Abstract
Background
Recently, some studies suggested that clinical diagnosis of fibromyalgia is inaccurate and does not reflect current definitions. However, this hypothesis has not been tested. We examined whether fibromyalgia was accurately diagnosed in the community.
Methods
We surveyed 3276 primary care patients to determine current fibromyalgia status by criteria (CritFM). We also determined whether the patients had a physician's diagnosis of fibromyalgia (MDFM) and the level of symptom severity as measured by the polysymptomatic distress scale (PSD).
Results
The prevalence of MDFM and CritFM was 6.1% (95% confidence interval [CI] 5.3%, 6.9%) and 5.5% (95% CI 4.8%, 6.3%), respectively. However, only 32.2% with MDFM met 2016 criteria (CritFM), and only 35.4% with CritFM also had MDFM. The kappa statistic for diagnostic agreement was 0.296 (minimal agreement). The mean PSD score was 12.4 and 18.4 in MDFM and CritFM, respectively. The odds ratio for being a woman compared with being a man was 3.2 for MDFM versus 1.9 for CritFM, P = 0.023. Of the patients with MDFM, 68.3% received specific fibromyalgia pharmacotherapy.
Conclusions
There is little agreement between MDFM and CritFM. Only one‐third of MDFM satisfy fibromyalgia criteria, and only one‐third of patients who meet the criteria have a clinical diagnosis of fibromyalgia. Physician diagnosis is biased and more likely in women. Fibromyalgia treatment is common in MDFM (70.7%). Overall, MDFM appears subjective and unrelated to fibromyalgia criteria. There appears to be no common definition of fibromyalgia in the community.
Introduction
Fibromyalgia is a common disorder but is one associated with controversies that extend to its nature, definition, and diagnosis 1, 2, 3. There are two methods used in the diagnosis of fibromyalgia. Criteria‐based diagnosis, which forms the basis of epidemiologic studies of prevalence, and can also be used in the clinic and in clinical trials, consists of applying published criteria to clinical symptoms 4, 5, 6. As a gold standard, criteria‐based diagnosis provides a case definition 5, 6 and a method for validating and understanding clinical diagnosis. Use of criteria‐based diagnosis, however, is uncommon in the clinic 7.
The idea of clinical diagnosis in the clinic is simple: the physician, who is presumed to have a wide understanding of the patient and medical history, listens to and examines the patient, then usually makes a gestalt diagnosis 8. Most clinical care for fibromyalgia is provided by primary care physicians 9. Recent reports that studied both methods of diagnosis have suggested that only 25% of patients with a clinical diagnosis would satisfy fibromyalgia criteria. In addition, 75% of criteria‐positive patients do not have a clinical diagnosis of fibromyalgia 10, 11.
There are complications to diagnosis, beyond the simple issues presented above, that enter the diagnostic process and may relate to the discordance between the methods noted above. First, despite the existence of criteria, “fibromyalgia has no binding definition … and no way of objectively testing for it” 12. Clinical diagnosis can “be a complex process, as medical diagnoses are ‘contested, socially created, framed and/or enacted’ and are influenced by ‘social, political, technological, cultural and economic forces’” 13, 14. Clinical diagnosis comes about “through a process of negotiation between patient and doctor, in which power is both wielded and ceded by each party” 14.
Problems arise when using criteria‐based diagnosis, as this method does not account for potential diagnostic exclusions 6, 15, 16. Moreover, criteria‐based diagnosis runs into the problem of diagnosis validity and meaning, as clinicians may deliberately choose not to diagnose fibromyalgia in some or all settings 6, 15, 16, and, for some clinicians, fibromyalgia legitimacy remains contentious 1.
To investigate issues surrounding clinical and criteria‐based diagnosis, we studied 3276 patients attending an educational network of primary care clinics in which each patient completed a fibromyalgia criteria questionnaire and also provided information about physician diagnosis and treatment of fibromyalgia. We report below prevalence rates, agreement between methods data, and the effect of diagnosis on treatment.
Methods
Over a 5‐week period beginning on 25 July 2018, second‐year medical students gave one‐page research questionnaires related to fibromyalgia to 3276 adult (21 years or older) patients attending 25 different primary care medical practices throughout the state of Kansas as part of their requirements for the Summer Training Option in Rural Medicine (STORM). The questionnaires were dispensed to patients before their physician visits. Except for provision of writing material, no help was given to the patients, and no student or medical staff member saw or was aware of the completed content of the questionnaire. No patient refused to complete the questionnaire. The medical students collected the completed deidentified questionnaires, which were then sent to the National Data Bank for Rheumatic Diseases for processing 17.
Questionnaire content
The questionnaire contained the 2016 modified American College of Rheumatology (ACR) fibromyalgia diagnostic items in check box format as specified in the published criteria 6. In addition, each patient provided information on age (21‐39, 40‐59, 60‐79, 80 years or older) and sex (male or female). Four additional questions regarding fibromyalgia were included:
Were you ever told by a physician or medical professional that you had fibromyalgia? Yes or no. If yes, how many years ago were you told by a physician or medical professional that you had fibromyalgia: less than 6 months, 6 months to 1 year, 1‐3 years, more than 3 years.
If you were diagnosed with fibromyalgia have you EVER taken any of the following medications (check all): [ ] Lyrica (pregabalin), [ ] Cymbalta (duloxetine), [ ] Savella (milnacipran).
If you were diagnosed with fibromyalgia are you NOW taking any of the following medications (check all): [ ] Lyrica (pregabalin), [ ] Cymbalta (duloxetine), [ ] Savella (milnacipran).
