Abstract
Objective
Immune checkpoint inhibitors (ICI) are transforming the field of oncology, leading to tumor regression in multiple advanced cancers. With this case series, we review the ultrasound imaging findings in a series of patients with ICI‐induced inflammatory arthritis (IA), a novel rheumatic disease that is caused by cancer immunotherapy.
Methods
We identified patients with rheumatologist‐diagnosed, ICI‐induced IA who had musculoskeletal ultrasound performed for clinical care. A retrospective chart review was done to obtain demographics, oncologic history, clinical presentation, imaging, and synovial fluid results. Ultrasound images were reviewed and scored for synovial and tendon pathology, presence of Doppler, and bony erosion.
Results
Nine patients were included in this study with a total of 18 joint regions assessed. The knees were the most commonly imaged joint followed by the hands, wrists, feet, and ankles. Synovitis was seen in 12 of the 18 joints with active Doppler in 50% of the cases. Tendon involvement was also frequently seen (13 of 18 joints) with tenosynovitis, tendinitis, and enthesophytes. Erosions were less frequent and seen in only three cases but were also an early finding.
Conclusion
Patients with ICI‐induced IA had a wide range of pathology affecting the synovium, tendons, and bones on musculoskeletal ultrasound. Further systematic study with imaging is needed for this group of diseases.
Introduction
The expansion of immunotherapies has introduced new treatment options for patients with advanced cancer. Immune checkpoint inhibitors (ICIs) target the inhibitory costimulatory molecules on T cells and their ligands, including cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4), programmed cell death‐1 (PD‐1), and programmed death ligand 1 (PD‐L1) 1, 2. Inhibition of these checkpoints by monoclonal antibodies permits nonspecific T‐cell activation and can result in a dramatic antitumor response.
As ICI use increases for a wider variety of cancers, immune‐related adverse effects (irAEs) are increasingly recognized as significant toxicities 3, 4. IrAEs manifest in a variety of organs, including skin rashes, colitis, myositis, sicca syndrome, and inflammatory arthritis (IA) 5, 6. IA appears to be the most common rheumatic irAE and can become chronic, persisting after ICI cessation 7. ICI‐induced IA shows notable clinical differences from “usual” rheumatoid arthritis. Most patients are seronegative for rheumatoid factor and anti‐cyclic citrullinated peptide (CCP) antibodies, many do not respond to low to moderate doses of corticosteroids, and there are several potential clinical subgroups, including small‐joint polyarthritis, reactive arthritis‐like disease, and large‐joint oligoarthritis with or without axial disease 8. The mechanism behind ICI‐induced IA is not well elucidated and may be due to direct T‐cell effects and/or downstream effects of T‐cell activation, like inflammation that is due to cytokines and effectors cells. The clinical response of ICI‐induced IA to tumor necrosis factor–inhibitor and IL‐6R inhibitors also suggests the importance of these cytokines 8, 9
Ultrasonography has become an important modality for the diagnosis and monitoring of IA, able to identify not only inflammatory features of synovitis, effusions, and tendonitis but also structural damage like bone erosions 10. There has been no systematic study using musculoskeletal ultrasound to evaluate patients with ICI‐induced IA and describe the imaging features of this condition. We describe ten cases of ICI‐induced IA evaluated with ultrasonography to highlight notable presentations and pathologic changes seen.
Materials and Methods
This is a retrospective case series of patients with ICI‐induced IA who were evaluated and treated at the Johns Hopkins Division of Rheumatology from October 2015 to January 2018. We identified and reviewed the medical records of those patients with ICI‐induced IA who had available sonographic imaging of their joints. The study was approved by the Johns Hopkins Institutional Review Board (IRB #00144789).
