 2).
Table 2.
Correlations between Clinicopathological Features and DNA Damage Response Proteins in Early-Onset or Familial Gastric Cancer
| BRCA1 | BRCA2 | MRE11 | Rad51C | γH2AX | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | ||
| Gender | Male | 22 (47.8) | 33 (55) | 0.464 | 14 (38.9) | 38 (58.5) | 0.059 | 12 (66.7) | 43 (49.4) | 0.182 | 8 (50) | 47 (52.2) | 0.87 | 17 (60.7) | 39 (49.4) | 0.302 |
| Female | 24 (52.2) | 27 (45) | 22 (61.1) | 27 (41.5) | 6 (33.3) | 44 (50.6) | 8 (50) | 43 (47.8) | 11 (39.3) | 40 (50.6) | ||||||
| Differentiation | Well differentiated | 0 (0) | 2 (4.2) | 0.462 | 1 (3.3) | 1 (2.1) | 0.195 | 1 (8.3) | 1 (1.4) | 0.336 | 0 (0) | 2 (2.9) | 0.272 | 0 (0) | 2 (3.2) | 0.672 |
| Moderately differentiated | 4 (11.4) | 6 (12.5) | 1 (3.3) | 8 (16.7) | 1 (8.3) | 9 (12.9) | 0 (0) | 10 (14.3) | 3 (14.3) | 7 (11.3) | ||||||
| Poorly differentiated | 31 (88.6) | 40 (83.3) | 28 (93.3) | 39 (81.3) | 10 (83.3) | 60 (85.7) | 13 (100) | 58 (82.9) | 18 (85.7) | 53 (85.5) | ||||||
| Lauren classification | Intestinal | 4 (11.4) | 8 (16.7) | 0.34 | 2 (6.7) | 9 (18.8) | 0.255 | 2 (16.7) | 10 (14.3) | 0.691 | 0 (0) | 12 (17.1) | 0.159 | 3 (14.3) | 9 (14.5) | 1 |
| Diffuse | 28 (80) | 39 (81.3) | 27 (90) | 36 (75) | 10 (83.3) | 56 (80) | 13 (100) | 54 (77.1) | 17 (81) | 50 (80.6) | ||||||
| Mixed | 3 (8.6) | 1 (2.1) | 1 (3.3) | 3 (6.3) | 0 (0) | 4 (5.7) | 0 (0) | 4 (5.7) | 1 (4.8) | 3 (4.8) | ||||||
| T stage | T1 | 8 (22.2) | 13 (26.5) | 0.891 | 8 (26.7) | 11 (22) | 0.833 | 1 (7.7) | 20 (28.2) | 0.115 | 6 (46.2) | 15 (20.8) | 0.083 | 2 (9.1) | 19 (30.2) | 0.154 |
| T2 | 2 (5.6) | 4 (8.2) | 1 (3.3) | 4 (8) | 0 (0) | 6 (8.5) | 2 (15.4) | 4 (5.6) | 2 (9.1) | 4 (6.3) | ||||||
| T3 | 15 (41.7) | 17 (34.7) | 12 (40) | 19 (38) | 5 (38.5) | 27 (38) | 2 (15.4) | 30 (41.7) | 8 (36.4) | 24 (38.1) | ||||||
| T4 | 11 (30.6) | 15 (30.6) | 9 (30) | 16 (32) | 7 (53.8) | 18 (25.4) | 3 (23.1) | 23 (31.9) | 10 (45.5) | 16 (25.4) | ||||||
| LN metastasis | Absent | 13 (35.1) | 15 (30.6) | 0.658 | 13 (41.9) | 13 (26) | 0.135 | 4 (30.8) | 24 (33.3) | 0.856 | 8 (57.1) | 20 (27.8) | 0.032 | 7 (31.8) | 21 (32.8) | 0.932 |
| Present | 24 (64.9) | 34 (69.4) | 18 (58.1) | 37 (74) | 9 (69.2) | 48 (66.7) | 6 (42.9) | 52 (72.2) | 15 (68.2) | 43 (67.2) | ||||||
| TNM stage | I | 8 (19.5) | 15 (27.8) | 0.561 | 8 (23.5) | 12 (21.4) | 0.945 | 1 (5.6) | 22 (28.9) | 0.015 | 7 (50) | 16 (19.8) | 0.044 | 2 (7.7) | 21 (30) | 0.062 |
| II | 19 (46.3) | 20 (37) | 14 (41.2) | 25 (44.6) | 6 (33.3) | 33 (43.4) | 3 (21.4) | 36 (44.4) | 14 (53.8) | 25 (35.7) | ||||||
| III | 14 (34.1) | 19 (35.2) | 12 (35.3) | 19 (33.9) | 11 (61.1) | 21 (27.6) | 4 (28.6) | 29 (35.8) | 10 (38.5) | 24 (34.3) | ||||||
In contrast, these DDR markers were significant correlated with clinicopathological variables in sporadic GC. Thus, BRCA1 negativity correlated with poor differentiation (p = 0.016), advanced T stage (p = 0.001), and positive lymph node metastasis (p < 0.001); BRCA2 negativity correlated with poor differentiation (p < 0.001); and RAD51C negativity correlated with poor differentiation (p < 0.001) (Table 3).
