Abstract
Objective
Retinoblastoma (Rb) protein is a nuclear protein with several important functions, including the ability to stabilize heterochromatin. Because antibodies against the nucleosome and chromatin are key in systemic lupus erythematosus (SLE), we sought to determine whether Rb was an autoantigen in SLE and to evaluate any associated clinical phenotypes.
Methods
Sera from 222 patients with SLE from the Hopkins longitudinal cohort were studied. Additional cohorts tested included sera from 100 patients with primary Sjögren syndrome (pSS) (disease controls) evaluated at the Johns Hopkins Jerome L. Greene Sjögren's Center and sera from 36 healthy individuals. Anti‐Rb antibodies were assayed by immunoprecipitation of 35S‐methionine–labeled Rb, which was generated by in vitro transcription/translation. Fisher's exact test was used for the univariate analysis. Multivariable exact logistic regression was used to model for the presence of proteinuria in patients with SLE.
Results
Anti‐Rb antibodies were present in 15 of 222 (6.8%) patients with SLE, in 3 of 100 patients with pSS (3%), and in 0 of 36 healthy individuals. Among patients with SLE, Rb antibodies were strongly negatively associated with proteinuria (P = 0.0031), renal involvement (odds ratio [OR] = 0.11; P = 0.01), and anemia (OR = 0.05; P < 0.0001) and were positively associated with stroke (OR = 7.65; P = 0.05). The negative association with lupus nephritis held true in multivariate models (adjusted OR = 0.11; P = 0.01).
Conclusion
Anti‐Rb antibodies are a novel specificity not previously described in SLE. These new data define a possible SLE subset that is protected against renal involvement, is positively associated with stroke, and is not associated with antiphospholipid antibodies.
Significance & Innovations.
This is the first autoantibody associated with protection against lupus nephritis.
This is a possible second pathogenetic pathway (separate from antiphospholipid syndrome) toward stroke in SLE.
Introduction
Systemic autoimmune diseases are characterized by remarkable phenotypic heterogeneity and overlapping clinical features. Autoantibodies are a hallmark feature of these diseases. Knowledge of these has proven to be of utility to classify patients, guide treatment decisions, enable predictions of outcomes, and provide insights into disease pathogenesis. An expanded set of well‐defined markers will be essential to effectively implement the emerging era of precision medicine in autoimmune rheumatic diseases. Historically, systemic lupus erythematosus (SLE) has been associated with several specific autoantibodies (eg, anti–double‐stranded DNA [dsDNA] and anti‐Smith [anti‐Sm]), but very few have been clearly linked to distinct clinical manifestations 1. More recently, several new SLE autoantigens have been discovered using new methodologies 2, 3. Of these, only anti–tubulin 1C and anti‐ficolin were closely associated with clinical features (nephritis and vasculitis, respectively), and validation studies are still pending 4, 5.
In the context of cancer‐induced autoimmunity, our group explored the role of tumor‐suppressor proteins in autoimmunity 6. In most cancers, there is inactivation and loss of retinoblastoma (Rb) function resulting from RB1 gene mutations or modifications of the Rb‐associated regulatory mechanisms 7. Rb is a 106‐kD nuclear protein, and its main tumor‐suppressor function is to control cell cycle through binding and repression of the E2F transcription factor family 8. Interestingly, Rb is highly expressed in many tissues of relevance in SLE as a target of the immune response, including in blood cells, bone marrow, the central nervous system (CNS), and breast. Based on these tissue‐expression sites and levels, it is interesting to speculate that Rb could potentially be a target of the immune‐system attack in this disease spectrum (https://www.genecards.org/cgi-bin/carddisp.pl?gene=RB1#expression). Of note, anti‐Rb antibodies have been previously identified only in patients with lung cancer 9.
Rb is also known to play a role in heterochromatin formation by maintaining overall constitutive chromatin structure 7. Because several of the well‐defined targets of the autoimmune response in SLE are nucleosome‐ and chromatin‐related (eg, antibodies against dsDNA or histones), we tested whether Rb itself was a target of the immune response in patients with SLE. In this brief report, we describe the prevalence of anti‐Rb antibodies in SLE and highlight their possible clinical significance as biomarkers of a new SLE disease subset.