Key study variables
From the 2016 criteria items 6, we calculated the presence or absence of the 2016 generalized pain criterion, the widespread pain index (WPI), the symptom severity scale (SSS), and the polysymptomatic distress scale (PSD)—also known as the fibromyalgia severity scale. We also determined the presence or absence of the widespread pain criterion used in the 1990 ACR fibromyalgia criteria definition 4. The WPI (on a scale of 0‐19) is a summary count of the number of 19 painful regions from the Regional Pain Scale, a self‐reported list of painful regions 18. The SSS (on a scale of 0‐12) is the sum of the severity scores of three symptoms (fatigue, waking unrefreshed, and cognitive symptoms) (0‐9) plus the sum (0‐3) of the number of the following symptoms by which the patient has been bothered that occurred during the previous 6 months: (1) headaches (0‐1), (2) pain or cramps in lower abdomen (0–1), and (3) depression (0–1). The PSD (0‐31) is the sum of the WPI and SSS. The PSD measures the magnitude and severity of fibromyalgia symptoms in those satisfying and not satisfying criteria. By definition, fibromyalgia criteria cannot be satisfied if the PSD is <12. PSD severity has also been categorized 19, and we used these categories to investigate severity and classification levels for physician and criteria diagnosis groups. Categories of PSD severity are as follows: 0 to 3 none, 4 to 7 mild, 8 to 11 moderate, 12 to 19 severe, and 20 to 31 very severe.
Criteria and physician diagnosis
Criteria‐based fibromyalgia was said to be present if the WPI was 7 or greater and the SSS score 5 or greater or if the WPI was between 4 and 6 and the SSS score 9 or greater. Generalized pain, defined as pain in at least 4 of 5 regions (right upper, right lower, left upper, left lower, axial), must be present 6. We did not analyze whether patients had symptoms for at least 3 months as this questionnaire item was meant for physician interview.
Physician diagnosed fibromyalgia was considered to be present if the patient reported ever being told by a physician or medical professional that he or she had fibromyalgia.
Statistical analysis
Data were analyzed using Stata version 15.0 20. Differences between physician diagnosis and clinic criteria groups were analyzed by linear and logistic regression, adjusted for age group. We also tested whether the odds ratio for being a woman compared with being a man, given a physician's diagnosis of fibromyalgia, was different from when diagnosis was by fibromyalgia criteria with the use of Stata's seemingly unrelated estimation (SUEST) command. The SUEST procedure combines information from the two models, then tests the null hypothesis that the odds ratios are equivalent across the two models. The kappa statistic was interpreted as None (0 to 0.20), Minimal (0.21‐0.39), Weak (0.40‐0.59), Moderate (0.60‐.079), Strong (0.80‐0.90), and almost perfect Above 0.90 21.
Ethics
This study was approved by the University of Kansas Medical Center Institutional Review Board (IRB) (Study 00142886).
Results
The prevalence of physician‐ and criteria‐diagnosed fibromyalgia in primary care was 6.1% (95% confidence interval [CI] 5.3%, 6.9%) and 5.5% (95% CI 4.8%, 6.3%), respectively (Table 1).
Table 1.
Characteristic of study participants by diagnostic group
| Group | N | MD Dx % | FM2016 % | Female % | PSD | WPI | SSS | WSP % | GP % | Treat Ever % | Treat Now % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | 3276 | 6.1 | 5.5 | 64.8 | 6.1 (5.1) | 2.4 (3.0) | 3.7 (2.9) | 15.0 | 9.9 | 4.7 | 2.9 |
| Dx groups | |||||||||||
| MD Dx | 199 | 100 | 32.2 | 84.0 | 12.4 (6.9) | 6.4 (4.6) | 6.0 (3.1) | 48.7 | 39.7 | 68.3 | 40.9 |
| 2016 Dx | 181 | 35.4 | 100 | 76.4 | 18.4 (4.4) | 10.4 (3.5) | 7.9 (2.0) | 97.2 | 100 | 27.6 | 12.7 |
| Dx subgroups | |||||||||||
| Crit 0 MD 0 | 2979 | 0.0 | 0.0 | 63.0 | 5.2 | 1.9 | 3.3 | 9.5 | 4.4 | 0.0 | 0.0 |
| Crit 0 MD 1 | 116 | 100 | 0.0 | 85.4 | 8.7 | 3.8 | 4.9 | 25.9 | 11.1 | 64.4 | 43.0 |
| Crit 1 MD 0 | 118 | 0.0 | 100 | 73.1 | 17.3 | 9.6 | 7.7 | 96.7 | 100 | 0.0 | 0.0 |
| Crit 1 MD 1 | 63 | 100 | 100 | 85.9 | 20.1 | 11.8 | 8.3 | 96.9 | 100 | 76.6 | 35.9 |
Differences between MDDX and 2016 Dx were significant at P < 0.05 for all study variables. The numbers are mean and (standard deviation). Crit 1 = fibromyalgia criteria positive; Crit 0 = fibromyalgia criteria negative; MD 1 = physician criteria positive; MD 0 = physician criteria negative.
Abbreviation: FM2016, diagnosis by FM criteria; GP, generalized pain criterion; MD Dx, diagnosis by physician; PSD, polysymptomatic distress; SSS, symptom severity scale; Treat, treated with fibromyalgia pharmacotherapy; WPI, widespread pain index; WSP, widespread pain criterion.
