ABSTRACT
Objective
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). This post hoc analysis evaluated tofacitinib persistence in patients with RA in long‐term extension (LTE) studies up to 9.5 years.
Methods
Data were pooled from two LTE studies: ORAL Sequel (NCT00413699) and Study A3921041 (NCT00661661). Patients received tofacitinib 5 or 10 mg twice daily (BID), as monotherapy or with background conventional synthetic disease‐modifying antirheumatic drugs. Kaplan‐Meier estimates for tofacitinib drug survival and reasons for discontinuation were evaluated. Baseline factors were analyzed as predictors of persistence.
Results
In 4967 tofacitinib‐treated patients entering LTE studies, mean (maximum) treatment duration was 3.5 (9.4) years. Median drug survival (95% confidence interval) was 4.9 (4.7, 5.1) years. Estimated 2‐ and 5‐year drug survival rates were 75.5% and 49.4%, respectively. Median drug survival was similar between the tofacitinib 5 and 10 mg BID groups, and slightly higher for patients receiving tofacitinib monotherapy versus combination therapy. Overall, 50.7% of patients discontinued tofacitinib; of these, 47.2% were due to adverse events and 7.1% for lack/loss of efficacy. An increased risk of discontinuation was associated with baseline diabetes, hypertension, negative anticyclic citrullinated peptide (anti‐CCP), negative rheumatoid factor (RF), and inadequate response to tumor necrosis factor inhibitors (TNFi‐IR).
Conclusion
Median drug survival of tofacitinib‐treated patients participating in LTE studies was approximately 5 years and was similar for tofacitinib dosed at 5 and 10 mg BID. Reduced drug survival was associated with negative anti‐CCP/RF status, TNFi‐IR, and certain comorbidities. These data support tofacitinib use for long‐term management of RA.
Keywords: clinical trial, DMARDs, rheumatoid arthritis, risk factors
Introduction
Time on treatment (also referred to as survival time, time to discontinuation, persistence, or durability of treatment) is a surrogate measure of the success of a treatment (effectiveness, tolerability, and safety) in the long‐term management of disease. Drug survival reflects the clinical effectiveness of treatment and is related to factors such as adverse events (AEs) and lack or loss of efficacy 1, 2. In long‐term extension (LTE) studies of biologic disease‐modifying antirheumatic drugs (bDMARDs) in patients with rheumatoid arthritis (RA), the proportion of patients remaining on treatment after 5 years ranges from 40% to 66% 3, 4, 5, 6. Survival of bDMARDs in RA has also been examined in observational studies and registries to determine how practical these treatments are for the long‐term management of RA. In a retrospective study, persistence of RA therapy (2‐year drug survival) was higher for tumor necrosis factor inhibitors (TNFis) than conventional synthetic DMARDs (csDMARDs; 38.7% and 29.5%, respectively) 7. A systematic review of studies from clinical practice indicated that the persistence of golimumab therapy (2‐year drug survival rates ranging from 40% to 77%) may be higher than for other TNFis 8. In a longitudinal observational study of patients with RA receiving bDMARDs between 1999 and 2013, discontinuations were mainly due to AEs (45.8%) and lack of efficacy (40.8%) 9. Similarly, an analysis of registry data for patients with RA treated with rituximab, infliximab, or etanercept also identified inadequate response (IR) to treatment (41.4%) and occurrence of serious AEs (22.2%) as the main reasons for discontinuation of these treatments 10.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib doses of 5 and 10 mg twice daily (BID) administered as monotherapy or in combination with csDMARDs, mainly methotrexate (MTX), in patients with moderately to severely active RA have been demonstrated in Phase 2 11, 12, 13, 14, 15 and Phase 3 16, 17, 18, 19, 20, 21 randomized controlled trials of up to 24 months' duration and in LTE studies with up to 114 months of observation 22, 23, 24. The objective of this post hoc analysis was to estimate tofacitinib drug survival based on pooled data from two LTE studies of up to 114 months (9.5 years) to determine the reasons for treatment discontinuation in these studies and to evaluate baseline clinical and demographic factors that may influence drug survival time.
Methods
Patients
Eligible patients were aged 18 years or older (ORAL Sequel; A3921024; NCT00413699) or 20 years or older (Study A3921041; NCT00661661) with a diagnosis of active moderate to severe RA, according to American College of Rheumatology 1987 revised criteria 25. Patients previously participated in a qualifying index study of tofacitinib comprising two Phase 1 studies (NCT01262118 26 and NCT01484561 27); nine Phase 2 studies (NCT00147498 12, NCT00413660 13, NCT00550446 11, NCT00603512 14, NCT00687193 15, NCT01164579 28, NCT00976599 29, NCT01059864 30, NCT01359150 31); and six Phase 3 studies (NCT00960440 16, NCT00847613 20, NCT00814307 17, NCT00856544 18, NCT00853385 21, NCT01039688 19). Patients who completed the qualifying studies or discontinued treatment in the qualifying studies for reasons other than treatment‐related AEs (Study A3921041) or treatment‐related serious AEs (ORAL Sequel) were eligible for LTE enrollment as long as they met the protocol eligibility criteria.
Study design
Data were pooled from two multicenter, open‐label LTE studies: ORAL Sequel, a global study (database not locked as of March 2017 data cutoff) and Study A3921041, which was conducted in Japan only (study completed April 2014). Patients received tofacitinib 5 or 10 mg BID, as monotherapy or with background csDMARDs, predominantly MTX. Most of the enrolled patients from Phase 2 index studies initiated open‐label treatment with tofacitinib 5 mg BID, and most of the patients from Phase 3 index studies initiated open‐label treatment with tofacitinib 10 mg BID, except for patients from China and patients from Korea who were newly enrolled after August 14 2014 and had initiated treatment with tofacitinib 5 mg BID as stipulated in the protocol. During the LTE, tofacitinib dose could be increased or decreased at the investigator's discretion (eg, increased in the case of inadequate control of RA symptoms [5 mg to 10 mg BID] or decreased in response to AEs or laboratory abnormalities [10 mg to 5 mg BID]).