Patients and Methods
Patient cohort and sera
All patients and healthy individuals who participated in the study were older than 18 years and signed an informed consent. Clinical data and sera collection was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Sera were aliquoted and stored at −80°C after collection. Clinical data were retrospectively retrieved after careful review of medical charts. The lupus cohort consisted of 222 patients consecutively evaluated at the Johns Hopkins Lupus Center from May 2013 to August 2013. The Hopkins Lupus Cohort is a prospective cohort in which 94% of the patients fulfill at least 4 of the 1982 revised criteria of the American College of Rheumatology, with clinical, laboratory, and outcome data recorded at every visit. The disease‐control cohort consisted of sera from 100 patients with primary Sjögren's syndrome (pSS) seen in the Johns Hopkins Jerome L. Greene Sjögren's Center (all met the 2002 American‐European Consensus Group criteria). Sera from 36 healthy individuals were also studied.
Immunoprecipitation using 35S‐methionine–labeled proteins generated by in vitro transcription/translation
Complementary DNA encoding full‐length human Rb protein was used to generate 35S‐methionine–labeled protein by in vitro transcription/translation (IVTT) according to the manufacturer's protocol (Promega). Immunoprecipitations were performed using these products as follows: IVTT products (2 μl) were diluted in 1 ml of ice‐cold lysis buffer (20 mM Tris pH 7.4/150 mM NaCl/1 mM EDTA pH 7.4/1% Nonidet P‐40 and a protease inhibitor cocktail); 1 μl of serum was added to each, and the mixture was rocked (1 hour at 4°C) before adding 35 μl of protein A agarose beads (Pierce) for 20 minutes at 4°C. After extensive washing, the immunoprecipitates were electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gels and visualized by autoradiography. A strong positive lupus serum was included as a positive reference in each data set; the immunoprecipitates were quantitated by densitometric scanning and were normalized to the reference included in each set 10. Specificity of the assay was confirmed by immunoprecipitating the 35S‐methionine–labeled Rb protein generated by IVTT using a commercial antibody against Rb (rabbit monoclonal antibody [EPR17512]; Abcam). Because the distribution of values in the SLE and pSS cohorts was bimodal, a cutoff (for assigning positive antibody status) of five normalized antibody units was chosen, which clearly separated both populations (see Figure 1B).
Figure 1.
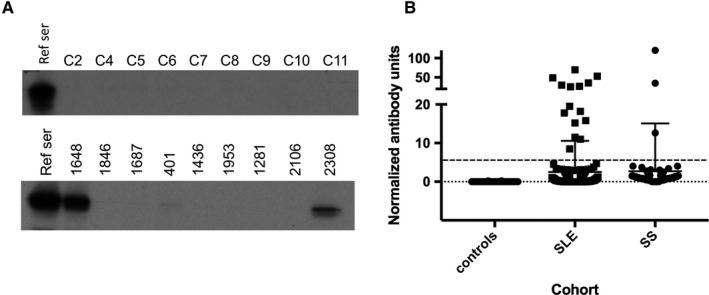
Identification of patients with antibodies against retinoblastoma (Rb) protein. A, Sera from nine healthy control subjects and nine patients with systemic lupus erythematosus (SLE) (upper and lower panels, respectively) were used to immunoprecipitate 35S‐methionine–radiolabeled Rb generated by in vitro transcription/translation. Two of the patients with SLE shown in the panel had antibodies against Rb (1648 and 2308). The remaining seven patients with SLE were anti‐Rb negative, as were all nine of the controls. The leftmost lane of each panel (reference serum [ref ser]) denotes immunoprecipitations (IPs) performed with the positive reference serum used as a calibrator in each set of IPs. B, Levels of anti‐Rb antibody in the three cohorts tested. Antibodies were assayed as described in the Methods section. Each symbol denotes an individual serum sample; horizontal and vertical lines show the mean and SD. The upper broken line denotes the cutoff for assigning antibody positivity. SS, Sjögren syndrome.
Statistical analysis
Fisher's exact test was used to assess the association between SLE manifestations/antibodies and anti‐Rb. SLE manifestations/antibodies were dichotomized as present versus absent. Unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. We further constructed a multivariable exact logistic regression to analyze the association between the presence of proteinuria and anti‐Rb. Covariates included in the model were presence of anti‐Rb, sex, ethnicity, presence of anti‐dsDNA, presence of anti‐Sm, and low complement levels. Adjusted ORs and 95% CIs were reported. All analyses were performed using the statistical software packages SAS version 9.4 (SAS Institute, Inc) and R version 3.5.1 (R Core Team, R Foundation for Statistical Computing). Tests of statistical significance were conducted at an alpha level of 0.05.
