Abstract
Objective
Primary cardiac involvement is presumed to account for a substantial part of disease‐related mortality in systemic sclerosis (SSc). Still, there are knowledge gaps on the evolution and total burden of systolic dysfunction in SSc. Here we evaluated prospective left ventricular (LV) and right ventricular (RV) systolic function in an unselected SSc cohort and assessed the burden of systolic dysfunction on mortality.
Methods
From the Oslo University Hospital cohort, 277 SSc patients were included from 2003‐2016 and compared with healthy controls. Serial echocardiographies were reevaluated in order to detect change in systolic function. Right heart catheterization was performed on patients suspected of pulmonary hypertension. Descriptive and regression analyses were conducted.
Results
At baseline, LV systolic dysfunction by ejection fraction less than 50%, or a global longitudinal strain greater than −17.0%, was found in 12% and 24%, respectively. RV systolic dysfunction measured by tricuspid annular plane systolic excursion (TAPSE) less than 17 mm was evident in 10%. Follow‐up echocardiography was performed after a median of 3.3 years (interquartile range [IQR] 1.5‐5.6). At follow‐up, LV systolic function remained stable, whereas RV function evaluated by TAPSE deteriorated (mean 23.1 to 21.7 mm, P = 0.005) equaling a 15% prevalence of RV systolic dysfunction. RV systolic function predicted mortality in multivariable models (hazard ratio 0.41, 95% confidence interval [CI] 0.19‐0.90, P value 0.027), whereas LV systolic function lost predictive significance when adjusted for TAPSE.
Conclusion
In this unselected and prospective study, systolic dysfunction of the LV and RV was a frequent complication of SSc. LV systolic function remained stable across the observation period, whereas RV function deteriorated and predicted mortality.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease hallmarked by autoimmunity, vasculopathy, and fibrosis of the skin and internal organs. Patients with SSc present high mortality, with cardiac and pulmonary affection as the most frequent causes of death 1.
Cardiac affection in SSc is thought to originate from disease‐related loss‐of‐function in smaller vessels. Presumably, vasospasms and luminal narrowing lead to hypoxia and reperfusion injury, promoting fibrosis 2. Fibrosis is further suggested to result from subclinical myocarditis 3. Recently, we showed diastolic dysfunction to be a frequent and deleterious complication of SSc 4. The impact of systolic dysfunction in SSc is less clear. Two large, cross‐sectional studies reported that left ventricular (LV) systolic dysfunction, defined by an LV ejection fraction (EF) of less than 45%, less than 50%, or less than 55% was present in not more than 5% of the SSc population 5, 6. However, the majority of previous studies on systolic function were performed on smaller cohorts 7, 8, 9, 10 and few have been prospective, evaluating mortality as an outcome measure 11, 12. Finally, as studies often excluded patients with known or suspected cardiac dysfunction 6, 7, 13, results may have failed to describe the entire SSc disease spectrum.
Strain analysis by speckle tracking is a novel method that measures myocardial deformation to evaluate LV systolic function. Global longitudinal strain (GLS) is a more sensitive measure of LV systolic function compared with EF and has been shown to be superior in predicting prognosis 14. Although considered complementary to EF in evaluation of systolic function, GLS has the advantage of higher reproducibility 15. Previous smaller studies have implied that patients with SSc have lower GLS compared with controls 16, 17 and that continuous deterioration of GLS in patients with normal EF has been reported in a small‐scale study with 2 years of follow‐up 18.
The right ventricle (RV) is vulnerable to increased afterload, a common feature of pulmonary affection in SSc. Reported frequencies of RV systolic dysfunction in SSc ranges from 4%‐38%, depending on the applied method 19, 20 Patients, irrespective of pulmonary pressures, seem to present lower values of RV systolic parameters than controls 19. Tricuspid annular plane systolic excursion (TAPSE) is a leading echocardiographic parameter of RV systolic function, and SSc patients have been shown to present lower values of TAPSE compared with healthy controls 21. RV free wall longitudinal strain (RVFWLS) is a novel method of evaluating RV systolic function by speckle tracking. In a recent study on 138 patients, RVFWLS was reduced among SSc patients as opposed to controls 22.
In this prospective echocardiographic study, we aimed to assess LV and RV systolic function by EF, GLS, TAPSE, and RVFWLS and to evaluate the impact of systolic function on mortality.
Materials and Methods
SSc study cohort
At the Oslo University Hospital (OUH), all SSc patients were enrolled in the prospective Oslo SSc cohort. Patients were followed annually, and data were recorded in the Norwegian Systemic Connective Tissue Disease and Vasculitis Registry (NOSVAR) 23. Echocardiographies are performed annually in order to screen for pulmonary hypertension (PH).
The SSc patients included in the current study cohort had at least one protocol echocardiography examination performed between 2003 and 2016 that was available for evaluation. All the study cohort patients fulfilled the 1980 American College of Rheumatology criteria for SSc 24 and/or the 2013 European League Against Rheumatism/American College of Rheumatology criteria 25. The SSc study cohort included both incident cases (diagnosed 2003‐2016; n = 202) and prevalent cases (diagnosed before 2003; n = 75). This study complied with the Declaration of Helsinki. The research protocol (No. 2017/1815) was approved by The Regional Committee of Health and Medical Research Ethics in southeast Norway. Written informed consent was obtained from included subjects.
Clinical data
Data on demographics, date of diagnosis, clinical presentation (including SSc subtype, modified Rodnan skin score [mRSS], digital ulcers, pulmonary fibrosis, anticentromere and anti‐topoisomerase antibodies), and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) were collected from our SSc database and electronic patient journals. Data on ischemic heart disease, heart failure, and medication were collected from electronic patient journals and registered as ever present during the observation period. Based on extent of skin involvement, patients were classified into limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc) 26. Extent of pulmonary fibrosis was reported as the percentage of total lung tissue affected on high‐resolution computed tomography 27.
