Abstract
Purpose of review:
The purpose of this paper was to address how sleep changes with aging, with the broader goal of informing how REM sleep and slow wave activity mechanisms interact to promote cognitive longevity.
Recent findings:
We conducted novel analyses based on the National Sleep Research Resource database. Over approximately five years, middle-to-older aged adults, on average, showed dramatically worse sleep fragmentation, a steady decrease in slow wave sleep, and yet a small increase in REM sleep. Averaging across participants, however, masked a major theme: Individuals differ substantially in their longitudinal trajectories for specific components of sleep. We considered this individual variability in light of recent theoretical and empirical work that has shown disrupted sleep and decreased slow wave activity to impair frontal lobe restoration, glymphatic system functioning, and memory consolidation. Based on multiple recent longitudinal studies, we contend that preserved or enhanced REM sleep may compensate for otherwise disrupted sleep in advancing age.
Summary:
The scientific community has often debated whether slow wave activity or REM sleep mechanisms are more important to cognitive aging. We propose that a more fruitful approach for future work will be to investigate how REM and slow wave processes dynamically interact to affect cognitive longevity.
Keywords: National Sleep Research Resource, older adults, dementia, memory consolidation, sleep spindles, sequential hypothesis
“Manifestly new, although evanescent combinations, are made during dreaming; but I contend that permanent rearrangements (internal evolutions) are made during so-called dreamless sleep.”
-John Hughlings Jackson, writing 70 years before the discovery of sleep stages [1•].
Introduction
With an aging population, increased prevalence of dementia, and rising healthcare costs, there has never been more interest in cognitive longevity. The scientific community has for decades communicated the importance of exercise and diet to maintaining cognitive functioning in older age, but recently, there has been a surge of interest in the role of sleep in cognitive health. For example, last year, Bubu et al. concluded that “15% of [Alzheimer’s disease] may be prevented should interventions be implemented to reduce sleep problems and disorders” [2]. Given such provocative claims, it is no surprise that the general public is similarly interested in sleep and cognitive health. When AARP surveyed 3,374 middle-aged and older adults, sleep was the number one brain-health topic that adults wanted to learn about, with 98% of respondents indicating that they believed sleep to be important to their brain health [3].
The purpose of this paper is to address how sleep typically changes with aging by conducting novel analyses on the data in the National Sleep Research Resource. We further aim to highlight recent advances in the mechanisms by which sleep affects cognitive longevity. Whereas the scientific community has debated whether slow wave sleep/activity (SWS/SWA) or REM-based mechanisms are more important to cognitive aging, in this paper, we argue a counterpoint based on the sequential hypothesis of memory processing: Both SWS and REM sleep interact dynamically to affect cognitive functioning with advancing age.
Sleep Changes with Aging Are Dynamic When Measured Longitudinally
More than 1,000 papers have been published on how sleep changes with aging [4-6]. In a meta-analysis, Ohayon and colleagues [7] evaluated the cross-sectional literature, reporting that nearly every measure of polysomnography (PSG) measured sleep steadily worsened across adulthood (nonlinear associations were sometimes observed when including children). They reported particularly large age-related changes for SWS and sleep fragmentation (wake after sleep onset).
What is striking from Ohayon and colleagues’ [7] meta-analysis, and from the sleep and aging literature in general, is how few studies have longitudinally assessed changes in PSG-defined features of sleep (there are several longitudinal studies that only used self-report sleep scales). Due to the costs of PSG and the difficulty of longitudinal assessments, almost all such studies have used small sample sizes. The now publicly available National Sleep Research Resource offers a solution [8, 9•] by including data from thousands of participants who underwent longitudinal PSG assessment in the Sleep Heart Health Study [10]. The traditional analytical approach has been to average sleep parameters for each age group at each time point; our goal was to re-analyze and plot individual trajectories to inform inter- and intra-individual variability in how aging impacts sleep.
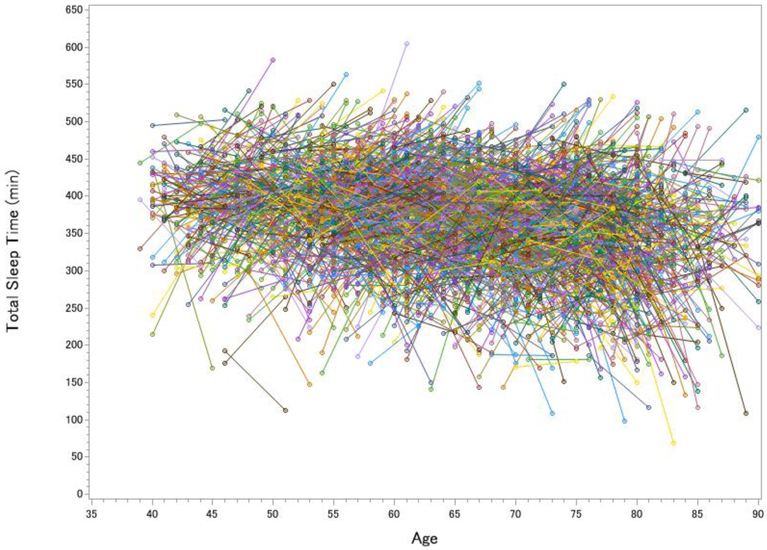
The Sleep Heart Health Study was a multi-center, longitudinal study with Wave 1 data collection occurring from 1995 to 1998 and Wave 2 data collection occurring from 2001 to 2003 [10]. Participants who were 40 years and older, without any history of treatment for sleep-disordered breathing were eligible for completing one night of in-home PSG at both visits. In addition to the numerous measures to assess sleep-disordered breathing, the montage included C3/A2 and C4/A1 EEG to score sleep stages (note that AASM later recommended including F3 and F4). Figure 1 displays the major results from the 2,643 participants who completed the second visit at least 4 years after the first visit (Mage = 62.34 ± 10.41 at visit 1, Mage = 67.56 at visit 2, 53.76% Female, 87.25% White).