Results
Antibodies against Rb are present in sera of patients with SLE
A striking and unique feature of SLE is that many of the autoantibodies are directed against the nucleosome and/or chromatin (eg, anti‐dsDNA and antihistone antibodies). Because Rb is known to play a role in heterochromatin formation by maintaining overall constitutive chromatin structure, we tested whether Rb itself was a target of the immune response in patients with SLE. Sera from 222 well‐characterized patients with SLE consecutively seen at the Johns Hopkins Lupus Center were tested for antibodies to Rb. Eighty‐five percent of the patients were women, and 94% were white; patients had a mean age of 51.1 ± 13.1 years at sample date. The mean disease duration was 15.4 ± 8.7 years. The patients with SLE exhibited the following manifestations: malar rash (48.2%), discoid rash (11.7%), photosensitivity (55.4%), oral/nasopharyngeal ulcers (62.6%), serositis (48.4%), arthritis (69.2%), renal disorder (34.2%), neurologic disorder (10.4%), hematologic disorder (63.1%), immunologic disorder (64.9%), and antinuclear antibody (95.5%). Antibodies to Rb were also assayed in sera from 100 patients with pSS in the Johns Hopkins Jerome L. Greene Sjögren's Center cohort and in sera from 36 healthy controls. Sera were designated as antibody positive if the absorbance value of the immunoprecipitate was more than five normalized units. Representative immunoprecipitations using sera from controls and patients with SLE are shown in Figure 1A (upper and lower panels, respectively), and the normalized levels of Rb antibodies detected in the three cohorts studied are shown in Figure 1B. Of 222 patients with SLE, 15 (6.8%) had antibodies against Rb, compared with 3 of 100 patients with pSS and 0 of 36 healthy controls.
Anti‐Rb antibodies associate with distinct clinical SLE features
The strongest finding was the negative association of anti‐Rb antibodies with lupus nephritis, which was shown in both univariate (Table 1) and multivariate (Table 2) analyses. The second finding was the positive association of anti‐Rb antibodies with stroke (near statistical significance) (Table 1). It is noteworthy that anti‐Rb antibodies were not associated with antiphospholipid antibodies (aPLs) (Table 1).
Table 1.
Association of Anti‐Rb antibodies with SLE manifestations
| Manifestations | Anti‐Rb Positive (N = 15), n (%) | Anti‐Rb Negative (N = 207), n (%) | P a | OR (95% CI) |
|---|---|---|---|---|
| Vasculitis ever | 0 (0.00) | 34 (16.43) | 0.1353 | N/Cb |
| Proteinuria ever | 0 (0.00) | 76 (36.71) | 0.0031 | N/Cb |
| Hematuria ever | 1 (6.67) | 44 (21.26) | 0.3155 | 0.27 (0.01‐1.72) |
| Renal SLE ever (hematuria or proteinuria) | 1 (6.67) | 82 (39.61) | 0.0111 | 0.11 (0.01‐0.69) |
| Stroke ever | 2 (13.33) | 4 (1.93) | 0.0547 | 7.65 (0.95‐45.25) |
| Anemia ever | 1 (6.67) | 126 (60.87) | <0.0001 | 0.05 (0.01‐0.29) |
| Heart murmur ever | 2 (13.33) | 83 (40.10) | 0.0525 | 0.23 (0.04‐1.06) |
| Moon facies ever | 0 (0.00) | 48 (23.19) | 0.0454 | N/Cb |
| Pulse steroid use ever | 1 (6.67) | 71 (34.30) | 0.0414 | 0.14 (0.01‐0.87) |
| Cytotoxic use ever | 3 (20.00) | 108 (52.17) | 0.0293 | 0.23 (0.05‐0.86) |
| Malignancy | 2 (13.33) | 36 (17.65) | 1.0000 | 0.72 (0.11‐3.08) |
| SLE antibodies | ||||
| Anti‐Ro | 4 (26.67) | 66 (32.04)c | 0.7801 | 0.77 (0.22‐2.65) |
| Anti‐La | 1 (6.67) | 32 (15.53)c | 0.7051 | 0.39 (0.02‐2.59) |
| Anti‐RNP | 1 (6.67) | 36 (17.39) | 0.4756 | 0.34 (0.02‐2.23) |
| Anti‐Smith | 1 (6.67) | 35 (16.91) | 0.4752 | 0.35 (0.02‐2.32) |
| Anti‐dsDNA | 6 (40.00) | 127 (61.35) | 0.1705 | 0.42 (0.14‐1.25) |
| RVVT | 2 (13.33) | 71 (34.30) | 0.1524 | 0.30 (0.05‐1.37) |
| Anticardiolipin | 9 (60.00) | 125 (60.39) | 1.0000 | 0.98 (0.33‐2.89) |
| Coombs | 1 (6.67) | 33 (15.94) | 0.4776 | 0.38 (0.02‐2.50) |
| Any of the selected antibodies | 11 (73.33) | 189 (91.30)d | 0.0477 | 0.26 (0.08‐0.98) |
Abbreviation: CI, confidence interval; dsDNA, double‐stranded DNA; N/C, not calculated; OR, odds ratio; Rb, retinoblastoma; RNP, ribonucleoprotein; RVVT, russell viper venom test; SLE, systemic lupus erythematosus.