Based on clinical presentation, echocardiography, or the DETECT algorithm, patients suspected of PH were evaluated by right heart catheterization 28. PH was defined as a mean pulmonary arterial pressure of 21 mmHg or greater and pulmonary vascular resistance 3.0 Wood units or greater according to recent definitions from the world symposium on PH 29. PH with a pulmonary capillary wedge pressure of 15 mmHg or less was defined as precapillary PH 30. Precapillary PH at baseline was defined as a diagnosis prior to, or at maximum 3 months after, baseline echocardiography.
Disease duration was defined as time from diagnosis to baseline echocardiography, whereas the observation period represented time from baseline echocardiography to date of death or study end (April 2017). Systemic hypertension was defined as the composite of i) presence of an International Classification of Diseases (ICD)‐10 code for hypertension and ii) a systolic blood pressure of 140 mmHg or greater at the time of baseline echocardiography. Vital status was available for all patients at study end.
Control group
We matched 65 healthy controls from the Nord‐Trøndelag Health Study 31, a population‐based cohort study containing echocardiographies from 1266 randomly assigned individuals with a Norwegian background population without recognized systemic hypertension, cardiovascular disease, or diabetes mellitus. Controls for the present study were matched to SSc patients with respect to sex, age, body mass index, and systolic blood pressure at the time of baseline echocardiography. As more than 90% of Norwegians are of Northern European descent, race was not adjusted for.
Echocardiographic analyses
The earliest available echocardiography from each patient was considered the baseline examination, whereas the most recent was considered follow‐up. Patients were examined in the left decubitus position. All echocardiographies were performed at Oslo University Hospital, Rikshospitalet, using GE Vivid 7 or Vivid E9 (GE Vingmed Ultrasound), and software analyses were performed using EchoPAC, v.201. All examinations were reanalyzed for the present study between September 2016 and July 2017 by the same investigator (A.H.T.), who was blinded to patient clinical status. In order to ensure adequate intra‐ and interobserver variability, 19 examinations were reanalyzed and 43 examinations were analyzed by a second observer (J.C.A.) as previously described 4. Inter‐ and intraclass coefficients more than 0.75 were considered to reflect good reliability.
LV systolic function was evaluated by 1) EF using the Simpson biplane method, 2) fractional shortening (FS) of the LV from the parasternal long axis view, and 3) by GLS using automatic algorithms of EchoPAC to average values of 17 segments from apical long axis and four‐ and two‐chamber views 32. Low EF was defined as values less than 50%. To date, there exists no recommendation for GLS cutoff, and earlier studies have often utilized different cutoffs. We conservatively defined low GLS as greater than −17.0% in order to minimize the number of false positives 33, 34.
RV systolic function was assessed by TAPSE, fractional area change (FAC), and RVFWLS. Peak systolic RVFWLS was calculated as an average of three RV free wall segments. RV parameters, including RV dimensions, were evaluated in the RV‐focused four‐chamber view. Tricuspid regurgitant pressure (TrP) is the main determinant of systolic pulmonary arterial pressure and was measured as a parameter of RV hemodynamics. Low TAPSE was defined as values less than 17 mm 32. As both TAPSE and GLS values are affected by the septal interventricular dependence, biventricular dysfunction was defined as simultaneous GLS greater than −17% and RVFWLS greater than −20% 32. Diastolic function was assessed as previously described 4.
Statistical analyses
Statistical analyses were performed using STATA v.15 (StataCorp LLC) and SPSS v.25 (IBM). Paired and independent t tests and χ2 tests were applied as appropriate for comparison of continuous and categorical data, respectively. Pearson and Spearman coefficients were applied for correlation analyses of continuous and ordinal variables, respectively. The associations between systolic dysfunction (GLS greater than −17% or TAPSE less than 17 mm) and clinical parameters were evaluated by logistic regressions with odds ratios (OR) and 95% confidence interval (CI). Univariable significant associations between variables and measures of systolic dysfunction were defined by a P value less than 0.05. Parameters significant at a univariable level were included in multivariable logistic regression models. Multivariable models were evaluated by area under the receiver operating characteristic curve (AUC), with values greater than 0.7 representing acceptable models. Cox regressions with hazard ratios (HR) with 95% CI were calculated for prediction of mortality. Clinical, serological, and baseline echocardiographic parameters of relevance, as evaluated by experts, were included in mortality analyses. Parameters presenting high correlation were not included in common models in order to avoid multicollinearity. Parameters presenting significance levels less than 0.2 at a univariable level, together with parameters of known clinical relevance, were included in multivariable Cox regression. We used manual backward stepwise elimination to identify independent predictors for mortality. Parameters presenting significance levels greater than 0.05 in the multivariable regression models were eliminated. C‐statistics were applied to compare multivariable Cox models, with C‐indexes greater than 0.7 representing acceptable models.
Results
Demographics
In total, 277 patients with SSc and baseline echocardiographies evaluable for systolic function were included in the study. Average age at baseline was 57 ± 13 years with median disease duration of 1.7 years (0.3‐7.6 interquartile [IQR] range). Median time from first non‐Raynaud symptom to echocardiography was 5.7 years (2.3‐10.7 IQR range). The cohort consisted of 227 (82%) women and 194 (70%) lcSSc patients (Table 1). Among included patients, baseline images eligible for evaluation of the LV systolic parameters EF and GLS were available in 258 (93%) and 194 (70%), whereas the RV parameter TAPSE was evaluable in 251 (91%). Inter‐ and intraclass correlation coefficients were 0.88 and 0.90 for GLS, respectively, whereas they were 0.79 and 0.94 for TAPSE, respectively. Two GLS measurements were excluded because of atrial fibrillation. Median observation period was 5.1 years (IQR 2.2‐7.2). Data on clinical parameters, serological parameters, cardiovascular comorbidities and medication, stratified by systolic function, are shown in Table 1.