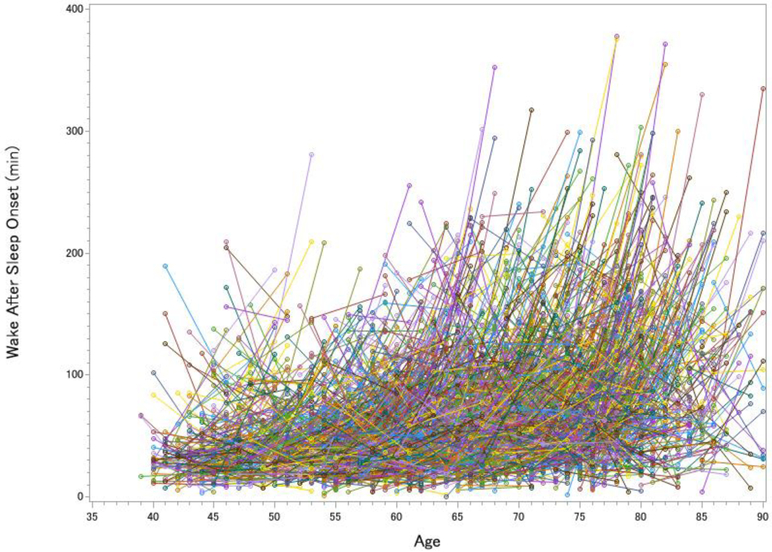
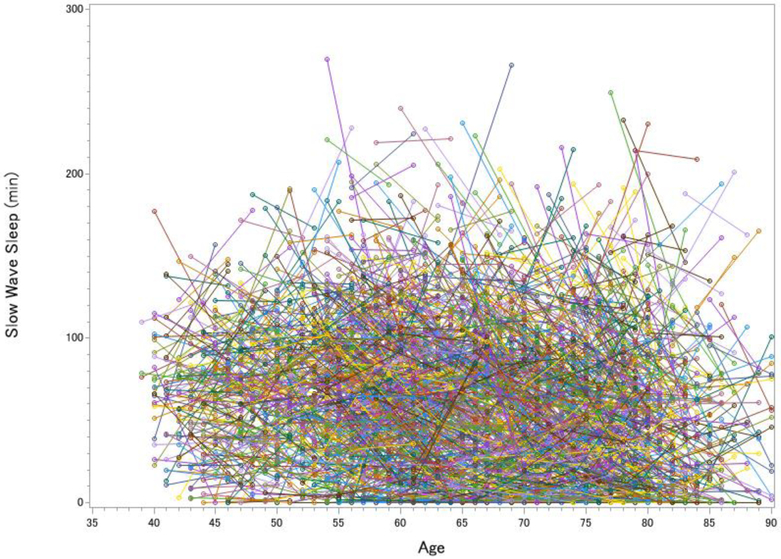
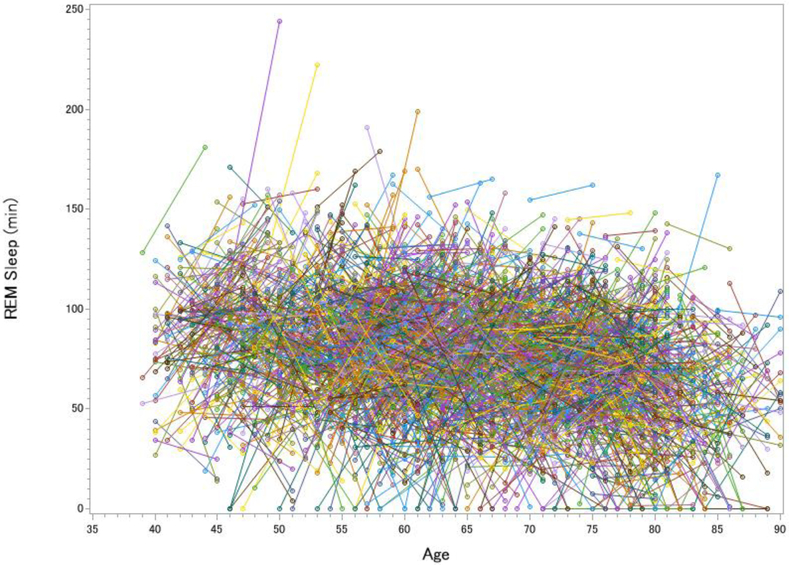
Figure 1. Longitudinal Changes in Total Sleep Time (A), Wake After Sleep Onset (B), Slow Wave Sleep (C) and REM sleep (D) in the Sleep Heart Health Study.
Each line represents one participant’s change in sleep from visit 1 to visit 2. The pre-to-post increase in total sleep time was significant but small, t(2642) = 3.12, p = .002, d = 0.06, whereas the increase in wake after sleep onset was large, t(2642) = 20.63, p < .001, d = 0.40. SWS declined over time by 9.0%, t(2642) = 8.51, p < .001, d = 0.17, whereas REM sleep actually increased over time by 3.6%, t(2642) = 3.99, p < .001, d = 0.08 (greater age at visits 1 and 2 was cross-sectionally associated with decreased REM, rs = −.220 and −.295).
All of the cross-sectional data from the Sleep Heart Health Study converged with Ohayon et al.’s [7] meta-analytic findings of chronological age being cross-sectionally associated with worse sleep. The pre-to-post longitudinal data showed a different story. Not only was there far more inter-individual and intra-individual variability than can be captured in a meta-analysis, but participants gained an average of 4.6 minutes of sleep in the five years between PSG assessments (Figure 1a). The gain in total sleep time was offset, however, by the severity of sleep fragmentation (Figure 1b). After five years, 31.4% of adults had doubled the amount of time they spent awake at night, with the largest increase being in adults 60 years old and older. By age 80, a WASO of more than 100 minutes per night was very common (50.5% of participants). These longitudinal findings indicated a more severe increase in WASO than cross-sectional studies observed.