However, only 32.2% of physician‐diagnosed cases met fibromyalgia 2016 criteria and only 35.4% of criteria‐positive cases were diagnosed as fibromyalgia by physicians. The kappa statistic for agreement beyond chance between physician diagnosis and diagnostic criteria was 0.296 (minimal agreement). Figure 1 shows that PSD scores for physician‐diagnosed fibromyalgia were unfocused and broadly distributed, dipping far below the PSD level of 12, the minimal level for diagnosis, and extending on the right into the distribution of fibromyalgia 2016 cases. We then split the PSD scale into five categories and examined percent in category, PSD, WPI, SSS, and number of painful regions according to the diagnostic method (Table 2). Categories of PSD severity for physician‐diagnosed fibromyalgia were None (0‐3) 8.5%, Mild (4‐7) 19.6%, Moderate (8‐11) 20.6%, Severe (12‐19) 35.2%, and Very severe (20‐31) 16.1%. Values for criteria‐defined fibromyalgia were Severe (12‐19) 68.0% and Very severe (20‐31) 32.0%. As fibromyalgia cannot be diagnosed if the PSD score is less than 12, the five categories allowed for a clearer understanding of physician‐reported fibromyalgia and PSD severity. The None and Mild categories constituted 29.1% of physician‐diagnosed fibromyalgia, and the Moderate category contained 20.6%. We also noted that the mean level of WPI for each of the None, Mild, and Moderate categories for physician‐diagnosed fibromyalgia was less than 7, whereas the mean SSS score was more than 5 at the moderate category. The values of 7 and 5 are one of the diagnostic cut points of the 2016 criteria. Although the data indicate that the physician positive cases had generally lower variable values than expected for positive cases, it appears that this finding was comparatively more common in WPI than SSS.
Figure 1.
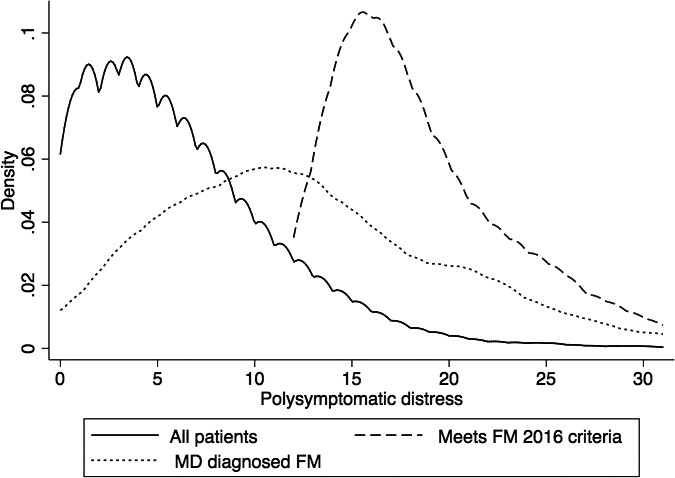
The distribution of PSD scores in all patients (solid line) and those diagnosed with fibromyalgia by criteria (dashed line) and physician diagnosis (dotted line)
Table 2.
PSD severity groups and severity variable means according to diagnostic methods
| Group | PSD Severity Category | N (%) | PSD | WPI | SSS | Regions |
|---|---|---|---|---|---|---|
| Physician diagnosis of FM | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| None (0‐3) | 17 (8.5) | 2.0 (1.1) | 0.8 (0.8) | 1.2 (1.0) | 0.7 (0.7) | |
| Mild (4‐7) | 39 (19.6) | 5.7 (1.0) | 2.4 (1.6) | 3.3 (1.5) | 1.3 (1.0) | |
| Moderate (8‐11) | 41 (20.6) | 9.7 (1.0) | 4.2 (1.9) | 5.5 (2.0) | 2.7 (1.2) | |
| Severe (12‐19) | 70 (35.2) | 14.9 (2.4) | 7.5 (3.0) | 7.3 (2.1) | 3.7 (1.2) | |
| Very severe (20‐31) | 32 (16.1) | 24.0 (3.3) | 14.4 (2.9) | 9.6 (1.9) | 5.0 (0.2) | |
| 2016 FM diagnosis by criteria | ||||||
| None (0‐3) | 0 | |||||
| Mild (4‐7) | 0 | |||||
| Moderate (8‐11) | 0 | |||||
| Severe (12‐19) | 123 (68.0) | 15.8 (1.9) | 8.7 (1.9) | 7.2 (1.7) | 4.6 (0.5) | |
| Very severe (20‐31) | 58 (32.0) | 23.7 (3.1) | 14.1 (3.1) | 9.6 (1.7) | 4.9 (0.3) | |
| All patients | ||||||
| None (0‐3) | 1202 (36.7) | 1.5 (1.2) | 0.4 (0.7) | 1.0 (1.1) | 0.4 (0.6) | |
| Mild (4‐7) | 1022 (31.2) | 5.4 (1.1) | 1.9 (1.4) | 3.5 (1.5) | 1.4 (1.1) | |
| Moderate (8‐11) | 593 (18.1) | 9.3 (1.1) | 3.4 (1.8) | 5.9 (1.8) | 2.2 (1.2) | |
| Severe (12‐19) | 396 (12.1) | 14.3 (2.1) | 6.7 (2.7) | 7.7 (2.3) | 3.4 (1.2) | |
| Very severe (20‐31) | 63 (1.9) | 23.5 (3.1) | 14.1 (3.3 | 9.4 (2.3) | 4.8 (0.6) |
Abbreviations: FM, fibromyalgia; PSD, polysymptomatic distress; Regions, number of regions (0‐5) of generalized pain fibromyalgia 2016 criterion; SSS, symptom severity scale; WPI, widespread pain index; WSP, widespread pain criterion.
