Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease with an average global prevalence rate of 238 per 100,000 in Europe, 319 per 100,000 in North America, and 167 per 100,000 in Asia 1. A very detailed review of its epidemiology was published early this year 2. Skeletal specimens in several museum collections testify that the disease has existed from the earliest times 3. An anatomical description of an ankylosed skeleton most likely resulting from AS was first published by Bernard Connor in 1695 4. In 1893, Vladimir Bechterev, a Russian neurologist‐psychiatrist, described a new disease that, in his opinion, was a neurologic illness characterized by stiffness of the spine, dorsal kyphosis, and symptoms of nerve root irritation, including thoracic girdle pain 3, 5. His name has been spelled sometimes as Bechterew, Bechtereff, and Bekhterew. In 1987, Adolph Strümpell from Germany described some patients with gradually progressive ankylosis of the spine and hip joints 3, 6. A year later, Pierre Marie from France reported ascending spinal ankylosis with early involvement of the sacroiliac joints 3, 7.
Nomenclature
Over the years, this disease was described using many eponyms and synonyms (see Footnote) before the term “ankylosing spondylitis” became a more acceptable descriptor of the disease 3, 8. However, in 1941, the American Rheumatism Association (ARA) selected the term “rheumatoid spondylitis,” considering it to be a spinal variant of rheumatoid arthritis (RA) 3. But in Europe, the disease was defined as an entity unrelated to RA because of male preponderance, younger age of onset, tendency to spinal ankylosis, no association with subcutaneous nodules and rheumatoid factor (seronegative), and lack of response to treatment with gold salts 3. Finally, in 1963, the ARA formally adopted the name “ankylosing spondylitis” 3, 9, accepting it to be a separate entity from RA. Interestingly, in German‐speaking countries, AS is still quite widely known as “Morbus Bechterew,” even though Bechterew always maintained during his lifetime that the disease he described was different from the one later reported by Strümpell and Marie 5. According to Wright and Moll 10, Marie, not Bechterew, deserves to have his name linked eponymously with the disease.
John Ball, in his 1970 Heberden Oration, reported that enthesitis is a prominent feature in AS, in contrast to RA 11; additionally, in 1971, McEwen et al 12 published a comparative study of AS as well as spondylitis accompanying ulcerative colitis and Crohn disease, psoriasis, and reactive arthritis (previously called Reiter's disease). In 1973, Moll and Wright 13 reported that “familial and clinical interrelationships exist between psoriatic arthritis and other seronegative arthritides,” particularly reactive arthritis, idiopathic AS, and enteropathic arthritis. In the same year, a remarkable association of HLA‐B27 with AS and these associated rheumatic diseases was discovered 14, 15. These advances have supported and validated the proposed grouping of these diseases under the term “spondyloarthropathies,” now more appropriately called “spondyloarthritides” (to emphasize their inflammatory aspect) or spondyloarthritis (SpA) 16, 17, 18.
Evolution of its classification criteria
At a symposium in Rome in 1960 that was sponsored by the World Health Organization through the Council for International Organizations of Medical Science, criteria were formulated (subsequently called the Rome criteria) to define AS for epidemiological studies 19. On their subsequent evaluation at a meeting in New York City in 1966, two items—thoracic pain and uveitis—were deleted, resulting in the New York criteria for AS 20, 21. Moll and Wright 22 published their critique of the New York criteria in 1973. Four years later, Calin et al 23 proposed a definition for chronic inflammatory back pain (IBP) to help differentiate it from many other causes of chronic back pain. Incorporation of these IBP components in place of the rather nonspecific clinical symptom of chronic low back pain that had been used in both the Rome and the New York criteria led to the modified New York (mNY) criteria 24, 25.