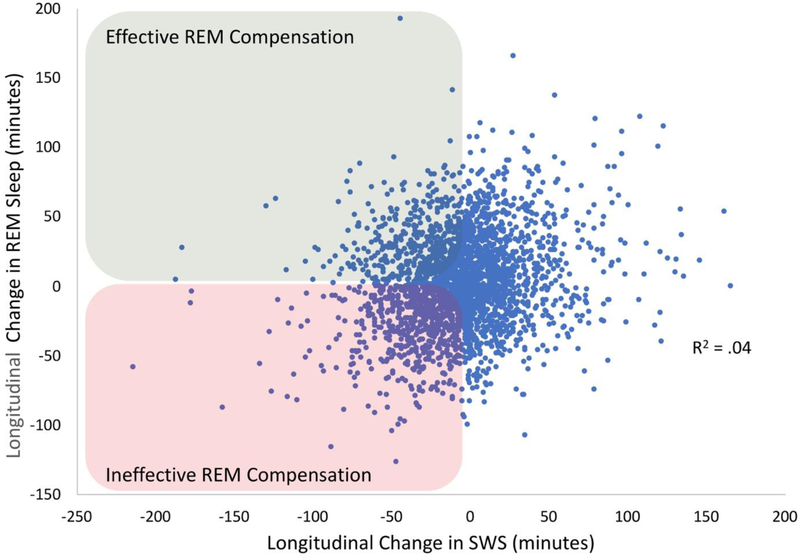
If sleep fragmentation increased more than total sleep time, then the prediction should be that sleep architecture is profoundly impacted. Figure 2 disfavored that notion. While the changes in sleep architecture were statistically significant (all ps < .001), the intra-individual variability rendered the average changes to be hardly visible to the eye, even for the expected decrease in SWS duration (6 minute, or 9%, average decrease; Figure 1c). For REM sleep, there was actually a 3 minute increase from Visit 1 to Visit 2, which was contrary to expectations from cross-sectional analyses, which are most typical in the literature (Figure 1d). One potential concern is that the longitudinal increase in REM simply reflected a “first night effect,” even though the assessments were five years apart [11]. However, two hallmarks of the first night effect—greater fragmentation and delayed REM latency on Night 1 [11]—were opposite of what we observed in our analyses (on Night 2, there were 22 more minutes of WASO and REM was delayed by 12 more minutes than Night 1). Lastly, we examined whether participants who showed a decline in SWS were also likely to show a decline in REM. Interestingly, there was only a weak association between the amount of change in REM sleep and change in SWS (R2 = .04; Figure 2).
Figure 2. Novel Analyses of the Changes in SWS and REM Sleep in the Sleep Heart Health Study.
Difference scores represent how much SWS and REM sleep were lost over approximately 5 years, with the correlation of loss being significant, but modest in size (r = .20, p < .001, R2 = .04). The effect size remained the same (r = .20) when controlling for chronological age. Highlighted in green are individuals whose SWS declined, but who showed an increase in REM sleep, which we theorize reflects compensation and should promote cognitive preservation. Highlighted in red are individuals who showed declining SWS, without any REM compensation, which we theorize should lead to cognitive decline and dementia. Increased SWS in the presence of increased/decreased REM is likely also relevant, and perhaps reflecting increases/decreases in exercise, diet, social/cognitive engagement, or other resiliency factors [79].
There are three take-home messages here. First, when looking at data averaged across individuals, the Sleep Heart Health Study [10] data converged with most “textbook” sleep knowledge: With increasing age, sleep tends to worsen, with the largest impact on measures of sleep fragmentation and sleep depth. Second, and much more importantly, averaging across individuals to determine how aging affects sleep is imprecise at-best, and misleading at-worst because of the substantial individual variability in longitudinal trajectories. Some adults’ sleep worsens dramatically, yet many adults’ sleep remains stable or even improves. Compared to consensus standards [12], many older adults maintained adequate or even optimal levels of SWS and REM sleep. Third, the weak association between the degree of loss of SWS and REM sleep over time raises intriguing possibilities. For example, some older adults may show resilience to the loss of SWS by compensating for the loss with increased REM sleep. From this view, some of the functions of SWS might be transferred to, or partially compensated by, processes during REM sleep.
In the following sections, we consider the implications of inter- and intra-individual variability in sleep for neurocognitive processes in aging adults. There are at least three pathways by which sleep disturbances with increasing age could accelerate cognitive aging: glymphatic metabolite clearance, frontal/executive restoration, and memory consolidation.
Mechanism 1: Glymphatic System Functioning
One mechanism by which sleep should be critical to cognitive aging is via glymphatic system functioning [13]. The glymphatic system is a waste clearance system that uses astroglial cells and increased cerebrospinal fluid (CSF) flow to remove metabolites such as amyloid beta with the purpose of minimizing neurotoxicity. The glymphatic system is suppressed during wakefulness, and turned on during sleep [14], perhaps specifically during SWS [13]. Aged mice show an 80% reduction in glymphatic function relative to young mice [15], and a mice model of Alzheimer’s disease showed glymphatic impairments even prior to significant amyloid beta accumulations [16].
In human studies, the connection between sleep, aging, and amyloid levels has mostly been correlational [17-21], with three recent exceptions [22•, 23, 24]. Ju and colleagues [22•] had 17 middle-aged and older adults undergo multiple overnight PSG recordings with morning lumbar punctures. On the experimental night, they delivered tones through earphones to wake the participant whenever the EEG spectral power exceeded 100 μV2 in the 0.5-4.0 Hz frequency band; thus, this procedure targets disrupting SWA. During the control night, participants wore earphones but did not deliver sleep-disrupting tones. The sleep-disrupting tones severely reduced both SWS and REM sleep during the experimental night, but there were no significant changes in CSF amyloid-β40 levels relative to the control night. One possible explanation is that the study was underpowered to detect a main effect on CSF amyloidβ40 levels, because the researchers identified a large association between the extent of change in amyloidβ40 in relation to the extent of change in delta spectral power.