Patients were allowed to maintain, discontinue, or add background arthritis therapy (including nonsteroidal anti‐inflammatory drugs, such as cyclooxygenase‐2 inhibitors, and opioids at 30 mg or less oral morphine/day potency), certain csDMARDs (MTX, leflunomide, sulfasalazine, anti‐malarials, auranofin, and injectable gold preparations at approved doses), and corticosteroids (10 mg or less prednisone or equivalent/day), with adjustments allowed at the investigator's discretion for reasons of inadequate efficacy, tapering/discontinuation with disease improvement, or toxicity. Intra‐articular injections were avoided during the 6 weeks prior to a study visit, after which they could be administered every 6 months.
The studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines, along with applicable local regulatory requirements and laws. Study protocols were approved by the Institutional Review Board and/or Independent Ethics Committee at each study center. All patients provided written, informed consent.
Outcomes and statistical analysis
Data were pooled for all patients in the LTE studies. Baseline values were obtained from the qualifying index studies. Analyses were conducted for all tofacitinib‐treated patients by tofacitinib treatment groups (5 versus 10 mg BID). Patients were assigned to treatment groups based on calculation of the average total daily dose (TDD) for each patient (ie, the sum of all doses received divided by the number of days treated): patients with TDD less than 15 mg were assigned to the average tofacitinib 5 mg BID group; patients with TDD 15 mg or greater were assigned to the average tofacitinib 10 mg BID group 32.
Patients were further classified into subgroups of patients who stayed on tofacitinib monotherapy or stayed on tofacitinib combination therapy with background csDMARDs (ie, patients remained on their study‐start therapy for the entire study duration, with the exception of a 28‐day or less break in csDMARD use allowed for stay‐on‐csDMARD patients) 33. Patients who changed their initial background csDMARD therapy were not included in these analyses; however, their data are included in the “All tofacitinib” cohort.
Kaplan‐Meier estimates of persistence (difference between the end‐of‐study date and first tofacitinib dose date + 1 day) were used to estimate the overall median drug survival (years) with 95% confidence intervals (CI). For all Kaplan‐Meier analyses, ongoing patients were censored at the LTE study cut‐off dates (March 2 2017, ORAL Sequel; April 24 2014, Study A3921041) and completers were censored at the end‐of‐study date. The percentages of patients achieving 2‐ and 5‐year survival were also calculated, with 100% of the study population considered as all patients who entered the LTE studies. Kaplan‐Meier methodology was used to estimate drug survival among tofacitinib‐treated patients who withdrew from the LTE studies for any reason, including lack/loss of efficacy or because of AEs. Categorical data (eg, reasons for treatment discontinuation) are presented descriptively (number, %).
Cox proportional hazards models were used to explore the potential effects of index baseline variables on risk of discontinuation. The models evaluated the following covariates of interest using backward selection: anticyclic citrullinated peptide (anti‐CCP) status (anti‐CCP+ versus anti‐CCP−), body mass index (less than 25 versus 25–30 and more than 30 kg/m2), cardiovascular disease (presence versus absence), csDMARD use (MTX ± other csDMARDs versus other csDMARDs alone), Disease Activity Score in 28 joints, erythrocyte sedimentation rate quartiles (Q2, Q3, Q4 versus Q1), diabetes (presence versus absence), disease duration (less than 1 year versus 1 year or more), glucocorticoid use (yes versus no), hypertension (presence versus absence), MTX dose (15 mg or less per week versus more than 15 mg/week), rheumatoid factor (RF) status (RF+ versus RF−), RF/anti‐CCP status (RF+/CCP+ versus RF−/CCP−), prior treatment (MTX‐IR versus TNFi‐IR), and smoking status (never smoked versus ever smoked). Hazard ratios, 95% CI, and P values were calculated.
Results
Patients
In the index studies 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 26, 27, 28, 29, 30, 31, 6427 patients received tofacitinib; of these, 3800 patients were from Phase 3 studies. In the LTE studies, 4967 patients received tofacitinib treatment. Although treatment group assignment was determined by average TDD, the majority of patients (76.4%) continued receiving their initial 5 or 10 mg BID dose throughout the LTE studies (by dose: 70.2% and 79.6%, respectively). The mean (median; maximum) treatment duration in the LTE studies was 3.5 (3.5; 9.4) years. Total LTE tofacitinib exposure was 17 738 patient‐years. Baseline patient demographics and characteristics were generally similar across treatment groups (Table 1). For all tofacitinib‐treated patients, the mean age (range) was 53.2 (18–86) years, 82.0% of patients were female, and the mean duration (range) of RA was 7.9 (0–55) years. Of the 4967 patients who were treated with tofacitinib in the LTE studies, 2604 (52.4%) stayed on combination therapy, 1525 (30.7%) stayed on monotherapy, and 838 (16.9%) changed background therapy. Baseline characteristics were also similar between patients who stayed on monotherapy compared with patients who stayed on combination therapy, with the exception of mean/median disease duration, prior nonbiologic DMARDs, including MTX, and prior TNFi, which were lower in the stay‐on‐monotherapy group.
Table 1.
