Abstract
Objective
Up to 30% of patients experience persistent pain and functional limitations following total knee replacement (TKR). Rapid symptom relief in the early postoperative period may be linked to longer‐term outcome improvements. We sought to identify early improvement trajectories and to identify risk factors for suboptimal outcomes.
Methods
We used data from the Adding Value in Knee Arthroplasty (AViKA) Cohort study, a prospective longitudinal study of patients with knee osteoarthritis who underwent TKR. We assessed pain and function using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). We used group‐based trajectory modeling to identify distinct patterns of pain and function improvement over 6 months. We assessed the association between these early improvement trajectories and 24‐month outcomes, including pain, function, and satisfaction.
Results
We analyzed data from 107 subjects. Mean baseline WOMAC pain and function scores were 42 (SD 17) and 44 (SD 15), respectively (0‐100; 100 = worst). We identified two pain‐improvement trajectories (suboptimal vs optimal improvement) and two function‐improvement trajectories (suboptimal vs optimal improvement). Greater pain catastrophizing, worse mental health status, and use of a supportive device prior to TKR were associated with being in a suboptimal trajectory. Recipients of TKR in the suboptimal trajectories had higher pain, high functional disability, and lower satisfaction at 24 months post‐TKR.
Conclusion
Patients with slower improvement over the first 6 months post‐TKR had worse outcomes at 24 months, suggesting that this early postoperative period may represent a window during which interventions aimed at speeding recovery may improve long‐term TKR outcomes.
INTRODUCTION
Knee osteoarthritis (OA) affects 14 million US adults and more than 300 million adults worldwide 1, 2. The disease is characterized by joint pain, swelling, and stiffness. OA of the lower extremities is associated with diminished quality of life and ranks among the highest contributors to disability globally 3, 4, 5. In the absence of an approved disease‐modifying OA drug, patients with advanced disease frequently turn to total knee replacement (TKR). TKR is an elective surgical procedure that can reduce pain and restore function in persons with knee OA 6, 7, 8. Use of TKR continues to increase rapidly, with more than 660 000 TKRs performed in 2014 in the United States 9, 10, 11.
Although TKR often relieves pain, improves quality of life, and is cost‐effective, evidence suggests that between 10% and 30% of patients undergoing TKR experience suboptimal outcomes, with persistent pain 1 or 2 years after the procedure 12, 13, 14. There is a critical need to understand which patients are at risk for suboptimal TKR outcomes and to determine whether any such risk factors are modifiable. Gandhi et al 15 recently published a study in which they demonstrated that early changes in pain and function over the first 3 months post‐TKR are strongly associated with outcomes at 2 years post‐TKR, suggesting that this early recovery period may be crucial to long‐term outcomes and that any intervention to improve outcomes must occur early. As the authors note, this early recovery period often involves follow‐up appointments with surgeons and physical therapists and may offer a potential opportunity for intervention.
The literature suggests that the improvement patterns over time may be variable, with some patients experiencing rapid improvement and others experiencing a slower and limited improvement 12, 13, 14. Conventional longitudinal models that estimate a mean population trend employ a 1‐size‐fits‐all approach, in which one improvement trend is estimated; covariates can determine how groups may differ from this mean population trajectory, but ultimately, all individuals are assumed to come from the same distribution 16, 17. Group‐based trajectory modeling (GBTM) allows for the modeling of separate, distinct subgroups based on longitudinal trajectory and may allow for distinct subgroups in a population to be uncovered.
Although previous work has used this methodology to understand improvement trajectories post‐TKR, the focus of these studies was either on defining long‐term improvement or on the acute post‐TKR recovery period in the days after surgery 18, 19, 20. Understanding recovery patterns and uncovering distinct trajectories in the early months post‐TKR could shed light on which patients may be at risk for suboptimal TKR when intervention is still possible.
In this analysis, we used patient‐reported data from the Adding Value in Knee Arthroplasty (AViKA) Cohort study to describe trajectories of pain and functional improvement over 6 months post‐TKR. We identified preoperative characteristics associated with suboptimal improvement trajectory over 6 months and assessed whether early recovery over 6 months was associated with long‐term patient‐reported outcomes at 24 months, including pain, function, and satisfaction.
PATIENTS AND METHODS
Sample
The AViKA Cohort study was a prospective cohort study of consecutive patients undergoing TKR by five orthopedic surgeons at a tertiary medical center. The surgical approaches or rehabilitation protocols were standardized across these five surgeons’ practices. Patients were enrolled between August 2010 and May 2011. Eligible patients were community‐dwelling adults aged 40 years or older, had a primary diagnosis of OA, and spoke English. We excluded patients with a diagnosis other than OA (eg, inflammatory arthritis), those with dementia, those with unicompartmental knee arthroplasty, those who lived in a nursing home, and those with plans for additional elective surgery within 6 months.
Data elements
Data collection
All participants completed a baseline questionnaire within 6 weeks prior to surgery and were followed postoperatively at weeks 1, 2.5, 4, 6, and 8 and at months 3, 4, 5, 6, and 24.
Outcomes
Primary outcomes include pain and functional status, which were assessed with the Western Ontario and McMaster Universities Arthritis Index (WOMAC). The WOMAC pain‐ and functional‐status scores were converted to a 0‐100 scale, with 100 being the worst. WOMAC was assessed at each visit with the exception of weeks 1 and 2.5. As a secondary outcome, we assessed overall satisfaction with surgery with the following question: “How satisfied are you with the results of your knee replacement surgery?” Overall satisfaction was classified as very satisfied vs not very satisfied (including somewhat satisfied, somewhat dissatisfied, and very dissatisfied). This stratification was determined by the distribution of responses and the general response to TKR. In this analysis, we sought to describe the short‐term recovery trajectory (over the first 6 months post‐TKR) and the association between short‐term recovery trajectory and long‐term outcomes at 24 months post‐TKR.
