Abstract
Background
Hydroxychloroquine (HCQ) is an antimalarial drug that is recommended as a safe, daily prophylactic intervention for individuals with systemic lupus erythematosus (SLE) based on previous studies that showed an association of HCQ use with reductions in flares compared with placebo. Our study aims to determine whether the discontinuation of HCQ leads to relapse of disease and whether the duration of HCQ use impacts the success of its eventual discontinuation.
Methods
A retrospective chart review was performed on the medical records of patients diagnosed with SLE between July 1, 2006, and June 30, 2016. The data gathered included demographic factors, diagnostic symptoms, laboratory values, and SLE medications. Additionally, HCQ usage and discontinuation rates were collected as well as the timing and prevalence of flares during and after HCQ usage. Patients who were diagnosed with SLE but never used HCQ were excluded from the study. The occurrence of flares, clinical characteristics, and duration of treatment with HCQ were compared between the group that continued HCQ and the group that discontinued HCQ.
Results
Of the 509 patients who met inclusion criteria, 66.2% (n = 337) continued HCQ throughout the duration of their treatment (median duration of HCQ treatment was 8.0 years), whereas 33.8% (n = 172) did not (median duration of HCQ treatment was 1.9 years). Patients who received HCQ for less than 1 year before discontinuation (median duration of HCQ treatment was 2.5 months) were more likely to experience SLE flares compared with those who continued HCQ for more than 1 year (13.1% vs 5.7%, P = 0.019). Patients who experienced a flare while on HCQ were more likely to have arthritis, oral ulcers, leukopenia, and thrombocytopenia.
Conclusion
With over 500 patient charts reviewed, this is the largest study comparing outcomes for patients on HCQ with those who discontinued it. Patients who discontinue HCQ after being on it for less than 1 year are at greater risk for flares compared with those who take HCQ for longer than 1 year. These findings should be used to guide treatment, educate patients on the role of continued treatment with HCQ, and ultimately reduce morbidity and mortality.
Introduction
Systemic lupus erythematous (SLE) is a systemic autoimmune disorder that predominantly affects females of reproductive age and has a strong predilection for ethnic minority groups. SLE presentation is variable, with the ability to affect any organ system, and symptoms accumulate over time. The disease course is characterized by flares and periods of remission. With rare exceptions, the unifying laboratory abnormality is the presence of circulating antinuclear antibodies (ANAs).
Hydroxychloroquine (HCQ) is an antimalarial drug that has been recommended for prophylactic intervention with minimal side effects for SLE patients 1. It was first synthesized in 1944, and its efficacy in treating rheumatic diseases was recognized during World War II. As one of the oldest prescribed drugs used in clinic, HCQ has been found to have versatile immunomodulatory and anti‐inflammatory activities. At present, different mechanisms of HCQ action on the immune system have been proposed, including alterations in lysosomal acidification and stability, interference with Toll‐like receptor signaling, and inhibition of T‐ and B‐cell receptor calcium signaling 2. Previous randomized drug withdrawal–based studies demonstrated significant decreases in SLE flares in individuals taking HCQ compared with those taking a placebo, albeit with a small sample size.
HCQ has a history of efficacy in the treatment of dermatologic manifestations of SLE 1 and is generally recommended as a daily maintenance drug for those with most forms of SLE 3. Approved for use in the United States and more than 50 other countries for several decades, HCQ is typically accepted as a safe medication and is usually well tolerated 4. Despite its widespread use, only a few small prospective randomized studies show that HCQ use is associated with significant reductions in lupus flares as compared with placebo use, and it has been found to have a protective effect on survival 1, 5. Additionally, there are limited data on the natural history of disease activity after the discontinuation of HCQ.
The objective of our study is to determine whether discontinuation of HCQ impacts the course of disease and whether the duration of HCQ affects the risk of patients experiencing more flares.
Methods
Patient chart records were reviewed at Loyola University Medical Center from July 1, 2006, to June 30, 2016. Patients who either met criteria for diagnosis of lupus or were diagnosed by their rheumatologist with SLE were included. The index date for the diagnosis was the date the physician entered the diagnosis in the electronic health record. Most patients (n = 486, 96.4%) met at least 4 of the 11 American College of Rheumatology (ACR) diagnostic criteria for SLE 6. A minority of patients met 3 or fewer ACR diagnostic criteria for SLE (n = 18, 3.6%). We excluded patients with a diagnosis of SLE who never received HCQ therapy.