The presence of radiographic sacroiliitis is an obligatory condition to fulfill the New York and the mNY criteria; therefore, these criteria will perform less well if they are used to classify patients with early disease in whom radiographically detectable damage (meeting the mNY criteria grading of bilateral grade 2 or unilateral grade 3 or 4) may not have yet occurred. Thus, in a study of first‐degree relatives of HLA‐B27–positive AS probands, presence of “spondylitic disease without radiologic evidence of sacroiliitis” in some of these first‐degree relatives, who were quite often females, was reported in 1985 26. These relatives had some of the clinical features of SpA but did not meet the mNY criteria 26. The use of magnetic resonance imaging (MRI) has now clearly demonstrated that the absence of radiographic sacroiliitis does not imply the absence of inflammation in the sacroiliac joints, whereas its presence implies structural damage that is associated with disease chronicity and/or severity.
In 2005, Rudwaleit et al 27 highlighted the prevailing difficulties in diagnosing and classifying patients with predominantly axial symptoms of AS but lacking sacroiliitis as defined by the mNY criteria. Such patients had previously been reported as having “spondylitic disease without radiologic evidence of sacroiliitis” 26, and it was emphasized that these patients form part of a wider disease spectrum called axial SpA (axSpA) 27. The patients who show structural damage on plain radiography that meets the mNY criteria definition of radiographic sacroiliitis are classified as having AS, whereas those without such changes were initially classified as having preradiographic axSpA 27. This latter term was subsequently changed to nonradiographic axSpA (nr‐axSpA) because not every such patient progresses to AS or what has now been termed radiographic axSpA (r‐axSpA) (see Figure 1). The decreasing sizes of the three chevrons from left to right in Figure 1 are meant to emphasize that only a portion of the patients with nr‐axSpA will progress to r‐axSpA/AS, estimated to be 5% in 5 years and 19% in 10 years 28, 29. Others may remain as nr‐axSpA, perhaps forever, and some patients can possibly have a self‐limiting disease course.
Figure 1.
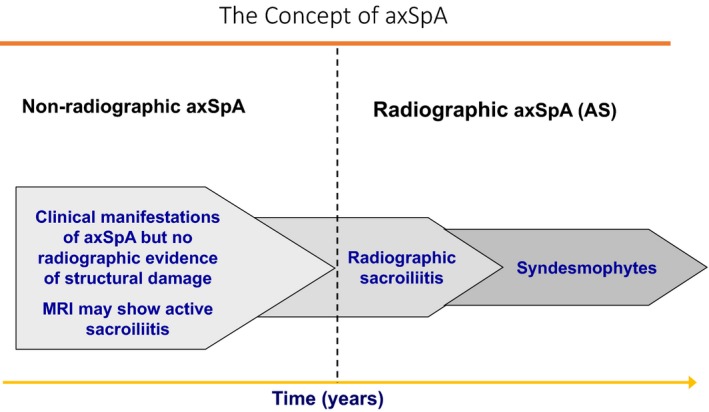
This figure schematically shows a unifying concept of axSpA that has a wide clinical spectrum. Inflammatory back pain is the leading symptom that may be present throughout the disease course without any occurrence of structural damage. As further explained in the text, the decreasing sizes of the three chevrons from the left to the right of this figure are meant to emphasize that only a portion of patients with nr‐axSpA will progress to r‐axSpA/AS, whereas others may remain as nr‐axSpA, perhaps forever or have a self‐limiting disease course. This figure also shows that not all patients with radiographic sacroiliitis progress to form syndesmophytes with resulting spinal ankylosis. This figure is adapted from figure 1 from the author's previous publication 17.
In 2009, the Assessment of Spondyloarthritis International Society (ASAS) proposed a new set of classification criteria to encompass this wider clinical spectrum of axSpA, that has an imaging arm and a clinical arm 30, 31. The imaging arm requires the presence of either radiographic sacroiliitis (meeting the mNY criteria grading of bilateral grade 2 or unilateral grade 3 or 4) or active inflammation of the sacroiliac joints detected by MRI, plus at least one other characteristic feature of SpA, whereas the clinical arm requires the presence of HLA–B27 plus at least two other characteristic features of SpA. The patients can be classified as having axSpA if they fulfill the imaging or the clinical arm, but always on the background of having chronic back pain for at least 3 months with an age at onset not exceeding 45 years 30, 31, 32.