Covariates
We assessed demographics and preoperative clinical characteristics of the cohort at baseline, including age, race, sex, body mass index (BMI), Charlson Comorbidity Index 21, preoperative medication use for knee pain, five‐item Mental Health Index (MHI‐5) 22, Pain Catastrophizing Scale (PCS) 23, use of assisted devices, and the Musculoskeletal (MSK) Functional Limitations Index 24. BMI was calculated from self‐reported height and weight. We then stratified BMI into four groups: BMI less than or equal to 25.0, BMI of 25.1‐30.0, BMI of 30.1‐35.0, and BMI greater than 35.0. The Charlson Comorbidity Index was grouped into none, 1, 2, and 3 or more. Medication use was categorized into three groups: none, occasional, and almost daily. The MHI‐5 was scaled from 0 to 100, with 100 being the best, and was dichotomized as less than 68 and greater than or equal to 68 25. PCS measured catastrophic thinking related to pain and was scaled from 0 to 52, with 52 being the worst. The MSK Functional Limitation Index aggregated self‐reported MSK problems in six anatomic regions that limit patients’ ability to perform daily activities and was scaled from 0 to 12, with 12 being the worst.
Statistical analysis
Descriptive statistics
We first conducted descriptive analysis to describe short‐term improvement after TKR. We assessed pain and function at each time point from baseline through 6 months using mean and SD.
Group‐based trajectory modeling
We used GBTM to determine whether there were latent classes (trajectory groups) with different patterns of pain and function over time 17, 26, 27. We determined trajectory groups separately for WOMAC pain and function, which were assessed at predefined time intervals from baseline to 6 months. The GBTM approach uses pain and function at each time point and uses multinomial modeling to identify multiple trajectory groups. Although conventional longitudinal modeling estimates a mean population trend, GBTM allows for the possibility that there are distinct subgroups within a population. We used a censored normal model, which allows for clustering at the minimum and maximum of scales. This is useful for scales with prespecified ranges, (eg, WOMAC). We considered between one and four trajectories and allowed for up to a fourth‐order polynomial in each trajectory group.
We used the Bayesian information criterion (BIC) as an indicator for the selection of the optimal model. Higher BIC values indicate better model fit. However, BIC values do not always clearly identify the optimal number of groups in GBTM. Therefore, we also took into account group size and stability during the model selection. We selected models with at least 10 participants (approximately 10% of sample) in each group and with similar trajectory shapes when we adjusted the order of polynomial. We then used posterior group‐membership probabilities as indicators of model fit. At the individual level, a subject is assigned a posterior group‐membership probability for each trajectory group, measuring the likelihood of belonging to that specific trajectory, with the probabilities summing to 1 across all groups. Probabilities close to 1 indicate more certainty about trajectory‐group membership; Nagin 17 suggests that an average posterior probability in each trajectory group greater than or equal to 0.7 indicates moderate to excellent model fit.
Association between preoperative characteristics and trajectories
After determining the number of trajectories and group membership, we evaluated the bivariate association between preoperative cohort characteristics and trajectory‐group membership using the t test for continuous variables and the χ2 test for categorical variables. We included variables shown to be statistically significant (P < 0.05) in a bivariate analysis in a multivariable logistic regression model.
Association between 24‐month outcomes and early improvement trajectories
We examined the associations between early improvement trajectory over 6‐ and 24‐month outcomes, including pain, function, and satisfaction. We used short‐term (first 6 months post‐TKR) trajectory‐group membership as the main exposure. We conducted separate analyses for each of these outcomes. First, we used the Wilcoxon rank sum test to compare 24‐month pain and function outcomes between the different trajectories (optimal vs suboptimal). Then we used generalized linear models to evaluate the association between 24‐month pain and function outcomes and early improvement trajectory, adjusting for preoperative covariates described previously that had significant bivariate associations with pain or function trajectory groups. For 24‐month satisfaction, we assessed its association with trajectory‐group membership using the χ2 test or Fisher's exact test. We built multivariate logistic regression to determine the independent factors associated with outcomes, adjusting for significant covariates described previously.
Sensitivity analysis for missing data
We assessed whether cohort characteristics differed between study completers vs noncompleters and compared the dropout rate between trajectory groups. We then used multiple imputation (MI) to augment missing 24‐month pain and function outcome data. MI uses a prediction equation to impute missing values, incorporating information about observed data 28. We accounted for factors that were different between study completers vs noncompleters in addition to observed pain and function. We reran the generalized linear models for the 24‐month pain and function outcomes, incorporating the imputed outcomes. To more accurately reflect the uncertainty in missing values, we created five imputed data sets and then used the MIANALYZE procedure in SAS (SAS Institute, Inc) to combine the results across the five imputations.
Data were collected and managed using Research Electronic Data Capture (REDCap), which is hosted by the Partners HealthCare, Research Computing, Enterprise Research Infrastructure & Services (ERIS) group 29. All analyses were conducted in SAS version 9.4 (SAS Institute, Inc). We used SAS PROC TRAJ, a custom SAS procedure available for free download, for GBTM analyses 26, 27.