Data from the 3276 primary care patients also provided useful community baseline measurements for study variables (Table 1). Widespread pain and generalized pain were present in 15.0% and 9.9% of participants, and the mean scores for PSD were 6.1 (SD 5.1), WPI 2.4 (3.0), and SSS 3.7 (2.9). By contrast, the scores for criteria‐positive fibromyalgia were PSD 18.4 (SD 4.4), WPI 10.4 (3.5), and SSS 7.9 (2.0), with 97.2% and 100% satisfying the widespread pain and generalized pain criteria. Values for physician‐diagnosed fibromyalgia were PSD 12.4 (SD 6.9), WPI 6.4 (4.6), and SSS 6.0 (3.1), with 48.7% and 39.7% satisfying the widespread pain and generalized pain criteria. Symptoms present for at least 3 months were reported by 91.3% of criteria‐positive patients and 88.2% of physician‐diagnosed patients. Differences between physician diagnosis and criteria diagnosis variables in Table 1 were significant at P < 0.05. Overall, 32.1% of patients attending the primary care clinics had PSD of 8 or more (Moderate or above).
To understand the probability of a criteria diagnosis of fibromyalgia in those who received a physician diagnosis, we examined the effect of the time from study assessment to first diagnosis of fibromyalgia by a physician. Table 3 shows that the likelihood of a criteria diagnosis in those receiving a physician diagnosis is directly related to the duration from first diagnosis time. In addition, PSD scores in physician‐diagnosed fibromyalgia increased rather than decreased with time from diagnosis. These data indicate that disagreement between physician and criteria diagnosis is unlikely to be related to time from diagnosis.
Table 3.
The relation of physician diagnosis and fibromyalgia severity (PSD) to time from initial diagnosis in physician diagnosed fibromyalgia
| Time of diagnosis | N | Physician‐diagnosed FM Patients Meeting FM Criteria % (95% CI) | Mean PSD Scores in Physician‐Diagnosed FMa |
|---|---|---|---|
| 0‐6 mo | 12 | 25.0 (5.5, 57.2) | 9.7 |
| 6 mo‐ <12 mo | 21 | 14.3 (3.0, 36.3) | 9.7 |
| 1‐3 y | 27 | 29.6 (13.8, 50.2) | 11.4 |
| >3 y | 129 | 38.0 (29.6, 46.9) | 13.7 |
| 0‐12 mo | 33 | 18.2 (7.0, 35.5) | 8.9 |
| All times | 189 | 33.3 (26.7, 40.5) | 12.4 |
P = 0.001 by nonparametric trend test.
Abbreviations: CI, confidence interval; FM, fibromyalgia; PSD, polysymptomatic distress.
Sex and fibromyalgia assessments
Of all study participants, 64.8% were women. As shown in Table 4, women were more likely to be diagnosed with fibromyalgia by physicians than by criteria (7.6% versus 2.7%) or to satisfy FM 2016 criteria (6.6% versus 3.7%). They were also more likely to have greater PSD, WPI, and SSS scores and more often to have WSP, generalized pain, and fibromyalgia treatments. We further explored whether the probability of a woman receiving a physician diagnosis of fibromyalgia was greater than the probability of a woman satisfying the FM 2016 criteria. We found that the odds ratio for being a woman compared with being a man, given a physician's diagnosis of fibromyalgia, was 3.2 (95% CI 2.2, 4.9). When we ran this model for satisfying FM 2016 criteria (instead of physician's diagnosis) the odds ratio was 1.9 (95% CI 1.4, 2.8). These differences in odds ratios were significant at P = 0.023.
Table 4.
The relation of sex to diagnostic and treatment variables
| Group | Sex % | MD Dx % | FM2016 % | PSD | WPI | SSS | WSP % | GP % | Treat Ever % | Treat Now % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patientsa | 3203 | 5.8 | 5.6 | 6.1 | 2.4 | 3.7 | 15.1 | 10.0 | 4.5 | 2.7 | |
| Women | 2075 | 64.8 | 7.6 | 6.6 | 6.5 | 2.6 | 3.9 | 15.8 | 10.6 | 5.7 | 3.3 |
| Men | 1128 | 35.2 | 2.7 | 3.7 | 5.4 | 2.2 | 3.1 | 13.8 | 8.8 | 2.6 | 1.6 |
Seventy‐three patients did not specify sex.
Differences in study variables according to sex were significant at P < 0.05, except for WSP and GP, which were not significantly different.
Abbreviations: GP, generalized pain criterion; M2016, diagnosis by fibromyalgia criterion; MD Dx, diagnosis by physician; PSD, polysymptomatic distress; SSS, symptom severity scale; Treat, treated with fibromyalgia pharmacotherapy; WPI, widespread pain index; WSP, widespread pain criterion.
We also examined the question of sex in fibromyalgia diagnosis by subgroup analysis of the 4 Crit MD groups described in Table 1. Compared with 63.0% women in Crit 0 MD 0, patients diagnosed by physicians who did not meet criteria were 22.4 (95% CI 15.8, 29.0) percentage points higher for being female than those not diagnosed with fibromyalgia by either method, and they were 20.6 (95% CI 14.6, 27.1) percentage points higher than the overall mean percentage of women. By contrast, those meeting only fibromyalgia criteria were 10.1 (95% CI 1.5, 18.7) and 8.5 (95% CI 0.2, 16.8) percentage points higher.
Current treatment and ever treatment were related to physician diagnosis (Figure 2 and Table 1). Of patients who received a physician diagnosis, 68.3% reported ever receiving the drug treatment, and 40.9% were currently receiving treatment. The probability of treatment was related to PSD scores in physician‐diagnosed patients, even when PSD levels were below the 12‐point fibromyalgia criteria threshold (Figure 2).
Figure 2.