Patient demographics and baseline characteristicsa in LTE studies
| All Tofacitinibb (N = 4966) | Tofacitinib 5 mg BID (N = 1535) | Tofacitinib 10 mg BID (N = 3431) | Stay on Tofacitinib Combination Therapyc (N = 2604) | Stay on Tofacitinib Monotherapyc (N = 1525) | |
|---|---|---|---|---|---|
| Female, n (%) | 4074 (82.0) | 1270 (82.7) | 2804 (81.7) | 2126 (81.6) | 1251 (82.0) |
| Age in years, mean (range) | 53.2 (18–86) | 53.7 (18–82) | 53.0 (18–86) | 53.3 (18–86) | 53.1 (19–85) |
|
Disease duration in years,d mean [median] (range) |
7.9 [5.3] (0.0–55.0) |
8.4 [6.0] (0.0–50.1) |
7.7 [5.0] (0.0–55.0) |
8.5 [6.0] (0.1–50.1) |
6.8 [4.2] (0.0–55.0) |
|
BMI in kg/m2, mean (range) [N1] |
26.9 (12.1–70.8) [4963] |
25.6 (15.5–50.7) [1532] |
27.5 (12.1–70.8) [3431] |
27.0 (13.9–58.1) [2601] |
26.6 (12.1–55.6) [1525] |
| Smoking status, n (%) | |||||
| Never smoked | 3145 (63.3) | 964 (62.8) | 2181 (63.6) | 1693 (65.0) | 977 (64.1) |
| Smoker | 875 (17.6) | 261 (17.0) | 614 (17.9) | 431 (16.6) | 286 (18.8) |
| Ex‐smoker | 871 (17.5) | 239 (15.6) | 632 (18.4) | 437 (16.8) | 254 (16.7) |
| Unknown | 75 (1.5) | 71 (4.6) | 4 (0.1) | 43 (1.7) | 8 (0.5) |
| RF+, n/N1 (%) | 3437/4644 (74.0) | 1131/1462 (77.4) |
2306/3182 (72.5) |
1784/2444 (73.0) |
1069/1426 (75.0) |
| Anti‐CCP+, n/N1 (%) | 2708/3537 (76.6) | 396/497 (79.7) |
2312/3040 (76.1) |
1551/2034 (76.3) |
734/951 (77.2) |
|
DAS28‐4(ESR), mean (SD) [N1] |
6.3 (1.0) [4421] |
6.2 (1.0) [1275] |
6.4 (1.1) [3146] |
6.3 (1.0) [2247] |
6.4 (1.1) [1444] |
| Prior MTX, n (%) | 3963 (79.8) | 1357 (88.4) | 2606 (76.0) | 2358 (90.6) | 887 (58.2) |
| Prior nonbiologic DMARD, n (%) | 4439 (89.4) | 1482 (96.5) | 2957 (86.2) | 2506 (96.2) | 1161 (76.1) |
| Number of prior nonbiologic DMARDs, mean (SD) [N1] |
1.8 (1.0) [4439] |
1.8 (1.0) [1482] |
1.8 (1.1) [2957] |
1.9 (1.1) [2506] |
1.6 (0.9) [1161] |
| Prior TNFi, n (%) | 877 (17.7) | 171 (11.1) | 706 (20.6) | 529 (20.3) | 158 (10.4) |
| Prior non‐TNFi DMARD, n (%) | 271 (5.5) | 49 (3.2) | 222 (6.5) | 140 (5.4) | 61 (4.0) |
| Number of prior biologic DMARDs, mean (SD) [N1] |
1.6 (0.9) [1003] |
1.4 (0.8) [201] |
1.6 (0.9) [802] |
1.6 (0.9) [590] |
1.5 (0.9) [193] |
| Baseline diabetes, n (%) | 367 (7.4) | 122 (7.9) | 245 (7.1) | 182 (7.0) | 106 (7.0) |
| Baseline hypertension, n (%) | 1706 (34.4) | 511 (33.3) | 1195 (34.8) | 899 (34.5) | 515 (33.8) |
| Baseline CV disease, n (%) | 2054 (41.4) | 600 (39.1) | 1454 (42.4) | 1058 (40.6) | 621 (40.7) |
Abbreviation: BID = twice daily; BMI = body mass index; CCP = cyclic citrullinated peptide; csDMARD = conventional synthetic DMARD; CV = cardiovascular; DAS28‐4(ESR) = Disease Activity Score in 28 joints, erythrocyte sedimentation rate; DMARD = disease‐modifying antirheumatic drug; LTE = long‐term extension; MTX = methotrexate; N1 = number of evaluable patients; RF = rheumatoid factor; SD = standard deviation; TNFi = tumor necrosis factor inhibitor.
Baseline values were obtained from the qualifying index studies.
Pooled population, including patients who received tofacitinib 5 and 10 mg BID as monotherapy or in combination with background csDMARDs, with data from one patient in the tofacitinib 10 mg group missing because of a database issue.
Stay‐on tofacitinib combination therapy and stay‐on tofacitinib monotherapy patients were defined as those patients who were assigned to, and remained on, tofacitinib (all doses) plus background DMARD or tofacitinib monotherapy (all doses), respectively, for their entire participation in the study.
Duration was defined as first diagnosis to day 1 of the qualifying study.
Drug survival
Median drug survival (95% CI) for all tofacitinib‐treated patients was 4.9 (4.7, 5.1) years (Table 2). Estimated 2‐ and 5‐year drug survival rates were 75.5% and 49.4%, respectively. Median drug survival (95% CI) was similar for the tofacitinib 5 and 10 mg BID treatment groups, 5.2 (4.9, 5.7) and 4.8 (4.5, 5.0) years, respectively (Figure 1A and Table 2). Overall, median drug survival (95% CI) was slightly higher for patients receiving tofacitinib monotherapy versus those receiving combination therapy (5.0 [4.6, 5.4] and 4.4 [4.2, 4.7] years, respectively), although CIs overlapped. Among patients treated with tofacitinib monotherapy, median drug survival (95% CI) was similar for tofacitinib 5 mg BID (5.0 [4.4, 6.2] years) and 10 mg BID (5.0 [4.5, 5.5] years). Median drug survival (95% CI) for patients receiving tofacitinib 5 mg BID combination therapy (5.0 [4.4, 5.4] years) was similar to those receiving either dose as monotherapy. A numerically lower median survival (95% CI) was observed for patients receiving tofacitinib 10 mg BID combination therapy (4.2 [4.0, 4.4] years; Figures 1B and C) versus all other groups.
Table 2.