Table 1.
Clinical and demographic parameters of the entire SSc cohort, and in patients stratified by GLS
| Clinical parameters | Total SSc Cohort, n = 277 | Normal GLS (≥17.0), n = 145 | Low GLS (>−17.0), n = 47 | P Value |
|---|---|---|---|---|
| Age at baseline, years | 57 (13) | 57 (14) | 57 (12) | 0.769 |
| Disease duration at baseline, years | 1.7 (0.3‐7.6) | 2.3 (0.4‐8.6) | 1.0 (0.3‐9.3) | 0.359 |
| Observation period, years | 5.1 (2.2‐7.2) | 4.8 (2.2‐7.1) | 4.9 (1.6‐6.8) | 0.554 |
| Female, n (%) | 227 (82) | 127 (88) | 30 (64) | <0.001 |
| BMI, kg/m2 (n = 275) | 24 (4) | 24 (4) | 24 (4) | 0.859 |
| History of smoking, n (%) (n = 256) | 154 (60) | 72 (53) | 34 (81) | 0.001 |
| Deceased, n (%) | 73 (26) | 29 (20) | 13 (28) | 0.270 |
| SSc parameters | ||||
| lcSSc, n (%) (n = 269) | 194 (70) | 106 (73) | 32 (68) | 0.235 |
| ACA, n (%) | 140 (51) | 75 (52) | 23 (49) | 0.740 |
| ATA, n (%) | 49 (18) | 29 (20) | 9 (19) | 0.899 |
| mRSS (n = 242) | 6 (4‐14) | 5 (3‐12) | 7 (4‐19) | 0.140 |
| Digital ulcers, n (%) (n = 270) | 119 (44) | 67 (47) | 18 (38) | 0.289 |
| Calcinosis, n (%) (n = 221) | 90 (41) | 49 (44) | 13 (34) | 0.302 |
| Pulmonary fibrosis, % (n = 256) | 150 (59) | 73 (54) | 29 (69) | 0.078 |
| Extent of pulmonary fibrosis, % (n = 256) | 7.9 (2.0‐22.8) | 9.6 (2.0‐22.2) | 6.6 (0.8‐34.5) | 0.724 |
| Cardiac parameters | ||||
| Hypertension, n (%) | 36 (13) | 17 (12) | 7 (15) | 0.568 |
| Heart failure, n (%) | 17 (6) | 4 (3) | 3 (6) | 0.365 |
| Ischemic heart disease, n (%) | 46 (17) | 17 (12) | 9 (19) | 0.196 |
| Diastolic dysfunction, n (%) (n = 205) | 32 (16) | 20 (17) | 5 (13) | 0.554 |
| Precapillary PH at baseline, n (%) | 29 (10) | 17 (12) | 5 (11) | 0.818 |
| NT‐proBNP, pmol/l (n = 238) | 15 (8‐47) | 14 (7‐40) | 12 (8‐76) | 0.734 |
| Creatinine, μmol/l (n = 274) | 65 (56‐78) | 67 (59‐78) | 63 (55‐81) | 0.407 |
| Medication | ||||
| Acetylsalicylic acid, n (%) | 103 (37) | 53 (37) | 15 (32) | 0.564 |
| Statins, n (%) | 102 (37) | 54 (37) | 15 (32) | 0.508 |
| Beta blockers, n (%) | 88 (32) | 36 (25) | 19 (40) | 0.040 |
| ACE‐I/ARBs, n (%) | 104 (38) | 49 (34) | 19 (40) | 0.409 |
| Diuretics, n (%) | 108 (39) | 48 (33) | 20 (43) | 0.239 |
| Calcium channel blockers, n (%) | 218 (79) | 118 (81) | 37 (79) | 0.688 |
| Anticoagulants, n (%) | 63 (23) | 25 (17) | 10 (21) | 0.533 |
Abbreviation: ACA, anti‐centromere antibodies; ACE‐I, angiotensin converting enzyme‐inhibitor; ARB, angiotensin receptor blocker; ATA, anti‐topoisomerase antibodies; BMI, body mass index; GLS, global longitudinal strain; lcSSc, limited cutaneous SSc; mRSS, modified Rodnan skin score; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PH, pulmonary hypertension; SSc, systemic sclerosis.
Assessed in all patients except otherwise stated (n =). Data are presented as means (SD), median (interquartile range), or number (percentage).
Reduced LV and RV function at baseline
To measure LV systolic function at baseline, EF, GLS, and FS were evaluated. SSc patients displayed lower EF and GLS at baseline when compared with controls (Table 2). GLS and EF were strongly correlated in SSc patients (r = 0.66, P < 0.001). A reduced EF at baseline was observed in 30 of 258 patients (12%), of whom 21 (8%) displayed a midrange EF between 40%‐49%, and 9 (3%) displayed an EF below 40%. All controls presented EF that was greater than 50% (Figure 1).
Table 2.