Fisher's exact test.
OR was not calculated because of zero cell frequencies.
One patient with negative anti‐Rb was dropped because the patient did not have anti‐Ro and anti‐La tests done.
Three patients with negative anti‐Rb had missing information on malignancy and were dropped.
Table 2.
Multivariable logistic regression for proteinuria
| Variables | aOR (95% CI)a | P |
|---|---|---|
| Anti‐Rb | 0.109 (0‐0.539) | 0.0139 |
| Female sex | 0.418 (0.172‐1.003) | 0.0509 |
| White race/ethnicity | 0.290 (0.060‐1.154) | 0.0859 |
| Anti‐dsDNA | 1.921 (0.933‐4.034) | 0.0795 |
| Anti‐Smith | 1.254 (0.496‐3.112) | 0.7412 |
| Low complement level | 2.154 (1.064‐4.452) | 0.0316 |
Abbreviation: aOR, adjusted odds ratio; CI, confidence interval; dsDNA, double‐stranded DNA; Rb, retinoblastoma.
aORs were estimated using exact logistic regression.
Careful review of the clinical characteristics of the three patients with pSS with antibodies against Rb yielded no readily apparent clinical features among this group because of the small numbers. Clinical characteristics of each patient with pSS who was anti–Rb antibody positive are listed in Supplemental Table 1.
Discussion
In this study, we tested sera from patients with SLE and pSS to determine whether Rb antibodies were found in these autoimmune diseases and, if so, whether there were any relevant clinical associations. Rb antibodies were detected in 15 of 222 (6.8%) patients with SLE, in 3 of 100 patients with pSS (3%), and in 0 of 36 healthy individuals. To our knowledge, this is the first report of anti‐Rb antibodies in systemic autoimmune diseases.
Among patients with SLE, Rb antibodies were negatively associated strongly with proteinuria (P = 0.0031) and renal involvement (P = 0.01). Furthermore, the absence of anti‐Rb was identified as an independent risk factor for proteinuria in the multivariate analysis model. It is noteworthy that the multivariate model included well‐known predictors of renal involvement, such as low C3 complement levels and other race as risk factors, further supporting the notion that the absence of anti‐Rb antibodies may indeed be associated with proteinuria and renal involvement. That an antibody could mediate protective functions for the kidney and for peripheral blood cells or bone marrow at the same time is unexpected. Because these findings are derived from a single‐center cohort, further studies are required to confirm and validate the clinical significance of anti‐Rb antibodies. Of note, earlier studies reported that in patients with SLE, those with rheumatoid factor were less likely to have severe SLE manifestations 11. Whether rheumatoid factor and Rb antibody levels correlate in any way may be informative to explore in future studies.
Interestingly, we found that among patients with SLE, Rb antibodies were positively associated with stroke (P = 0.0547). This finding is especially noteworthy because Rb protein levels are high in the brain (https://www.genecards.org/cgi-bin/carddisp.pl?gene=RB1#expression). In this setting, it is interesting to consider the possibility that an immune attack against CNS structures or modifications of Rb protein in the context of tumorigenesis or a chronic inflammatory process could potentially lead to the production of anti‐Rb antibodies. It is also interesting that despite the role Rb plays in heterochromatin formation 7, no correlation was found between antibodies against dsDNA and Rb in the patients studied with SLE. This may indicate that Rb antibodies are not part of a broader response to chromatin and could be consistent with the antitumor response proposed previously.