Median drug survival across tofacitinib groups by selected index baseline characteristics
| Total Patient Number | Median Drug Survival, years (95% CI) | |||
|---|---|---|---|---|
| Tofacitinib 5 mg BID (N = 1535) | Tofacitinib 10 mg BID (N = 3432) | All tofacitinib (N = 4967) | ||
| All patients | 4967 | 5.2 (4.9, 5.7) | 4.8 (4.5, 5.0) | 4.9 (4.7, 5.1) |
| RF+ | 3437 | 5.3 (4.9, 6.0) | 5.0 (4.6, 5.2) | 5.1 (4.8, 5.3) |
| RF− | 1207 | 4.2 (3.5, 4.9) | 4.8 (4.4, 5.2) | 4.6 (4.3, 5.0) |
| Anti‐CCP+ | 2533 | 5.1 (4.5, NE) | 5.0 (4.5, 5.2) | 5.0 (4.6, 5.2) |
| Anti‐CCP− | 764 | 3.3 (2.7, 4.5) | 4.3 (3.9, 4.8) | 4.2 (3.8, 4.7) |
| Disease duration | ||||
| <1 year | 732 | 5.1 (4.4, NE) | 4.4 (4.0, 5.0) | 4.5 (4.3, 5.1) |
| ≥1 year | 4234 | 5.2 (4.8, 5.7) | 4.8 (4.5, 5.1) | 5.0 (4.7, 5.2) |
| DAS28‐4(ESR)a | ||||
| Q1 | 1106 | 5.4 (4.5, 6.5) | 4.7 (4.3, 5.3) | 5.0 (4.4, 5.4) |
| Q2 | 1104 | 5.0 (4.5, 6.6) | 4.8 (4.4, 5.4) | 4.9 (4.5, 5.2) |
| Q3 | 1106 | 6.0 (5.0, 7.6) | 5.2 (4.6, 5.9) | 5.5 (4.9, 6.0) |
| Q4 | 1105 | 4.5 (3.8, 5.7) | 4.6 (4.3, 5.0) | 4.6 (4.3, 5.0) |
| MTX‐IR | 4246 | 5.2 (4.9, 5.7) | 5.0 (4.8, 5.4) | 5.1 (4.9, 5.4) |
| TNFi‐IR | 720 | 5.6 (3.5, 7.2) | 4.0 (3.6, 4.3) | 4.0 (3.7, 4.3) |
| Baseline csDMARD use | ||||
| MTX alone | 2639 | 5.5 (5.1, 5.9) | 4.8 (4.5, 5.1) | 5.0 (4.8, 5.2) |
| MTX combinationb | 253 | 5.1 (3.0, NE) | 4.7 (4.2, 5.9) | 4.7 (4.2, 5.9) |
| Other csDMARDc | 322 | 4.0 (3.3, 5.2) | 4.2 (3.5, 4.4) | 4.2 (3.6, 4.4) |
| Index MTX category | ||||
| ≤15 mg/week | 1772 | 5.3 (4.9, 6.0) | 5.1 (4.7, 5.5) | 5.2 (4.9, 5.5) |
| >15 mg/week | 877 | 5.0 (4.0, 5.9) | 4.3 (3.9, 4.8) | 4.4 (4.0, 4.9) |
| BMI category | ||||
| <25 kg/m2 | 2226 | 5.4 (4.9, 6.7) | 5.1 (4.7, 5.5) | 5.2 (4.9, 5.7) |
| 25–<30 kg/m2 | 1427 | 5.4 (4.5, 6.0) | 4.9 (4.5, 5.2) | 5.0 (4.6, 5.3) |
| ≥30 kg/m2 | 1312 | 4.5 (3.7, 5.3) | 4.4 (4.0, 4.8) | 4.4 (4.0, 4.8) |
| Smoking status | ||||
| Smoker | 875 | 5.2 (4.1, 6.6) | 4.9 (4.2, 5.4) | 5.0 (4.3, 5.4) |
| Ex‐smoker | 871 | 4.7 (3.7, 6.0) | 4.3 (4.0, 4.9) | 4.5 (4.0, 4.9) |
| Never smoked | 3145 | 5.2 (4.8, 5.7) | 5.0 (4.7, 5.2) | 5.0 (4.8, 5.2) |
| Unknown | 75 | 5.7 (4.0, 7.9) | 4.4 (2.0, 6.0) | 5.7 (4.0, 7.5) |
| Baseline diabetes | ||||
| Yes | 367 | 3.3 (2.7, 4.4) | 3.5 (2.8, 4.2) | 3.3 (2.8, 3.8) |
| No | 4599 | 5.4 (5.0, 5.9) | 4.9 (4.6, 5.2) | 5.0 (4.9, 5.2) |
| Baseline hypertension | ||||
| Yes | 1706 | 5.0 (4.4, 5.5) | 4.2 (3.9, 4.5) | 4.4 (4.0, 4.7) |
| No | 3260 | 5.5 (4.9, 6.3) | 5.2 (4.9, 5.5) | 5.2 (5.0, 5.5) |
| Baseline CV disease | ||||
| Yes | 2054 | 5.0 (4.5, 5.6) | 4.3 (4.0, 4.6) | 4.5 (4.2, 4.8) |
| No | 2912 | 5.4 (4.9, 6.2) | 5.2 (4.9, 5.5) | 5.2 (5.0, 5.5) |
Abbreviation: BID = twice daily; BMI = body mass index; CCP = cyclic citrullinated peptide; CI = confidence interval; csDMARD = conventional synthetic disease‐modifying antirheumatic drug; CV = cardiovascular; DAS28‐4(ESR) = Disease Activity Score in 28 joints, erythrocyte sedimentation rate; IR = inadequate response; MTX = methotrexate; NE = not estimated; Q = quartile; RF = rheumatoid factor; TNFi = tumor necrosis factor inhibitor.
DAS28‐4(ESR) quartiles were based on all tofacitinib‐treated patients: Q1 = 5.67, Q2 = 6.36, Q3 = 7.05, Q4 = 9.04.
With other csDMARD.
Without MTX.
Figure 1.