Baseline echocardiographic features of SSc patients and healthy controls
| Echocardiographic parameters | SSc Patients | Healthy Controls | P Value |
|---|---|---|---|
| Interventricular septum thickness, mm (n = 254) | 8.6 (1.8) | 7.8 (1.1) | <0.001 |
| Left ventricular parameters | |||
| Ejection fraction, % (n = 258) | 58 (8) | 62 (4) | <0.001 |
| Global longitudinal strain, % (n = 192) | −18.4 (3.0) | −20.3 (2.1) | <0.001 |
| Fractional shortening, % (n = 241) | 37.1 (7.2) | 38.6 (4.5) | 0.112 |
| Right ventricular parameters | |||
| Tricuspid annular plane systolic excursion, mm (n = 251) | 23.1 (5.0) | 25.2 (4.3) | 0.005 |
| Right ventricular free wall longitudinal strain, % (n = 150) | −23.7 (5.4) | … | … |
| Fractional area change of the right ventricle, % (n = 213) | 40 (9) | 41 (5) | 0.287 |
| Right ventricular basal diameter, mm (n = 249) | 3.9 (0.6) | 3.7 (0.5) | 0.012 |
Figure 1.
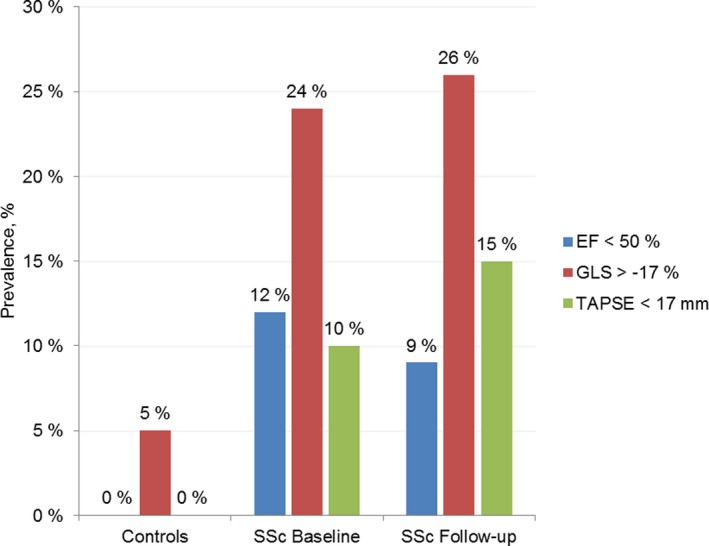
Prevalence of ventricular systolic dysfunction in systemic sclerosis (SSc) patients and healthy controls; evaluated by ejection fraction (EF) in blue, global longitudinal strain (GLS) in red, and tricuspid annular plane systolic excursion (TAPSE) in green, at baseline and follow‐up.
A low GLS (greater than −17.0%) was observed in 47 of 192 patients (24%) and 2 of 38 controls (5%) (Figure 1). Regarding FS, we observed a trend toward lower values in SSc patients (37% vs 39%, P = 0.112). Frequency of LV and RV systolic dysfunction was similar in the incident and prevalent SSc patients (data not shown).
In univariable and multivariable logistic regression, low GLS was associated with male sex, smoking, higher NT‐proBNP, lower values of diffusing capacity for carbon monoxide (DLCO), and forced vital capacity (FVC). In the final multivariable logistic regression model, low GLS was independently associated with sex (OR 4.07, 95% CI 1.65‐10.06, P value 0.002), NT‐proBNP (OR 1.01, 95% CI 1.01‐1.01, P value 0.039), and FVC (OR 0.98, 95% CI 0.96‐0.99, P value 0.022) when adjusted for age (AUC = 0.74).
For evaluation of RV systolic function, TAPSE, FAC, and RVFWLS were measured, whereas RV basal diameter (RVBD) estimated RV dimension. Compared with controls, SSc patients displayed deviant TAPSE and RVBD (Table 2). Lower TAPSE was, as expected, observed in SSc patients with precapillary PH at baseline (n = 28, 20.0 vs 25.2 mm, P < 0.001), but also in patients without PH during the study period (n = 188, 23.6 vs 25.2 mm, P = 0.028. Patients did not differ significantly from controls with respect to FAC. However, because of methodological limitations, FAC was mainly intended for validation of RV function.
At baseline, 24 of 251 (10%) SSc patients had RV dysfunction evaluated by TAPSE that was less than 17 mm. In contrast, all the controls had normal TAPSE (Figure 1). Eleven of the 24 patients with low baseline TAPSE already had established PH (seven precapillary and four postcapillary). In the 13 remaining patients with reduced baseline TAPSE, there was one case of new‐onset PH (postcapillary) during the study observation period.
In univariable logistic regression, low TAPSE was associated with male sex, diastolic dysfunction, GLS, precapillary PH, pulmonary fibrosis, DLCO, and FVC. Adjusting for age and sex, PH, DLCO, FVC, pulmonary fibrosis, and GLS remained associated with low TAPSE. Male sex was associated with low TAPSE when adjusted for age (Table 3). Because of a few events, further multivariable analyses were not performed.
Table 3.
Factors associated with tricuspid annular plane systolic excursion (TAPSE) values less than 17 mm at baseline, evaluated by multivariable logistic regression, adjusted for age and sex
| Factors associated with low TAPSE | OR | 95% CI | P Value |
|---|---|---|---|
| Male sex | 2.69 | 1.06‐6.82 | 0.037 |
| Precapillary PH | 3.54 | 1.19‐10.52 | 0.023 |
| DLCO, % | 0.97 | 0.95‐0.99 | 0.019 |
| FVC, % | 0.97 | 0.95‐0.99 | 0.010 |
| Pulmonary fibrosis, % | 1.03 | 1.01‐1.06 | 0.006 |
| GLS, % | 0.78 | 0.64‐0.94 | 0.011 |
Abbreviation: CI, confidence interval; DLCO, diffusion capacity of the lung for carbon monoxide; FVC, functional vital capacity; GLS, global longitudinal strain; OR, odds ratio; PH, pulmonary hypertension.