Table 3.
Correlations between Clinicopathological Features and DNA Damage Response proteins in Sporadic Gastric Cancer
| BRCA1 | BRCA2 | MRE11 | Rad51C | γH2AX | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | ||
| Gender | Male | 70 (68) | 142 (79.3) | 0.033 | 90 (70.3) | 153 (77.7) | 0.136 | 85 (72.6) | 163 (75.8) | 0.526 | 111 (75) | 129 (74.1) | 0.86 | 130 (71.4) | 81 (79.4) | 0.14 |
| Female | 33 (32) | 37 (20.7) | 38 (29.7) | 44 (22.3) | 32 (27.4) | 52 (24.2) | 37 (25) | 45 (25.9) | 52 (28.6) | 21 (20.6) | ||||||
| Differentiation | Well differentiated | 2 (1.9) | 28 (15.6) | < 0.001 | 8 (6.3) | 22 (11.2) | < 0.001 | 7 (6) | 24 (11.2) | 0.116 | 13 (8.8) | 18 (10.3) | < 0.001 | 10 (5.5) | 16 (15.7) | 0.001 |
| Moderately differentiated | 37 (35.9) | 77 (43) | 39 (30.5) | 97 (49.2) | 44 (37.6) | 92 (42.8) | 43 (29.1) | 88 (50.6) | 71 (39) | 50 (49) | ||||||
| Poorly differentiated | 64 (62.1) | 74 (41.3) | 81 (63.3) | 78 (39.6) | 66 (56.4) | 99 (46) | 92 (62.2) | 68 (39.1) | 101 (55.5) | 36 (35.3) | ||||||
| Lauren classification | Intestinal | 37 (35.9) | 96 (53.6) | 0.016 | 36 (28.1) | 117 (59.4) | < 0.001 | 52 (44.4) | 102 (47.4) | 0.734 | 44 (29.7) | 104 (59.8) | < 0.001 | 77 (42.3) | 56 (54.9) | 0.008 |
| Diffuse | 46 (44.7) | 56 (31.3) | 66 (51.6) | 52 (26.4) | 47 (40.2) | 77 (35.8) | 74 (50) | 45 (25.9) | 78 (42.9) | 25 (24.5) | ||||||
| Mixed | 20 (19.4) | 27 (15.1) | 26 (20.3) | 28 (14.2) | 18 (15.4) | 36 (16.7) | 30 (20.3) | 25 (14.4) | 27 (14.8) | 21 (20.6) | ||||||
| T stage | T1 | 20 (19.4) | 72 (40.2) | 0.001 | 58 (45.3) | 52 (26.4) | 0.003 | 26 (22.2) | 87 (40.5) | 0.003 | 60 (40.5) | 48 (27.6) | 0.008 | 40 (22) | 47 (46.1) | < 0.001 |
| T2 | 64 (62.1) | 89 (49.7) | 55 (43) | 119 (60.4) | 78 (66.7) | 100 (46.5) | 65 (43.9) | 106 (60.9) | 109 (59.9) | 49 (48) | ||||||
| T3 | 19 (18.4) | 16 (8.9) | 15 (11.7) | 24 (12.2) | 13 (11.1) | 26 (12.1) | 23 (15.5) | 18 (10.3) | 33 (18.1) | 4 (3.9) | ||||||
| T4 | 0 (0) | 2 (1.1) | 0 (0) | 2 (1) | 0 (0) | 2 (0.9) | 0 (0) | 2 (1.1) | 0 (0) | 2 (2) | ||||||
| LN metastasis | Absent | 39 (37.9) | 102 (57) | 0.002 | 75 (58.6) | 92 (46.7) | 0.036 | 50 (42.7) | 123 (57.2) | 0.012 | 78 (52.7) | 89 (51.1) | 0.781 | 77 (42.3) | 64 (62.7) | 0.001 |
| Present | 64 (62.1) | 77 (43) | 53 (41.4) | 105 (53.3) | 67 (57.3) | 92 (42.8) | 70 (47.3) | 85 (48.9) | 105 (57.7) | 38 (37.3) | ||||||