To further test whether altered sleep and SWS affects amyloid production or clearance, Lucey and colleagues [23] sampled CSF every two hours for 36 hours across three conditions. Eight middle-aged to older adults underwent this intensive procedure during periods of total sleep deprivation, normal sleep, or SWS-enhanced sleep (via sodium oxybate). Total sleep deprivation increased amyloid production by 25-30%. However, even though they pharmacologically increased SWS from 14.2 min (normal sleep) to 69.3 min (sodium oxybate), it had no effect on amyloid production or clearance.
Based on the Ju et al. [22•] and Lucey et al.’s [23] experimental findings, SWS probably does not account for glymphatic clearance of amyloid in human adults, at least not by itself. Researchers should address whether the increased stress, which is common in studies of sleep deprivation/disruption, explains alterations in amyloid [25]. Subjective moodiness has already been implicated in an experimental sleep deprivation and PET amyloid neuroimaging study at the National Institutes of Health [24]. In 20 cognitively normal adults (ages 22-72), a night of total sleep deprivation caused an increase in amyloid beta burden in the hippocampus and thalamus. The amount of increase in amyloid beta burden was strongly associated with the amount of increase in moodiness. If the role of stress can be successfully addressed, then based on the clear effects of total sleep deprivation (cf. nonconclusive effects of selective sleep deprivation), another fruitful approach may be to investigate how SWS and REM sleep interact dynamically in determining glymphatic and cognitive functioning.
Mechanism 2: Frontal Restoration and Executive Function
A second mechanism by which poor sleep may affect cognitive aging is through restoration of frontal lobe and attention/executive functioning. In healthy young adults, a night of total sleep deprivation impairs frontoparietal network functioning and default mode network connectivity [26]. Studies of sleep, neuroimaging, and aging are covered elsewhere in this issue [27], but briefly, numerous studies now indicate that self-reported sleep quality and quantity are associated with faster cortical thinning with aging [28]. One line of thinking is that SWS and/or REM sleep are needed to restore the functioning of the frontal and medial temporal lobe regions on a nightly basis [29, 30]. Thus, if one sleeps poorly across their lifespan, then that may have cumulative negative effects on tests of attention, memory encoding/retrieval, and/or executive function.
Poor sleep patterns are associated with worse performance on tests of attention and executive function in cross-sectional studies that included broad age ranges or clinical groups [6]. For example, Dijk and colleagues [31] conducted PSG recordings in 206 healthy adults ages 20-84 years old who performed several tests of attention, processing speed, and executive function. After controlling for demographic variables, SWS was associated with processing speed, whereas REM sleep and measures of sleep fragmentation (e.g., awakenings, sleep efficiency) were significantly related to measures of executive functioning. Moreover, in the HypnoLaus study that compared 289 cognitively healthy adults to 291 adults with mild cognitive impairment or dementia, nearly every measure of PSG-defined poor sleep (i.e., increased stage 1 sleep and WASO; and decreased SWS, REM, and sleep efficiency) was associated with poorer cognitive functioning as determined by a neuropsychological test battery [32].
The most striking evidence for PSG measures predicting cognitive decline arises from three recent longitudinal studies. First, in the Osteoporotic Fractures in Men Study (MrOS)[33], more than two thousand community-dwelling older men underwent PSG recording at home. Greater time spent in N1, and shorter REM duration, were predictive over 3.4 years of faster decline on the mini mental state examination (MMSE) and Trail Making Test Part B. Second, similar findings emerged in the Framingham Heart Study that included 321 older participants who underwent PSG recording at home and repeated MMSE testing [34•]. Greater wake after sleep onset and shorter REM duration were predictive of cognitive decline 12 years later. Third, in the Study of Oseoporotic Fractures [35], researchers conducted quantitative EEG analyses on women who developed mild cognitive impairment or dementia five years later (n = 85) versus those who remained cognitively healthy (n = 85). Surprisingly, greater cognitive decline on the MMSE was associated with greater baseline SWA (see also [36]). However, consistent with the MrOS and Framingham Heart Study, greater sleep fragmentation (i.e., greater alpha power during both NREM and REM sleep) was strongly predictive of later cognitive decline (see also [37]).
Despite several reported correlations between cognitive decline and sleep fragmentation, we would be remiss if we did not also call attention to the UK Biobank study’s findings. Kyle et al. [38•] analyzed data from 477,529 middle-aged and older adults who performed five cognitive tasks assessing processing speed, short term memory, prospective memory, and reasoning. Participants also answered questions about their total sleep time and whether they had difficulty falling asleep or staying asleep. In initial analyses, they observed worse performance on all five cognitive tasks in participants who slept fewer than 7 hours, just as the field predicted (presumably, participants who sleep <7 hours should have less REM sleep). Surprisingly, however, after adjusting for demographic and health variables, participants who reported difficulty falling asleep or staying asleep showed significantly better processing speed, short term memory, and prospective memory than those who did not report sleep difficulties. Thus, within a single, large-sample study, poor sleep could be interpreted as leading to worse cognition or better cognition in older adults.
As we have noted in the past [6], a major challenge for the field is to account for the scores of null and unexpectedly negative associations between sleep and behavioral cognitive outcomes, with the complexity of findings possibly rising with advancing age. A potentially fruitful direction for the field emerges from the idea that sleep-based physiological processes do not decline uniformly, but instead change dynamically ,with aging (Figure 2). Based on this idea, the variability in the literature may reflect that individual studies vary in whether they have a disproportionately large (or small) number of older adults who show effective (or ineffective) REM compensation in the presence of declining SWS.
Mechanism 3: Memory Consolidation
A third mechanism by which poor sleep may affect cognitive aging is through memory consolidation. Studies have repeatedly shown that sleep promotes memory consolidation in healthy young adults [39]. Many of these studies have emphasized the memory role of SWS [40], with a focus on slow oscillations and spindle activity [5].
