Abstract
Background
Exercise capacity and raised heart rate (HR) are important prognostic markers in patients with heart failure (HF). There has been significant interest in wrist-worn devices that track activity and HR.
Objective
We aimed to assess the feasibility and accuracy of HR and activity tracking of the Fitbit and Apple Watch.
Methods
We conducted a two-phase study assessing the accuracy of HR by Apple Watch and Fitbit in healthy participants. In Phase 1, 10 healthy individuals wore a Fitbit, an Apple Watch, and a GE SEER Light 5-electrode Holter monitor while exercising on a cycle ergometer with a 10-watt step ramp protocol from 0-100 watts. In Phase 2, 10 patients with HF and New York Heart Association (NYHA) Class II-III symptoms wore wrist devices for 14 days to capture overall step count/exercise levels.
Results
Recorded HR by both wrist-worn devices had the best agreement with Holter readings at a workload of 60-100 watts when the rate of change of HR is less dynamic. Fitbit recorded a mean 8866 steps/day for NYHA II patients versus 4845 steps/day for NYHA III patients (P=.04). In contrast, Apple Watch recorded a mean 7027 steps/day for NYHA II patients and 4187 steps/day for NYHA III patients (P=.08).
Conclusions
Both wrist-based devices are best suited for static HR rate measurements. In an outpatient setting, these devices may be adequate for average HR in patients with HF. When assessing exercise capacity, the Fitbit better differentiated patients with NYHA II versus NYHA III by the total number of steps recorded. This exploratory study indicates that these wrist-worn devices show promise in prognostication of HF in the continuous monitoring of outpatients.
Keywords: MeSH: exercise physiology, heart rate tracker, wrist worn devices, Fitbit, Apple watch, heart failure, steps
Introduction
Exercise capacity and raised heart rate (HR) are important prognostic markers in patients with heart failure (HF) [1,2]. In clinic, we rely on patients’ self-reported exercise capacity and classify their symptoms based on the New York Heart Association (NYHA) scale. Although widely used, this classification is subjective and poorly reproducible [2]. Furthermore, clinicians are exposed to only a snapshot of the patients’ HR in the ambulatory setting. There has been significant growth in wrist-worn fitness devices that track activity and HR [3]. These wearable devices use infrared and green light emitting diodes to track HR using the photoplethysmography (PPG) method [4]. The aim of this study was to validate the accuracy of HR monitoring using Fitbit and Apple Watch at rest and during structured cardiopulmonary exercise testing in healthy individuals and to then examine the relationship of physical activity in patients with HF.
Methods
We conducted a two-phase study assessing the accuracy of HR using Apple Watch and Fitbit wrist-based devices in healthy participants and then as continuous HR monitoring in patients with HF. In Phase 1, 10 healthy individuals wore a Fitbit, an Apple Watch, and a GE SEER Light 5-electrode Holter monitor while exercising on a cycle ergometer with a 10-watt step ramp protocol. During the first 60 seconds of the test, the workload was set to 0 watts and followed by increments of 10 watts with a maximum workload of 100 watts. In the recovery period, the workload was decreased to 10 watts. In Phase 1, two participants were excluded as data from one device could not be recorded. In Phase 2, 10 patients with HF with NYHA Class II-III symptoms wore both wrist devices for 14 days to capture overall step count/exercise levels. Two patients were excluded due to incomplete data recorded.
For Phase 1, we calculated a single measures intraclass correlation (ICC) and a 95% confidence interval, specific to each exercise workload, between HR measured by Holter (gold standard) and HR measured by the Fitbit and Apple Watch devices. The mean ICC and 95% confidence interval for each device against the Holter as the gold standard was plotted against exercise workload.
For Phase 2, we used a Kruskal-Wallis rank test to compare the mean number of steps, calculated by either device, between patients in NYHA Class II or III. We used STATA 13.1 and SPSS 22 statistical packages for our statistical analysis. The study protocol was approved by the University Health Network Research Ethics Board.
