Abstract
Objective
The objective of this study is to investigate the clinical characteristics and treatment of patients with early‐onset gout.
Methods
We retrospectively reviewed 327 adult patients with a first diagnosis of gout from 2008 to 2016 using the database of a multispecialty group practice in New England. Patients were classified into the following groups: age 30 years or younger at first diagnosis (group 1), age 31‐40 years (group 2), and age over 40 years (group 3). The clinical characteristics and treatment of gout were compared among the three groups.
Results
We identified 87 patients in group 1 and 140 patients in group 2. Group 3 included 100 patients randomly chosen from the 7216 patients with a first diagnosis at age over 40 years. Patients within group 1 had significantly higher serum uric acid (sUA) levels at the time of diagnosis and a more prominent family history of gout. Younger patients (groups 1 and 2) had a significantly higher body mass index than patients over 40 years of age (group 3). A substantial number of younger patients also had hypertension or hyperlipidemia. The majority of younger patients met the 2012 American College of Rheumatology (ACR) guidelines for initiating urate‐lowering therapy (ULT) on the basis of frequency of gout attacks, whereas the majority of patients over 40 years of age met the guidelines for ULT on the basis of chronic kidney disease. Patients over 40 years of age were more likely to achieve an sUA level less than 6.0 mg/dl.
Conclusion
Patients with a first diagnosis of gout at age 40 years or younger frequently had cardiovascular risk factors and were less likely to achieve an sUA level less than 6.0 mg/dl compared with patients over 40 years of age who were treated in routine clinical practice. Clinicians should be aware that patients with early‐onset gout may be an undertreated population with poor adherence to ULT and increased risk of recurrent gout and cardiovascular diseases.
Introduction
Gout is the most common inflammatory arthritis in adults, affecting primarily men over 45 years of age 1, 2. The prevalence of gout among US adults more than doubled between the 1960s and the 1990s 3 and was estimated at 3.9% in a national study in 2007‐2008 4.
In recent decades, data have shown that the first attack of gout occurs at a younger age than previously found 5, 6, 7. In 2007‐2008, the prevalence of gout was 0.4% among US adults aged 20‐29 years and was 1.3% among those aged 30‐39 years 4. Similar data for the US population within the past decade are not available. Meanwhile, the distribution of gout varies among different populations; for example, in a retrospective study in Taiwan, Yu and Luo 6 found that 25% of gout patients had their first attack before age 30.
Although, to date, there has been no consensus on the definition of early‐onset gout 8, 9, 10, distinct features in the younger patient population have been reported. A cross‐sectional French national cohort study (GOSPEL) revealed that patients with gout onset before age 40 years often had polyarticular flares, a family history of gout, longer urate‐lowering treatment, higher serum uric acid (sUA) levels, and the metabolic syndrome 10. Chen and Shen 11 found that gout onset before age 20 years was associated with obesity and a positive family history of gout. In a cross‐sectional observational study from a Chinese gout clinic, Zhang et al 12 reported that patients with gout onset before 40 years of age had more frequent flares, more joints clinically affected, longer disease duration, and a lower proportion of cardiovascular, cerebrovascular, and renal comorbidities at presentation. However, patients with early‐onset gout were at higher risk of severe gout and future cardiovascular events 12.
The clinical characteristics of gout, including comorbidities, concomitant medications, treatment adherence, and outcome, have been extensively investigated, mainly in elderly patients. Limited data are available for characterizing early‐onset gout 13, especially in the US population. Given the potential aggressiveness of gout progression and the clinical consequences that would warrant early recognition and intervention, we conducted this retrospective study to investigate the clinical characteristics and treatment of young patients with gout.
Patients and Methods
We retrospectively reviewed the medical records of 327 adult patients who received a diagnosis of gout from January 1, 2008, to December 31, 2016, using the database of a multispecialty group practice in New England. The group practice provides care in Central and MetroWest Massachusetts. All medical records are shared within this group.