Biventricular systolic dysfunction
Patients who presented RV dysfunction defined by reduced TAPSE values less than 17 mm at baseline (n = 24) more often showed LV dysfunction, defined as reduced GLS values greater than −17%, than patients with normal TAPSE (n = 227) (57% vs 22%, P = 0.007). Although TAPSE is a leading marker of RV systolic function, both GLS and TAPSE depend on the longitudinal contraction of the interventricular septum. RVFWLS measures the contraction of the isolated RV free wall and is potentially less affected by septal contraction. RVFWLS was therefore utilized for biventricular evaluation together with GLS. Concurrent evaluation of GLS and RVFWLS at baseline was possible in 126 patients, and 12 (10%) of these met the preliminary definition of biventricular dysfunction (low RVFWLS and low GLS). These 12 patients were more often male, had higher mRSS, and presented higher mortality, but no difference in frequency of PH was observed (Table 4). Because of low numbers, no regression analyses were performed.
Table 4.
Demographic and clinical features of patients stratified by biventricular dysfunction, evaluated by a combined low global longitudinal strain (GLS) and right ventricular free wall longitudinal strain (RVFWLS)
| Clinical parameters | Combined Low GLS and RVFWLS (n = 12) | Normal Function of One or Both Ventricles (n = 114) | P value |
|---|---|---|---|
| Age at echo, years | 60 (53‐67) | 58 (47‐67) | 0.445 |
| Disease duration, years | 1.7 (0.6‐9.6) | 2.0 (0.4‐9.0) | 0.997 |
| Observation period, years | 1.5 (0.2‐3.5) | 4.3 (1.8‐7.1) | 0.006 |
| Male sex, n (%) | 7 (58) | 15 (13) | 0.001 |
| Mortality, n (%) | 7 (58) | 22 (19) | 0.006 |
| lcSSc, n (%) | 7 (58) | 87 (78) | 0.152 |
| ACA, n (%) | 5 (42) | 60 (53) | 0.470 |
| ATA, n (%) | 4 (33) | 16 (14) | 0.098 |
| mRSS | 12 (6‐29) | 5 (2‐11) | 0.018 |
| Pulmonary fibrosis, n (%) | 9 (82) | 59 (56) | 0.117 |
| Extent of pulmonary fibrosis, n (%) | 6.6 (1.3‐25.5) | 7.2 (2.0‐17.0) | 0.986 |
| Precapillary PH at baseline, n (%) | 2 (17) | 14 (12) | 0.608 |
| Ischemic heart disease, n (%) | 1 (8) | 16 (14) | 1.000 |
| Hypertension, n (%) | 1 (8) | 18 (16) | 0.691 |
Abbreviation: ACA, anti‐centromere antibodies; ATA, anti‐topoisomerase I antibodies; lcSSc, limited cutaneous systemic sclerosis; GLS, global longitudinal strain; mRSS, modified Rodnan skin score; PH, pulmonary hypertension; RVFWLS, right ventricular free wall longitudinal strain.
Data are number (%) or median (interquartile range).
Prospective LV and RV systolic function
A follow‐up echo was available in 173 of the SSc study cohort patients (62%), 3.3 years (IQR 1.5‐5.6) after baseline echocardiography. LV systolic parameters EF and GLS remained stable in patients with two consecutive measurements, whereas TAPSE deteriorated from 23.1 to 21.7 mm (Table 5 and Figure 1). Patients with two consecutive GLS measurements had a longer observation period (5.8 vs 3.4 years, P < 0.001) and more frequent precapillary PH at baseline (20% vs 9%, P = 0.044), compared with patients with only baseline GLS measurement. There were no differences with respect to age, gender, body mass index, disease duration at baseline echocardiography, SSc subtype, pulmonary fibrosis, ischemic heart disease, diastolic dysfunction, or mortality.
Table 5.
Systolic parameters in patients with available measurements at both baseline and follow‐up echocardiography
| Echocardiographic parameters | Baseline Echo | Follow‐up Echo | P Value |
|---|---|---|---|
| Left ventricular parameters | |||
| EF, % (n = 128) | 58 (8) | 59 (8) | 0.447 |
| GLS, % (n = 76) | 18.7 (2.3) | 18.7 (2.1) | 0.923 |
| FS, % (n = 131) | 37.4 (7) | 37.5 (8) | 0.840 |
| Right ventricular parameters | |||
| TAPSE, mm (n = 143) | 23.1 (0.5) | 21.7 (0.6) | 0.005 |
| RVFWLS, % (n = 42) | 24.5 (4.5) | 23.9 (5.9) | 0.447 |
| FAC, % (n = 56) | 40 (10) | 39 (10) | 0.498 |
| RVBD, cm (n = 135) | 4.0 (0.6) | 4.1 (0.8) | 0.014 |
| TrP, mmHg (n = 103) | 33.0 (17.6) | 38.6 (21.0) | 0.001 |
Abbreviation: EF, ejection fraction; FAC, fractional area change of the right ventricle; FS, fractional shortening of the left ventricle; GLS, global longitudinal strain; RVBD, basal diameter of the right ventricle; RVFWLS, right ventricular free wall strain; TAPSE, tricuspid annular plane systolic excursion; TrP, tricuspid regurgitant pressure.
Values are mean (SD).
Further analyses of the 143 patients with baseline and follow‐up TAPSE measurements showed that patients with PH presented a significant decline of TAPSE (21.6‐18.8 mm, P = 0.007, n = 36), whereas patients without PH presented only a trend of declining TAPSE (23.8‐22.9 mm, P = 0.135, n = 100). We also found reduction of TAPSE in patients with dcSSc (n = 37; mean difference −3.0 mm, P = 0.005) and anti‐topoisomerase I antibodies (n = 24; mean difference −3.9 mm, P = 0.005). Among the 18 patients who developed low TAPSE between baseline and follow‐up, three had established precapillary PH at baseline and a further five had developed precapillary PH by the time of follow‐up echocardiography. We did not observe any change in RVFWLS or FAC over time; however, few patients had consecutive measurements of RVFWLS and FAC (n = 42 and n = 56, respectively).