RESULTS
Sample description
A total of 185 participants were eligible for the study; 132 (71%) agreed to participate, and of these, 116 (88%) completed a baseline visit. A total of 110 patients underwent TKR; 3 patients had bilateral TKR and were excluded, leaving an analytic cohort of 107 participants. The 78 patients eligible and not included in the analytic cohort were slightly older than the 107 patients included (65.6 vs 64.7 years) and were more likely to be women (51% vs 61%). The demographic difference did not reach statistical differences (P = 0.53 for age; P = 0.21 for sex).
The average age was 64.7 years (SD 8.8), 51% were women, and 88% were white (Table 1). The average BMI was 31.2 (SD 6.6). There were 21 (20%) participants with a BMI less than 25, 31 (29%) participants had a BMI of 25‐29.9, 31 (29%) had a BMI of 30‐34.9, and 23 (22%) had a BMI greater than or equal to 35. The mean Charlson Comorbidity Index score was 1.4 (SD 1.8), and 21 (20%) participants had a score higher than 2. Forty‐three percent reported taking medication for knee pain almost every day. The average PCS score was 12.2 (SD 11.3). There were 25 (23%) participants with an MHI‐5 less than 68. The overall mean MSK index score was 3.5 (SD 2.1). Thirty‐two percent reported use of a supportive device in the last week.
Table 1.
Cohort characteristics
| Cohort Characteristics | Overall (N = 107) |
|---|---|
| WOMAC function score, mean (SD)a | 43.68 (14.92) |
| WOMAC pain score, mean (SD)a | 42.45 (17.43) |
| Index of MSK functional limitations, mean (SD)b | 3.52 (2.10) |
| Use of any supportive device in the past week, n (%) | |
| No | 73 (68.2) |
| Yes | 34 (31.8) |
| Age at surgery, mean (SD), y | 64.72 (8.84) |
| Race/ethnicity, n (%) | |
| Multiracial: Black, Hispanic, mixed‐race | 13 (12.1) |
| White | 94 (87.9) |
| Sex, n (%) | |
| Male | 52 (48.6) |
| Female | 55 (51.4) |
| BMI group, n (%) | |
| <25 | 21 (19.8) |
| ≤25‐30 | 31 (29.2) |
| ≤30‐35 | 31 (29.3) |
| ≥35 | 23 (21.7) |
| Charlson Comorbidity Index group, n (%) | |
| 0 | 43 (40.6) |
| 1 | 22 (20.7) |
| 2 | 20 (18.9) |
| 3+ | 21 (19.8) |
| Pain medication use for knee, n (%) | |
| No | 25 (23.4) |
| Yes, occasionally | 36 (33.6) |
| Yes, almost every day | 46 (43.0) |
| Pain Catastrophizing Scale, mean (SD)c | 12.22 (11.34) |
| MHI‐5 category: <68 vs ≥68, n (%)d | |
| <68 | 25 (23.4) |
| ≥68 | 82 (76.6) |
Abbreviation: BMI, body mass index; MHI‐5, five‐item Mental Health Index; MSK, musculoskeletal; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Scaled at 0‐100, with 100 being the worst.
Scaled at 0‐12, with 12 being the worst.
Scaled at 0‐52, with 52 being the worst.
Scaled at 0‐100, with 100 being the best; the cutoff is at 68.
Longitudinal WOMAC pain and function at 6 months
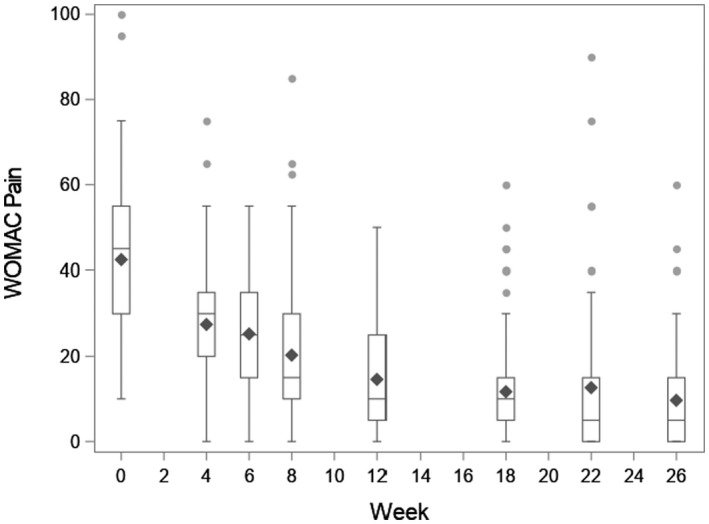
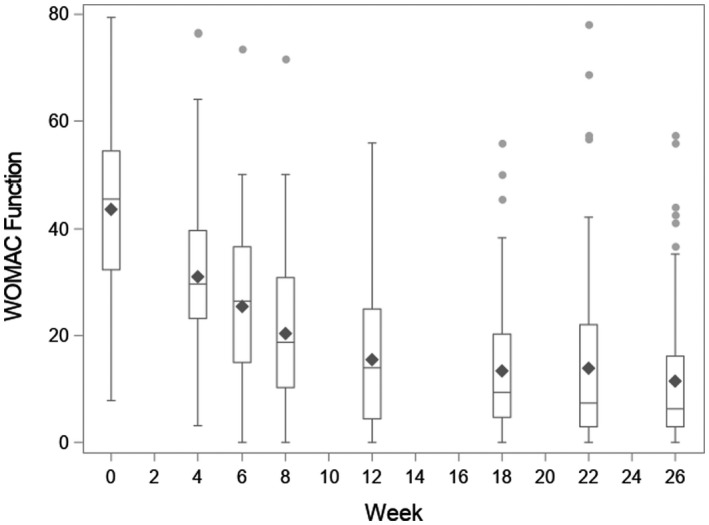
The average preoperative WOMAC pain and function scores were 42.5 (SD 17.4) and 43.7 (SD 14.9), respectively. Both improved substantially in the 6 months post‐TKR, with 6‐month pain and function scores of 9.7 (SD 11.8) and 11.5 (SD 12.8), respectively (Appendix Figures 1 and 2).