Inclusion criteria included the following: 1) age 18 years or older; 2) patient had 2 or more diagnoses of gout per International Classification of Diseases, 10th Revision (ICD‐10) code M10.xx or International Classification of Diseases, Ninth Revision (ICD‐9) code 274.xx recorded on 2 or more dates (office visit only), or patient had 1 diagnosis of gout (office visit only) and 1 or more pharmacy prescriptions of gout‐related medication (allopurinol, febuxostat, or colchicine); and 3) patient was treated by the primary care provider or rheumatologist within the multispecialty group practice at the time of first diagnosis of gout.
Exclusion criteria included the following: 1) patients who did not fulfill the 2015 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) gout classification criteria 14 based on a review of available medical records, 2) patients who took allopurinol, febuxostat, or colchicine for medical conditions other than gout, 3) inadequate information from available medical records to evaluate the validity of the diagnosis of gout or assess treatment, 4) patients who discontinued medical care from the multispecialty group practice prior to the chart review, and 5) patients who were deceased at the time of the chart review.
Patient groups
Patients were classified into the following groups: age 30 years or younger at first diagnosis of gout (group 1), age 31‐40 years (group 2), and age >40 years (group 3). Age at the first diagnosis of gout was initially determined based on the onset date documented in the problem list in the electronic medical database but was adjusted if needed based on a subsequent chart review.
Data collection
All charts of patients age 40 years or younger by the date when gout was first diagnosed were reviewed; 100 of 7216 patients first diagnosed with gout at over 40 years of age were randomly selected and defined as group 3 (Figure 1). All data were extracted from electronic medical records: demographic features, comorbidities, gout diagnosis–related information, and laboratory and radiological findings at the time of diagnosis and follow‐up. Assessment of the indication for treatment was based on 2012 ACR guidelines for the management of gout 15, 16. We compared the clinical characteristics among the three groups. The study protocol was approved by the local institutional review board.
Figure 1.
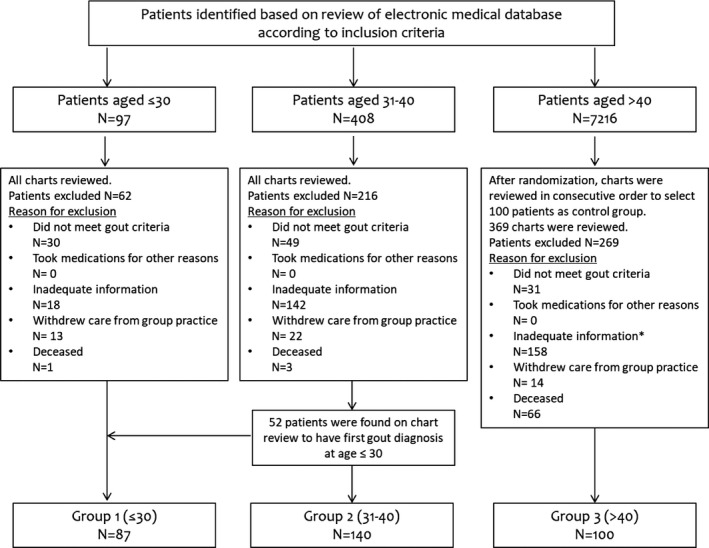
Patient inclusion flowchart. According to the inclusion criteria, 97, 408, and 7216 patients were identified to have a first diagnosis of gout at age 30 years or younger, at age 31‐40 years, and at over 40 years of age, respectively, during the initial review of the electronic medical database based on the age documented when gout was first listed on the problem list. After a detailed chart review and after applying the exclusion criteria, 87 and 140 patients were identified in groups 1 and 2, respectively; 100 patients were randomly selected from patients aged over 40 years and defined as group 3. *Ten patients were found on the chart review to have a first gout diagnosis at age 40 years and younger; however, all were excluded because of inadequate information.
Statistical analysis
Continuous variables were described as the mean and SD. Categorical variables were displayed as the fraction and percentage. Comparisons between any two groups were conducted by one‐way analysis of variance, followed by Tukey's honest significant difference test for continuous variables and the χ2 tests for categorical variables. P < 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics version 22.0 software (IBM Corporation).