During the observation period, 73 patients (26%) died. In multivariable Cox regression analyses, baseline LV dysfunction measured by GLS predicted mortality independently when adjusted for age and sex (HR 0.89, 95% CI 0.80‐0‐99, P = 0.027, C‐index: 0.76), whereas baseline EF was not predictive (HR 0.98, 95% CI 0.95‐1.00, P = 0.106). However, GLS lost its predictive ability when adjusted for RV systolic function evaluated by TAPSE (HR 0.96, 95% CI 0.85‐1.08, P = 0.499). RV systolic dysfunction evaluated by TAPSE predicted mortality when adjusted for sex and age (HR 0.38, 95% CI 0.22‐0‐64, P < 0.001, C‐index: 0.75) and was also included in the final multivariable Cox regression model adjusted for age, sex, diastolic dysfunction, NT‐proBNP, mRSS, and DLCO, as shown previously in our study on diastolic dysfunction 4. One millimeter increase of TAPSE equaled a mortality risk reduction of 9%.
Discussion
In this study we investigated multiple LV and RV systolic function parameters in a prospective unselected cohort of SSc patients and in healthy controls. Major findings were i) a higher than expected prevalence of LV, RV, and biventricular systolic dysfunction, ii) stable LV systolic function across the study observation, and iii) deterioration of RV systolic function during the disease course, with impact on mortality. To our knowledge, this is the largest echocardiography study evaluating prospective systolic function of both LV and RV in SSc.
Previous studies on cardiac SSc were often performed on small cohorts 7, 9, 10 and excluded patients with potential cardiac disease 6, 7, 13. Such cohorts may underestimate the true burden of systolic dysfunction in SSc. Echocardiography studies on larger SSc cohorts have reported LV systolic dysfunction evaluated by EF to be uncommon (1%‐5%) 5, 6, although they lack GLS data. In our study, 12% had reduced systolic function measured by EF and 24% when measured by GLS. Many studies report comparable EF between SSc patients and healthy controls 7, 13, 16, but in a recent report, EF was lower among SSc patients compared with controls (58.5 vs 62.9, P < 0.001) 22. Furthermore, in an unselected SSc cohort, EF less than 45% was observed in 12 of 52 patients (23%) by magnetic resonance imaging (MRI). However, echocardiography did not reveal the same frequency of cardiac pathology as MRI 35. Our study supports the notion that LV systolic function by EF is more deteriorated in SSc than earlier presumed.
The relatively few studies on GLS in SSc indicate reduced values of this LV strain parameter in patients with SSc 12, 16. Our patients presented a mean GLS of −18.4%, compared with −20.3% among controls; additionally, 24% of patients had a GLS greater than −17.0% at baseline compared with 5% of the matched control population.
GLS has been reported to be an independent predictor of prognosis in several cardiovascular diseases 36, 37, but reports from SSc cohorts are conflicting 7, 12. In a large Danish cohort of heart failure patients, GLS was considered the superior echocardiographic predictor of mortality, even though it lost its significance in female patients 38. In our study, LV systolic function measured by GLS predicted mortality at a univariable level, but lost significance when adjusted for RV systolic function. Previously, we have described LV diastolic dysfunction to be the most significant predictor of mortality. LV systolic parameters were not thematized because of a lack of mortality predictive abilities. In this article, we embellish these results, which are further supported by stable LV systolic function at follow‐up echocardiography. This is in concordance with the work of Saito et al 12 where longitudinal strain failed to predict adverse outcome in SSc.
Little is known about LV systolic function during the course of SSc. GLS was reported to deteriorate in a small‐scale prospective study on 22 patients with SSc 18. Over a 3.3‐year observation period, we did not find any deterioration of LV function. This was surprising, yet results were consistent for EF, GLS, and FS, which may support the notion that most organ manifestations develop early in the course of SSc 39. One may further speculate whether this stabilization is due to the frequent administration of calcium channel blockers in SSc, which are shown to increase cardiovascular perfusion and function 40.
Our RV systolic function data are in line with smaller previously conducted studies that show lower baseline values of TAPSE in SSc, irrespective of pulmonary vascular disease and TAPSE as an unfavorable prognostic factor 21, 41, 42. TAPSE was elected the main parameter of RV systolic function as it is easily evaluable and presents high reproducibility. Although patients differ significantly from controls, mean TAPSE is still within normal values. This is not surprising as SSc‐driven cardiac affliction is often subclinical 43. As TAPSE further deteriorates along the disease course, one may speculate whether the RV is more susceptible to SSc‐specific disease processes than the LV. However, in patients without PH, we could only show a trend of TAPSE deterioration (23.8‐22.9 mm, P = 0.135). It was evident that TrP, reflecting pulmonary arterial pressure, increased at follow‐up (Table 5). However, this increase was not significant in patients without PH. Our data suggest RV systolic function to be a valuable predictor of mortality; however, to what degree this is due to the contribution from PH is still unclear. We have previously shown TAPSE to independently predict mortality in a model including age, sex, LV diastolic dysfunction, mRSS, DLCO, and NT‐proBNP 4.
Because the heart acts as a functional unit, contraction of the ventricular septum will always contribute to LV and RV contraction. This makes it challenging to isolate LV function from RV function by echocardiography. Furthermore, as a failing LV leads to congestion of pulmonary vasculature, RV workload increases. Given these ventricular interdependences, it appears highly challenging to identify echocardiographic parameters suitable for evaluation of isolated RV systolic function. In this study, we applied RVFWLS as a proxy for RV systolic function. This parameter assesses deformation of the right ventricular free wall, and may therefore be more closely related to the RV contraction.