Ultrasound assessments
Symptomatic joints were assessed in each patient based on clinical need. Studies were carried out using a GE Logiq e (GE Healthcare), which had a 12L linear phased array transducer or hockey stick probe, by one examiner (JA), a rheumatologist with 6 years of musculoskeletal ultrasound experience. For each joint region scanned, orthogonal views of symptomatic areas were obtained. The images were then reviewed, assessing for the presence/absence of joint pathology (synovial hypertrophy, Doppler signal, effusion), tendon pathology (tenosynovitis, enthesopathy), and bone changes (erosion). Definitions for ultrasound pathology are as described by the Outcome Measures in Rheumatology (OMERACT) network 11. For enthesopathy, findings of thickening or hypoechoic irregularity of the tendons and presence of Doppler signal were specified. The presence of enthesophytes was also recorded for more granularity. A semiquantitative scoring system for synovial hypertrophy and Doppler assessments was used according to the EULAR/OMERACT score 12. The most severe finding/grade was recorded for each section. For all other pathology, presence or absence was recorded as either positive (+) or negative (−).
Results
Demographics
Nine patients with IA due to ICIs were included in this study (Table 1). They ranged in age from 47 to 81 (mean 61.6) and less than half were female (Table 1). Underlying cancer diagnoses included lung cancer (n = 4), melanoma (n = 2), basal cell carcinoma (n = 1), prostate cancer (n = 1), and colorectal cancer (n = 1). Five of nine patients (55%) received combination therapy with ipilumimab and nivolumab; four received a PD‐1 inhibitor as single agent (nivolumab or pembrolizumab). Four of the nine patients (44%) had other irAE symptoms preceding, including colitis, sicca syndrome, and pancreatitis. Patients developed symptoms of IA anywhere from 1 to 23 months after starting ICI therapy (average 7.8 months).
Table 1.
Patient demographics and clinical variables
| Pt | Age | Sex | Cancer Dx | Tx | Duration of Symptoms Before US (mos) | IS at Time of US | Prednisone (mg) dose | Other IS prior to US | Other irAE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | F | Melanoma | N/Ip | 20 | M/E | … | I/Pr | C, S, Th |
| 2* | 69 | M | Prostate cancer | N/Ip | 2 | Pr | 5 | … | C |
| 3 | 59 | F | NSCLC | N | 2 | … | … | … | … |
| 4 | 59 | M | Basal cell carcinoma | N/Ip | 0 | Pr | 20 | … | … |
| 5 | 62 | M | NSCLC | N/Ip | 3 | Pr | 5 | … | … |
| 6 | 56 | F | Colon cancer | P | 0 | … | … | … | Pa |
| 7 | 47 | F | NSCLC | N | 12 | … | … | Pr | … |
| 8 | 74 | M | NSCLC | N/Ip | 0.5 | … | … | … | … |
| 9 | 81 | M | Melanoma | N | 2 | Pr | 40 | … | C |
Abbreviation: C, colitis; Dx, diagnosis; E, etanercept; Ip, ipilumimab; irAE, immune‐related adverse event; IS, immunosuppression; I, infliximab; M, methotrexate; N, nivolumab; NSCLC, non–small cell lung cancer; Pa, pancreatitis; Pe, pembrolizumab; Pr, prednisone; S, sicca; Th, thyroid disease; Tx, treatment; US, ultrasound.
*Correction added after online publication 11 July 2019: the patients in Table 1 have been renumbered.
Clinically affected joints
Three patients presented with an inflammatory monoarthritis that affected the knee; all other patients (n = 6) had at least three joints involved over their clinical course. Most patients with polyarthritis had both upper and lower extremities involved (n = 7), with one patient having only upper extremity involvement (wrists, fingers, shoulders) and another having only lower extremity involvement (knees, metatarsophalangeal joints).
Ultrasound findings
The most frequently imaged joint was the knee, with seven out of nine patients undergoing an ultrasound of this joint (Table 2) because of their symptoms. In order of decreasing frequency, the other joints assessed included the hands, wrists, ankles, elbows, and feet.
Table 2.