Results
We identified 87 patients with a first diagnosis of gout at age 30 years or younger (group 1) and 140 patients with a first diagnosis of gout at age 31‐40 years (group 2) in addition to the randomly selected 100 patients diagnosed with gout after age 40 years (group 3), as shown in Figure 1.
Demographic features
The demographic features of the three groups of patients are summarized in Table 1. The majority of patients were white. The male/female ratio and body mass index (BMI) at the time of diagnosis were similar between groups 1 and 2; the male/female ratio and BMI in both groups were significantly higher than those in group 3. The percentage of patients with obesity was higher in group 1, although this did not show statistical difference among the three groups. A positive family history of gout was more frequently identified in group 1. More than half of the patients were managed by primary care providers, and about 40% were seen at least once by a rheumatologist within the group practice (Table 1).
Table 1.
Demographic features of patients in different age groups
| Group 1 (n = 87) | Group 2 (n = 140) | Group 3 (n = 100) | P 1 (Group 1 vs 2)a | P 2 (Group 1 vs 3)b | P 3 (Group 2 vs 3)c | |
|---|---|---|---|---|---|---|
| Male, n (%) | 86 (99) | 136 (97) | 71 (71) | 0.652 | <0.001 | <0.001 |
| Race, n (%) | 0.911 | 0.117 | 0.046 | |||
| White | 69 (79) | 107 (76) | 89 (89) | … | … | … |
| Asian | 6 (7) | 11 (8) | 2 (2) | … | … | … |
| African American | 3 (3) | 3 (2) | 1 (1) | … | … | … |
| Hispanic | 0 (0) | 1 (1) | 2 (2) | … | … | … |
| Unknown | 9 (10) | 180 (13) | 6 (6) | … | … | … |
| Rheumatology consultation, n (%) | 34 (39) | 44 (31) | 49 (49) | 0.253 | 0.187 | 0.007 |
| BMI, x̄ ± SDd | 34.6 ± 7.2 | 33.6 ± 7.5 | 31.5 ± 6.1 | 0.530 | 0.007 | 0.058 |
| With obesity, n (%) | 59 (68) | 88 (63) | 55 (55) | 0.478 | 0.098 | 0.233 |
| Alcohol use, n (%) | 44 (51) | 68 (49) | 48 (48) | 0.786 | 0.770 | 1.000 |
| Family history of gout, n (%) | 26 (30) | 20 (14) | 11 (11) | 0.006 | 0.002 | 0.559 |
Abbreviation: BMI, body mass index.
P 1 results are from comparisons between group 1 and group 2.
P 2 results are from comparisons between group 1 and group 3.
P 3 results are from comparisons between group 2 and group 3.
One‐way analysis of variance P = 0.007.
In groups 1, 2, and 3, respectively, 16, 17, and 20 patients underwent arthrocentesis; 13, 14, and 7 patients in groups 1, 2, and 3, respectively, were reported to be positive for monosodium urate crystals. One patient in group 1 had concomitant calcium pyrophosphate dehydrate reported. The synovial‐fluid white blood cell (WBC) count was not available in all patients given a clinical decision to treat empirically or an inadequate amount of synovial fluid sometimes obtained during arthrocentesis. Twenty‐four patients had an available synovial‐fluid WBC count ranging from few to 67 020 cells per ml.
Features of first gout attack
Patients within group 1 had significantly higher sUA levels at the time of diagnosis (9.2 ±1.5 mg/dl) compared with those in Group 2 (8.4 ± 1.3 mg/dl, p=0.001) and Group 3 (8.4 ± 1.6 mg/dl, p=0.002) (Table 2). The most common sites of the first gout attack in all age groups were the first metatarsophalangeal joint, foot, and ankle. X‐ray reports at the time of gout diagnosis documented degenerative changes in 31%, 23%, and 47% of patients in groups 1, 2, and 3, respectively; erosive changes consistent with gout were found in only a few patients.
Table 2.