Disruptions of memory consolidation may be evident as early as middle age [41]. For example, Spencer and colleagues [42] asked 20 young adults (18-30 years old) and 20 middle-aged adults (35-50 years) to encode emotionally negative and emotionally neutral pictures. After a 12-hour daytime wake retention interval or a 12-hour nocturnal sleep retention interval, participants completed recognition tests. The young adults who slept performed better than the young adults who did not sleep, whereas middle-aged adults who slept performed similarly to those in the wake group (regardless of memory valence). On the contrary, Payne and colleagues [43] argued that the ability to consolidate emotional memory does not deteriorate by middle age. They had young adults (18-39 years old) and middle-aged adults (40-64 years old) encode 100 scenes with emotionally neutral or negative objects placed on neutral backgrounds. Participants then took a nap or stayed awake. In both age groups, a nap immediately following encoding led to significantly better retention of emotional objects at the expense of retention of neutral background scenes. A possible, but admittedly not fully satisfying, explanation of these mixed findings is that in middle-age, memory consolidation starts to decline in only some individuals.
In older adults (e.g., ages 60 and beyond), researchers often observe a substantial reduction in memory consolidation [44-47]. Recent studies have shown that older adults are less likely than young adults to consolidate memories in the directed forgetting task [48], a vocabulary learning task [49], the knowledge-insight number reduction task [50], and a motor-memory finger sequence task [51]. Interestingly, however, a meta-analysis found that the age-related decline in memory consolidation was larger for declarative/episodic memory consolidation than for non-declarative/procedural memory consolidation [39]. Given this seeming dissociation, it becomes conceptually important to determine whether what is being interpreted as a memory-consolidation deficit can be more parsimoniously explained by older adults’ difficulty with encoding new declarative memories and retrieving those declarative memories. Because memory consolidation is always measured by a retrieval test, consolidation processes will always be somewhat conflated with encoding and retrieval processes, and in implicit memory the attentional demands of encoding and retrieval are much lower for older adults [52].
If one must infer memory consolidation from a test of memory retrieval, then it becomes important to connect sleep architecture to memory task performance if one is to conclude that memory consolidation is sleep-dependent. This has not always been easy to show. For example, in a study of nearly one thousand participants, there were no significant correlations between PSG measures and memory consolidation for pictorial stimuli [53], and behavioral evidence for a decline in memory consolidation has been observed without any observed age-related reductions in SWS or REM sleep duration [42].
Quantitative EEG analysis may offer a solution [5]. Rather than counting how much time an individual spends in the four sleep stages, fast Fourier transform and automated spindle detectors can assist in identifying the microevents within sleep stages that are hypothesized to represent memory reactivation and consolidation. One candidate microevent is the coupling between slow-wave oscillations and sleep spindles during NREM sleep [54, 55]. Walker and colleagues [56] found that when a sleep spindle occurred close to the slow oscillation up-state peak, memory consolidation was promoted, but that older adults experience impairments in slow oscillation-spindle coupling, with the degree of impairment predicting lower memory consolidation. Such age-related changes have been associated with a reduction in gray matter in the hippocampus and medial prefrontal cortex [28, 56, 57]. The unresolved, “million dollar question,” is whether sleep micro- and macro-architecture determine how well the brain/cognition are preserved in older age, or instead, whether brain/cognition preservation determines how well sleep quality/depth are preserved.
To causally test if SWS or REM processes determine memory functioning, researchers have experimentally augmented these sleep processes and tested memory outcomes in older adults. One trial of cholinesterase inhibitors increased both REM sleep and memory functioning in older adults [58•]. To our knowledge, there have been no replications of this important work. Instead, the majority of recent work has focused on enhancing SWA [59]. For example, Ladenbauer and colleagues [60, 61] applied transcranial slow oscillatory stimulation (so-tDCS) during an afternoon nap in 18 healthy older adults and 16 mild cognitive impairment patients, with a sham-control nap cross-over design. The so-tDCS increased slow oscillations and fast spindle activity and boosted retention of pictures encoded before the nap (but not word pairs). Similar positive findings have been reported for verbal memory retention following so-tDCS in 19 older adults [62] and acoustic stimulation of slow oscillations in 13 older adults [63]. These positive observations notwithstanding, limitations of this literature include the studies excluding 23-45% of recruited participants from analyses and a lack of memory correlations with spindles and SWA (except for [63]). Furthermore, one must consider that attempts to improve memory functioning in older adults via nocturnal sleep tDCS stimulation and targeted memory reactivation have not been successful [49, 64, 65]. Therefore, at present, we cannot definitively conclude that sleep-specific processes were the mechanisms of action for memory improvements in the napping studies [66, 67]. Our interpretation of the literature on sleep interventions for cognitive aging is that either 1) the methodologies have not been perfected to yield consistently positive outcomes, 2) sleep processes are not a viable target for enhancing cognition in older age, or 3) existing interventions have limited themselves by solely targeting SWA mechanisms or solely targeting REM mechanisms. We again point to the data in Figure 2 that many older adults appear to show REM sleep compensation in the midst of declining SWS, and future interventions might target that compensatory response with the goal of promoting cognitive longevity.
SWS and REM Sleep “Sequential” Dynamics
Several fundamentals of sleep make it difficult to pinpoint how any individual sleep process relates to cognitive aging. For example, many aspects of sleep are intercorrelated [68-69]. Moreover, sleep stages repeatedly cycle, with the amplitude, duration, and frequency of sleep events changing across the night. Furthermore, even though sleep clearly changes across the lifespan, the longitudinal changes in sleep are sometimes nonlinear and deficits in one component of sleep (e.g., SWS) may co-occur with augmentation of other components of sleep (e.g., REM; Figure 2). We theorize that these “dynamic” hallmarks of sleep are important for understanding the role of sleep in cognitive functioning. But, we are certainly not the first to note this possibility. This line of reasoning dates back at least to the 19th century writings of John Hughlings Jackson [1•] and John Addington Symonds [70], as illustrated by the quote in the introduction section. Their early observations that “dream sleep” and “dreamless sleep” have interactive cognitive functions are remarkable when considering that at the time of their writings there were no published empirical studies on human memory [71], and REM sleep would not be discovered until the next century [72].