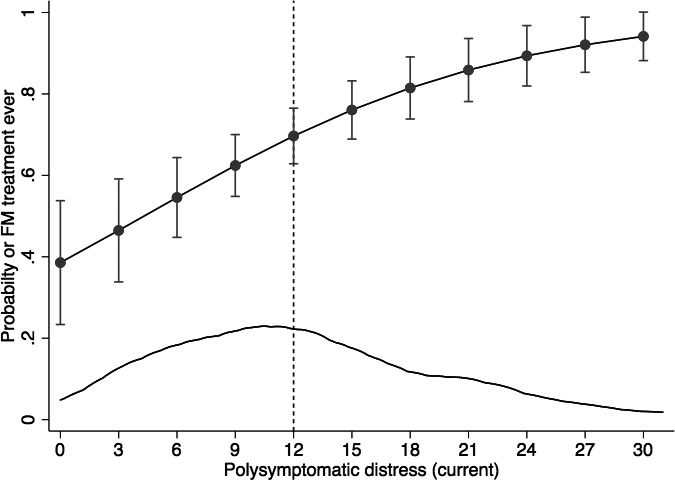
The relation of polysymptomatic distress (PSD) to the probability of treatment in physician‐diagnosed fibromyalgia. The lower curve represents the distribution of PSD scores in this group. Fibromyalgia criteria cannot be satisfied at PSD scores less than 12 (vertical line). FM, fibromyalgia.
Discussion
There are four important results of this study and a number of extrapolations from our results that are important to understanding the fibromyalgia idea: 1) Two‐thirds of patients with a physician's diagnosis of fibromyalgia do not satisfy fibromyalgia criteria. 2) Two‐thirds of patients who satisfy criteria have not received a diagnosis of fibromyalgia. 3) Treatment is related to PSD levels in physician‐diagnosed patients even when criteria are not met. 4) Physician diagnosis of fibromyalgia is biased with respect to sex.
One important effect of these observations is to confirm and validate the work of Walitt and colleagues 10, 11. Using ad hoc fibromyalgia criteria, these authors found under‐ and overdiagnosis of 75% as well as the presence of gender bias. Because their data created a population‐weighted sample of 8446 persons from the US general population (the US National Health Interview Survey [NHIS]) and explored many medical and social issues, their important findings now take on additional meaning. They found that persons satisfying fibromyalgia criteria had severe comorbid medical and psychological problems that increased with increasing PSD scores, whereas those who were misdiagnosed (or overdiagnosed) had real but lesser problems and a diagnosis that was linked to gender and social factors. This finding is key. The persons who received the fibromyalgia diagnosis but did not satisfy criteria “had real but lesser problems.” That is, they had less fibromyalgia. According to the authors of the NHIS report, “Examination of the surrogate polysymptomatic distress scale (PSD) of the 2010 ACR criteria found fibromyalgia symptoms extending through the full length of the scale”10.
Some other studies have also looked at the concordance issue. In a university rheumatology clinic, 50.4% of 104 consecutive patients with a fibromyalgia clinical diagnosis also satisfied fibromyalgia criteria, and 24.3% of 121 criteria‐positive patients received a clinical diagnosis of fibromyalgia 22. Agreement beyond chance was only fair: kappa 0.41. In a 2012 study of 312 women with incident interstitial cystitis/bladder pain syndrome and matched population‐based controls, 23% of patients with reported fibromyalgia diagnoses satisfied ad hoc criteria based on 1990 fibromyalgia criteria 4, 23.
Among the strengths of our study was its large and unbiased nature—including 25 centers and 3276 patients in primary care. We were also able to provide normative data for fibromyalgia, PSD, and widespread and generalized pain. Our study also had a number of weaknesses. We did not have fibromyalgia measurements at the time of initial physician diagnosis, and we did not have medical records to elucidate diagnostic consideration in those who met fibromyalgia criteria but were not diagnosed. Our study went for unbiasedness, rapidity, simplicity, presence of fibromyalgia measurements and low cost. What we missed out on was data and diagnostic considerations at the time of a physician diagnosis. In reality, such data are usually not recorded in sufficient detail in medical records, or even recoded at all. Although clearly desirable, they are almost impossible to obtain without changing the diagnosis process. IRB rules for obtaining such data would have required IRB course training for the geographically separate 25 physicians and their staffs, signed informed consents from each subject, and major disruption of the clinical practices—factors that would not allow us to obtain such data. Finally, we thought it important to not influence patients’ responses by breaking privacy and seeking access to medical records in this study.
Why do patients who do not meet criteria get a diagnosis of fibromyalgia? Is it that they met criteria at the time of diagnosis and then improved? If that were the case, we would have expected PSD levels and agreement with fibromyalgia criteria to fall over time as they continued to improve. What we found, however, was that PSD levels and the proportions meeting fibromyalgia criteria increased with time. Although our data were insufficient to answer the above questions, they suggest—as did the Walitt data—that patients probably did not meet criteria at the time of diagnosis. Studies that have followed fibromyalgia patients tend to suggest a stability in severity measures rather than improvement 24. Our data are insufficient to settle the question of current diagnosis discordance, but we believe that at least a substantial proportion of patients diagnosed with fibromyalgia did not meet criteria at the time of diagnosis.