Clinical characteristics of first gout attack in different age groups
| Group 1 (n = 87) | Group 2 (n = 140) | Group 3 (n = 100) | P 1 (Group 1 vs 2)a | P 2 (Group 1 vs 3)b | P 3 (Group 2 vs 3)c | |
|---|---|---|---|---|---|---|
| Age at gout diagnosis, x̄ ± SDe | 26 ± 3 | 35 ± 3 | 61 ± 12 | <0.001 | <0.001 | <0.001 |
| sUA at gout diagnosis, x̄ ± SDe | 9.2 ± 1.5 | 8.4 ± 1.3 | 8.4 ± 1.6 | 0.001 | 0.002 | 0.999 |
| sUA >8.0 mg/dl at gout diagnosis, n (%) | 75/87 (86) | 100/136 (74) | 73/99 (74) | 0.030 | 0.045 | 1.000 |
| Arthrocentesis available, n (%) | 16 (18) | 17 (12) | 20 (20) | 0.245 | 0.853 | 0.106 |
| Crystal‐proven gout, n (%) | 13 (15) | 14 (10) | 7 (7) | 1.000 | 0.008 | 0.007 |
| Site of first gout attack, n (%) | 0.732 | 0.026 | 0.207 | |||
| First MTP joint | 49/84 (58)d | 85/138 (62)d | 64/100 (64) | … | … | … |
| Foot and ankle | 29/84 (35) | 41/138 (30) | 20/100 (20) | … | … | … |
| Knee | 5/84 (6) | 6/138 (4) | 5/100 (5) | … | … | … |
| Hand | 0/84 (0) | 2/138 (1) | 4/100 (4) | … | … | … |
| Multiple sites | 1/84 (1) | 4/138 (3) | 7/100 (7) | … | … | … |
Abbreviation: MTP, metatarsophalangeal; sUA, serum uric acid.
P 1 results are from comparisons between group 1 and group 2.
P 2 results are from comparisons between group 1 and group 3.
P 3 results are from comparisons between group 2 and group 3.
Three and two patients in groups 1 and 2, respectively, did not have detailed information of involved joints during the first gout attack.
One‐way analysis of variance P < 0.001.
Medical comorbidities
The concurrence of coronary artery disease and other cardiovascular risk factors, namely hypertension, hyperlipidemia, and diabetes, were significantly higher in patients over age 40 years (group 3), as shown in Figure 2. It is noteworthy that a substantial number of younger patients (both groups 1 and 2) also had hypertension (41% in both groups) or hyperlipidemia (48% and 46% in groups 1 and 2, respectively). Two known risk factors for gout, namely low‐dose aspirin (81 mg daily) and diuretic use, were more prevalent among patients in group 3.
Figure 2.
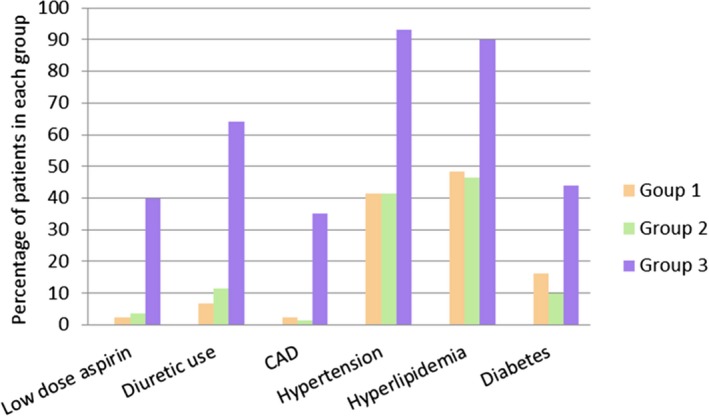
Medical comorbidities, aspirin, and diuretic use in each group. The y‐axis represents the percentage of patients within each age group having each condition.
Treatment
In groups 1 and 3, respectively, 74% and 78% of patients met the 2012 ACR guidelines for urate‐lowering therapy (ULT); only 48% of patients in group 2 warranted ULT. The majority of patients in groups 1 and 2 met the guidelines for ULT on the basis of frequency of gout attacks, as shown in Table 3. The majority of patients in group 3 met the guidelines for ULT on the basis of chronic kidney disease; one‐third of patients had more than one indication for ULT.