Group‐based trajectory modeling
Pain
We identified two distinct early pain‐improvement trajectories (Figure 1): 67 (63%) participants in group 1 (optimal improvement) improved from a mean preoperative pain score of 39.3 (SD 16.3) to a mean score of 3.8 (SD 5.7) at 6 months, and 40 (37%) participants in group 2 (suboptimal improvement) had higher preoperative pain (mean 47.8 [SD 18.2]) and a higher 6‐month score (mean 19.2 [SD 13.0]). The average posterior group‐membership probability was 0.93 for group 1 and 0.92 for group 2, indicating good model fit.
Figure 1.
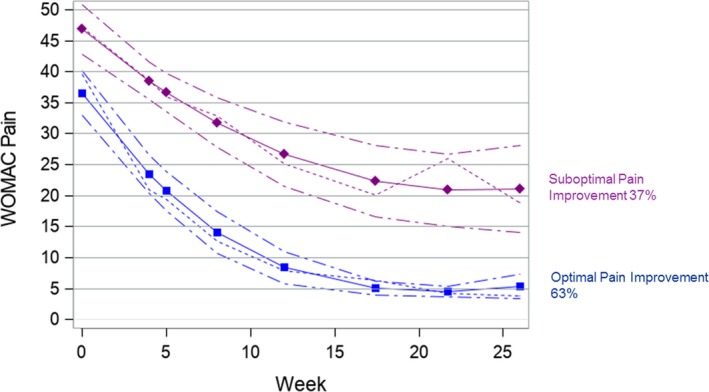
Group‐based early improvement trajectories for the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score. The WOMAC pain score is along the y‐axis, and follow‐up week is along the x‐axis. The short dashed lines depict the mean WOMAC pain score over 6 months post surgery. The solid lines depict the predicted trajectory, and the medium dashed lines represent 95% confidence intervals.
Fifty percent of participants who reported use of a supportive device at baseline were in the suboptimal pain trajectory group, compared with 32% of those who did not require a supportive device (P = 0.066). Patients in the suboptimal pain trajectory group had a higher PCS compared with the optimal pain trajectory group (14.8 vs 10.7; P = 0.072). The MHI‐5 was significantly associated with the pain‐improvement trajectory group: 56% of patients who reported an MHI‐5 less than 68 were in the suboptimal pain trajectory group, compared with 32% of those with an MHI‐5 greater than or equal to 68 (P = 0.028) (Table 2). Associations were similar in the multivariable model, with higher pain catastrophizing, a lower MHI‐5, and use of a supportive device associated with increased odds of being in the suboptimal pain trajectory, although none of the covariates reached statistical significance.
Table 2.
Associations between patient characteristics and early improvement trajectory
| Cohort Characteristics | WOMAC Pain | WOMAC Function | ||||
|---|---|---|---|---|---|---|
| Group 1: Optimal Early Pain Improvement (n = 67)a | Group 2: Suboptimal Early Pain Improvement (n = 40) | P | Group 1:Optimal Early Functional Improvement (n = 66) | Group 2: Suboptimal Early Functional Improvement (n = 41) | P | |
| Age group, y, n (%) | 0.705 | 0.578 | ||||
| <65 | 36 (61.0) | 23 (39.0) | … | 35 (59.3) | 24 (40.7) | … |
| 65+ | 31 (64.6) | 17 (35.4) | … | 31 (64.6) | 17 (35.4) | … |
| Sex, n (%) | 0.823 | 0.976 | ||||
| Male | 32 (61.5) | 20 (38.5) | … | 32 (61.5) | 20 (38.5) | … |
| Female | 35 (63.6) | 20 (36.4) | … | 34 (61.8) | 21 (38.2) | … |
| Race/ethnicity, n (%) | 0.191 | 0. 535 | ||||
| Multiracial: Black, Hispanic, mixed race | 6 (46.2) | 7 (53.8) | … | 7 (53.8) | 6 (46.2) | … |
| White | 61 (64.9) | 33 (35.1) | … | 59 (62.8) | 35 (37.2) | … |
| BMI group, n (%) | 0.403 | 0.465 | ||||
| <25 | 15 (71.4) | 6 (28.6) | … | 14 (66.7) | 7 (33.3) | … |
| ≤25‐30 | 20 (64.5) | 11 (35.5) | … | 19 (61.3) | 12 (38.7) | … |
| ≤30‐35 | 20 (64.5) | 11 (35.5) | … | 21 (67.7) | 10 (32.3) | … |
| ≥35 | 11 (47.8) | 12 (52.2) | … | 11 (47.8) | 12 (52.2) | … |
| Charlson Comorbidity Index group, n (%) | 0.181 | 0.736 | ||||
| 0 | 31 (72.1) | 12 (27.9) | … | 27 (62.8) | 16 (37.2) | … |
| 1 | 15 (68.2) | 7 (31.8) | … | 15 (68.2) | 7 (31.8) | … |
| 2 | 9 (45.0) | 11 (55.0) | … | 13 (65.0) | 7 (35.0) | … |
| 3+ | 12 (57.1) | 9 (42.9) | … | 11 (52.4) | 10 (47.6) | … |
| Pain Catastrophizing Scale, mean (SD)b | 10.7 (10.0) | 14.8 (13.0) | 0.072 | 9.7 (10.4) | 16.2 (11.8) | 0.003 |
| Index of MSK functional limitations, mean (SD)c | 3.4 (2.0) | 3.7 (2.2) | 0.566 | 3.3 (1.9) | 4.0 (2.4) | 0.097 |
| MHI‐5 category: <68 vs ≥68, n (%) | 0.028 | 0.003 | ||||
| <68 | 11 (44.0) | 14 (56.0) | … | 9 (36.0) | 16 (64.0) | … |
| ≥68 | 56 (68.3) | 26 (31.7) | … | 57 (69.5) | 25 (30.5) | … |
| Use of any supportive device in the past week, n (%) | 0.066 | 0.001 | ||||
| No | 50 (68.5) | 23 (31.5) | … | 53 (72.6) | 20 (27.4) | … |
| Yes | 17 (50.0) | 17 (50.0) | … | 13 (38.2) | 21 (61.8) | … |
Abbreviation: BMI, body mass index; MHI‐5, five‐item Mental Health Index; MSK, musculoskeletal; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
t tests were performed for continuous variables; χ2 tests were performed for categorical variables.