Also noteworthy is that anti‐Rb antibodies were not associated with aPLs, a major cause of stroke among patients with SLE. To date, traditional risk factors for coronary artery and cerebrovascular disease (lupus anticoagulant and other aPLs) have been implicated in the pathogenesis of stroke in SLE 12. In addition, SLE disease activity has been identified as an independent risk factor for stroke because of endothelial dysfunction or other, yet unknown, SLE disease–specific causes. Anti‐Rb antibodies, if pathogenic, could potentially represent a novel pathway associated with cerebrovascular events in SLE. Further studies that investigate whether Rb antibodies are pathogenic or represent an epiphenomenon associated with stroke will be insightful.
Interestingly, patients with SLE have a lower prevalence of breast cancer compared with the general population 13. Because Rb is highly expressed in breast tissue (https://www.genecards.org/cgi-bin/carddisp.pl?gene=RB1#expression), it is tantalizing to speculate that a potential link might exist between the antitumor response against breast cancer and this specific SLE phenotype. Additional studies will be needed to investigate this.
Rb antibodies are a novel specificity not previously described in patients with SLE. These initial findings raise the possibility that these antibodies may be a biomarker of a novel SLE protective phenotype for renal involvement, proteinuria, and anemia. Intriguingly, and for reasons that are presently unclear, these antibodies are also positively associated with stroke in the absence of an association with aPLs. Future studies that validate these findings in other SLE cohorts and that address the mechanism of these findings are warranted.
Author Contributions
All authors drafted the article, revised it critically for important intellectual content, approved the final version to be published, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Goules, Rosen, Petri, Casciola‐Rosen.
Acquisition of data
Goules, Petri, Casciola‐Rosen.
Analysis and interpretation of data
Goules, Li, Antiochos, Goldman, Rosen, Petri, Casciola‐Rosen.
Supporting information
Funded by the Donald B. and Dorothy L. Stabler Foundation and the Jerome L. Greene Foundation. The Rheumatic Diseases Research Core Center, where the autoantibodies were assayed, was supported by NIH grant P30‐AR‐070254. Dr. Antiochos is a Jerome L. Greene Scholar, and his work was funded by the Rheumatology Research Foundation. Drs. Petri and Casciola‐Rosen's work was funded in part by grants from the NIH (grants R01‐AR‐069572 and R01‐AR‐073208, respectively).
Drs. Goules and Li contributed equally to this work. Drs. Petri and Casciola‐Rosen contributed equally to this work.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 2004;34:501–37. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Hao C, Deng Y, Liu Y, Hu S, Peng Y, et al. Screening epitopes on systemic lupus erythematosus autoantigens with a peptide array. Oncotarget 2017;8:85559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis MJ, McAndrew MB, Wheeler C, Workman N, Agashe P, Koopmann J, et al. Autoantibodies targeting TLR and SMAD pathways define new subgroups in systemic lupus erythematosus. J Autoimmun 2018;91:1–12. [DOI] [PubMed] [Google Scholar]
- 4. Zhao X, Cheng Y, Gan Y, Jia R, Zhu L, Sun X. Anti‐tubulin‐α‐1C autoantibody in systemic lupus erythematosus: a novel indicator of disease activity and vasculitis manifestations. Clin Rheumatol 2018;37:1229–37. [DOI] [PubMed] [Google Scholar]
- 5. Colliard S, Jourde‐Chiche N, Clavarino G, Sarrot‐Reynauld F, Gout E, Deroux A, et al. Autoantibodies targeting ficolin‐2 in systemic lupus erythematosus patients with active nephritis. Arthritis Care Res (Hoboken) 2018;70:1263–68. [DOI] [PubMed] [Google Scholar]
- 6. Shah AA, Casciola‐Rosen L, Rosen A. Review: cancer‐induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol 2015;67:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol 2013;14:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev 2016;30:1492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsumoto S, Teramoto H, Nakamoto M, Igishi T, Kawasaki Y, Shimizu E. Presence of antibodies against retinoblastoma tumor suppressor protein in patients with lung cancer. Int J Oncol 2001;19:1035–9. [DOI] [PubMed] [Google Scholar]
- 10. Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola‐Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM‐140): a retrospective study. J Am Acad Dermatol 2011;65:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldman D, Feldman D, Ginzler E, Kaplan D. Rheumatoid factor in patients with systemic lupus erythematosus. J Rheumatol 1989;16:618–22. [PubMed] [Google Scholar]
- 12. de Amorim LC, Maia FM, Rodrigues CE. Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus 2017;26:529–36. [DOI] [PubMed] [Google Scholar]
- 13. Choi MY, Flood K, Bernatsky S, Ramsey‐Goldman R, Clarke AE. A review on SLE and malignancy. Best Pract Res Clin Rheumatol 2017;31:373–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