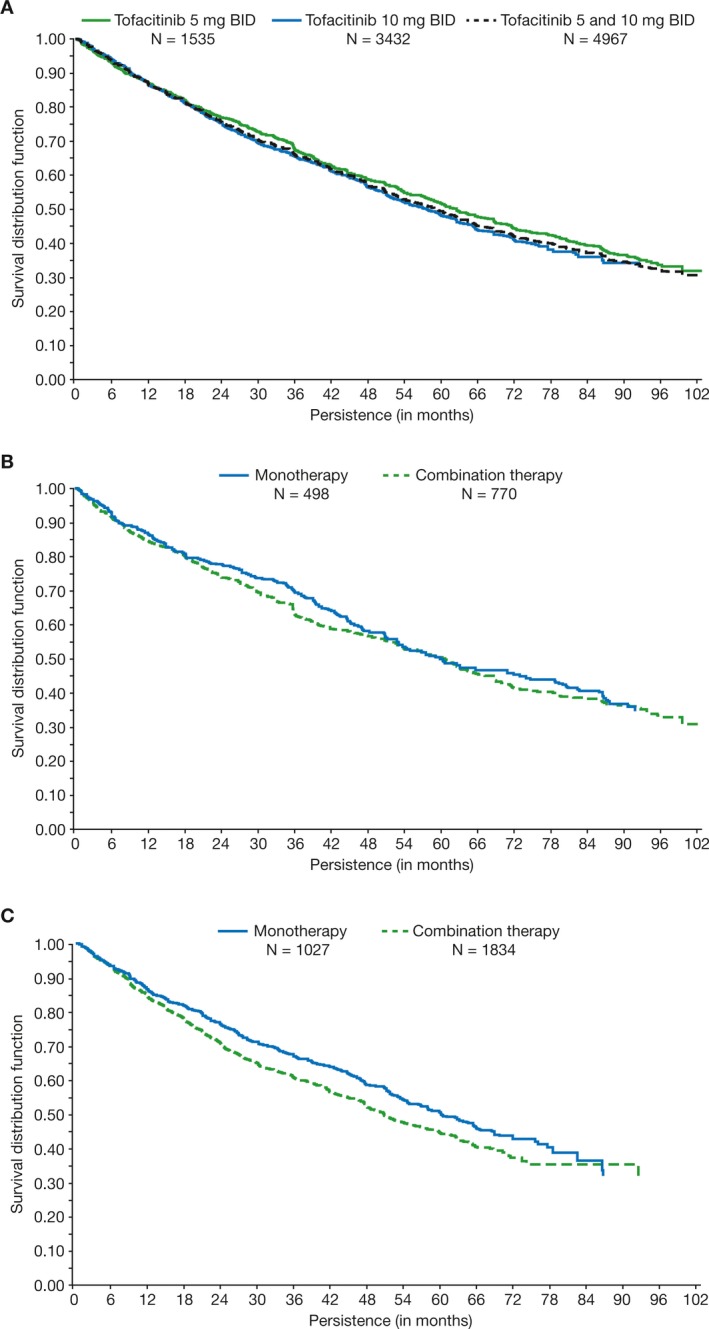
Kaplan‐Meier drug survival estimates for all tofacitinib‐treated patients (A); patients receiving tofacitinib 5 mg BID monotherapy versus combination therapy (B); and patients receiving tofacitinib 10 mg BID monotherapy versus combination therapy (observed data) (C). Persistence/drug survival was defined as the difference between the end‐of‐study date and first tofacitinib dose date + 1 day. Ongoing patients were censored at the cut‐off date (March 2 2017 for ORAL Sequel; April 24 2014 for Study A3921041). LTE completers were censored at the end‐of‐study date. Data include all patients who entered the LTE. BID = twice daily; LTE = long‐term extension.
Numerical differences in median drug survival times were observed for some of the selected patient baseline characteristics (Table 2). Positive RF, positive anti‐CCP, low body mass index (BMI less than 25 kg/m2 compared with 30 kg/m2 or greater), MTX monotherapy, or MTX dose 15 mg or less per week, and absence of specific comorbidities (diabetes, hypertension, or cardiovascular disease) appeared to be associated with increased drug survival (Table 2).
The Cox regression analysis demonstrated that diabetes, hypertension, and negative anti‐CCP at index baseline were associated with an increased risk of discontinuation versus comparator baseline values (Table 3). Prior TNFi‐IR was associated with an increased risk of discontinuation versus MTX‐IR. Also, absence of both RF and anti‐CCP at baseline was associated with an increased risk of discontinuation versus RF+/CCP+ status.
Table 3.
Cox proportional hazard regression analysis to estimate risk of tofacitinib discontinuation with respect to baseline covariates: variables selected in the final modela
| Baseline Variable (Index Baseline) | Comparison | Hazard Ratio (95% CI) | P value |
|---|---|---|---|
| Diabetes | Yes vs No | 1.3 (1.1, 1.5) | 0.0041 |
| Hypertension | Yes vs No | 1.2 (1.1, 1.3) | 0.0002 |
| Glucocorticoid use | Yes vs No | 1.1 (1.0, 1.2) | 0.0528 |
| Anti‐CCP status | CCP+ vs CCP− | 0.8 (0.7, 0.9) | 0.0001 |
| Prior treatmentb | MTX‐IR vs TNFi‐IR | 0.8 (0.7, 0.9) | <0.0001 |
| RF and anti‐CCP statusb | RF+/CCP+ vs RF−/CCP− | 0.8 (0.7, 0.9) | 0.0003 |
Abbreviation: CCP = cyclic citrullinated peptide; CI = confidence interval; IR = inadequate response; MTX = methotrexate; RF = rheumatoid factor; TNFi = tumor necrosis factor inhibitor.
Analysis conducted in 3534 patients with 1727 events. Data for significant (P < 0.05) or near significant (glucocorticoid use) variables are presented.
Significance estimated from univariate Cox regression analysis fitted to avoid multicollinearity in the backward selection models.
Reasons for treatment discontinuation
Overall, 50.7% (2518/4967) of patients discontinued tofacitinib treatment during the LTE studies (Table 4). The most common reasons for discontinuation were AEs (n = 1189; 23.9%), lack of patient willingness to participate (n = 504; 10.1%), “other” reasons (n = 307; 6.2%, ie, any reason not otherwise classified), and lack/loss of efficacy (n = 179; 3.6%).
Table 4.