Demographic factors that were assessed included age, sex, ethnicity, and family history. The comorbidities that were assessed included smoking and body mass index (BMI). The ACR diagnostic criteria for SLE included malar rash, discoid rash, photosensitivity, oral ulcers, arthritis, serositis, renal disorder, neurologic disorder, hematologic disorder, immunologic disorder, and antinuclear antibody (ANA) status. The laboratory tests included ANA, rheumatoid factor, anti‐double‐stranded DNA (dsDNA), anti‐Sjögren's syndrome–related antigen A, anti‐Sjögren's syndrome type B, anti‐Smith, U1‐ribonucleoprotein, lupus anticoagulant, anti‐cardiolipin, anti‐beta‐2‐glycoprotein, complement 3, complement 4, erythrocyte sedimentation rate, C‐reactive protein, excretion of urine protein and creatinine, urinalysis, and a complete blood count. The immunomodulatory medications monitored included methotrexate, leflunomide, prednisone, azathioprine, mycophenolate, rituximab, cyclophosphamide, and belimumab.
HCQ start dates (indicated by the date that the physician entered the prescription in the electronic health record), discontinuation dates, and length of treatment were collected as well as the timing and prevalence of SLE flares during HCQ usage or when HCQ was discontinued.
Primary outcome
The primary outcome was defined as the time to development of a flare‐up that is defined by new or worsening symptoms that required the addition or increase in the dose of immunosuppressive medications.
Secondary outcomes
The secondary outcomes were defined as the specific manifestations of the flares, which include the following: arthritis, malar rash, bullous lupus, subacute cutaneous lupus, lupus panniculitis, photosensitivity, discoid rash, oral ulcers, alopecia, pleuritis, pericarditis, lupus nephritis, seizure, psychosis, mononeuritis multiplex, myelitis, neuropathy, vasculitis, hemolytic anemia, leukopenia, lymphopenia, thrombocytopenia, or hospitalization from exacerbation.
Statistical analysis
Patient demographics and clinical characteristics are presented as means (SDs) or counts (percentages). Statistical significance of differences between those who discontinued versus did not discontinue HCQ was assessed with t tests for continuous variables and chi‐square or Fisher's exact test for nominal variables. Time to first flare was calculated and displayed visually using the Kaplan‐Meier method. Analyses were performed using SAS 9.4 (SAS Institute).
Results
A total of 509 patients met the study criteria between July 1, 2006, and June 30, 2016. Of those, 337 (66.2%) patients continued HCQ for the entire study period, whereas 172 (33.8%) patients chose to discontinue HCQ at some point during that same period of time. Patients who never received HCQ and patients who did not follow up in rheumatology clinic after the first clinic visit were excluded. The mean age was 40.8 years (SD = 15.3). The majority of patients were female (90.4%). Most patients were Caucasians (n = 223, 43.8%), followed by African Americans (156, 30.6%), Hispanics (101, 19.8%), then Asians (19, 3.7%). Overall, demographics were similar between the group that continued HCQ and the group that discontinued HCQ (Table 1).
Table 1.