Mean (SD) is presented for continuous variables; n (%) is presented for categorical variables.
Scaled at 0‐52, with 52 being the worst.
Scaled at 0‐12, with 12 being the worst.
Function
We identified two distinct early function‐improvement trajectories (Figure 2): 66 (62%) participants in group 1 (optimal) improved from a mean pre‐TKR score of 39.2 (SD 14.4) to a score of 5.3 (SD 6.0) at month 6; 41 (38%) participants in the suboptimal functional‐improvement trajectory group changed from a pre‐TKR score of 50.9 (SD 12.9) to a score of 23.5 (SD 14.1) at 6 months post‐TKR. The average posterior group‐membership probability was 0.973 for group 1 and 0.937 for group 2, indicating good model fit.
Figure 2.
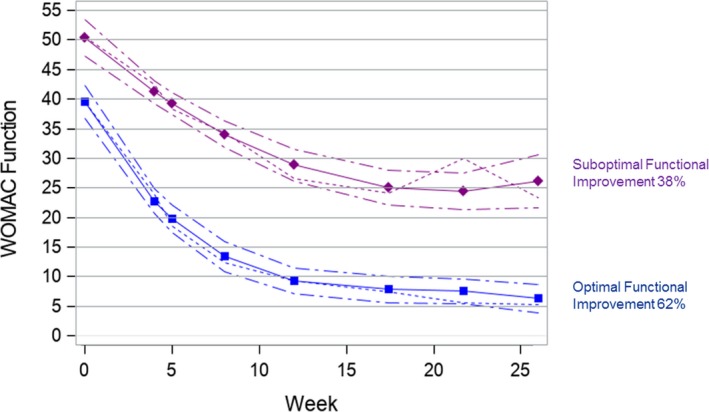
Group‐based early improvement trajectories for the Western Ontario and McMaster Universities Arthritis Index (WOMAC) function score. WOMAC function score is along the y‐axis, and follow‐up week is along the x‐axis. The short dashed lines depict the mean WOMAC function score over 6 months post surgery. The solid lines depict the predicted trajectory, and the medium dashed lines represent 95% confidence intervals.
The PCS, the MHI‐5, and pre‐TKR supportive device use were significantly associated with function‐improvement trajectory. Sixty‐two percent of participants who reported use of a supportive device at baseline were in the suboptimal function‐improvement trajectory group, compared with 27% of those who reported not using a supportive device (P = 0.001). Patients in the suboptimal function‐improvement trajectory group had a higher PCS compared with those in the optimal function‐improvement trajectory group (16.2 vs 9.7; P = 0.003). Sixty‐four percent of patients who reported an MHI‐5 less than 68 were in the suboptimal function‐improvement trajectory group, compared with 31% of those with an MHI‐5 greater than or equal to 68 (P = 0.003) (Table 2). The MHI‐5 and use of supportive device remained significantly associated with function‐improvement trajectory in the multivariable logistic regression. The adjusted odds ratio of being in the suboptimal functional trajectory for users of pre‐TKR supportive device compared with nonusers was 4.0 (95% confidence interval [CI]: 1.5‐10.2), whereas the adjusted odds ratio for those with an MHI‐5 less than 68 compared with an MHI‐5 greater than or equal to 68 was 3.8 (95% CI: 1.3‐11.8). The PCS was not found to be significantly associated with function‐improvement trajectory (adjusted odds ratio 1.0; 95% CI: 1.0‐1.1).
Pain and function
Fifty‐six (52%) participants were in both the optimal pain‐improvement trajectory and the optimal functional‐improvement trajectory, whereas 30 (28%) participants were in suboptimal trajectories for both pain and functional status. Ten participants (9%) were in the suboptimal pain‐improvement trajectory but the optimal functional‐improvement trajectory, and 11 (10%) participants were in the optimal pain‐improvement trajectory but the suboptimal functional‐improvement trajectory.