Reasons for tofacitinib discontinuation in LTE studies
| n (%) | Tofacitinib 5 mg BID (N = 1535) | Tofacitinib 10 mg BID (N = 3432) | All Tofacitinib (N = 4967) |
|---|---|---|---|
| Total discontinuations | 792 (51.6) | 1726 (50.3) | 2518 (50.7) |
| AE | 398 (25.9) | 791 (23.0) | 1189 (23.9) |
| Related to study drug | 273 (17.8) | 542 (15.8) | 815 (16.4) |
| Not related to study drug | 125 (8.1) | 249 (7.3) | 374 (7.5) |
| No longer willing to participate | 133 (8.7) | 371 (10.8) | 504 (10.1) |
| Other | 92 (6.0) | 215 (6.3) | 307 (6.2) |
| Lack or loss of efficacy | 63 (4.1) | 116 (3.4) | 179 (3.6) |
| Lost to follow‐up | 31 (2.0) | 102 (3.0) | 133 (2.7) |
| Protocol violation | 38 (2.5) | 89 (2.6) | 127 (2.6) |
| Death | 25 (1.6) | 30 (0.9) | 55 (1.1) |
| Withdrawn because of pregnancy | 10 (0.7) | 8 (0.2) | 18 (0.4) |
| Does not meet entrance criteria | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Medication error without associated AE | 0 | 1 (<0.1) | 1 (<0.1) |
| Study terminated by sponsor | 0 | 1 (<0.1) | 1 (<0.1) |
Abbreviation: AE = adverse event; BID = twice daily; LTE = long‐term extension.
Of 1189 patients who discontinued tofacitinib because of AEs, the most common classes of AEs were infections and infestations (n = 475, 39.9%), investigations (ie, laboratory parameter abnormalities; n = 227, 19.1%), and neoplasms that were benign, malignant, and unspecified (including cysts and polyps) (n = 183, 15.4%). The most common infections (0.3% or more of all patients) resulting in tofacitinib discontinuation were pneumonia (n = 89; 1.8%), herpes zoster (n = 44; 0.9%), urinary tract infection (n = 24; 0.5%), diverticulitis (n = 19; 0.4%), and cellulitis (n = 16; 0.3%).
Among all tofacitinib‐treated patients, median drug survival (95% CI) for patients who discontinued because of AEs was 2.0 (1.8, 2.1) years (n = 1189) and for patients who discontinued because of a lack/loss of efficacy was 1.3 (1.2, 1.8) years (n = 179). Patients in the tofacitinib 5 mg BID group had a slightly numerically higher median drug survival (95% CI) for discontinuations that were due to AEs (2.2 [1.9, 2.4] years) versus the tofacitinib 10 mg BID group (1.8 [1.7, 2.0] years). Median drug survival (95% CI) times for discontinuations that were due to AEs did not appear to differ between those patients who received tofacitinib as monotherapy (1.7 [1.4, 1.9] years) or combination therapy (1.8 [1.6, 2.0] years).
Discussion
Following an initial response, many patients with RA stop responding to treatment over time, and this loss of efficacy and poor patient tolerability frequently leads to treatment discontinuation. Furthermore, there is no reliable method to predict which patients will persist on a given treatment. Understanding time on treatment and factors influencing drug survival is important for the long‐term management of patients with RA.
Kaplan‐Meier estimates of time to discontinuation demonstrated that median drug survival for all tofacitinib‐treated patients was 4.9 years, with 2‐ and 5‐year estimated drug survival rates of 75.5% and 49.4%, respectively. Median drug survival was generally similar between tofacitinib 5 and 10 mg BID and for patients who received tofacitinib 5 mg BID as monotherapy or with background csDMARDs. Time on treatment for patients who received tofacitinib 10 mg BID with background csDMARDs was numerically lower than all other groups.
Previously published drug survival data from LTE studies for adalimumab 34, etanercept 5, certolizumab 3, golimumab 4, and abatacept 35 were similar to that reported here for tofacitinib. A higher 5‐year estimated survival rate (66%; patients completing 5 years' treatment) was observed for tocilizumab monotherapy compared with tofacitinib (49.4%), although median drug survival was comparable (5.6 versus 4.9 years for tocilizumab and tofacitinib, respectively) 6. The median drug survival of tofacitinib in these LTE studies was similar to previous reports on registry data for adalimumab (5.7 years), higher than abatacept (3.9 years), and lower than etanercept (6.6 years) 36. However, as these data are from registries with different inclusion criteria and follow‐up methods, and therefore include different populations to those of the LTE studies, direct comparisons should not be made.
Patients with RA tend to discontinue treatment with bDMARDs mainly because of AEs and lack or loss of clinical efficacy 9, with a similar proportion of LTE patients discontinuing because of AEs and lack of efficacy 4, 5, 34, 37. Discontinuations that are due to lack/loss of efficacy with tofacitinib were low (3.6%) compared with LTE studies of bDMARDs used in the treatment of RA (8%–23%) 4, 5, 34, 37. Slightly more patients discontinued because of AEs (23.9%; 16.4% related to study drug; 7.5% not related to study drug) with tofacitinib in this study compared with LTE studies of bDMARDs (12%–19%) 4, 5, 34. In this study, infections and infestations, investigations, and neoplasms that are benign, malignant, and unspecified (including cysts and polyps) were the most common classes of AEs associated with tofacitinib discontinuation. Overall, tofacitinib has demonstrated a consistent safety profile for up to 114 months in LTE studies 22, 23, 24, with incidence rates for serious AEs, serious infections, and malignancies, excluding nonmelanoma skin cancer, being generally consistent across data‐cuts over time 22, 23, 24. In an integrated analysis of safety 32, types and rates of AEs observed following up to 8.5 years of tofacitinib exposure were similar to those observed in Phase 3 trials 16, 17, 18, 19, 20, 21.
Concomitant use of csDMARDs had limited impact on drug survival with tofacitinib. Although drug survival with tofacitinib 5 mg BID was similar regardless of background treatment, slightly lower drug survival was observed with tofacitinib 10 mg BID combination therapy versus all other groups. This may be as a result of increased rates of AEs in this subgroup; in a recent analysis by Fleischmann et al, numerically higher incidence rates for discontinuations that are due to AEs, serious AEs, and serious infections were observed for tofacitinib 10 mg BID with combination csDMARD therapy versus all other groups (tofacitinib 10 mg BID monotherapy, tofacitinib 5 mg BID monotherapy, and tofacitinib 5 mg BID with combination csDMARD therapy) 38. In that analysis, the efficacy of tofacitinib as monotherapy or as combination therapy was sustained from baseline to month 72 38. The reduced persistence of tofacitinib 10 mg BID with concomitant use of csDMARDs contrasts with results from a registry study of TNFi in patients with RA, in which treatment with TNFi in combination with MTX was associated with longer drug survival than TNFi monotherapy 39. In an observational prospective cohort study, antidrug antibody development was associated with a lack of concomitant MTX in patients receiving adalimumab, also supporting that TNFi with MTX is associated with longer drug survival 40.