Patient demographics and baseline clinical characteristics by HCQ discontinuation
| Patient characteristics | Overall n = 509 | Continued HCQ n = 172 (33.8%) | Discontinued HCQ n = 337 (66.2%) | P value |
|---|---|---|---|---|
| Age, mean (SD) | 40.8 (15.3) | 40.6 (14.7) | 40.9 (15.7) | 0.97 |
| Female, n (%) | 460 (90.4) | 156 (90.7) | 304 (90.2) | 0.86 |
| Race/Ethnicity, n (%) | ||||
| Caucasian | 223 (43.8) | 63 (36.6) | 160 (47.5) | 0.029 |
| Hispanic | 101 (19.8) | 39 (22.7) | 62 (18.4) | |
| African American | 156 (30.6) | 59 (34.3) | 97 (28.8) | |
| Asian | 19 (3.7) | 10 (5.8) | 9 (2.7) | |
| Unknown | 10 (2.0) | 1 (0.6) | 9 (2.7) | |
| BMI (kg/m²), mean (SD) | 29.3 (8.2) | 29.6 (8.0) | 29.1 (8.2) | 0.55 |
| Smoking status, n (%) | ||||
| Current/Past | 159 (32.2) | 50 (29.1) | 109 (32.4) | 0.45 |
| Never | 350 (68.8) | 122 (70.9) | 228 (67.7) | |
| Family history of SLE, n (%) | 46 (9.0) | 14 (8.1) | 32 (9.5) | 0.61 |
| SLE Criteria | ||||
| ≤3 | 18 (3.6) | 4 (2.4) | 14 (4.2) | 0.29 |
| >3 | 486 (96.4) | 166 (97.6) | 320 (95.8) | |
| Diagnostic symptoms, n (%) | ||||
| Malar rash | 184 (36.1) | 66 (38.4) | 118 (35.0) | 0.46 |
| Discoid rash | 81 (15.9) | 30 (17.4) | 51 (15.1) | 0.50 |
| Photosensitivity | 90 (17.7) | 30 (17.4) | 60 (17.8) | 0.92 |
| Oral ulcers | 75 (14.7) | 28 (16.3) | 47 (13.9) | 0.48 |
| Arthritis | 353 (69.4) | 108 (62.8) | 245 (72.7) | 0.022 |
| Serositis | 91 (17.9) | 31 (18.0) | 60 (17.8) | 0.95 |
| Renal disorder | 122 (24.0) | 37 (21.5) | 85 (25.2) | 0.35 |
| Neurological disorder | 51 (10.0) | 17 (9.9) | 34 (10.1) | 0.94 |
| Hematologic disorder | 144 (28.3) | 53 (30.8) | 91 (27.0) | 0.37 |
| Immunologic disorder | 468 (91.9) | 162 (94.2) | 306 (90.8) | 0.18 |
| Anti‐nuclear antibody | 491 (96.5) | 170 (98.8) | 321 (95.3) | 0.038 |
| Medications, n (%) | ||||
| Prednisone | 352 (69.2) | 125 (72.7) | 227 (67.4) | 0.22 |
| Mycophenolate | 165 (32.4) | 63 (36.6) | 102 (30.3) | 0.15 |
| Azathioprine | 128 (25.1) | 56 (32.6) | 72 (21.4) | 0.006 |
| Methotrexate | 45 (8.8) | 14 (8.1) | 31 (9.2) | 0.69 |
| Cyclophosphamide | 41 (8.1) | 14 (8.1) | 27 (8.0) | 0.96 |
| Leflunomide | 14 (2.8) | 7 (4.1) | 7 (2.1) | 0.25 |
| Rituximab | 19 (3.7) | 11 (6.4) | 8 (2.4) | 0.024 |
| Belimumab | 5 (1.0) | 4 (2.3) | 1 (0.3) | 0.047 |
Abbreviation: BMI, body mass index; HCQ, hydroxychloroquine; SLE, systemic lupus erythematosus.
Those who remained on HCQ and those who discontinued HCQ had similar clinical manifestations and laboratory abnormalities at baseline, but patients who discontinued HCQ had a higher rate of arthritis at the time of presentation (62.8% vs 72.7% in patients who continued HCQ and patients who discontinued HCQ, respectively, P = 0.022). The majority of patients in both groups were on corticosteroids (72.7% and 67.4% in patients who continued HCQ and patients who discontinued HCQ, respectively, P = 0.22) (Table 1). For those who discontinued HCQ, some of the individuals were already on or were started on disease‐modifying antirheumatic drugs after discontinuing HCQ as illustrated by the following: 30.3% on mycophenolate, 21.4% on azathioprine, 9.2% on methotrexate, 8% on cyclophosphamide, 2.4% on rituximab, 2.1% on leflunomide, and 0.3% on belimumab. Also, there was no significant difference in laboratory findings between the two groups (Table 2).
Table 2.