Results
Recorded HR by both devices was not significantly related with the Holter HR at rest (Fitbit ICC=.263, 95% CI 0.257-0.447, Apple Watch ICC=.218, 95% CI 0.010-0.408). However, with cycle ergometer workloads of 60-100 watts, both devices had stronger agreement with the Holter HR (Figure 1).
Figure 1.
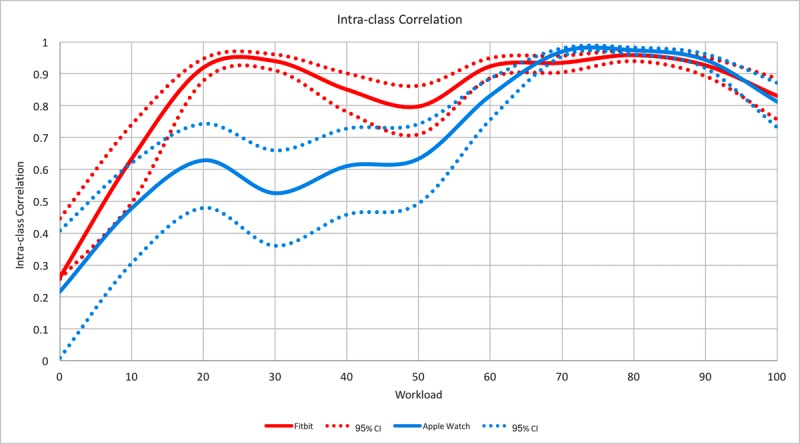
Intraclass correlation curves (solid) with 95% confidence intervals (dotted) for workload comparing Fitbit to Holter and Apple Watch to Holter in healthy individuals.
Table 1 shows the baseline demographics of the patients included in Phase 2. Patients were predominantly male (5/8, 63%), with an average age of 58 years and ischemic cardiomyopathy (5/8, 63%). All patients were on guideline-directed medical therapy including a betablocker and either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin-receptor blocker (ARB) when indicated. As shown in Figure 2, Fitbit recorded a mean 8866 steps/day for NYHA II patients versus 4845 steps/day for NYHA III patients (P=.04). In contrast, Apple Watch recorded a mean 7027 steps/day for NYHA II patients and 4187 steps/day for NYHA III patients (P=.08).
Table 1.
Demographics and baseline data.
|
Number |
Age (years) |
Gender |
LVEFa, % | Etiology of HFb | NYHAc class | Medicationsd | ||
| Betablocker | Amiodarone | Other | ||||||
| 1 | 67 | Male | 40 | Ischemic | 3 | Bisoprolol 2.5 mg | None | Candesartan 8 mg |
| 2 | 68 | Male | 18 | Ischemic | 2 | Bisoprolol 10 mg | 200 | Irbesartan 300 mg |
| 3 | 63 | Male | 25 | Ischemic | 3 | Bisoprolol 10 mg | None | Perindopril 8 mg |
| 4 | 61 | Female | 27 | Non-ischemic | 2 | Bisoprolol 10 mg | None | Perindopril 4 mg |
| 5 | 52 | Male | 25 | Ischemic | 2 | Bisoprolol 10 mg | None | Perindopril 8 mg |
| 6 | 57 | Female | 27 | Non-ischemic | 3 | Carvedilol 25 mg | None | Ramipril 2.5 mg |
| 7 | 58 | Female | 60 | Familial | 2 | None | None | None |
| 8 | 35 | Male | 33 | Hypertrophic | 3 | Carvedilol 50 mg | None | Ramipril 10 mg |
aLVEF: left ventricular ejection fraction.
bHF: heart failure.
cNYHA: New York Heart Association.
dDrug doses are total daily dose.
Figure 2.