Table 3.
Indications and performance of ULT in different age groups
| Group 1 (n = 87) | Group 2 (n = 140) | Group 3 (n = 100) | P 1 (Group 1 vs 2)a | P 2 (Group 1 vs 3)b | P 3 (Group 2 vs 3)c | |
|---|---|---|---|---|---|---|
| ULT indicated by 2012 ACR guidelines, n (%) | 64 (74) | 67 (48) | 78 (78) | <0.001 | 0.479 | <0.001 |
| Flare: >2 per year | 60 (94) | 61 (91) | 42 (54) | 0.745 | <0.001 | <0.001 |
| Tophi | 9 (14) | 4 (6) | 9 (12) | 0.150 | 0.801 | 0.383 |
| CKD (≥stage 2 CKD) | 4 (6) | 7 (10) | 58 (74) | 0.532 | <0.001 | <0.001 |
| Nephrolithiasis | 2 (3) | 8 (12) | 8 (10) | 0.275 | 0.185 | 1.000 |
| Patients who had ≥1 indication for ULT, n (%) | 10 (11) | 9 (6) | 31 (31) | 0.220 | 0.001 | <0.001 |
| ULT initiated/ULT indicated, (%) | 59/64 (92) | 55/67 (82) | 53/78 (68) | 0.086 | <0.001 | 0.051 |
| Prophylaxis initiated/ULT initiated, (%) | 36/59 (61) | 33/55 (60) | 27/53 (51) | 1.000 | 0.341 | 0.439 |
| sUA <6.0mg/dl achieved/ULT initiated, (%) | 19/59 (32)d | 21/54 (39)d | 24/45 (53)d | 0.555 | 0.044 | 0.163 |
Abbreviation: CKD, chronic kidney disease; sUA, serum uric acid; ULT, urate‐lowering therapy.
P 1 results are from comparisons between group 1 and group 2.
P 2 results are from comparisons between group 1 and group 3.
P 3 results are from comparisons between group 2 and group 3.
Zero, one, and eight patients in groups 1, 2, and 3, respectively, did not have follow‐up sUA tests.
A higher percentage of patients in group 1 initially received recommended ULT, whereas only 68% of patients in group 3 who met guidelines for ULT initiated treatment. Among patients who started ULT, more than half received prophylaxis in all three groups. Although not consistently tested, the last available sUA during the study time frame (January 1, 2008, to December 31, 2016) was used to assess the achievement of an sUA level less than6.0 mg/dl. Compared with younger patients (groups 1 and 2), patients in group 3 were more likely to achieve an sUA level less than 6.0 mg/dl.
Discussion
Our study demonstrated that patients with gout onset at age 30 years or younger had significantly higher sUA levels at the time of diagnosis and a more prominent family history of gout compared with patients with gout onset after age 30 years (groups 2 and 3). Patients in group 2 had similar clinical features to those in group 1, including a significantly higher BMI than that of patients in group 3. The prevalence of cardiovascular risk factors (hypertension and hyperlipidemia) was unexpectedly high among younger patients (both groups 1 and 2; Figure 2).
Although it did not reach statistical significance, we found that the prevalence of obesity was higher in patients with early‐onset gout. Obesity has previously been found to be associated with earlier gout onset. In a community‐based cohort study, DeMarco et al 17 found that the overall mean age at gout onset was 59.3 years. The onset of gout was 3.1 years earlier in those with obesity at baseline and was 11.0 years earlier in participants with obesity at age 21 compared with participants without obesity.