Laboratory values at baseline comparing the group that discontinued HCQ and the group that did not discontinue HCQ
| Patient characteristics | Did not discontinue HCQ n = 172 (33.8%) | Discontinued HCQ n = 337 (66.2%) |
|---|---|---|
| Laboratory values, n (%) | ||
| Anti‐nuclear antibody + | 166 (98.8) | 308 (95.7) |
| Rheumatoid factor + | 11 (37.9) | 18 (39.1) |
| Anti‐double stranded DNA + | 63 (42.6) | 106 (38.1) |
| Anti‐SSA + | 62 (44.9) | 107 (38.5) |
| Anti‐SSB + | 26 (18.8) | 43 (15.5) |
| Anti‐Smith + | 39 (28.1) | 57 (20.9) |
| Anti‐ribonuclear protein antibody + | 55 (39.6) | 90 (32.6) |
| Low complement 3 | 45 (29.2) | 66 (21.9) |
| Low complement 4 | 70 (47.9) | 115 (39.2) |
Abbreviation: BMI, body mass index; HCQ, hydroxychloroquine; SLE, systemic lupus erythematosus; SSA, Sjögren's syndrome–related antigen A; SSB, Sjögren's syndrome type B.
Tables 3 and 4 describe the differences in clinical and laboratory findings between the two groups of patients. Table 3 compares clinical and laboratory manifestations of the first SLE flare between the two groups, and Table 4 describes clinical and laboratory manifestations of any SLE flare between the two groups. Between the two groups, there was not a statistically significant difference in the proportion of patients who experienced any flares. However, when evaluating both groups during their time on HCQ, fewer patients who eventually discontinued HCQ had flares compared with those who continued HCQ (27.9% vs 40.7%, P = 0.004). Additionally, 8% of patients who discontinued HCQ developed flares after discontinuation.
Table 3.
HCQ discontinuation and first flare manifestations
| Patient characteristics | Did not discontinue HCQ n = 172 (33.8%) | Discontinued HCQ n = 337 (66.2%) | P value |
|---|---|---|---|
| Flares, n (%) | |||
| Any flare | 70 (40.7) | 114 (33.9) | 0.13 |
| Flare while taking HCQ | 70 (40.7) | 94 (27.9) | 0.004 |
| Flare after discontinuation | 27 (8.0) | … | |
| First flare symptoms, n (%) | |||
| Arthritis | 32 (18.6) | 41 (12.2) | 0.050 |
| Malar rash | 7 (4.1) | 15 (4.5) | 0.84 |
| Bullous lupus | 0 (0.0) | 1 (0.3) | 0.99 |
| Subacute cutaneous lupus | 2 (1.2) | 6 (1.8) | 0.72 |
| Lupus panniculitis | 1 (0.6) | 2 (0.6) | 0.99 |
| Photosensitivity | 3 (1.7) | 4 (1.2) | 0.69 |
| Discoid rash | 4 (2.3) | 13 (3.9) | 0.44 |
| Oral ulcers | 6 (3.5) | 6 (1.8) | 0.23 |
| Alopecia | 9 (5.2) | 17 (5.0) | 0.93 |
| Pleuritis | 6 (3.5) | 20 (5.9) | 0.24 |
| Pericarditis | 0 (0.0) | 1 (0.3) | 0.99 |
| Lupus nephritis | 7 (4.1) | 8 (2.4) | 0.28 |
| Seizure | 2 (1.2) | 4 (1.2) | 0.99 |
| Psychosis | 0 (0.0) | 3 (0.9) | 0.55 |
| Mononeuritis multiplex | 0 (0.0) | 2 (0.6) | 0.55 |
| Peripheral or cranial neuropathy | 1 (0.6) | 3 (0.9) | 0.99 |
| Vasculitis | 2 (1.2) | 4 (1.2) | 0.99 |
| Hemolytic anemia | 1 (0.6) | 5 (1.5) | 0.67 |
| Leukopenia | 6 (3.5) | 1 (0.3) | 0.007 |
| Lymphopenia | 2 (1.2) | 0 (0.0) | 0.11 |
| Thrombocytopenia | 6 (3.5) | 4 (1.2) | 0.095 |
| Hospitalization | 14 (8.1) | 17 (5.0) | 0.17 |
Abbreviation: HCQ, hydroxychloroquine.
Table 4.