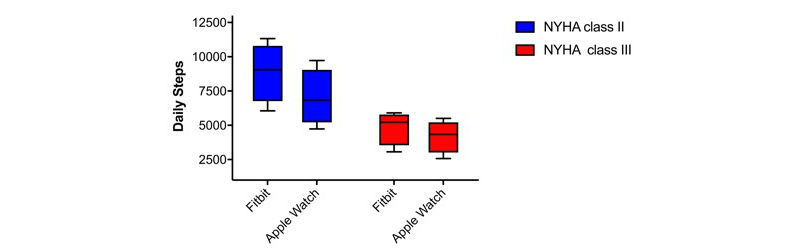
Total daily steps recorded (with 95% confidence interval) by Fitbit and Apple watch over 14 days for patients with New York Heart Association (NYHA) Class II and III symptoms .
Discussion
Principal Findings
In this study, both PPG-based monitors had difficulty predicting HR when compared to a 5-lead electrode electrocardiogram (ECG) Holter as the gold standard in a cardiopulmonary study where HR is expected to change quickly over a 10-minute period. A recent study by Wang et al reported similar variability among four popular wrist-worn devices in relation to standard ECG limb leads and a Polar H7 chest strap monitor [5]. The HR underestimation was due to the inherent limitation of PPG that requires a longer settling time processing and averaging to eliminate optical and motion artifact. As a result, PPG monitors underestimated HR and showed poor correlation until the latter stages of the ramp study where HR would tend to saturate and the settling time was sufficient for the PPG HR estimation to compare favorably to the ECG gold standard. Although this indicates that PPG is less suited for dynamic HR measurements, in an outpatient monitoring context, PPG may be suitable for long-term static measurement of HR over long periods of time where settling time would not be an issue [6].
Strengths and Limitations
When assessing exercise capacity, the Fitbit better differentiated patients with NYHA II versus III by the total number of steps recorded. The limitations in this study include the small sample size; larger studies will be needed to confirm these findings. This is the first study in the literature that suggests the possibility of better classifying patients, quantitatively, and potentially remotely, rather than the current practice of determining class through self-reported symptoms with its inherent limitations. Reductions in daily step counts may herald the onset of progressive NYHA symptoms alerting physicians to assess patients in a timely manner.
Conclusion
This exploratory study indicates that wrist-worn devices show promise in prognostication of HF in the continuous monitoring of outpatients, but they require further validation.
Abbreviations
- ACE
angiotensin-converting enzyme
- ARB
angiotensin-receptor blocker
- ECG
electrocardiogram
- HF
heart failure
- HR
heart rate
- ICC
intraclass correlation
- NYHA
New York Heart Association
- PPG
photoplethysmography
Footnotes
Conflicts of Interest: None declared.
References
- 1.Castagno D, Skali H, Takeuchi M, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pfeffer MA, McMurray JJV, Solomon SD, CHARM Investigators Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol. 2012 May 15;59(20):1785–1795. doi: 10.1016/j.jacc.2011.12.044. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(12)00797-8. [DOI] [PubMed] [Google Scholar]
- 2.Raphael C, Briscoe C, Davies J, Ian WZ, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007 Apr;93(4):476–482. doi: 10.1136/hrt.2006.089656. http://europepmc.org/abstract/MED/17005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015 Feb 10;313(6):625–626. doi: 10.1001/jama.2014.17841. [DOI] [PubMed] [Google Scholar]
- 4.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007 Mar;28(3):R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Blackburn G, Desai M, Phelan D, Gillinov L, Houghtaling P, Gillinov M. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol. 2017 Jan 01;2(1):104–106. doi: 10.1001/jamacardio.2016.3340. [DOI] [PubMed] [Google Scholar]
- 6.Kroll R, Boyd JG, Maslove DM. Accuracy of a wrist-worn wearable device for monitoring heart rates in hospital inpatients: a prospective observational study. J Med Internet Res. 2016 Sep 20;18(9):e253. doi: 10.2196/jmir.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]