Our study has some limitations. First, this study is based on echocardiographic recordings from 2003 to 2016. As speckle tracking is a novel method, not all examinations had evaluable GLS, and only a subset of patients had two consecutive GLS examinations, slightly limiting the ability to describe how GLS changes over time.
Naturally, only patients with 2 or more echocardiographies were included in prospective analyses. Excluding patients with only one echocardiography may have introduced survival bias, reducing the generalizability of our findings on stable LV systolic function. Survival bias should most likely not affect our mortality prediction analyses, as these are based on baseline parameters.
Unfortunately, as too few controls presented adequate images for analyzation of TrP and RVFWLS, comparisons between patients and controls for these parameters were not performed.
The study possesses several significant strengths compared with earlier studies. This is, to our knowledge, the largest follow‐up study on systolic function in SSc. All patient and control echocardiographies were evaluated by the same investigator, reducing bias from interobserver variability. Furthermore, as vital status was available for all patients at study end, conditions were ideal for analyzing predictor variables for mortality.
In conclusion, in our large and unselected SSc cohort, LV and RV systolic dysfunction presented frequently. Although RV systolic function deteriorated at follow‐up, no alteration could be shown for the LV. Opposed to LV systolic function, RV systolic function was an independent predictor of mortality. However, it remains uncertain to what degree RV systolic function is independent from PH.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Tennøe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Tennøe, Murbræch, Aakhus, Molberg, Hoffmann‐Vold.
Acquisition of data. Tennøe, JC Andreassen, Fretheim, Midtvedt, Garen, Dalen, Gude, A Andreassen, Aakhus, Molberg, Hoffmann‐Vold.
Analysis and interpretation of data. Tennøe, Hoffmann‐Vold.
Acknowledgments
All authors thank The Nord‐Trøndelag Health study (The HUNT Study), which is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU)), Nord‐Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
This project was made possible by the Norwegian Extra Foundation for Health and Rehabilitation, Oslo, Norway, and the Norwegian Women's Public Health Association, Oslo, Norway.
Dr. Tennøe has received consulting fees or other remuneration from GlaxoSmithKline (GSK) and Actelion. Dr. Fretheim has received consulting fees or other remuneration from GSK and Actelion. Dr. Dalen held position in Medical Imaging Laboratory, an institutional collaboration between St Olav's University Hospital, Norwegian University of Science and Technology, and industrial partners. GE Ultrasound was one of the industrial partners, contributing to 6% of the total budget. Dr. Hoffmann‐Vold has received research funding and/or consulting fees or other remuneration from Boehringer Ingelheim, GSK, and Actelion. No other disclosures relevant to this article were reported.
References
- 1. Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir‐Gurman A, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. [DOI] [PubMed] [Google Scholar]
- 2. Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010;28 Suppl 62:S48–53. [PubMed] [Google Scholar]
- 3. Pieroni M, De Santis M, Zizzo G, Bosello S, Smaldone C, Campioni M, et al. Recognizing and treating myocarditis in recent‐onset systemic sclerosis heart disease: potential utility of immunosuppressive therapy in cardiac damage progression. Semin Arthritis Rheum 2014;43:526–35. [DOI] [PubMed] [Google Scholar]
- 4. Tennøe AH, Murbræch K, Andreassen JC, Fretheim H, Garen T, Gude E, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018;72:1804–13. [DOI] [PubMed] [Google Scholar]
- 5. Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010;69:218–21. [DOI] [PubMed] [Google Scholar]
- 6. De Groote P, Gressin V, Hachulla E, Carpentier P, Guillevin L, Kahan A, et al. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008;67:31–6. [DOI] [PubMed] [Google Scholar]
- 7. Piccione MC, Zito C, Bagnato G, Oreto G, Di Bella G, Bagnato G, et al. Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovasc Ultrasound 2013;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Luca G, Bosello SL, Gabrielli FA, Berardi G, Parisi F, Rucco M, et al. Prognostic role of ventricular ectopic beats in systemic sclerosis: a prospective cohort study shows ECG indexes predicting the worse outcome. PLoS One 2016;11:e0153012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hekimsoy V, Kaya EB, Akdogan A, Sahiner L, Evranos B, Canpolat U, et al. Echocardiographic assessment of regional right ventricular systolic function using two‐dimensional strain echocardiography and evaluation of the predictive ability of longitudinal 2D‐strain imaging for pulmonary arterial hypertension in systemic sclerosis patients. Int J Cardiovasc Imaging 2018;34:883–92. [DOI] [PubMed] [Google Scholar]
- 10. Karna SK, Rohit MK, Wanchu A. Right ventricular thickness as predictor of global myocardial performance in systemic sclerosis: a Doppler tissue imaging study. Indian Heart J 2015;67:521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faludi R, Költő G, Bartos B, Csima G, Czirják L, Komócsi A. Five‐year follow‐up of left ventricular diastolic function in systemic sclerosis patients: determinants of mortality and disease progression. Semin Arthritis Rheum 2014;44:220–7. [DOI] [PubMed] [Google Scholar]
- 12. Saito M, Wright L, Negishi K, Dwyer N, Marwick TH. Mechanics and prognostic value of left and right ventricular dysfunction in patients with systemic sclerosis. Eur Heart J Cardiovasc Imaging. 2017. [DOI] [PubMed] [Google Scholar]
- 13. D'Andrea A, D'Alto M, Di Maio M, Vettori S, Benjamin N, Cocchia R, et al. Right atrial morphology and function in patients with systemic sclerosis compared to healthy controls: a two‐dimensional strain study. Clin Rheumatol 2016;35:1733–42. [DOI] [PubMed] [Google Scholar]