Recent studies have shown that nr‐axSpA is a more heterogeneous entity than AS. For example, patients with nr‐axSpA are female at a relatively higher proportion, confirming the original report 26, have a lower burden of inflammation, and a slower disease course that can sometimes be self‐limiting, as compared with patients with AS 32, 33, 34, 35. The primary goal of any valid classification criteria for any disease is to provide a homogeneous study population with a common etiopathogenesis, similar prognosis, and similar response to identical treatment 36, 37, 38. All criteria are dynamic concepts that need to be updated as our knowledge advances. Thus, there is a need for further research to improve the ASAS criteria, based on better knowledge of the natural history, etiopathogenesis, and response to treatment of patients with axSpA 36, 37, 38. Availability of reliable biomarkers are needed to help identify patients with nr‐axSpA who are more likely to progress to AS and to facilitate therapeutic drug trials designed to prevent or retard such progression 36, 37, 38.
The specificity and sensitivity of the ASAS classification criteria for axSpA were found to be 84.4% and 82.9%, respectively, based on the experts’ opinions 30, 31, 32. However, these performance characteristics were not obtained in an independent set of patients and therefore may be even lower when tested in independent new sets of patients that include non‐Europeans. Ideally, the classification criteria should have at least a 90% specificity (in order to decrease their false‐positivity) while retaining a sensitivity of at least 80%. It is now generally agreed that a re‐evaluation of the ASAS classification criteria is needed. Therefore, ASAS and Spondyloarthritis Research and Treatment Network (SPARTAN) have embarked on a study named Classification of Axial Spondyloarthritis Inception Cohort (CLASSIC) to validate the current criteria in independent prospective cohorts and plan for its subsequent improvement if its specificity is found to be below 90% (CLASSIC: Background and Introduction. http://www.spartangroup.org). In the meantime, the mNY criteria for AS continue to be very useful for defining a relatively homogenous group of cases for clinical research and genetic studies 36.
It needs to be emphasized that there are no validated diagnostic criteria for axSpA/AS, and clinicians are inappropriately using the mNY criteria and more recently the ASAS criteria as an aid to clinical diagnosis in daily practice. This also results from a widespread lack of understanding of the differences between diagnostic and the classification criteria 27. We admit that, in hindsight, we had wrongly labeled the mNY criteria as diagnostic criteria in our publications 24, 25. This error occurred because, at the time, there was no concept of classification criteria and diagnostic criteria as two distinct entities. For example, Watson Buchanan, a famous rheumatologist, stated in 1980 that the Rome criteria were diagnostic criteria and that the New York criteria should be used in clinical rheumatology practice and in epidemiological surveys 3. Moreover, Alvin Feinstein, a well‐known epidemiologist and statistician of his time, discussed the revised Jones criteria for acute rheumatic fever as diagnostic criteria 39.
Conclusion
In clinical medicine, use of the term axSpA should be preferred over AS because the word “ankylosing” has a negative prognostic connotation for patients as that degree of structural damage may take a long time to develop or may not occur at all. The introduction of the mNY and the ASAS classification criteria has been very important steps forward for clinical research, earlier disease recognition, and for conducting treatment trials that have led to approval of biologic therapies for patients with axSpA, including AS and nr‐axSpA. The performance characteristics of the ASAS criteria are now being re‐evaluated in independent prospective cohorts of axSpA patients prior to any decision to further improve them in order to achieve a specificity of at least 90%.
The published studies on the efficacy of the current treatment of patients with axSpA are frequently difficult to compare because some investigators use the term AS and others axSpA or r‐axSpA. However, the patients who fulfill the mNY criteria for AS and those who fulfill the ASAS criteria for r‐axSpA are not fully comparable 37, 38. Therefore, we propose that to promote quality of reporting and comparability of results of future studies of this intriguing disease, authors should clearly indicate important study characteristics, such as age, sex, disease duration, HLA‐B27 status, and proportions of patients with AS, nr‐axSpA, and r‐axSpA.
Lastly, we have a new name—axSpA—for an old disease 32, and to paraphrase William Shakespeare (who had stated in a different context “What we call a rose would smell as sweet by any other name”): What we call a disease is the same by another name.