HCQ discontinuation and any flare manifestations
| Patient characteristics | Did not discontinue HCQ n = 172 (33.8%) | Discontinued HCQ n = 337 (66.2%) | P value |
|---|---|---|---|
| Any flare symptoms, n (%) | |||
| Arthritis | 38 (22.1) | 56 (16.6) | 0.13 |
| Malar rash | 12 (7.0) | 26 (7.7) | 0.76 |
| Bullous lupus | 0 (0.0) | 1 (0.3) | 0.99 |
| Subacute cutaneous lupus | 7 (4.1) | 9 (2.7) | 0.39 |
| Lupus panniculitis | 2 (1.2) | 2 (0.6) | 0.61 |
| Photosensitivity | 4 (2.3) | 9 (2.7) | 0.99 |
| Discoid rash | 6 (3.5) | 18 (5.3) | 0.35 |
| Oral ulcers | 12 (7.0) | 11 (3.3) | 0.057 |
| Alopecia | 15 (8.7) | 22 (6.5) | 0.37 |
| Pleuritis | 10 (5.8) | 25 (7.4) | 0.50 |
| Pericarditis | 3 (1.7) | 2 (0.6) | 0.34 |
| Lupus nephritis | 11 (6.4) | 11 (3.3) | 0.11 |
| Seizure | 3 (1.7) | 5 (1.5) | 0.99 |
| Psychosis | 0 (0.0) | 5 (1.5) | 0.17 |
| Mononeuritis multiplex | 0 (0.0) | 3 (0.9) | 0.55 |
| Peripheral or cranial neuropathy | 2 (1.2) | 3 (0.9) | 0.99 |
| Vasculitis | 4 (2.3) | 6 (1.8) | 0.74 |
| Hemolytic anemia | 3 (1.7) | 5 (1.5) | 0.99 |
| Leukopenia | 10 (5.8) | 2 (0.6) | <0.001 |
| Lymphopenia | 3 (1.7) | 1 (0.3) | 0.11 |
| Thrombocytopenia | 9 (5.2) | 6 (1.8) | 0.029 |
| Hospitalization | 20 (11.6) | 23 (6.8) | 0.065 |
Abbreviation: HCQ, hydroxychloroquine.
The most common clinical symptom during flares in both groups was arthritis. For first flare symptoms, patients who did not discontinue HCQ experienced arthritis and had leukopenia on laboratory workup (18.6% and 3.5%, respectively). Those who discontinued HCQ experienced arthritis in 12.2% (P = 0.05) and leukopenia in 0.3% (P = 0.007). For any flare symptoms, the most common laboratory finding in patients who did not discontinue HCQ was leukopenia (5.8%) and thrombocytopenia (5.2%), whereas those who discontinued HCQ were found to have leukopenia in 0.6% (P < 0.001) and thrombocytopenia in 1.8% (P = 0.029).
Our results also showed that within the group of patients who discontinued HCQ, there was a significant difference in frequency of flares and the duration of HCQ treatment. More specifically, patients who took HCQ for 1 year or more experienced fewer flares compared with those who took HCQ for less than a year (13.1% vs 5.7%, P = 0.019) (Figure 1).
Figure 1.
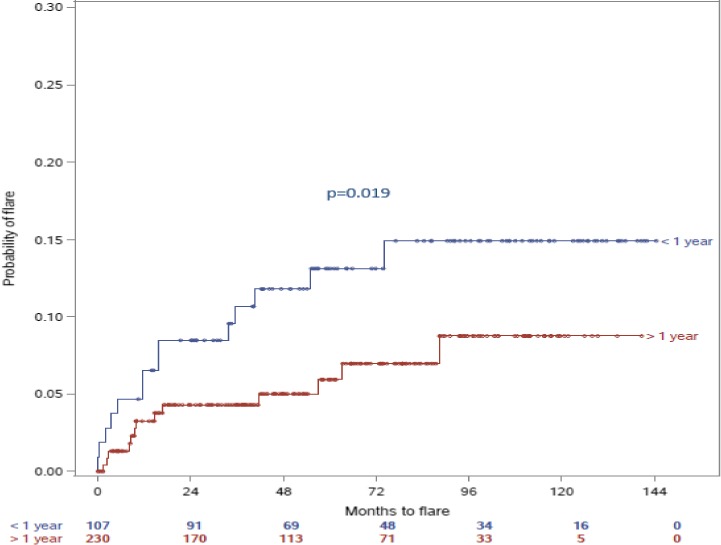
Comparison of number of months to flare up of SLE between the patients treated with HCQ for greater than 1 year before discontinuation, and those who received HCQ for less than 1 year. Time “zero” represents the time when HCQ was discontinued. The blue line represents the group that discontinued HCQ after being on it for less than 1 year, whereas the red line represents the group that discontinued HCQ after being on it for more than one year. The index date for comparisons of those who discontinued versus those who did not discontinue is the first HCQ start date at our institution. Among those who discontinued, Kaplan‐Meier curves were constructed to estimate time to first flare following the date of discontinuation, stratified by duration of HCQ.