Jackson’s theory [1•], when viewed through the lens of contemporary cognitive neuroscience, was that individual memories are strengthened during N3, with additional cognitive associations being formed and integrated during REM sleep. A related view is Giuditta’s sequential hypothesis [73, 74], which argued that SWS first weakens memories encoded while awake, which he called a “cleaning operation” to remove irrelevant information. Then, during REM sleep, he hypothesized that the retained memories were integrated into associative networks. Llewelyn and Hobson [75] similarly contend that during NREM sleep, certain memories are selectively reactivated, and then, if the memory has particular survival or other future relevance, then it undergoes a preplay process during REM sleep.
Though two-stage, sequential theories of sleep-based cognitive processing have long existed, most work on sleep and cognitive aging has taken the approach that either-SWA-or-REM promote glymphatic, frontal restoration, and memory consolidation functioning with aging. We view the recent literature as indicating that both REM and SWA contribute to cognitive outcomes in older adults. Experimental sleep studies that reliably altered amyloid outcomes did so by disrupting both SWS and REM sleep [23, 24]. Cross-sectional studies that related PSG variables to attention/executive function often identified roles for both SWS and REM sleep [31]. Longitudinal PSG studies have typically identified roles for both REM sleep and NREM sleep fragmentation (and, less commonly, for SWS [76]). Experiments that aimed to increase memory consolidation were sometimes effective when enhancing SWA, though the most effective intervention augmented both REM and SWA [77]. What is critically missing from this literature is work that targets how compensatory dynamics of SWS and REM impact cognitive longevity.
Conclusions
Outside of Alzheimer’s disease drug discovery [78], sleep is at the forefront of public and scientific interest in cognitive longevity. Over the last five years, we have learned that 1) sleep is important to glymphatic functioning, but that glymphatic function is impaired with aging; 2) sleep is important to frontal lobe functioning, and adults with sleep fragmentation and minimal REM sleep typically show faster attention/executive function declines; and 3) sleep is important to memory consolidation, but older adults tend to show minimal or no evidence for sleep-dependent memory consolidation. What the field has not yet definitively addressed is the provocative idea that Alzheimer’s disease or normal age-related cognitive decline can be prevented or slowed by implementing sleep interventions [2, 3]. The existing evidence for improving glymphatic, memory consolidation, and overall cognitive functioning with hypnotic medications, transcranial direct current stimulation, and targeted memory reactivation are mixed.
The field needs large, multisite, pre-registered randomized controlled trials to inform whether improving sleep improves cognitive longevity. Our theoretical perspective is that any interventions that focus solely on SWS, solely on REM sleep, or solely on sleep fragmentation will continue to produce modest or null results. Building on the theoretical view that SWS and REM sleep interact dynamically to preserve cognitive health, future intervention studies should target multiple sleep mechanisms simultaneously.
Acknowledgments
This work was supported in part by NIH AG053161 (M.K.S.). The National Sleep Research Resource is supported by NIH HL114473. The authors are appreciative to Yo-El Ju for helpful discussions on glymphatic functioning during the preparation of this manuscript.
Footnotes
Conflict of Interest
Michael K. Scullin reports a grant for research on memory and aging by NIH AG053161. Chenlu Gao declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance.
- 1.Jackson JH. The Croonian lectures on evolution and dissolution of the nervous system. BMJ 1884;1:703–7.• He theorized about dissociable, interactive sleep-based cognitive processes one year before Ebbinghaus’ seminal work on the forgetting curve, and nearly 70 years before the discovery of sleep stages.
- 2.Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, et al. Sleep, cognitive impairment and Alzheimer's disease: A systematic review and meta-analysis. Sleep 2017;40:zsw032 Doi: 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- 3.Global Council on Brain Health. The brain-sleep connection: GCBH recommendations on sleep and brain health. American Association of Retired Persons. 2016. https://www.aarp.org/content/dam/aarp/health/healthy-living/2017/01/gcbh-recommendations-sleep-and-brain-health-aarp.pdf. Accessed 7 Sept 2018.
- 4.Miles LE, Dement WC. Sleep and aging. Sleep 1980;3(2):1–220. [PubMed] [Google Scholar]
- 5.Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron 2017;94(1):19–36. Doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: Integrating a half-century of multidisciplinary research. Perspect Psychol Sci 2015;10(1):97–137. Doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004;27(7):1255–74. Doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 8.Zhang GQ, Cui L, Mueller R, Tao S, Kim M, Rueschman M, et al. The National Sleep Research Resource: Towards a sleep data commons. J Am Med Inform Assoc 2018. May 31 Doi: 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean DA 2nd, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, et al. Scaling up scientific discovery in sleep medicine: The National Sleep Research Resource. Sleep 2016;39(5): 1151–64. Doi: 10.5665/sleep.5774.• A publicly available resource for researchers to analyze polysomnography data in thousands of participants across the lifespan.