Author Contributions
Drs. Khan and van der Linden drafted the article and critically reviewed it for important intellectual content.
Footnote
Eponyms of AS: Morbus Bechterew, Bekhterev's syndrome, Bechterew's disease, Bekhterev‐Strümpell‐Marie disease, Marie's disease, Marie‐Strümpell arthritis, (Marie‐Strumpell‐Bechterew disease, Pierre‐Marie's disease 3, 4, 5, 6, 7, 8.
Synonyms of AS: Pelvospondylitis, pelvospondylitis ossificans, spondylitis atrophica ligamentosa, rheumatismal ossifying pelvospondylitis, spondylose rhizomélique, spondylosis ankylopoëtica, pelvospondylitis ossificans, spondylitis ossificans ligamentosa, spondylitis ankylopoetica, spondyloarthritis ankylopoëtica, bamboo spine, poker back, atrophic ligamentous spondylitis; ossifying ligamentous spondylitis, rhizomelic spondylosis, spondylitis deformans, rheumatoid ossifying pelvispondylitis, and rheumatoid spondylitis 3, 4, 5, 6, 7, 8.
Muhammad A. Khan, MD, FRCP, MACP: Case Western Reserve University, Cleveland, Ohio; Sjef van der Linden, MD, PhD: Maastricht University Medical Center, Maastricht, The Netherlands, and University of Bern, Bern, Switzerland.
No potential conflicts of interest relevant to this article were reported.
References
Correction added after online publication 4 September 2019: the title of reference 26 has been updated.
- 1. Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–7. [DOI] [PubMed] [Google Scholar]
- 2. Akkoc N, Khan MA. Epidemiology of axial spondyloarthritis In: Mease P, Khan MA, editors. Axial Spondyloarthritis. 1st ed Amsterdam: Elsevier; 2019. p. 31–56. [Google Scholar]
- 3. Spencer DG, Sturrock RD, Buchanan WW. Ankylosing spondylitis: yesterday and today. Med Hist 1980;24:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumberg BS, Blumberg JL. Bernard Connor (1666‐1698): and his contribution to the pathology of ankylosing spondylitis. J Hist Med Allied Sci 1958;13:349–66. [DOI] [PubMed] [Google Scholar]
- 5. Von Bechterew W. Steifigkeit der wirbelsäule und ihre verkrümmung als besondere erkrankungsform. Neurologischer Zentralblatt 1893;12:426. [Google Scholar]
- 6. Strümpell A. “Bemerkung über die chronische ankylosierende entzündung der wirbelsäule und der hüftgelenke.” Dtsch Z Nervenheilkd 1897;11:338–42. [Google Scholar]
- 7. Marie P. Sur la spondylose rhizomélique. Revue de Médecine 1898;18:285–315. [Google Scholar]
- 8. Matteson EL, Woywodt A. Eponymophilia in rheumatology. Rheumatology (Oxford) 2006;45:1328–30. [DOI] [PubMed] [Google Scholar]
- 9. Hollander JL, McCarthy DJ. Arthritis and allied conditions. 8th ed Philadelphia (PA): Lea & Febiger; 1974. p. 701. [Google Scholar]
- 10. Wright V, Moll JM. Ankylosing spondylitis. Br J Hosp Med 1973;9:331–41. [Google Scholar]
- 11. Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis 1971;30:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McEwen C, DiTata D, Lingg C, Porini A, Good A, Rankin T. Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter's disease. A comparative study. Arthritis Rheum 1971;14:291–318. [DOI] [PubMed] [Google Scholar]
- 13. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. [DOI] [PubMed] [Google Scholar]
- 14. Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL‐A 27. Lancet 1973;1:904–7. [DOI] [PubMed] [Google Scholar]
- 15. Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL‐A antigen, W27, with ankylosing spondylitis. N Engl J Med 1973;288:704–6. [DOI] [PubMed] [Google Scholar]
- 16. Khan MA, editor. Ankylosing spondylitis and related spondyloarthropathies. Philadelphia (PA): Hanley & Belfus, Inc.; 1990. [Google Scholar]
- 17. Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005;52:1000–8. [DOI] [PubMed] [Google Scholar]
- 18. Mease M, Khan MA, editors. Axial spondyloarthritis. 1st ed Amsterdam: Elsevier; 2019. [Google Scholar]