Finally, we performed a sensitivity analysis to model the duration of HCQ as a time‐varying exposure in a multivariable Cox proportional hazards regression model. Importantly, the index time for this model is HCQ start date, and the event of interest is time to first flare following HCQ start date (as opposed to our original approach, with time to first postdiscontinuation flare as the event of interest among those who discontinued only). In this model that adjusts for patient age, sex, race/ethnicity, arthritis, and ANAs, the hazard ratio for flare was 0.44 (95% confidence interval [CI]: 0.28‐0.68) comparing those who discontinued with those who did not discontinue HCQ. This mirrors the univariable finding that those who discontinued HCQ were less likely to have experienced a flare while taking HCQ.
Discussion
As the largest study comparing outcomes in patients who remain on HCQ compared with those who discontinue the drug, our findings support those noted in other studies 1. We observed that patients who discontinued HCQ are more likely to have flares, particularly those who have taken HCQ for less than a year compared with those who are treated for longer than a year.
Likewise, in a 6‐month‐long randomized, prospective, placebo‐controlled trial looking specifically at withdrawal of HCQ in 47 patients with SLE, the Canadian Hydroxychloroquine Study Group found that patients who discontinued HCQ are more likely to experience flares and had shorter times leading up to flares. In addition, in that study, those who withdrew HCQ had higher rates of severe exacerbation of SLE (defined by central nervous system issues [CNS], vasculitis, or myositis) and were 6.1 times more likely to have severe exacerbation than those who continued HCQ 1. Of note in our study, patients who stopped HCQ before 1 year of treatment did not have significantly different rates of certain severe CNS and vascular manifestations, such as psychosis or vasculitis, as compared with those who had a treatment duration of longer than 1 year. It is possible that these differences are related to different study designs or treatment durations (6 months vs 1 year) among our studies, or differences in other SLE medications prescribed. Also, the incidence of SLE flares and hospitalization rate at follow up were lower in the group that discontinued HCQ before 1 year compared with the group that continued HCQ (Table 3). A possible explanation for these findings is that patients who had fewer SLE flares thought their disease was mild and therefore were more comfortable discontinuing HCQ.
In our investigation, we also elucidate further information on the natural history of SLE flares, finding that patients with specific manifestations, such as arthritis, and hematologic abnormalities are more likely to flare when discontinuing the drug than patients with other manifestations. In addition, we have found that patients who discontinued HCQ are more likely to have low complements (C4) as part of their flare, which may reflect a more severe manifestation of SLE as compared with those who remain on HCQ. The notion that those with low complements and hematologic derangements are more prone to flare has been indirectly explored in other studies 7. For example, Wilhelm et al postulate that durable remission in SLE is rare and that those with low C3 at baseline, hematologic abnormalities, and African American patients were at risk for delayed remission 7. Further prospective studies are needed on this topic, but these findings suggest that patients with hematologic derangements, such as leukopenia and thrombocytopenia, may benefit more than others from a prolonged course of HCQ. Furthermore, because our study distinguishes between less than 1 year and longer than 1 year treatment plans, HCQ use for more than 1 year could be considered in these selected patients, despite resolution of clinical and biochemical manifestations.
Other studies have confirmed that HCQ is protective against flares in SLE. In a large case control study of 465 patients with SLE, older age at diagnosis of SLE and HCQ use were the only protective variables amongst numerous examined variables 8. Similarly, in a randomized, placebo‐controlled trial of 24 patients, Meinão et al found that HCQ reduced disease exacerbation rates, prednisone dose, and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores in SLE 9. However, in that study, only patients with nonlifethreatening disease were included. In our study, we find that longer use of HCQ use is associated with reduced rates of flare in a diverse population of patients with varying manifestations.