| TNM stage | I | 42 (40.8) | 108 (60.3) | 0.002 | 80 (62.5) | 97 (49.2) | 0.062 | 54 (46.2) | 128 (59.5) | 0.064 | 84 (56.8) | 92 (52.9) | 0.526 | 81 (44.5) | 67 (65.7) | 0 |
| II | 23 (22.3) | 37 (20.7) | 24 (18.8) | 48 (24.4) | 32 (27.4) | 43 (20) | 28 (18.9) | 42 (24.1) | 40 (22) | 24 (23.5) | ||||||
| III | 38 (36.9) | 34 (19) | 24 (18.8) | 52 (26.4) | 31 (26.5) | 44 (20.5) | 36 (24.3) | 40 (23) | 61 (33.5) | 11 (10.8) | ||||||
Early-onset or familial cancer and patient outcomes
A Kaplan–Meier analysis of overall survival showed slightly higher survival rates for sporadic GC than for early-onset or familial GC, but the difference was not significant. In a univariate survival analysis according to DDR expression, BRCA1 negativity was associated with a decreased overall survival only in sporadic GC (p = 0.002), and MRE11 negativity was associated with a decreased overall survival only in sporadic GC (p = 0.012) (Figure 2).
Figure 2.
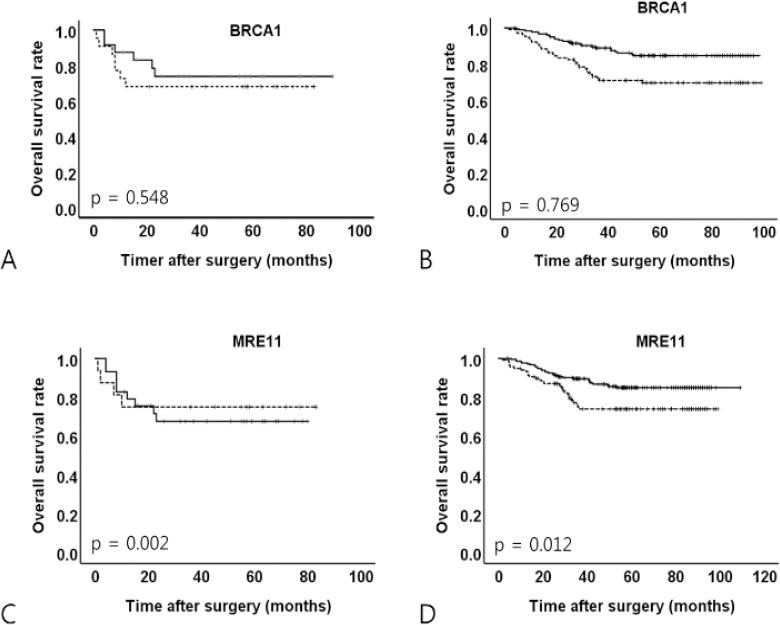
Kaplan–Meier Analyses of Overall Survival According to BRCA1 and MRE11 Expression in Early-Onset or Familial and Sporadic Gastric Cancers. A, BRCA1 in early-onset or familial gastric cancer; B, BRCA1 in sporadic gastric cancer; C, MRE11 in early-onset or familial gastric cancer; D, MRE11 in sporadic gastric cancer. Dotted line, negative expression of protein; solid line, positive expression of protein
Multivariate analyses showed that the DDR is not correlated with survival in early-onset or familial and sporadic GC (data not shown).
Discussion
GC is classified into sporadic and familial forms; although most GC cases are sporadic, aggregation within families accounts for roughly 10% of cases. Mutations in CDH1 and CTNNA1 are associated with familial GC risk (Oliveira et al., 2015). Molecular expression profiles of early-onset GC and conventional GC have been found to differ. Early-onset GC less frequently expresses cyclooxygenase-2 (COX-2) and Trefoil Factor-1 (TFF-1) (Milne et al., 2006) and more frequently aberrantly expresses E-Cadherin regardless of the histological type (Lim et al., 2003).
Our results showed that early-onset or familial GC is substantially more aggressive than sporadic GC. These results are concordant with those of previous studies reporting greater aggressiveness and lethality for early-onset GC compared to that in the general population (Smith and Stabile, 2009; Kong et al., 2012; Lee et al., 2016).