Twenty‐four–month outcomes
At 24 months post‐TKR, the mean WOMAC pain and function scores were 5.8 (SD 11.9) and 7.9 (SD 12.8), respectively. The mean twenty‐four–month pain score was 11.7 (SE 3.6) in the early suboptimal pain trajectory and 3.1 (SE 0.9) in the early optimal pain trajectory (P = 0.005) (Table 3). After adjusting for the PCS, the MHI‐5, and use of a supportive device, the difference in pain scores between the two early pain‐improvement trajectories remained statistically significant (11.6 vs 4.0; P = 0.004). We observed similar results for function: after adjusting for the PCS, the MHI‐5, and use of a supportive device, 24‐month function scores differed between 6‐month suboptimal and optimal trajectories (15.1 vs 5.6; P = 0.001).
Table 3.
Associations between 24‐month outcomes and 6‐month improvement trajectories
| Analyses | 24‐mo WOMAC Pain | 24‐mo WOMAC Function | ||||
|---|---|---|---|---|---|---|
| Group 1: Optimal Early Pain Improvement (n = 67) | Group 2: Suboptimal Early Pain Improvement (n = 40) | P | Group 1: Optimal Early Functional Improvement (n = 66) | Group 2: Suboptimal Early Functional Improvement (n = 41) | P | |
| Unadjusted analysis, mean (SE), median | 3.1 (0.9), 0 | 11.7(3.6), 5 | 0.005 | 3.7 (1.0), 0.7 | 16.9 (3.4), 13.2 | 0.000 |
| Adjusted analysis, mean (SE) | 4.0 (1.5) | 11.6 (2.1) | 0.004 | 5.6 (1.5) | 15.1 (2.1) | 0.001 |
| Adjusted analysis after MI, mean (SE) | 8.0(3.0) | 14.2 (3.2) | 0.033 | 10.9 (2.2) | 17.9 (2.1) | 0.008 |
Abbreviation: MI, multiple imputation.
Satisfaction and early improvement trajectory group
Seventy‐one percent of the study participants reported that they were very satisfied with surgery at 6 months, and 82% reported that they were very satisfied at 24 months. Ninety percent of the participants in the early optimal pain trajectory group were very satisfied at 24 months, compared with 64% in the early suboptimal pain trajectory group (P = 0.010). After adjusting for the PCS, the MHI‐5, and use of a supportive device, satisfaction remained significantly associated with the pain trajectory group, with participants in the early optimal pain trajectory group having 5.2 times the odds (95% CI: 1.4‐18.9) of being very satisfied with surgery at 24 months, compared with those in the early suboptimal pain trajectory group (Table 4). We found a similar pattern for 24‐month satisfaction in the early function trajectory group.
Table 4.
Associations between 24‐month satisfaction and 6‐month improvement trajectories
| Analyses | 24‐mo Satisfaction | |||||||
|---|---|---|---|---|---|---|---|---|
| Group 1: Suboptimal Early Pain Improvement, n (%) | Group 2: Suboptimal Early Pain Improvement, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | Group 1: Optimal Early Functional Improvement, n (%) | Group 2: Suboptimal Early Functional Improvement, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Very satisfied | 47 (74.6) | 16 (25.4) | 5.3 (1.5‐18.1) | 5.2 (1.4‐18.9) | 48 (76.2) | 15 (23.8) | 8.0 (2.2‐29.2) | 8.0 (2.0‐32.4) |
| Somewhat satisfied/somewhat or very dissatisfied | 5 (35.7) | 9 (64.3) | … | … | 4 (28.6) | 10 (71.4) | … | … |
Abbreviation: CI, confidence interval; OR, odds ratio.
Impact of missing data
Of 107 participants, 99 (93%) completed the 6‐month visit, and 79 (74%) completed the 24‐month visit. Among the 28 participants who dropped out of the study before 24 months, 15 (54%) participants were lost to follow‐up, 5 (18%) had surgery on the contralateral knee and were discontinued, 2 (7%) died, and 6 (21%) dropped out because of other reasons. There were more dropouts in the early suboptimal pain‐ and function‐improvement groups: 15 (38%) participants in the suboptimal pain trajectory group dropped out before 24 months, compared with 13 (19%) participants in the optimal pain trajectory group. Sixteen (39%) participants in the suboptimal function trajectory group dropped out before 24 months, compared with 12 (18%) participants in the optimal function trajectory group. Compared with participants who did not complete the 24‐month visit, study completers tended to have a lower preoperative PCS (mean 10.5 for completers vs 17.2 for dropouts), a higher proportion had MHI‐5 scores greater than or equal to 68 (82% for completers vs 61% for dropouts), and a lower proportion used supportive device (28% for completers vs 43% for dropouts) preoperatively.
We accounted for observed pain and function, demographics (age, sex, and race), and potential factors for dropout (mental health and use of supportive device) in the MI. After MI, differences in 24‐month outcome between trajectory groups were slightly attenuated (Table 3). The adjusted mean 24‐month pain score was 8.0 (SE 3.0) for the early optimal pain trajectory group compared with 14.2 (SE 3.2) for the suboptimal pain trajectory group (P = 0.033). The adjusted mean 24‐month function score was 10.9 (SE 2.2) for the optimal functional‐improvement trajectory group compared with 17.9 (SE 2.1) for the suboptimal functional‐improvement trajectory group (P = 0.008).
DISCUSSION
We identified two distinct trajectories of pain and function recovery in the first 6 months following TKR. We found that accelerated improvement over the first 6 months is predictive of optimized pain relief, functional status, and satisfaction with TKR at 24 months after surgery. For both pain and function, approximately one‐third of participants were in the early suboptimal improvement trajectories. Higher pain catastrophizing, worse mental health status, and use of a supportive device prior to TKR were associated with being in a suboptimal improvement trajectory.