Ultrasound findings
| Patient | Location | Joint | Tendon | Bone | ||||
|---|---|---|---|---|---|---|---|---|
| GS | D | Ef | TS | En | Enp | Er | ||
| 1 | Elbow | 2 | 1 | + | − | − | − | + |
| Hand | 1 | 0 | + | − | − | − | ‐ | |
| 2 | Wrist | 2 | 2 | − | + (Wrist extensors comp 3/4) | − | − | ‐ |
| Knee | 2 | 1 | + | − | + (Thickening of distal P) | − | ‐ | |
| 3 | Knee | 1 | 1 | + | − | + (Thickening of Q/proximal and distal P) | + At Q and proximal P) | + |
| 4 | Knee | 1 | 2 | + | − | + (Thickening of Q insertion with doppler) | − | ‐ |
| Hand | 0 | 0 | − | − | + (Thickening of extensor tendon at PIP) | − | ‐ | |
| Foot | 2 | 1 | − | + (MTP extensor tendon) | − | − | ‐ | |
| Ankle | 0 | 0 | + | + (Peroneus tendons) | − | − | + | |
| 5 | Elbow a | * | * | + | * | * | * | * |
| Knee | 0 | 0 | − | + (Thickening of Q insertion) | + (At distal P) | ‐ | ||
| 6 | Knee | 2 | 3 | + | − | + (Hypoechoic proximal P) | − | ‐ |
| 7 | Knee | 0 | 0 | − | − | + (Hypoechoic Q/distal P) | − | ‐ |
| Ankle | 0 | 0 | + | + (Peroneus tendons) | − | − | ‐ | |
| Foot | 2 | 0 | + | − | − | − | ‐ | |
| 8 | Hand | 1 | 0 | + | + (Flexor tendons at MCPs) | − | + (At extensor tendon at PIP) | ‐ |
| Wrist | 2 | 1 | − | − | − | − | ‐ | |
| 9 | Knee | 2 | 2 | + | − | + (Thickening of Q) | + (At Q/proximal P) | ‐ |
Abbreviation: D, Doppler; Ef, effusion; En, enthesopathy; Enp, enthesophyte; Er, erosion or bone irregularity; GS, gray scale synovial hypertrophy; MCP, metacarpophalangeal joint; MTP, metatarsophalangeal joint; P, patellar tendon; Q, quadriceps tendon; TS, tenosynovitis.
Note that asterisks (*) indicate metastasis.
Out of 18 joint regions assessed, 12 were found to have synovitis, with active Doppler signal in 9 of those joints (one example: Figure 1A and 1G). Effusions were most commonly seen in the knees and ankles (Figure 1B) but were also noted in elbows and hands. Tendon involvement with either tenosynovitis (Figure 1A, 1E, and 1F) or enthesopathy was seen in 13 of the joint regions and spanned the range of both large and small joints. Enthesophytes were noted most commonly at knee tendons (Figure 1H) but were also seen at an extensor tendon of the finger (Figure 1C and 1D). Erosions and bony cortical irregularities were only seen in three joints but were noted even in cases with a symptom duration of only 2‐3 months. Metastatic joint involvement was found in one case at an elbow, as previously described 13.
Figure 1.