In addition, studies evaluating serum levels of HCQ found that SLE patients with low HCQ blood concentration were at greater risk for flare 10. Another study by Mok et al studied the relationship between serum HCQ concentrations and flares of SLE in a longitudinal cohort of patients. He concluded that in patients in remission, higher HCQ concentrations were associated with a trend showing fewer flares over time 11.
Arthritis and tenosynovitis in SLE are significant causes of morbidity among SLE patients, and pain is often out of proportion to clinical examination findings 12, 13, 14, 15. Although HCQ has been specifically studied in the setting of arthritis in SLE, previous studies have yet to elucidate optimal treatment duration for SLE patients with bothersome joint symptoms. Our study reveals that those who take HCQ for more than 1 year are less likely to have arthritis than those who do not. As HCQ is a relatively safe medication, one might consider continuation of HCQ in patients with history of SLE arthritis for more than 1 year, even in the setting of otherwise inactive SLE.
It is important to compare our findings to previous literature on HCQ use in patients with cutaneous SLE. HCQ is often used to treat certain cutaneous manifestations of SLE, including discoid lupus, bullous lupus, and subacute cutaneous lupus 16. There are several studies assessing the efficacy of HCQ in discoid subacute cutaneous SLE 17, 18, 19.
In a double‐blind, randomized, parallel‐group clinical trial of patients with active cutaneous SLE, Yokogawa et al randomized 103 patients 3:1 to receive HCQ or placebo during the 16‐week double‐blind period and found that HCQ significantly improved patient and provider's global assessment of their skin 3. However, there was no significant difference among the two groups in terms of Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score. Alternatively, we found no significant difference in cutaneous SLE findings such discoid rash, malar rash, or photosensitivity among our patients who were treated for less than 1 year compared with those who were treated for more than a year. These data suggest that perhaps HCQ may improve skin findings but lacks the capacity to completely eradicate or prevent certain severe cutaneous flares. Also, it may be that the optimal treatment duration for HCQ in cutaneous SLE is not yet clear, or that HCQ may have a threshold effect on skin findings (ie, 4 months or more is necessary, but 6 months may be no different than 1 year). Further prospective data are necessary to clarify this matter.
Of note, other specific markers of SLE activity, such as anti‐dsDNA antibodies and anti‐Smith antibodies were not significantly different among those who continued HCQ vs those who discontinued the drug, and therefore it is not clear if patients with these immunologic markers would benefit distinctly from longer HCQ duration.
Our study has some limitations, the most important being the retrospective nature of the study. In addition, we did not specifically compare outcomes amongst multiple distinct treatment durations. In future studies, it would be helpful to compare outcomes in patients on various durations of HCQ in a prospective, randomized trial. Another limitation of our study is that the data on HCQ start date and length of treatment were estimated based on documented electronic medical records and documented medication prescriptions. If the medication was taken irregularly or stopped before the end of prescription, these data may not be captured.
Although there are instruments available to measure SLE disease activity such as SLEDAI, PGA, and SELENA‐SLEDAI as described by Thanou et al 20, a limitation in our study is that the endpoint was based on clinical determination of the treating rheumatologist. In future studies, use of disease activity measures such as SLEDAI can certainly enhance the outcomes captured.
With over 500 patient charts reviewed, ours is the largest study comparing outcomes for patients on HCQ with the outcomes of those who discontinued it. Based on the results of our study, patients that discontinued HCQ after being on it for less than 1 year are at greater risk for flares compared with those who were on it for longer than 1 year. In addition, we have cited specific SLE characteristics that may be at greater risk for flare when HCQ is discontinued, including patients with arthritis, leukopenia, and thrombocytopenia. Future prospective, randomized trials are needed in order to explore these patient populations and to further elucidate specific subsets of patients who would benefit from a longer duration of treatment with HCQ. In addition, we hope to use this information to further identify the optimal treatment duration with HCQ.
Author Contributions
Drs. Aouhab and Ostrowski had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; they are responsible for administrative, technical, or material support; and critically revised the manuscript for important intellectual content. Drs. Aouhab and Tarplin as well as Hong and Felicelli drafted the manuscript.