Predictors of discontinuation with bDMARDs previously identified from registries and administrative databases in RA include lack of concomitant DMARD use, female sex, longer disease duration (for discontinuation that is due to AEs only) and concomitant glucocorticoid use (for discontinuation that is due to lack of efficacy only) 41. In this post hoc analysis, characteristics associated with longer tofacitinib drug survival included positive RF status, positive anti‐CCP, low BMI, MTX monotherapy, or MTX dose 15 mg or less per week, and no diabetes, hypertension, or cardiovascular disease.
The Cox regression analysis indicated that patients with positive RF and anti‐CCP status, and absence of diabetes and hypertension, are less likely to discontinue tofacitinib treatment. Additionally, it showed that the risk of tofacitinib discontinuation was greater in patients with TNFi‐IR compared with those with MTX‐IR. Factors for which no significant differences were found included smoking status and disease duration. The TNFi‐IR finding is consistent with previous studies in which increased drug survival with golimumab for TNFi‐naïve patients has been observed 8 and seems to be generalizable to many bDMARDs in RA. Similar to the results for tofacitinib, high BMI has previously been reported as a predictor for discontinuation of etanercept treatment in RA 42. Furthermore, patients with fewer comorbidities may experience fewer AEs, which could account for the increased drug survival observed in these patients.
Limitations of this analysis include differences in the study designs and patient populations enrolled in each index study. Moreover, the LTE analysis included both patients treated with tofacitinib in the index studies and had demonstrated acceptable efficacy and tolerability as well as those treated with placebo or comparator treatments who switched to tofacitinib at LTE study entry; the latter category of patients would, therefore, not have been previously exposed to tofacitinib, which may have impacted median drug survival times. All LTE studies may be biased, as patients exposed to active treatment have tolerated it and likely have a perceived benefit, whereas patients on placebo who switch to active treatment may encounter AEs and their response to treatment should be similar to that of the active treatment group during the randomized part of the index trial. Additionally, if there are differential dropout rates in the index trials, those entering the LTE study may be a selected population. Another limitation of this analysis is that prior tofacitinib treatment in the qualifying studies differed between patients (from 4 weeks to 24 months), thus drug survival based only on LTE study data does not fully reflect the total treatment duration of tofacitinib. Although the study population included patients receiving tofacitinib 5 or 10 mg BID, many of the patients were on the 10 mg BID dose which, in many countries, has not been approved.
Overall, these LTE data demonstrate long‐term persistence of tofacitinib in patients with RA. Discontinuation of tofacitinib treatment was most commonly due to AEs in those who enrolled in the LTE, with far fewer patients discontinuing because of a lack/loss of efficacy. Increased risk of discontinuation was associated with baseline diabetes or hypertension, anti‐CCP−/RF− status, and prior TNFi‐IR. Together, these data support the use of tofacitinib for the long‐term management of patients with RA.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Pope reviewed all analyses and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Pope, Keystone, Fallon, Woolcott, Haraoui.
Acquisition of data
Wang, Fallon, Woolcott, Lazariciu, Chapman.
Analysis and interpretation of data
Pope, Keystone, Jamal, Fallon, Woolcott, Haraoui, Wang, Lazariciu, Chapman.
Data sharing
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Acknowledgments
These studies were funded by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Kristina Harrison, PhD, at CMC CONNECT, a division of McCann Health Medical Communications Ltd, Macclesfield, UK, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461‐464).
This study was sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Kristina Harrison, PhD, at CMC CONNECT, a division of McCann Health Medical Communications Ltd., Macclesfield, UK, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines.
1Janet E. Pope, MD, MPH, FRCPC: Western University, London, Ontario, Canada; 2Edward Keystone, MD, FRCPC: University of Toronto, Ontario, Canada; 3Shahin Jamal, MD, FRCPC, MSc: University of British Columbia, Vancouver, British Columbia, Canada; 4Lisy Wang, MD: Pfizer Inc, Groton, Connecticut; 5Lara Fallon, PhD: Pfizer Inc, Montreal, Quebec, Canada, John Woolcott, PhD: Pfizer Canada Inc, Montreal, Quebec, Canada; 6Irina Lazariciu, MSc: IQVIA, Montreal, Quebec, Canada; 7Douglass Chapman, MS: Pfizer Inc, New York, New York; 8Boulos Haraoui, MD: Institut de Rhumatologie de Montréal, Montreal, Quebec, Canada.
Dr. Pope has received grant and/or research support from AbbVie, Amgen, BMS, Celltrion, Lilly, Merck, Novartis, Pfizer Inc, Roche, Sandoz, Sanofi, and UCB, and has received consulting fees from AbbVie, Amgen, BMS, Celltrion, Merck, Novartis, Roche, Sandoz, and UCB (less than $10,000 each), and from Lilly, Pfizer Inc, and Sanofi (more than $10,000 each). Dr. Keystone has received grant and/or research support from Pfizer Inc, and has received consulting fees and speaker honoraria from Pfizer Inc (more than $10,000). Dr. Jamal has received grant and/or research support from Pfizer Inc, and has received consulting fees from Amgen, BMS, Merck, Novartis, Pfizer Inc, Sandoz, Sanofi, and UCB (less than $10,000 each), and from Abbvie and Lilly (more than $10,000 each). Drs. Wang, Fallon, Woolcott, and Chapman are employees and shareholders of Pfizer Inc. I. Lazariciu is an employee of IQVIA, contracted to Pfizer Inc. Dr. Haraoui has received grant and/or research support from Pfizer Inc, and has received consulting fees from Pfizer Inc (less than $10,000). No other disclosures relevant to this article were reported.