Several studies have investigated recovery trajectories following joint replacement surgery. For instance, Page et al 19 examined WOMAC pain trajectories over the first year following TKR using growth‐mixture modeling. The authors identified four trajectories, with three trajectories exhibiting marked pain improvement and one trajectory (9% of the cohort) demonstrating persistently high pain levels. Poorer preoperative lower‐extremity functioning, higher anxiety scores, and higher levels of functioning difficulty were associated with being in the persistent pain group. Similarly, Dowsey et al 18 used latent class growth analysis to classify groups of subjects according to their trajectory of knee pain and function over 1‐5 years post‐TKR using the Knee Society Score. The authors identified three pain trajectories based on the profile of the Knee Society Score over the 5‐year period, which they named “no pain” (33%), “mild pain” (45%), and “moderate pain” (22%). The authors found that the preoperative 12‐Item Short Form Health Survey (SF‐12) mental component summary and physical component summary and a higher Charlson Comorbidity Index were associated with being in a worse recovery trajectory.
Across the studies by Page et al 19 and Dowsey et al 18 and our study, distinct pain and function recovery trajectories have been consistently identified despite the postoperative time frame considered. In addition, the studies by Page et al 19 and Dowsey et al 18 and our study found that poorer baseline physical and mental function were associated with a worse recovery trajectory. The association between baseline levels of pain and functional status and 6‐ and 24‐month pain and function has been demonstrated previously 30, 31.
Another study used short‐term WOMAC scores over 3 to 12 months to predict long‐term outcomes at 24 months 15. The study found that 80.3% and 79.9% of changes in pain and function, respectively, over the 2 years occurred within the first 3 months. Early changes were associated with final outcomes: changes in pain and function from baseline to 3 months post‐TKR explained the greatest proportion of variability of pain and function at 2 years (16% for pain; 23% for function). A higher comorbidity count and a worse presurgery score were found to be significantly associated with worse 2‐year pain and function scores. These results were consistent with results of our analyses that showed strong association between early pain and function trajectories and outcome at 2 years.
A major strength of our study is the frequent and standardized data collection during the early postoperative period, with WOMAC scores assessed at seven time points between week 4 and month 6. This may have enhanced our ability to separate trajectory from pain flare. We also know that the recovery trend over time for TKR is nonlinear 15, 32; these multiple time points allowed us to model the early rapid recovery over months 0‐3 and the later slower recovery over months 3‐6.
Our analysis had several limitations. Decisions regarding the number and shape of trajectories are somewhat subjective 17. We used BIC and posterior group‐membership probabilities to guide our decisions and sought stable groups with at least 10% of the cohort (approximately 10 participants). It is possible that our modest sample size of 107 limited our ability to uncover a small persistently high pain trajectory or a high disability trajectory, as seen, for example, in the work by Page et al 19. Another possible limitation is biased losses to follow‐up. Our study had 93% completion at the month 6 visit; thus, we do not think that potential missing not at random data would greatly impact our observed trajectories. However, we did note important differences in follow‐up at 24 months, with more dropouts in the high pain‐ and functional‐impairment groups and important differences in the PCS, the MHI‐5, and functional impairment between the study completers vs dropouts. To assess the impact of this dropout on our 24‐month outcome analysis, we used an MI approach and found that the differences in 24‐month pain and function between short‐term trajectory groups were attenuated in this analysis. These data come from one academic medical center in the Northeast United States and may not be generalizable to other TKR populations.
Our cohort represented 58% of eligible patients receiving TKR, and we found differences in age and sex between those who agreed to participate and were included in the analysis vs those who refused and/or were excluded. In addition, although we did not find an association between preoperative medication use and recovery trajectories, we did not have data on specific medication use. Although we also believe postsurgery complications, including infection and implant, are highly correlated with pain, we do not have data on them. Lastly, individual pain thresholds would be an informative covariate in identifying recovery trajectories, but we did not ascertain this information in the current study sample.
We identified two distinct pain recovery trajectories and two distinct function recovery trajectories in the 6 months following TKR. Pain or function trajectories diverged as early as 6‐8 weeks post‐TKR. These early recovery trajectories were associated with differential outcome at 2 years, with those patients in the slower recovery trajectories having higher pain, higher functional limitations, and less satisfaction compared with those patients in the faster recovery trajectories. This early recovery period in the first months after TKR may represent a window for intervention aimed at improvement of TKR outcomes.
AUTHOR CONTRIBUTIONS
All authors drafted the article, revised it critically for important intellectual content, approved the final version to be published, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Yang, Losina, Collins.
Acquisition of data
Yang, Losina, Collins.
Analysis and interpretation of data
Yang, Losina, Lange, Katz, Collins.
ACKNOWLEDGMENTS
The authors acknowledge the editorial assistance of Emma Lape, BA.
Appendix Figure 1. Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain over time. WOMAC pain is along the y‐axis and week post surgery is along the x‐axis. Week 0 represents the preoperative questionnaire. Boxplots display the raw data at each time point: the diamond is the mean; the box corresponds to the 25th percentile (lowest line), the 50th percentile (middle line), and the 75th percentile (top line), with whiskers extended to the most extreme observations that are not outliers. Outliers are represented by circles.
Appendix Figure 2. Western Ontario and McMaster Universities Arthritis Index (WOMAC) function over time. WOMAC function is along the y‐axis, and week post surgery is along the x‐axis. Week 0 represents the preoperative questionnaire. Boxplots display the raw data at each time point: the diamond is the mean; the box corresponds to the 25th percentile (lowest line), the 50th percentile (middle line), and the 75th percentile (top line), with whiskers extended to the most extreme observations that are not outliers. Outliers are represented by circles.