We detected differences in the expression profiles of RAD51C and γH2AX between early-onset or familial GC and sporadic GC. DDR has been implicated in tumorigenesis and progression in human cancers. The impairment of DDR causes genetic instability and may affect prognosis and susceptibility to chemotherapy in GC (Bartkova et al., 2005). In GC, elevated levels of γH2AX and pATM are adverse factors for progression-free and overall survival (Ronchetti et al., 2017). Additionally, the expression of the MRN complex is associated with a poor prognosis and negatively associated with the expression of the DNA damage marker γH2AX in the nucleus (Altan et al., 2016).
DSB repair is mediated by two principal mechanisms: non-homologous end-joining and HR (Jackson and Bartek, 2009). In mammals, the core of the HR pathway includes the MRE11-RAD50-NBS1 complex and RAD51 and RAD51 paralogs (Carvalho and Kanaar, 2014). HR-defective tumors are more sensitive to DNA-damaging agents, such as cisplatin and IR. Mutations in HR-associated genes, such as BRCA1 and BRCA2, are responsible for approximately 40% of the genetic predisposition to familial breast and ovarian cancers (Somyajit et al., 2010). HR-defective tumors may be more sensitive to chemoradiotherapeutic strategies that promote DNA damage. PARP inhibitors targeting DDR pathways exhibit potent anticancer activity in preclinical models and clinical studies of GC, especially in those with low ATM or RAD51 expression (Kim et al., 2013). RAD51C-deficient cancer cells are highly sensitive to the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib (Min et al., 2013). RAD51C has been characterized as a cancer susceptibility gene (Somyajit et al., 2010). Biallelic mutations in RAD51C lead to Fanconi anemia-like disorder, and monoallelic mutations in RAD51C are associated with an increased risk of breast and ovarian cancer (Somyajit et al., 2010).
Our study is a retrospective clinical study using IHC and tissue microarray. Intratumoral heterogeneity has become an important issue in cancer therapeutics (Gerlinger et al., 2012). For clinical application of the DDR-related proteins identified in this study as tumor biomarkers, further investigation regarding intratumoral heterogeneity would be required.
Our results showed that early-onset or familial GC and sporadic GC have significantly different clinicopathologic characteristics and DDR marker expression patterns, especially with respect to RAD51C and γH2AX. In particular, early-onset or familial GC is significantly associated with RAD51C and γH2AX. The observation that early-onset or familial GC has higher rates of DDR deficiency could contribute to the prediction of the response to chemotherapy. These results suggest that early-onset or familial GC might have a higher degree of deregulation of DDR, raising the possibility that this form of GC has higher chemotherapy sensitivity than sporadic GC.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2014R1A1A2057925).
Acknowledgments
We appreciate Ms. Hye Jung Lee and Seung Hee Jung for help with tissue array construction and immunohistochemical staining.
References
- Altan B, Yokobori T, Ide M, et al. High expression of MRE11–RAD50–NBS1 is associated with poor prognosis and chemoresistance in gastric cancer. Anticancer Res. 2016;36:5237–47. doi: 10.21873/anticanres.11094. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Brandt S, Samartzis EP, Zimmermann AK, et al. Lack of MRE11-RAD50-NBS1 (MRN) complex detection occurs frequently in low-grade epithelial ovarian cancer. BMC Cancer. 2017;17:44. doi: 10.1186/s12885-016-3026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho JFS, Kanaar R. Targeting homologous recombination-mediated DNA repair in cancer. Expert Opin Ther Targets. 2014;18:427–58. doi: 10.1517/14728222.2014.882900. [DOI] [PubMed] [Google Scholar]
- Chen X, Qian D, Cheng J, et al. High expression of Rad51c predicts poor prognostic outcome and induces cell resistance to cisplatin and radiation in non-small cell lung cancer. Tumour Biol. 2016;37:13489–98. doi: 10.1007/s13277-016-5192-x. [DOI] [PubMed] [Google Scholar]