- 10.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, et al. The Sleep Heart Health Study: Design, rationale, and methods. Sleep 1997;20(12):1077–85. [PubMed] [Google Scholar]
- 11.Agnew HW, Webb WB, Williams RL. The first night effect: An EEG study of sleep. Psychophysiology 1966;2(3):263–6. [DOI] [PubMed] [Google Scholar]
- 12.Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, et al. National Sleep Foundation's sleep quality recommendations: First report. Sleep Health 2017;3(1):6–19. Doi: 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–7. Doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: A beginner’s guide. Neurochem Res 2015;40(12):2583–99. Doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014;76(7):845–61. Doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol Dis 2016;93:215–25. Doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease?. Trends Neurosci 2016;39(8):552–66. Doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JY, Byun MS, Choe YM, Lee JH, Yi D, Choi JW, et al. Moderating effect of APOE ε4 on the relationship between sleep-wake cycle and brain β-amyloid. Neurology 2018;90(13):e1167–73. Doi: 10.1212/WNL.0000000000005193. [DOI] [PubMed] [Google Scholar]
- 19.Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YE. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol 2018;75(5):582–90. Doi: 10.1001/jamaneurol.2017.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga AW, Wohlleber ME, Giméenez S, Romero S, Alonso JF, Ducca EL, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep 2016;39(11):2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilckens KA, Tudorascu DL, Snitz BE, Price JC, Aizenstein HJ, Lopez OL, et al. Sleep moderates the relationship between amyloid beta and memory recall. Neurobiol Aging 2018;71:142–8. Doi: 10.1016/j.neurobiolaging.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju YE, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 2017;140(8):2104–11. Doi: 10.1093/brain/awx148.• They cleverly manipulated sleep disruption via earphone-tones that responded to EEG spectral power. Though sleep disruption did not produce main effects on amyloid levels, there were dynamic changes in SWS and REM activity (in response to the disruption manipulation) that correlated with changes in amyloid levels.
- 23.Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann Neurol 2018;83(1):197–204. Doi: 10.1002/ana.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci 2018;115(17):4483–8. Doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-β via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci 2007;104(25):10673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, et al. The sleep-deprived human brain. Nat Rev Neurosci 2017;18(7):404 Doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reference placeholder for paper to be published in CSMR.
- 28.Scullin MK. Do older adults need sleep? A review of neuroimaging, sleep, and aging studies. Curr Sleep Med Rep 2017;3(3):204–14. Doi: 10.1007/s40675-017-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilckens KA, Aizenstein HJ, Nofzinger EA, James JA, Hasler BP, Rosario-Rivera BL, et al. The role of non-rapid eye movement slow-wave activity in prefrontal metabolism across young and middle-aged adults. J Sleep Res 2016;25:296–306. Doi: 10.1111/jsr.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci 2009;12:122–3. [DOI] [PubMed] [Google Scholar]
- 31.della Monica C, Johnsen S, Atzori G, Groeger JA, Dijk DJ. Rapid eye movement sleep, sleep continuity and slow wave sleep as predictors of cognition, mood, and subjective sleep quality in healthy men and women, aged 20–84 years. Front Psychiatry 2018;9:255 Doi: 10.3389/fpsyt.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haba-Rubio J, Marti-Soler H, Tobback N, Andries D, Marques-Vidal P, Waeber G, et al. Sleep characteristics and cognitive impairment in the general population The HypnoLaus study. Neurology 2017;88:463–9. Doi: 10.1212/WNL.0000000000003557. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL, et al. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep 2015;38:411–21. Doi: 10.5665/sleep.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pase MP, Himali JJ, Grima NA, Beiser AS, Satizabal CL, Aparicio HJ, et al. Sleep architecture and the risk of incident dementia in the community. Neurology 2017;89:1244–50. Doi: 10.1212/WNL.0000000000004373.• They conducted the longest longitudinal PSG and cognition study (that included a strong sample size). In so doing, they revealed that baseline REM and sleep fragmentation were predictive of faster cognitive decline.
- 35.Djonlagic I, Aeschbach D, Harrison SL, Dean D, Yaffe K, Ancoli-Israel S, et al. Associations between quantitative sleep EEG and subsequent cognitive decline in older women. J Sleep Res 2018;e12666 Doi: 10.1111/jsr.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tranah GJ, Yaffe K, Nievergelt CM, Parimi N, Glymour MM, Ensrud KE, et al. APOEε4 and slow wave sleep in older adults. PLoS ONE 2018;13(1):e0191281 Doi: 10.1371/journal.pone.0191281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brayet P, Petit D, Frauscher B, Gagnon JF, Gosselin N, Gagnon K, et al. (2016). Quantitative EEG of rapid-eye-movement sleep: a marker of amnestic mild cognitive impairment. Clin EEG Neurosci 2016;47(2):134–41. Doi: 10.1177/1550059415603050. [DOI] [PubMed] [Google Scholar]
- 38.Kyle SD, Sexton CE, Feige B, Luik AI, Lane J, Saxena R, et al. Sleep and cognitive performance: Cross-sectional associations in the UK Biobank. Sleep Med 2017;38:85–91. Doi: 10.1016/j.sleep.2017.07.001.• This study included nearly half a million participants, and their results highlighted the variability in positive, null, and negative results that are pervasive when looking across individual behavioral studies in the sleep, cognition and aging literature.