If some patients are diagnosed with fibromyalgia though they do not meet criteria, why is that? Clearly most physicians do not know or pay attention to criteria 7, 25, 26. Next, we have to pay attention to the admonition that “fibromyalgia has no binding definition … and no way of objectively testing for it” 12. That is, the fibromyalgia definition could shift and the examination data could change during the “process of negotiation between patient and doctor …” 14. Diagnosis can go beyond medical issues and can be “socially created … and influenced by ‘social, political, technological, cultural and economic forces’” 13, 14, and psychosocial reasons might be a very important component of diagnosis. A fibromyalgia diagnosis offers an explanation for difficult‐to‐explain symptoms, and an explanation that bypasses the issues of psychogenicity 27, 28. It is an “easy” diagnosis for the physician and for the patient. The mechanisms involved in such a clinical interaction require a change in the severity or interpretation of the severity of symptoms together with an alteration of the boundaries between disease and nondisease.
A second possibility that is linked to the “no binding definition … and no way of objectively testing” observation is the role played by pharmaceutical companies in expanding diagnosis 27, 29, 30, 31. If two‐thirds of diagnosed patients do not have (satisfy criteria) fibromyalgia and diagnosis is linked to treatment, then extensive advertising, including direct‐to‐patient advertising may play an important role in recruiting physicians and patients to the diagnosis. Table 1 provides evidence that a clinical diagnosis of fibromyalgia in the United States leads to Food and Drug Administration–approved therapy. The mechanism of influence is to suggest to many persons that they may have fibromyalgia (changing the definition of fibromyalgia) and emphasizing the benefits of receiving a diagnosis. A second suggested mechanism relates to off‐label prescription. Clinicians may implicitly use the fibromyalgia indication to justify off‐label prescribing … for ill‐defined pain that appears similar to fibromyalgia pain, but also for more defined conditions such as low back pain and pain from osteoarthritis”31.
However, there is another potentially important reason that is usually not considered. The 1990 and 2010‐2016 criteria diagnosis point, the point at which nonfibromyalgia changes to fibromyalgia, was set by an expert committee but may have set the severity level too high, leaving some patients who have bothersome somatic symptom problems in the diagnosis‐negative group. This possibility can be considered after looking at Figure 1. A PSD score of 12 is the lower limit of PSD scores in the 2016 fibromyalgia criteria. If that limit were lowered, for example, to 8, the misclassification rate might fall by as much as 30%. In a previous study, we proposed, based on data, that a PSD score of 8‐11 could be considered “moderate” from the point of view of PSD severity 19. In the current study, 20.6% of physician‐diagnosed fibromyalgia had such moderate scores. If a PSD score of 8‐11 was considered also to be a measure of criteria positivity, then the percentage of physician‐diagnosed fibromyalgia meeting criteria would rise from 32.2% to 52.8%.
To summarize, “misdiagnosis’ (overdiagnosis) of fibromyalgia as described above can be a result of (1) ignorance of the disorder and its definition, (2) a negotiated decision between patient and doctor to satisfy psychosocial needs, (3) influence of pharmaceutical company advertising, and/or (4) an attempt to put a name on real, bothersome, but subthreshold fibromyalgia symptoms. The last consideration (option 4) in effect uses the fibromyalgia diagnostic language to identify what others would call bodily distress or somatic symptom disorders 32, 33, terminology widely used in the psychologic and psychosomatic literature but far less commonly among clinicians, particularly in the United States.
The other side of the diagnostic dilemma reflects the reality that two‐thirds of patients who met fibromyalgia criteria did not receive a fibromyalgia diagnosis. Essentially all epidemiological studies take satisfying criteria as the measure of fibromyalgia prevalence 34. That most people who meet criteria are not diagnosed by physicians as having fibromyalgia challenges that assumption. Ehrlich pointed out that irrespective of a clinical diagnosis one has tuberculosis, cancer, rheumatoid arthritis, hookworm infestation—the gamut of diseases, but not fibromyalgia, “No one has FM until it is diagnosed”35. That is, fibromyalgia represents an interpretation of symptoms that could be characterized in other ways. Use of the term, for some, endorses the concept of fibromyalgia. One might expect fibromyalgia advocates to equate fibromyalgia criteria positivity as measure of fibromyalgia legitimacy, whereas doubters would avoid using the term.
Clinicians have reasons for nondiagnosis: “There is no such thing as fibromyalgia” and “much of the medical profession is highly skeptical that the disease fibromyalgia—at least in purely physical terms—actually exists” 36. “The fibromyalgia symptoms are produced by another disease” 37. “The patient's disease is better explained by another diagnosis” 6. “The patient's medical problem does not require that I consider fibromyalgia.” “A diagnosis of fibromyalgia conveys no benefit to my patient”38. “I didn't think of it.” There are data regarding some of these reasons.
A common reason given for nondiagnosis is that the patient may have another diagnosed disease that is concomitant or dominant. Walitt reported that “many persons who satisfied NHIS criteria for fibromyalgia reported receiving alternative diagnoses, such as rheumatoid arthritis (15.3%), gout (3.3%), lupus (1.4%), low back pain (21.7%), and nonspecific “arthritis” (47.5%) 11. Similarly, a population study of fibromyalgia criteria noted that 28%‐45% of persons satisfying a version of the ACR 2010 criteria 5, 39 were told by health care providers that they had one of the following diseases: “osteoarthritis, rheumatoid arthritis, osteoporosis, lupus, scleroderma, ankylosing spondylitis, gout, or fibromyalgia” 40. Although the authors of the 2016 fibromyalgia criteria stated that if “patients … satisfy fibromyalgia criteria, they may be diagnosed as having fibromyalgia using fibromyalgia criteria: the current criteria definition of fibromyalgia does not exclude patients with coexistent conditions. … [A diagnosis] is only an acknowledgement that the patient has symptoms of fibromyalgia and satisfies fibromyalgia criteria”6.