Study concept and design
Aouhab, Ostrowski, Felicelli.
Acquisition of data
Aouhab, Ostrowski.
Analysis and interpretation of data
Aouhab, Ostrowski.
Acknowledgments
All authors thank Cara Joyce, PhD (Loyola University Medical Center, Maywood, Illinois) for the statistical analysis.
No potential conflicts of interest relevant to this article were reported.
References
- 1. Canadian Hydroxychloroquine Study Group . A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991;324:150–4. [DOI] [PubMed] [Google Scholar]
- 2. Hu C, Lu L, Wan JP, Wen C. The pharmacological mechanisms and therapeutic activities of hydroxychloroquine in rheumatic and related diseases. Curr Med Chem 2017;24:2241–9. [DOI] [PubMed] [Google Scholar]
- 3. Gatto M, Saccon F, Zen M, Bettio S, Iaccarino L, Punzi L, et al. Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. J Autoimmun 2016;74:94–105. [DOI] [PubMed] [Google Scholar]
- 4. Yokogawa N, Eto H, Tanikawa A, Ikeda T, Yamamoto K, Takahashi T, et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double‐blind, randomized, parallel‐group trial. Arthritis Rheumatol 2017;69:791–9. [DOI] [PubMed] [Google Scholar]
- 5. Alarcón GS, McGwin G, Bertoli AM, Fessler BJ, Calvo‐Alén J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINAL). Ann Rheumatic Dis 2007;66:1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm TR, Magder LS, Petri M. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis 2017;76:547–53. [DOI] [PubMed] [Google Scholar]
- 8. Ugarte‐Gil MF, Wojdyla D, Pastor‐Asurza CA, Gamboa‐Cárdenas RV, Acevedo‐Vásquez EM, Catoggio LJ, et al. Predictive factors of flares in systemic lupus erythematosus patients: data from a multiethnic Latin American cohort. Lupus 2018;27:536–44. [DOI] [PubMed] [Google Scholar]
- 9. Meinão IM, Sato EI, Andrade LE, Ferraz MB, Atra E. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus 1996;5:237–41. [DOI] [PubMed] [Google Scholar]
- 10. Costedoat‐Chalumeau N, Amoura Z, Hulot JS, Hammoud HA, Aymard G, Cacoub P, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:3284–90. [DOI] [PubMed] [Google Scholar]
- 11. Mok CC, Penn HJ, Chan KL, Tse SM, Langman LJ, Jannetto PJ. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res (Hoboken) 2016;68:1295–302. [DOI] [PubMed] [Google Scholar]
- 12. Cronin ME. Musculoskeletal manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am 1988;14:99–116. [PubMed] [Google Scholar]
- 13. Ostendorf B, Scherer A, Specker C, Mödder U, Schneider M. Jaccoud's arthropathy in systemic lupus erythematosus: differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis Rheum 2003;48:157–65. [DOI] [PubMed] [Google Scholar]
- 14. Van Vugt RM, Derksen RH, Kater L, Bijlsma JW. Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. Ann Rheum Dis 1998;57:540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol 2009;23:495–506. [DOI] [PubMed] [Google Scholar]
- 16. Chasset F, Bouaziz JD, Costedoat‐Chalumeau N, Francès C, Arnaud L. Efficacy and comparison of antimalarials in cutaneous lupus erythematosus subtypes: a systematic review and meta‐analysis. Br J Dermatol 2017;177:188–96. [DOI] [PubMed] [Google Scholar]
- 17. Callen JP. Chronic cutaneous lupus erythematosus. Clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol 1982;118:412–6. [DOI] [PubMed] [Google Scholar]
- 18. Ruzicka T, Sommerburg C, Goerz G, Kind P, Mensing H. Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br J Dermatol 1992;127:513–8. [DOI] [PubMed] [Google Scholar]
- 19. Chang AY, Piette EW, Foering KP, Tenhave TR, Okawa J, Werth VP. Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis. Arch Dermatol 2011;147:1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thanou A, Chakravarty E, James JA, Merrill JT. How should lupus flares be measured? Deconstruction of the safety of estrogen in lupus erythematosus national assessment‐systemic lupus erythematosus disease activity flare index. Rheumatology (Oxford) 2014;53:2175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