- 39.Gui WJ, Li HJ, Guo YH, Peng P, Lei X, Yu J. Age-related differences in sleep-based memory consolidation: a meta-analysis. Neuropsychologia 2017;97:46–55. Doi: 10.1016/j.neuropsychologia.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci 2010;11(2):114 Doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 41.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem 2007;14(5):336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones BJ, Mackay A, Mantua J, Schultz KS, Spencer RM. The role of sleep in emotional memory processing in middle age. Neurobiol Learn Mem 2018;155:208–15. Doi: 10.1016/j.nlm.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alger SE, Kensinger EA, Payne JD. Preferential consolidation of emotionally salient information during a nap is preserved in middle age. Neurobiol Aging 2018;68:34–47. Doi: 10.1016/j.neurobiolaging.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem 2007;14(7):480–4. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 2012;33(5):991–1000. Doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci 2013;16(3):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scullin MK. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging 2013;28(1):105 Doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scullin MK, Fairley J, Decker M, Bliwise DL. The effects of an afternoon nap on episodic memory in young and older adults. Sleep 2017;40: zsx035 Doi: 10.1093/sleep/zsx035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordi MJ, Schreiner T, Rasch B. No effect of vocabulary reactivation in older adults. Neuropsychologia 2018;119:253–61. Doi: 10.1016/j.neuropsychologia.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Debarnot U, Rossi M, Faraguna U, Schwartz S, Sebastiani L. Sleep does not facilitate insight in older adults. Neurobiol Learn Mem 2017;140:106–13. Doi: 10.1016/j.nlm.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, et al. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp 2014;35(8):3625–45. Doi: 10.1002/hbm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleischman DA, Wilson RS, Gabrieli JD, Bienias JL, Bennett DA. A longitudinal study of implicit and explicit memory in old persons. Psychol Aging 2004;19(4):617. [DOI] [PubMed] [Google Scholar]
- 53.Ackermann S, Hartmann F, Papassotiropoulos A, de Quervain DJ, Rasch B. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep 2015;38(6):951–9. Doi: 10.5665/sleep.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Latchoumane CV, Ngo HVV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 2017;95(2):424–35.e6. Doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 55.Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci 2015;18(11):1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MP. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron 2018:97(1):221–30. Doi: 10.1016/j.neuron.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fogel S, Vien C, Karni A, Benali H, Carrier J, Doyon J. Sleep spindles: A physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol Aging 2017;49:154–64. Doi: 10.1016/j.neurobiolaging.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Hornung OP, Regen F, Danker-Hopfe H, Schredl M, Heuser I. The relationship between REM sleep and memory consolidation in old age and effects of cholinergic medication. Biol Psychiatry 2007;61(6):750–7.• Perhaps the largest intervention study for memory consolidation in older adults. They discovered that cholinesterase inhibitors improved memory consolidation, with the improvements potentially explained by augmenting both REM sleep and SWA (see ref 77).
- 59.Wilckens KA, Ferrarelli F, Walker MP, Buysse DJ. Slow-Wave Activity Enhancement to Improve Cognition. Trends Neurosci 2018;41(7):470–82. Doi: 10.1016/j.tins.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ladenbauer J, Külzow N, Passmann S, Antonenko D, Grittner U, Tamm S, et al. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. Neuroimage 2016;142:311–23. Doi: 10.1016/j.neuroimage.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 61.Ladenbauer J, Ladenbauer J, Külzow N, de Boor R, Avramova E, Grittner U, et al. Promoting sleep oscillations and their functional coupling by transcranial stimulation enhances memory consolidation in mild cognitive impairment. J Neurosci 2017;37:7111–24. Doi: 10.1523/JNEUROSCI.0260-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westerberg CE, Florczak SM, Weintraub S, Mesulam MM, Marshall L, Zee PC, et al. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging 2015;36(9):2577–86. Doi: 10.1016/j.neurobiolaging.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub S, Paller KA, et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci 2017;11:109 Doi: 10.3389/fnhum.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimulation 2013;6(6):938–45. Doi: 10.1016/j.brs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Paßmann S, Külzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, et al. Boosting slow oscillatory activity using tDCS during early nocturnal slow wave sleep does not improve memory consolidation in healthy older adults. Brain Stimulation 2016;9(5):730–739. Doi: 10.1016/j.brs.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 66.Manenti R, Sandrini M, Gobbi E, Cobelli C, Brambilla M, Binetti G, et al. Strengthening of existing episodic memories through non-invasive stimulation of prefrontal cortex in older adults with subjective memory complaints. Front Aging Neurosci 2017;9:401 Doi: 10.3389/fnagi.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lafon B, Henin S, Huang Y, Friedman D, Melloni L, Thesen T, et al. Low frequency transcranial electrical stimulation does not entrain sleep rhythms measured by human intracranial recordings. Nat Commun 2017;8(1):1199 Doi: 10.1038/s41467-017-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bliwise DL. Sleep in normal aging and dementia. Sleep 1993;16:40–81. [DOI] [PubMed] [Google Scholar]
- 69.Lyamin OI, Kosenko PO, Korneva SM, Vyssotski AL, Mukhametov LM, Siegel JM. Fur seals suppress REM sleep for very long periods without subsequent rebound. Curr Biol 2018;28(12):2000–5. Doi: 10.1016/j.cub.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Symonds JA. Sleep and dreams, two lectures. Murray; 1851. [Google Scholar]
- 71.Ebbinghaus H Ueber das Gedächtnis. Leipzig: Drucker & Humblat; 1885. [Google Scholar]
- 72.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953;118(3062):273–4. [DOI] [PubMed] [Google Scholar]
- 73.Giuditta A, Ambrosini MV, Montagnese P, Mandile P, Cotugno M, Zucconi GG, et al. The sequential hypothesis of the function of sleep. Beh Brain Res 1995;69:157–66. [DOI] [PubMed] [Google Scholar]
- 74.Giuditta A Sleep memory processing: the sequential hypothesis. Front Syst Neurosci 2014; 16:219 Doi: 10.3389/fnsys.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Llewellyn S, Hobson JA. Not only… but also: REM sleep creates and NREM Stage 2 instantiates landmark junctions in cortical memory networks. Neurobiol Learn Mem 2015;122:69–87. Doi: 10.1016/j.nlm.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Gelber RP, Redline S, Ross GW, Petrovitch H, Sonnen JA, Zarow C, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015;84:296–303. Doi: 10.1212/WNL.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hornung OP, Regen F, Dorn H, Anghelescu I, Kathmann N, Schredl M, Danker-Hopfe H, Heuser I. The effects of donepezil on postlearning sleep EEG of healthy older adults. Pharmacopsychiatry. 2009;42(01):9–13. [DOI] [PubMed] [Google Scholar]
- 78.Kaiser J The Alzheimer’s gamble: Can the National Institute on Aging turn a funding windfall into a treatment for the dreaded brain disease? Science 2018;361:839–841. [Google Scholar]
- 79.Horne J Sleeplessness: Assessing sleep need in society today. Leicestershire: Palgrave Macmillan; 2016. [Google Scholar]