Although the prevalence of cardiovascular risk factors was understandably higher in patients over age 40 years, it was surprising that nearly half of patients under age 30 years had been diagnosed with hypertension or hyperlipidemia and that 30% of patients had both. Similar findings were also seen in patients with gout onset age 31‐40 years. The prevalence of cardiovascular risk factors among younger patients with gout is substantially higher than the risk in the general population. Based on Centers for Disease Control and Prevention data from 2015‐2016, the prevalence of hypertension in the US general population was 7.5% in those age 18‐39 years and 33.2% in those age 40‐59 years 18. Sullivan et al 19 reported that the prevalence of hypertension in the general population was 0.4%, 2.2%, and 6.5% in age groups 18‐19, 20‐29, and 30‐39 years, respectively, and the prevalence of hyperlipidemia was 0.1%, 0.4%, and 2.3% in age groups 18‐19, 20‐29, and 30‐39 years, respectively. Along with obesity and hyperuricemia, lifestyle changes in recent decades 20, including sedentary habits, a diet rich in red meat and fructose, and increased alcohol consumption, could play a role in the shift toward a younger population with gout and warrant early intervention.
Gout is an independent risk factor for all‐cause and cardiovascular mortality 21. A higher sUA level has been confirmed to pose an increased risk of incident and recurrent gout 22. Hyperuricemia has also been suggested as an integral part of metabolic syndrome and an independent risk factor for both cardiovascular disease and renal disease 23, 24. The potential mutual benefit of ULT on gout and cardiovascular disease has been proposed; however, previous studies have not shown consistent results 25, 26, 27, 28. Additional studies are warranted to determine whether treatment of gout or hyperuricemia in patients younger than age 40 decreases the risk of cardiovascular disease.
More than 90% of patients in group 1 met criteria for ULT initiated treatment, but only 32% achieved a target sUA level less than 6.0 mg/dl. By comparison, 68% of patients in group 3 met criteria for ULT initiated treatment, of whom 53% achieved the target sUA level (Table 3). Group 2 had an intermediate percentage of patients who achieved the target sUA level. Previous studies have shown that adherence to ULT is poor; among the treatment of seven common chronic diseases, including hypertension and diabetes, adherence to gout therapy was the lowest 29, 30. A lack of education on lifestyle changes and gout‐related medications, particularly the risks of gout flares with initiation of ULT 31, likely contributes to poor adherence. Patients with early‐onset gout appear to be a particularly high‐risk group for poor adherence.
Our study has several limitations. The retrospective chart review was subject to incomplete data and clinical documentation. For example, we could not quantify alcohol consumption, provide details of any family history of gout, and specify reasons ULT or prophylaxis was or was not prescribed or taken. The achievement of an sUA level less than 6.0 mg/dl was based on the last available sUA, which was not consistently tested.
Patients with a first diagnosis of gout at age 40 years or younger frequently had cardiovascular risk factors and were less likely to achieve an sUA level less than 6.0 mg/dl compared with patients over age 40 who were treated in routine clinical practice. Clinicians should be aware that patients with early‐onset gout may be an undertreated population with poor adherence to ULT and increased risk for recurrent gout and cardiovascular diseases.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Li, Piranavan, Yood.
Acquisition of data
Sundaresan.
Analysis and interpretation of data
Li, Piranavan, Yood.
Acknowledgments
The authors give special thanks to Joel Popkin, MD, for critical review and interpretation of the study results.
No potential conflicts of interest relevant to this article were reported.