Keeping Ehrlich's interpretation in mind, such data suggest the possibility of physicians dividing fibromyalgia nondiagnosis in persons with symptoms sufficient to meet fibromyalgia criteria into several groups: (1) The concomitant disease is too important or urgent to care about fibromyalgia, like, for example, a serious infection or advanced cancer. (2) Fibromyalgia is recognized but not diagnosed or coded. That is, it is not judged important enough to receive a separate diagnosis, or other conditions adequately explain the patient's problems. (3) Fibromyalgia‐like symptoms are recognized but differently diagnosed or coded 36. For example, somatoform pain disorders, ie, somatic symptoms or bodily distress disorders, are coded instead 41. (4) Fibromyalgia is not considered or recognized by the physician, ie, the physician did not think of it. (5) The physician does not recognize fibromyalgia as a legitimate or useful concept. Studies that identify nondiagnosed but criteria‐positive fibromyalgia are needed to explore this diagnostic problem.
Our data make clear that issues of diagnosis and prevalence are not simple and, at the community level, that diagnosis is uncertain. Are the criteria for fibromyalgia as promulgated by experts and written about in texts and medical articles a measure of the gold standard? Or does it fall to the marginally reliable, negotiated, socially constructed fibromyalgia that appears in the clinic and in public discourse to actually define the disorder? We think the data are clear on this point. Fibromyalgia in community practice does not follow the rules of published criteria. Rather, it is a marginally reliable, negotiated, and socially constructed diagnosis that is less severe than the fibromyalgia of published criteria. It reflects the needs of patients and physicians to identify and manage a broader spectrum of physical and somatic symptom distress.
The broad range of fibromyalgia severity should raise questions concerning putative mechanisms of disease and the nature of fibromyalgia 33. At one end of the spectrum, criteria‐based fibromyalgia blends with comorbid physical and mental disorders in variable ways such that what is or should be fibromyalgia is uncertain. At the other end of the spectrum, many physicians diagnose fibromyalgia at low levels of severity for reasons that are not entirely clear but probably reflect, at least in part, psychosocial factors. There are many experts who see and define fibromyalgia as part of an inclusive single bodily distress disorder 42, 43, 44. Problems in diagnosis and definition suggest caution in defining and interpreting results of clinical trials and neurobiological studies as if fibromyalgia was a single, well‐circumscribed disorder. The use of the PSD provides a method to quantify severity and may be a way to overcome the difficulties and uncertainty of binary diagnosis in research settings.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Wolfe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Srinivasan, Wolfe, Häuser, Rasker.
Acquisition of data. Srinivasan, Maloney, Wright, Kennedy, Kallail.
Analysis and interpretation of data. Srinivasan, Wolfe, Häuser, Rasker.
Acknowledgements
The authors thank the staff of the National Data Bank for Rheumatic Diseases who processed the study questions and created the research database. We also gratefully acknowledge the faculty, staff, and students of the Summer Training Option in Rural Medicine (STORM) program at the University of Kansas School of Medicine.
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. The authors declare that they have no financial or other interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to this manuscript.
References
- 1. Dumit J. Illnesses you have to fight to get: facts as forces in uncertain, emergent illnesses. Soc Sci Med 2006;62:577–90. [DOI] [PubMed] [Google Scholar]
- 2. Hazemeijer I, Rasker JJ. Fibromyalgia and the therapeutic domain. A philosophical study on the origins of fibromyalgia in a specific social setting. Rheumatology (Oxford) 2003;42:507–15. [DOI] [PubMed] [Google Scholar]
- 3. Häuser W, Ablin J, Fitzcharles M‐A, Littlejohn G, Luciano JV, Usui C, et al. Fibromyalgia. Nature Reviews Disease Primers 2015;1:15022. [DOI] [PubMed] [Google Scholar]
- 4. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 5. Wolfe F, Clauw D, Fitzcharles MA, Goldenberg D, Katz RS, Mease P, et al. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 6. Wolfe F, Clauw DJ, Fitzcharles M‐A, Goldenberg DL, Häuser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- 7. Perrot S, Choy E, Petersel D, Ginovker A, Kramer E. Survey of physician experiences and perceptions about the diagnosis and treatment of fibromyalgia. BMC Health Serv Res 2012;12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Littlejohn G. Fibromyalgia: honing fibromyalgia diagnosis. Nat Rev Rheumatol 2014;10:267–9. [DOI] [PubMed] [Google Scholar]
- 9. Choy E, Perrot S, Leon T, Kaplan J, Petersel D, Ginovker A, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res 2010;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10:e0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walitt B, Katz RS, Bergman MJ, Wolfe F. Three‐quarters of persons in the US population reporting a clinical diagnosis of fibromyalgia do not satisfy fibromyalgia criteria: the 2012 National Health Interview Survey. PLoS One 2016;11:e0157235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reed M, Herrmann M. New Insights into Fibromyalgia. InTech.com: InTech; 2012.