- 14. Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non‐ischaemic stage B heart failure in the community. Eur J Heart Fail 2016;18:1331–9. [DOI] [PubMed] [Google Scholar]
- 15. Medvedofsky D, Kebed K, Laffin L, Stone J, Addetia K, Lang RM, et al. Reproducibility and experience dependence of echocardiographic indices of left ventricular function: side‐by‐side comparison of global longitudinal strain and ejection fraction. Echocardiography 2017;34:365–70. [DOI] [PubMed] [Google Scholar]
- 16. Yiu KH, Schouffoer AA, Marsan NA, Ninaber MK, Stolk J, Vlieland TV, et al. Left ventricular dysfunction assessed by speckle‐tracking strain analysis in patients with systemic sclerosis: relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum 2011;63:3969–78. [DOI] [PubMed] [Google Scholar]
- 17. Spethmann S, Dreger H, Schattke S, Riemekasten G, Borges AC, Baumann G, et al. Two‐dimensional speckle tracking of the left ventricle in patients with systemic sclerosis for an early detection of myocardial involvement. Eur Heart J Cardiovasc Imaging 2012;13:863–70. [DOI] [PubMed] [Google Scholar]
- 18. Spethmann S, Rieper K, Riemekasten G, Borges AC, Schattke S, Burmester GR, et al. Echocardiographic follow‐up of patients with systemic sclerosis by 2D speckle tracking echocardiography of the left ventricle. Cardiovasc Ultrasound 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meune C, Allanore Y, Devaux JY, Dessault O, Duboc D, Weber S, et al. High prevalence of right ventricular systolic dysfunction in early systemic sclerosis. J Rheumatol 2004;31:1941–5. [PubMed] [Google Scholar]
- 20. Meune C, Khanna D, Aboulhosn J, Avouac J, Kahan A, Furst DE, et al. A right ventricular diastolic impairment is common in systemic sclerosis and is associated with other target‐organ damage. Semin Arthritis Rheum 2016;45:439–45. [DOI] [PubMed] [Google Scholar]
- 21. Schattke S, Knebel F, Grohmann A, Dreger H, Kmezik F, Riemekasten G, et al. Early right ventricular systolic dysfunction in patients with systemic sclerosis without pulmonary hypertension: a Doppler Tissue and Speckle Tracking echocardiography study. Cardiovasc Ultrasound 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukherjee M, Chung SE, Ton VK, Tedford RJ, Hummers LK, Wigley FM, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging 2016;9:e003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann‐Vold AM, Hesselstrand R, Fretheim H, Ueland T, Andreassen AK, Brunborg C, et al. CCL21 as a potential serum biomarker for pulmonary arterial hypertension in systemic sclerosis. Arthritis Rheumatol 2018;70:1644–53. [DOI] [PubMed] [Google Scholar]
- 24. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee . Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 25. Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 27. Hoffmann‐Vold AM, Aaløkken TM, Lund MB, Garen T, Midtvedt Ø, Brunborg C, et al. Predictive value of serial high‐resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015;67:2205–12. [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann‐Vold AM, Fretheim H, Midtvedt Ø, Kilian K, Angelshaug M, Chaudhary A, et al. Frequencies of borderline pulmonary hypertension before and after the DETECT algorithm: results from a prospective systemic sclerosis cohort. Rheumatology (Oxford) 2018;57:480–7. [DOI] [PubMed] [Google Scholar]
- 29. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 31. Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr 2010;11:176–83. [DOI] [PubMed] [Google Scholar]
- 32. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 33. Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, et al. Normal range of left ventricular 2‐dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J 2012;76:2623–32. [DOI] [PubMed] [Google Scholar]
- 34. Pun SC, Landau HJ, Riedel ER, Jordan J, Yu AF, Hassoun H, et al. Prognostic and added value of two‐dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J Am Soc Echocardiogr 2018;31:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hachulla AL, Launay D, Gaxotte V, de Groote P, Lamblin N, Devos P, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross‐sectional observational study of 52 patients. Ann Rheum Dis 2009;68:1878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biering‐Sørensen T, Biering‐Sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, et al. Global longitudinal strain by echocardiography predicts long‐term risk of cardiovascular morbidity and mortality in a low‐risk general population: the Copenhagen City Heart study. Circ Cardiovasc Imaging 2017;10:e005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- 38. Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz‐Hansen T, et al. Global longitudinal strain is a superior predictor of all‐cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015;8:1351–9. [DOI] [PubMed] [Google Scholar]
- 39. Jaeger VK, Wirz EG, Allanore Y, Rossbach P, Riemekasten G, Hachulla E, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PLoS One 2016;11:e0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vignaux O, Allanore Y, Meune C, Pascal O, Duboc D, Weber S, et al. Evaluation of the effect of nifedipine upon myocardial perfusion and contractility using cardiac magnetic resonance imaging and tissue Doppler echocardiography in systemic sclerosis. Ann Rheum Dis 2005;64:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Durmus E, Sunbul M, Tigen K, Kivrak T, Ozen G, Sari I, et al. Right ventricular and atrial functions in systemic sclerosis patients without pulmonary hypertension. Speckle‐tracking echocardiographic study. Herz 2015;40:709–15. [DOI] [PubMed] [Google Scholar]
- 42. Mathai SC, Sibley CT, Forfia PR, Mudd JO, Fisher MR, Tedford RJ, et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis‐associated pulmonary arterial hypertension. J Rheumatol 2011;38:2410–8. [DOI] [PubMed] [Google Scholar]
- 43. Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis–a clinical study using myocardial T1‐mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]