References
- 1. Dirken‐Heukensfeldt KJ, Teunissen TA, van de Lisdonk H, Lagro‐Janssen AL. “Clinical features of women with gout arthritis.” A systematic review. Clin Rheumatol 2010;29:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh JA. Research priorities in gout: the patient perspective. J Rheumatol 2014;41:615–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007‐2008. Arthritis Rheum 2011;63:3136–41. [DOI] [PubMed] [Google Scholar]
- 5. Lu CC, Wu SK, Chen HY, Chung WS, Lee MC, Yeh CJ. Clinical characteristics of and relationship between metabolic components and renal function among patients with early‐onset juvenile tophaceous gout. J Rheumatol 2014;41:1878–83. [DOI] [PubMed] [Google Scholar]
- 6. Yu KH, Luo SF. Younger age of onset of gout in Taiwan. Rheumatology (Oxford) 2003;42:166–70. [DOI] [PubMed] [Google Scholar]
- 7. Chen SY, Chen CL, Shen ML, Kamatani N. Trends in the manifestations of gout in Taiwan. Rheumatology (Oxford) 2003;42:1529–33. [DOI] [PubMed] [Google Scholar]
- 8. Matsuo H, Ichida K, Takada T, Nakayama A, Nakashima H, Nakamura T, et al. Common dysfunctional variants in ABCG2 are a major cause of early‐onset gout. Sci Rep 2013;3:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li QH, Liang JJ, Chen LX, Mo YQ, Wei XN, Zheng DH, et al. Clinical characteristics and renal uric acid excretion in early‐onset gout patients. Zhonghua Nei Ke Za Zhi 2018;57:185–90. In Chinese. [DOI] [PubMed] [Google Scholar]
- 10. Pascart T, Norberciak L, Ea HK, Guggenbuhl P, Liote F. Patients with early‐onset gout and development of earlier severe joint involvement and metabolic comorbid conditions: results from a cross‐sectional epidemiologic survey. Arthritis Care Res (Hoboken) 2019;71:986–92. [DOI] [PubMed] [Google Scholar]
- 11. Chen SY, Shen ML. Juvenile gout in Taiwan associated with family history and overweight. J Rheumatol 2007;34:2308–11. [PubMed] [Google Scholar]
- 12. Zhang B, Fang W, Zeng X, Zhang Y, Ma Y, Sheng F, et al. Clinical characteristics of early‐ and late‐onset gout: a cross‐sectional observational study from a Chinese gout clinic. Medicine (Baltimore) 2016;95:e5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamanaka H. Gout and hyperuricemia in young people. Curr Opin Rheumatol 2011;23:156–60. [DOI] [PubMed] [Google Scholar]
- 14. Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative [published erratum appears in Arthritis Rheumatol 2016;68:515]. Arthritis Rheumatol 2015;67:2557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1. Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2. Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 2012;64:1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeMarco MA, Maynard JW, Huizinga MM, Baer AN, Kottgen A, Gelber AC, et al. Obesity and younger age at gout onset in a community‐based cohort. Arthritis Care Res (Hoboken) 2011;63:1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon‐Moran D. Hypertension prevalence and control among adults: United States, 2015‐2016. NCHS Data Brief 2017;1–8. [PubMed] [Google Scholar]
- 19. Sullivan PW, Ghushchyan VH, Ben‐Joseph R. The impact of obesity on diabetes, hyperlipidemia and hypertension in the United States. Qual Life Res 2008;17:1063–71. [DOI] [PubMed] [Google Scholar]
- 20. Doherty M. New insights into the epidemiology of gout. Rheumatology (Oxford) 2009;48 Suppl 2:ii2–8. [DOI] [PubMed] [Google Scholar]
- 21. Kuo CF, See LC, Luo SF, Ko YS, Lin YS, Hwang JS, et al. Gout: an independent risk factor for all‐cause and cardiovascular mortality. Rheumatology (Oxford) 2010;49:141–6. [DOI] [PubMed] [Google Scholar]
- 22. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol 2017;44:388–96. [DOI] [PubMed] [Google Scholar]
- 23. Wei CY, Sun CC, Wei JC, Tai HC, Sun CA, Chung CF, et al. Association between hyperuricemia and metabolic syndrome: an epidemiological study of a labor force population in Taiwan. Biomed Res Int 2015;2015:369179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk [published erratum appears. ]. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT‐HF) Study. Circulation 2015;131:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med 2015;128:653.e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis 2016;75:1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Givertz MM. Treating gout in patients with cardiovascular disease: mutual benefit or unintended consequences? [editorial]. J Am Coll Cardiol 2018;71:1005–8. [DOI] [PubMed] [Google Scholar]
- 29. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008;28:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheepers LE, van Onna M, Stehouwer CD, Singh JA, Arts IC, Boonen A. Medication adherence among patients with gout: a systematic review and meta‐analysis. Semin Arthritis Rheum 2018;47:689–702. [DOI] [PubMed] [Google Scholar]
- 31. Harrold LR, Mazor KM, Peterson D, Naz N, Firneno C, Yood RA. Patients’ knowledge and beliefs concerning gout and its treatment: a population based study. BMC Musculoskelet Disord 2012;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]