References
- 1. Aletaha D, Smolen JS. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. J Rheumatol 2002;29:1631–8. [PubMed] [Google Scholar]
- 2. Fries JF. Effectiveness and toxicity considerations in outcome directed therapy in rheumatoid arthritis. J Rheumatol Suppl 1996;44:102–6. [PubMed] [Google Scholar]
- 3. Keystone E, Landewé R, van Vollenhoven R, Combe B, Strand V, Mease P, et al. Long‐term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5‐year results from the RAPID 1 trial and open‐label extension. Ann Rheum Dis 2014;73:2094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smolen JS, Kay J, Doyle M, Landewé R, Matteson EL, Gaylis N, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double‐blind, placebo‐controlled, phase 3 GO‐AFTER study. Arthritis Res Ther 2015;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klareskog L, Gaubitz M, Rodriguez‐Valverde V, Malaise M, Dougados M, Wajdula J. Assessment of long‐term safety and efficacy of etanercept in a 5‐year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol 2011;29:238–47. [PubMed] [Google Scholar]
- 6. Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long‐term safety and efficacy of tocilizumab, an anti‐IL‐6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5‐year extension study. Ann Rheum Dis 2009;68:1580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Áliva Machado MA, de Moura CS, Ferré F, Bernatsky S, Rahme E, de Assis Acurcio F. Treatment persistence in patients with rheumatoid arthritis and ankylosing spondylitis. Rev Saude Publica 2016;50:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svedbom A, Storck C, Kachroo S, Govoni M, Khalifa A. Persistence with golimumab in immune‐mediated rheumatic diseases: a systematic review of real‐world evidence in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Patient Prefer Adherence 2017;11:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leon L, Rodriguez‐Rodriguez L, Rosales Z, Gomez A, Lamas JR, Pato E, et al. Long‐term drug survival of biological agents in patients with rheumatoid arthritis in clinical practice. Scand J Rheumatol 2016;45:456–60. [DOI] [PubMed] [Google Scholar]
- 10. Narongroeknawin P, Chevaisrakul P, Kasitanon N, Kitumnuaypong T, Mahakkanukrauh A, Siripaitoon B, et al. Drug survival and reasons for discontinuation of the first biological disease modifying antirheumatic drugs in Thai patients with rheumatoid arthritis: analysis from the Thai Rheumatic Disease Prior Authorization registry. Int J Rheum Dis 2018;21:170–8. [DOI] [PubMed] [Google Scholar]
- 11. Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease‐modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 12. Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double‐blind, placebo‐controlled phase IIa trial of three dosage levels of CP‐690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 13. Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez‐Reino J, et al. A phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators . Phase II study of tofacitinib (CP‐690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12‐week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burmester GR, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 17. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 18. Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin‐Mola E, et al. Tofacitinib in combination with nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 19. Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 20. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four‐month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 21. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 22. Wollenhaupt J, Silverfield J, Lee EB, Terry K, Kwok K, Strengholt S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open‐label, long‐term extension studies over 9 years [Abstract]. Arthritis Rheumatol 2017;69 Suppl 10 URL: https://acrabstracts.org/abstract/tofacitinib-an-oral-janus-kinase-inhibitor-in-the-treatment-of-rheumatoid-arthritis-safety-and-efficacy-in-open-label-long-term-extension-studies-over-9-years/. [Google Scholar]
- 23. Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open‐label, long‐term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open‐label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 25. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 26. Charles‐Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015;67:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kremer JM, Kivitz AJ, Simon‐Campos JA, Nasonov EL, Tony HP, Lee SK, et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Res Ther 2015;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conaghan PG, Østergaard M, Bowes MA, Wu C, Fuerst T, van der Heijde D, et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate‐naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis 2016;75:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1‐STAT signalling in rheumatoid arthritis. Ann Rheum Dis 2015;74:1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McInnes IB, Kim HY, Lee SH, Mandel D, Song YW, Connell CA, et al. Open‐label tofacitinib and double‐blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis 2014;73:124–31. [DOI] [PubMed] [Google Scholar]
- 31. Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long‐term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleischmann R, Wollenhaupt J, Cohen S, Wang L, Fan H, Bandi V, et al. Effect of discontinuation or initiation of methotrexate or glucocorticoids on tofacitinib efficacy in patients with rheumatoid arthritis: a post hoc analysis. Rheumatol Ther 2018;5:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, Segurado OG. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended study. Ann Rheum Dis 2006;65:753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kremer JM, Russell AS, Emery P, Abud‐Mendoza C, Szechinski J, Westhovens R, et al. Long‐term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3‐year results from the AIM trial. Ann Rheum Dis 2011;70:1826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts L, Tymms K, de Jager J, Littlejohn G, Griffiths H, Nicholls D, et al. The CEDAR study: a longitudinal study of the clinical effects of conventional DMARDs and biologic DMARDs in Australian rheumatology practice. Int J Rheumatol 2017;2017:1201450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Westhovens R, Kremer JM, Emery P, Russell AS, Alten R, Barré E, et al. Long‐term safety and efficacy of abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: a 7‐year extended study. Clin Exp Rheumatol 2014;32:553–62. [PubMed] [Google Scholar]
- 38. Fleischmann R, Wollenhaupt J, Takiya L, Maniccia A, Kwok K, Wang L, et al. Safety and maintenance of response for tofacitinib monotherapy and combination therapy in rheumatoid arthritis: an analysis of pooled data from open‐label long‐term extension studies. RMD Open 2017;3:e000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aaltonen KJ, Joensuu JT, Pirilä L, Kauppi M, Uutela T, Varjolahti‐Lehtinen T, et al. Drug survival on tumour necrosis factor inhibitors in patients with rheumatoid arthritis in Finland. Scand J Rheumatol 2017;46:359–63. [DOI] [PubMed] [Google Scholar]
- 40. Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long‐term follow‐up. JAMA 2011;305:1460–8. [DOI] [PubMed] [Google Scholar]
- 41. Souto A, Maneiro JR, Gómez‐Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta‐analysis of drug registries and health care databases. Rheumatology (Oxford) 2016;55:523–34. [DOI] [PubMed] [Google Scholar]
- 42. Elkayam O, Lidar M, Reitblat T, Balbir‐Gurman A, Almog R. Increased body mass index and biologics drug survival in patients with inflammatory rheumatic diseases. Ann Rheum Dis 2015;74:326 https://ard.bmj.com/content/74/Suppl_2/326.2.abstract.25351522 [Google Scholar]