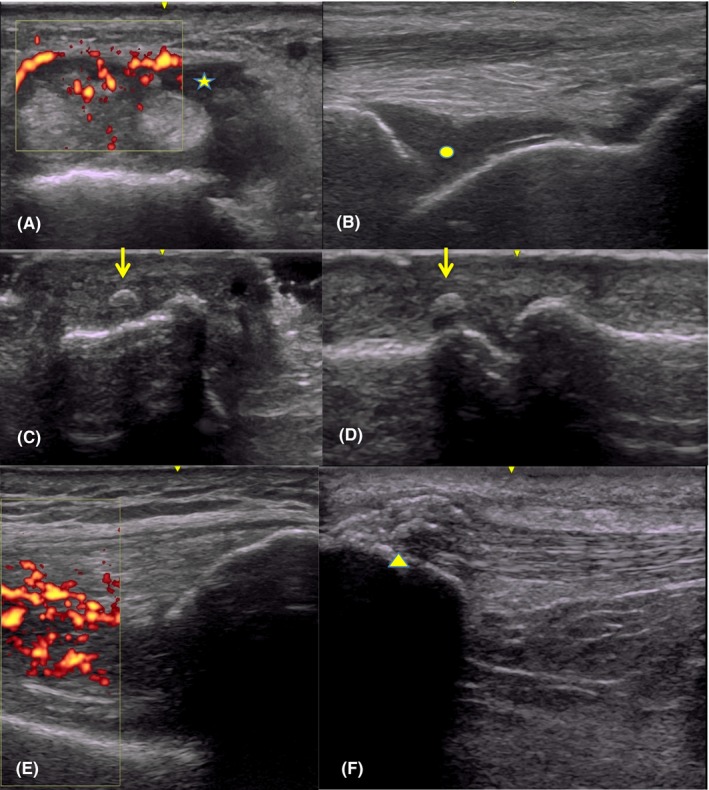
Transverse view of wrist extensors with tenosynovitis (*) and Doppler signal (A); effusion at the tibiotalar joint of the ankle ( ● ) (B); orthogonal dorsal views of a third PIP showing enthesophyte (arrows (C, D); synovitis with grade 3 Doppler signal at the knee (E); and bulky enthesophytes at proximal insertion of patellar tendon of knee (arrowhead) (F).
Synovial studies
Fluid aspiration was performed for six knees to rule out alternative etiologies to irAE. All knee aspirates were sent for bacterial culture and resulted in no positive cultures. Five aspirates were also sent for cell count and crystal evaluation. One case showed the presence of monosodium urate crystals so colchicine was initiated for therapy, which yielded minimal clinical response; prednisone, and ultimately methotrexate, were effective for this patient's ICI‐induced IA. The white blood cell count of the crystalline‐negative aspirates ranged from 49 to 11 400 cells/cu mm (mean 5037). Two of the six patients were on prednisone (5 and 40 mg, respectively) at the time of joint aspiration, with white blood cell counts of 4900 and 3800, respectively. The patient with a low white blood cell count (n = 49) had received intra‐articular corticosteroids several weeks before the aspiration.
Discussion
Rheumatic complications of ICI therapy have been increasingly recognized in the last few years, with IA being one of the more common manifestations. ICI‐induced IA can be a complex entity, with varied presentations and some similarities with primary rheumatic diseases. An additional layer of difficulty is posed by its occurrence in the background of active malignancy, requiring a quick and judicious response from the evaluating clinician as to whether the manifestation is an irAE or cancer progression. Imaging provides an important additive assessment to the clinical examination, allowing for objective characterization of the anatomic and inflammatory nature of the process that can help guide management.
In studies detailing the occurrence of joint‐related complications from ICIs, a spectrum of findings has been described, including synovitis, tenosynovitis, and enthesitis 3, 14, 15, 16. In our study, we show that the most common findings were synovial hypertrophy with or without Doppler signal, as well as tendon involvement. The tendon involvement was one of the more interesting findings. It spanned the range of the more classic tenosynovitis seen with IA, to tendinopathy with only decreased echogenicity and thickening of the involved tendon, which could be interpreted as mechanically induced in a different clinical setting. However, a more unusual presentation was that of enthesophytes, such as at extensor tendons in the small joints of the hands and bulky ones at the knee tendons. Like bone erosions, enthesophytes are considered structural changes, indicative of damage and are usually seen in more long‐standing disease. Hence, finding enthesophytes and erosions in these early cases (symptom duration between 2‐20 months) raises further questions about underlying mechanisms, particularly in terms of the potential impact of ICIs on bone.
The retrospective nature of our study and targeted ultrasound assessments pose limitations on potential conclusions. Unfortunately, some patients were already on steroids by the time of sonographic evaluation, and this may have already affected changes, such as decreasing the amount of Doppler signal and inflammatory synovial or tendon changes seen. In one case, the elbow involvement was later determined to be due to metastatic disease, hence other findings in the elbow (like tendon abnormalities or effusions) were not counted toward ICI‐induced IA as has been described elsewhere 13.