- 13. Jutel A, Nettleton S. Towards a sociology of diagnosis: reflections and opportunities. Soc Sci Med 2011;73:793–800. [DOI] [PubMed] [Google Scholar]
- 14. Madden S, Sim J. Acquiring a diagnosis of fibromyalgia syndrome: the sociology of diagnosis. Sociol Theory Health 2016;14:88–108. [Google Scholar]
- 15. Häuser W, Perrot S, Sommer C, Shir Y, Fitzcharles M‐A. Diagnostic confounders of chronic widespread pain: not always fibromyalgia. Pain Rep 2017;2:e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzcharles MA, Perrot S, Häuser W. Comorbid fibromyalgia: a qualitative review of prevalence and importance. Eur J Pain 2018;22:1565–76. [DOI] [PubMed] [Google Scholar]
- 17. Wolfe F, Michaud K. The National Data Bank for rheumatic diseases: a multi‐registry rheumatic disease data bank. Rheumatology (Oxford) 2010;50:16–24. [DOI] [PubMed] [Google Scholar]
- 18. Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol 2003;30:369–78. [PubMed] [Google Scholar]
- 19. Wolfe F, Walitt BT, Rasker JJ, Katz RS, Häuser W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J Rheumatol 2015;42:1494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stata statistical software: release 15.0. College Station (TX): Stata Corp; 2017. [Google Scholar]
- 21. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfe F, Schmuckler J, Jamal S, Castrejon I, Gibson K, Srinivasan S, et al. Diagnosis of fibromyalgia: disagreement between fibromyalgia criteria and clinician based fibromyalgia diagnosis in a university clinic. Arthritis Care Res (Hoboken). 2019. [DOI] [PubMed] [Google Scholar]
- 23. Warren JW, Clauw DJ. Functional somatic syndromes: sensitivities and specificities of self‐reports of physician diagnosis. Psychosom Med 2012;74:891–5. [DOI] [PubMed] [Google Scholar]
- 24. Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Häuser W, Wolfe F. The longitudinal outcome of fibromyalgia: a study of 1555 patients. J Rheumatol 2011;38:2238–46. [DOI] [PubMed] [Google Scholar]
- 25. Vandvik P, Aabakken L, Farup P. Diagnosing irritable bowel syndrome: poor agreement between general practitioners and the Rome II criteria. Scand J Gastroenterol 2004;39:448–53. [DOI] [PubMed] [Google Scholar]
- 26. Kumbhare D, Ahmed S, Sander T, Grosman‐Rimon L, Srbely J. A survey of physicians’ knowledge and adherence to the diagnostic criteria for fibromyalgia. Pain Med 2018;19:1254–64. [DOI] [PubMed] [Google Scholar]
- 27. Barker KK. Listening to Lyrica: contested illnesses and pharmaceutical determinism. Soc Sci Med 2011;73:833–42. [DOI] [PubMed] [Google Scholar]
- 28. Rasmussen EB. Balancing medical accuracy and diagnostic consequences: diagnosing medically unexplained symptoms in primary care. Sociol Health Illn 2017;39:1227–41. [DOI] [PubMed] [Google Scholar]
- 29. Jeffery DD, Bulathsinhala L, Kroc M, Dorris J. Prevalence, health care utilization, and costs of fibromyalgia, irritable bowel, and chronic fatigue syndromes in the military health system, 2006‐2010. Mil Med 2014;179:1021–9. [DOI] [PubMed] [Google Scholar]
- 30. Moynihan RN, Cooke GP, Doust JA, Bero L, Hill S, Glasziou PP. Expanding disease definitions in guidelines and expert panel ties to industry: a cross‐sectional study of common conditions in the United States. PLoS Med 2013;10:e1001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017;377:411–4. [DOI] [PubMed] [Google Scholar]
- 32. Henningsen P, Zipfel S, Sattel H, Creed F. Management of functional somatic syndromes and bodily distress. Psychother Psychosom 2018;87:12–31. [DOI] [PubMed] [Google Scholar]
- 33. Henningsen P, Gündel H, Kop WJ, Löwe B, Martin A, Rief W, et al. Persistent physical symptoms as perceptual dysregulation: a neuropsychobehavioral model and its clinical implications. Psychosom Med 2018;80:422–31. [DOI] [PubMed] [Google Scholar]
- 34. Marques AP, Santo ASDE, Berssaneti AA, Matsutani LA, Yuan SLK. Prevalence of fibromyalgia: literature review update. Rev Bras Reumatol Engl Ed 2017;57:356–63. [DOI] [PubMed] [Google Scholar]
- 35. Ehrlich GE. Pain is real; fibromyalgia isn't. J Rheumatol 2003;30:1666–7. [PubMed] [Google Scholar]
- 36. Tavel ME. Somatic symptom disorders without known physical causes: one disease with many names? [review]. Am J Med 2015;128:1054–8. [DOI] [PubMed] [Google Scholar]
- 37. Di Franco M, Iannuccelli C, Bazzichi L, Atzeni F, Consensi A, Salaffi F, et al. Misdiagnosis in fibromyalgia: a multicentre study. Clin Exp Rheumatol 2011;29:S104–8. [PubMed] [Google Scholar]
- 38. Bass C, Henderson M. Fibromyalgia: an unhelpful diagnosis for patients and doctors. BMJ 2014;348:g2168. [DOI] [PubMed] [Google Scholar]
- 39. Wolfe F, Clauw D, Fitzcharles MA, Goldenberg D, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–22. [DOI] [PubMed] [Google Scholar]
- 40. Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi‐Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol 2015;67:568–75. [DOI] [PubMed] [Google Scholar]
- 41. Häuser W, Henningsen P. Fibromyalgia syndrome: a somatoform disorder? [review]. Eur J Pain 2014;18:1052–9. [DOI] [PubMed] [Google Scholar]
- 42. Wessely S, White PD. There is only one functional somatic syndrome. Br J Psychiatry 2004;185:95–6. [DOI] [PubMed] [Google Scholar]
- 43. Fink P, Schröder A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J Psychosom Res 2010;68:415–26. [DOI] [PubMed] [Google Scholar]
- 44. Fink P. Syndromes of bodily distress or functional somatic syndromes ‐ where are we heading. Lecture on the occasion of receiving the Alison Creed award 2017. J Psychosom Res 2017;97:127–30. [DOI] [PubMed] [Google Scholar]