The funding sources did not have a role in the in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Ms. Yang’s work was supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant P30‐AR‐072577 and by Partners HealthCare. Dr. Losina's work was supported by NIH NIAMS grants K24‐AR‐057827 and P30‐AR‐072577 and by Partners HealthCare. Dr. Lange’s work was supported by Partners HealthCare. Dr. Katz’s work was supported by NIH NIAMS grant P30‐AR‐072577 and by Partners HealthCare. Dr. Collins’ work was supported by NIH NIAMS grant P30‐AR‐072577, by Partners HealthCare and by the Rheumatology Research Foundation Investigator Award.
Dr. Losina has received research grants to Brigham and Women's Hospital from Genentech and Samumed, served as a consultant for Samumed and TissueGene more than 12 months ago, and serves as The Journal of Bone and Joint Surgery Deputy Editor for Methodology and Statistics. Dr. Lange has received payment for lectures from Flexion Therapeutics and travel/accommodations/meeting expenses from Stryker and from Smith & Nephew. Dr. Kats has received research grants to Brigham and Women's Hospital from Samumed and Flexion Therapeutics and serves as The Journal of Bone and Joint Surgery Deputy Editor for Methodology and Statistics. Dr. Collins has served as a consultant to Boston Imaging Core Labs. No other disclosures relevant to this article were reported.
REFERENCES
- 1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken) 2016;68:1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salaffi F, Carotti M, Stancati A, Grassi W. Health‐related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging Clin Exp Res 2005;17:255–63. [DOI] [PubMed] [Google Scholar]
- 4. Arthritis Foundation . Arthritis by the Numbers: Book of Trusted Facts and Figures. Atlanta: Arthritis Foundation; 2018. [Google Scholar]
- 5. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- 6. Daigle ME, Weinstein AM, Katz JN, Losina E. The cost‐effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol 2012;26:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paxton EW, Namba RS, Maletis GB, Khatod M, Yue EJ, Davies M, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community‐based registry in the United States. J Bone Joint Surg Am 2010;92 Suppl 2:117–32. [DOI] [PubMed] [Google Scholar]
- 8. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health‐related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 2004;86‐A:963–74. [DOI] [PubMed] [Google Scholar]
- 9. Weinstein AM, Rome BN, Reichmann WM, Collins JE, Burbine SA, Thornhill TS, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am 2013;95:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- 11. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624–30. [DOI] [PubMed] [Google Scholar]
- 12. Harris WH, Sledge CB. Total hip and total knee replacement (1). N Engl J Med 1990;323:725–31. [DOI] [PubMed] [Google Scholar]
- 13. Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost‐effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med 2009;169:1113–21; discussion 1121‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beswick AD, Wylde V, Gooberman‐Hill R, Blom A, Dieppe P. What proportion of patients report long‐term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gandhi R, Mahomed NN, Cram P, Perruccio AV. Understanding the relationship between 3‐month and 2‐year pain and function scores after total knee arthroplasty for osteoarthritis. J Arthroplasty 2018;33:1368–72. [DOI] [PubMed] [Google Scholar]
- 16. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed Hoboken (NJ): John Wiley & Sons; 2011. [Google Scholar]
- 17. Nagin DS. Group‐Based Modeling of Development. Cambridge (MA): Harvard University Press; 2005. [Google Scholar]
- 18. Dowsey MM, Smith AJ, Choong PF. Latent Class Growth Analysis predicts long term pain and function trajectories in total knee arthroplasty: a study of 689 patients. Osteoarthritis Cartilage 2015;23:2141–9. [DOI] [PubMed] [Google Scholar]
- 19. Page MG, Katz J, Romero Escobar EM, Lutzky‐Cohen N, Curtis K, Fuss S, et al. Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. Pain 2015;156:460–8. [DOI] [PubMed] [Google Scholar]
- 20. Page MG, Katz J, Curtis K, Lutzky‐Cohen N, Escobar EM, Clarke HA. Acute pain trajectories and the persistence of post‐surgical pain: a longitudinal study after total hip arthroplasty. J Anesth 2016;30:568–77. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 22. Rumpf HJ, Meyer C, Hapke U, John U. Screening for mental health: validity of the MHI‐5 using DSM‐IV Axis I psychiatric disorders as gold standard. Psychiatry Res 2001;105:243–53. [DOI] [PubMed] [Google Scholar]
- 23. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- 24. Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskeletal Disord 2009;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelly MJ, Dunstan FD, Lloyd K, Fone DL. Evaluating cutpoints for the MHI‐5 and MCS using the GHQ‐12: a comparison of five different methods. BMC Psychiatry 2008;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001;29:374–93. [Google Scholar]
- 27. Jones BL, Nagin DS. Advances in group‐based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007;35:542–71. [Google Scholar]
- 28. Molenberghs G, Kenward MG. Missing Data in Clinical Sciences. Hoboken (NJ): John Wiley & Sons; 2007. [Google Scholar]
- 29. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum 1999;42:1722–8. [DOI] [PubMed] [Google Scholar]
- 31. Lingard AE, Katz NJ, Wright AE, Sledge BC. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am 2004;86‐A: 2179–86. [DOI] [PubMed] [Google Scholar]
- 32. Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther 2008;88:22–32. [DOI] [PubMed] [Google Scholar]