There is much more to understand about ICI‐induced IA. Ultrasound imaging may help further define clinically relevant subgroups of ICI‐induced IA, and differences in affected structures might reflect differences in pathogenesis. Musculoskeletal ultrasound can also evaluate for alternate causes of joint pain and has the added advantage of bedside availability, allowing evaluation of as many joints as desired, which makes it suitable for the assessment of this novel rheumatic disease.
In summary, musculoskeletal ultrasound showed a variety of pathology involving the synovium, tendons, and bones in patients who develop IA because of ICIs. Further systematic studies are needed to fully characterize the imaging features of this disease and to direct guidelines for the use of ultrasound in diagnosis and monitoring of response to therapy.
Author Contributions
Drs. Albayda and Capelli drafted the article. Drs. Dein, Shah, and Bingham were responsible for article revision.
Study conception and design
Albayda, Cappelli.
Acquisition of data
Albayda, Dein, Cappelli.
Analysis and interpretation of data
Shah, Bingham.
This work was supported by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (P30‐ AR070254 to Drs. Albayda, Bingham, Shah, Cappelli; R01‐AR073208 to Dr. Shah) as well as the Jerome L. Greene Foundation (Drs. Albayda and Cappelli). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Dr. Bingham has served as a consultant to and received research funding from Bristol Myers Squibb. Dr. Cappelli has received research funding from bristol Myers Squibb. Dr. Shah has served on a Pan Tumour Rheumatology Advisory Board for Bristol Myers Squibb. No other disclosures relevant to this article were reported.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calabrese C, Kirchner E, Kontzias K, Velcheti V, Calabrese LH. Rheumatic immune‐related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 2017;3:e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappelli LC, Gutierrez AK, Bingham CO III, Shah AA. Rheumatic and musculoskeletal immune‐related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken) 2017;69:1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman CF, Proverbs‐Singh TA, Postow MA. Treatment of the immune‐related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2:1346–53. [DOI] [PubMed] [Google Scholar]
- 6. Lidar M, Giat E, Garelick D, Horowitz Y, Amital H, Steinberg‐Silman Y, et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018;17:284–9. [DOI] [PubMed] [Google Scholar]
- 7. Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, et al. Clinical presentation of immune checkpoint inhibitor‐induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum 2018;48:553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim ST, Tayar J, Trinh VA, Suarez‐Almazor M, Garcia S, Hwu P, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76:2061–4. [DOI] [PubMed] [Google Scholar]
- 10. Sudoł‐Szopińska I, Schueller‐Weidekamm C, Plagou A, Teh J. Ultrasound in arthritis. Radiol Clin North Am 2017;55:985–96. [DOI] [PubMed] [Google Scholar]
- 11. Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485–7. [PubMed] [Google Scholar]
- 12. D'Agostino MA, Terslev L, Aegerter P, Backhaus M, Balint P, Bruyn GA, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR‐OMERACT ultrasound taskforce – part 1: definition and development of a standardised, consensus‐based scoring system. RMD Open 2017;3:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albayda J, Bingham CO III, Shah AA, Kelly RJ, Cappelli L. Metastatic joint involvement or inflammatory arthritis? A conundrum with immune checkpoint inhibitor‐related adverse events. Rheumatology (Oxford) 2018;57:760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan MM, Kefford RF, Carlino M, Clements A, Manolios N. Arthritis and tenosynovitis associated with the anti‐PD1 antibody pembrolizumab in metastatic melanoma. J Immunother 2015;38:37–9. [DOI] [PubMed] [Google Scholar]
- 15. Inamo J, Kaneko Y, Takeuchi T. Inflammatory tenosynovitis and enthesitis induced by immune checkpoint inhibitor treatment. Clin Rheumatol 2018;37:1107–10. [DOI] [PubMed] [Google Scholar]
- 16. Mekki A, Dercle L, Lichtenstein P, Marabelle A, Michot J‐M, Lambotte O, et al. Detection of immune‐related adverse events by medical imaging in patients treated with anti‐programmed cell death 1. Eur J Cancer 2018;96:91–104. [DOI] [PubMed] [Google Scholar]