- Correa P, Shiao Y-h. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941–3. [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JD, Cawkwell L, Sue-Ling H, et al. Assessment of microsatellite alterations in young patients with gastric adenocarcinoma. Cancer. 1997;79:684–7. doi: 10.1002/(sici)1097-0142(19970215)79:4<684::aid-cncr4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Ho V, Chung L, Singh A, et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer. 2018;18:869. doi: 10.1186/s12885-018-4776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein UK, Park HS, Bae JS, et al. Expression of oxidized protein tyrosine phosphatase and gammaH2AX predicts poor survival of gastric carcinoma patients. BMC Cancer. 2018;18:836. doi: 10.1186/s12885-018-4752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Cho HJ, Kim M, et al. Differing effects of adjuvant chemotherapy according to BRCA1 nuclear expression in gastric cancer. Cancer Chemother Pharmacol. 2013;71:1435–43. doi: 10.1007/s00280-013-2141-x. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yamaguchi T, Yamamoto K, et al. Clinicopathological analysis of gastric cancer in young adults. Hepatogastroenterology. 1996;43:1273–80. [PubMed] [Google Scholar]
- Kokkola A, Sipponen P. Gastric carcinoma in young adults. Hepatogastroenterology. 2001;48:1552–5. [PubMed] [Google Scholar]
- Kong X, Wang JL, Chen HM, et al. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma:a meta analysis. J Surg Oncol. 2012;106:346–52. doi: 10.1002/jso.23004. [DOI] [PubMed] [Google Scholar]
- Lai JF, Kim S, Li C, et al. Clinicopathologic characteristics and prognosis for young gastric adenocarcinoma patients after curative resection. Ann Surg Oncol. 2008;15:1464–9. doi: 10.1245/s10434-008-9809-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee MA, Kim IH, et al. Clinical characteristics of young-age onset gastric cancer in Korea. BMC Gastroenterol. 2016;16:110. doi: 10.1186/s12876-016-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Lee HS, Kim HS, et al. Alteration of E-cadherin-mediated adhesion protein is common, but microsatellite instability is uncommon in young age gastric cancers. Histopathology. 2003;42:128–36. doi: 10.1046/j.1365-2559.2003.01546.x. [DOI] [PubMed] [Google Scholar]
- McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–74. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- Milne AN, Carvalho R, Morsink FM, et al. Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Mod Pathol. 2006;19:564–72. doi: 10.1038/modpathol.3800563. [DOI] [PubMed] [Google Scholar]
- Milne AN, Sitarz R, Carvalho R, et al. Early onset gastric cancer:On the road to unraveling gastric carcinogenesis. Curr Mol Med. 2007;7:15–28. doi: 10.2174/156652407779940503. [DOI] [PubMed] [Google Scholar]
- Min A, Im SA, Yoon YK, et al. RAD51C-Deficient Cancer Cells Are Highly Sensitive to the PARP Inhibitor Olaparib. Mol Cancer Ther. 2013;12:865–77. doi: 10.1158/1535-7163.MCT-12-0950. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–60. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer:genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:60–70. doi: 10.1016/S1470-2045(14)71016-2. [DOI] [PubMed] [Google Scholar]
- Park SH, Noh SJ, Kim KM, et al. Expression of DNA damage response molecules PARP1, gammaH2AX, BRCA1, and BRCA2 predicts poor survival of breast carcinoma patients. Transl Oncol. 2015;8:239–49. doi: 10.1016/j.tranon.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Ronchetti L, Melucci E, De Nicola F, et al. DNA damage repair and survival outcomes in advanced gastric cancer patients treated with first-line chemotherapy. Int J Cancer. 2017;140:2587–95. doi: 10.1002/ijc.30668. [DOI] [PubMed] [Google Scholar]
- Rothkamm K. From the Cover:Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–62. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabudhe R, Lott P, Bohorquez M, et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology. 2017;152(983-6):e6. doi: 10.1053/j.gastro.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg (Chicago, Ill 1960) 2009;144:506. doi: 10.1001/archsurg.2009.77. [DOI] [PubMed] [Google Scholar]
- Somyajit K, Subramanya S, Nagaraju G. RAD51C:a novel cancer susceptibility gene is linked to Fanconi anemia and breast cancer. Carcinogenesis. 2010;31:2031–8. doi: 10.1093/carcin/bgq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu Y, Hiki N, Nunobe S, et al. Clinicopathological features of gastric cancer in young patients. Gastric Cancer. 2016;19:472–8. doi: 10.1007/s10120-015-0484-1. [DOI] [PubMed] [Google Scholar]
- Vilar E, Bartnik CM, Stenzel SL, et al. MRE11 deficiency increases sensitivity to Poly (ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers. Cancer Res. 2011;71:2632–42. doi: 10.1158/0008-5472.CAN-10-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-H, Zhao C-M, Huang Y, et al. BRCA1 and BRCA2 expression patterns and prognostic significance in digestive system cancers. Hum Pathol. 2018;71:135–44. doi: 10.1016/j.humpath.2017.10.032. [DOI] [PubMed] [Google Scholar]