- 19. Kellgren JH, Jeffrey MR, Ball J. The epidemiology of chronic rheumatism. Vol. I Oxford: Blackwell Scientific Publications; 1963. p. 326–7. [Google Scholar]
- 20. Gofton JP, Lawrence JS, Bennett PH, Burch TA. Sacro‐iliitis in eight populations. Ann Rheum Dis 1966;25:528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett PH, Wood PH. Population studies of the rheumatic diseases. 1st ed Amsterdam: Excerpta Medica Foundation; 1968. p. 456–7. [Google Scholar]
- 22. Moll JM, Wright V. New York clinical criteria for ankylosing spondylitis. A statistical evaluation. Ann Rheum Dis 1973;32:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calin A, Porta J, Fries JF, Schurmann DJ. Clinical history as a screening test for ankylosing spondylitis. JAMA 1977;237:2613–4. [PubMed] [Google Scholar]
- 24. Van der Linden S. Spondylitis ankylopoetica, een familie en bevolkingsonderzoek en toetsing van diagnostische criteria [MD thesis]. Leiden, The Netherlands: Leiden University; 1982. [Google Scholar]
- 25. Van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 26. Khan MA, van der Linden SM, Kushner I, Valkenburg HA, Cats A. Spondylitic disease without radiologic evidence of sacroiliitis in relatives of HLA‐B27 positive ankylosing spondylitis patients. Arthritis Rheum 1985;28:40–3. [DOI] [PubMed] [Google Scholar]
- 27. Mease PJ. Spondyloarthritis update: new insights regarding classifcation, pathophysiology, and management. Bull NYU Hosp Jt Dis 2008;66:203–9. [PubMed] [Google Scholar]
- 28. Wang R, Gabriel SE, Ward MM. Progression of nonradiographic axial spondyloarthritis to ankylosing spondylitis: a population‐based cohort study. Arthritis Rheumatol 2016;68:1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang R, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol 2018;30:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of Spondyloarthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 31. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of Spondyloarthritis International Society classification criteria for axial spondylarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 32. Sieper J, van der Heijde D. Nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013;65:543–51. [DOI] [PubMed] [Google Scholar]
- 33. Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. Do patients with non‐radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res 2012;64:1415–22. [DOI] [PubMed] [Google Scholar]
- 34. Poddubnyy D, Brandt H, Vahldiek J, Spiller I, Song IH, Rudwaleit M, et al. The frequency of non‐radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann Rheum Dis 2012;71:1998–2001. [DOI] [PubMed] [Google Scholar]
- 35. Poddubnyy D, Sieper J. Similarities and differences between nonradiographic an radiographic axial spondyloarthritis: a clinical, epidemiological and therapeutic assessment. Curr Opin Rheumatol 2014;26:377–83. [DOI] [PubMed] [Google Scholar]
- 36. Robinson PC, Wordsworth BP, Reveille JD, Brown MA. Axial spondyloarthritis: a new disease entity, not necessarily early ankylosing spondylitis. Ann Rheum Dis 2013;72:162–4. [DOI] [PubMed] [Google Scholar]
- 37. Van der Linden S, Akkoc N, Brown MA, Robinson PC, Khan MA. The ASAS criteria for axial spondyloarthritis: strengths, weaknesses, and proposals for a way forward. Curr Rheumatol Rep 2015;17:62. [DOI] [PubMed] [Google Scholar]
- 38. Akkoc N, Khan MA. Looking into the new ASAS classification criteria for axial spondyloarthritis through the other side of the glass. Curr Rheumatol Rep 2015;17:515. [DOI] [PubMed] [Google Scholar]
- 39. Feinstein AR. Clinical biostatistics. XLV. The purposes and functions of criteria. Clin Pharmacol Ther 1978;24:779–92. [PubMed] [Google Scholar]


