Abstract
Objective
Variants in the SLCO1B1 gene, encoding a hepatic methotrexate (MTX) transporter, affect clearance of high‐dose MTX. We tested whether in the *14 and *15 alleles of SLCO1B1 influenced the response to low‐dose MTX in juvenile idiopathic arthritis (JIA) patients.
Methods
The study included 310 JIA patients genotyped for three single nucleotide polymorphisms (SNPs) in SLCO1B1 (rs4149056, rs2306283, and rs11045819). A patient's SLCO1B1 diplotype was determined by combining the SNPs into the *1a, *1b, *4, *5, *14, and *15 alleles. Number of active joints at follow‐up (visit closest to 6 months of treatment and prior to starting a tumor necrosis factor inhibitor) was used as the dependent variable in a negative binomial regression model that included active joint count at baseline as a covariate.
Results
The SLCO1B1*14 allele was associated with less response to MTX (P = 0.024) and the *15 allele was not associated with response to MTX (P = 0.392).
Conclusion
SLCO1B1 alleles may be associated with poor response to MTX in JIA patients. The *14 allele has been associated with fast clearance (low exposure) after high‐dose MTX in patients with leukemia. Thus, the SLCO1B1 gene may be informative for precision dosing of MTX in JIA patients. Patients carrying the *14 allele may require a higher dose than noncarriers to achieve a similar response to MTX.
Introduction
Juvenile idiopathic arthritis (JIA), the most common pediatric rheumatic condition, manifests as a chronic inflammatory arthritis that can result in morbidity and disability when inadequately treated 1. For children with multiple affected joints, initial treatment often consists of nonsteroidal anti‐inflammatory drugs in conjunction with methotrexate (MTX), a slow‐acting disease‐modifying antirheumatic drug (DMARD) 2. As a result of the newly recognized early “window of opportunity” for treatment 3, rapid and effective disease control is an aim of clinicians and an expectation of patients. However, MTX can take several months before the full effect is realized, and even though about 30% of these children will achieve a good response within the first 3 months and 60%‐70% by 6 months on MTX, waiting this time without a guarantee of response is suboptimal when long‐term outcomes are at stake 3.
Variants in the SLCO1B1 gene, encoding a hepatic MTX transporter, were the top findings in a genome‐wide association study focused on MTX clearance in pediatric leukemia patients treated with high‐dose MTX 4, 5. As is conventional with pharmacogenes, star nomenclature is used for SLCO1B1 to indicate the alleles, which are categorized by the Clinical Pharmacogenetics Implementation Consortium by function 6. There are three no‐function alleles: *5, *15, and *17, and one increased function allele, *14. Normal function alleles are *1a and *1b. Given the influence of SLCO1B1 on the pharmacokinetics of high‐dose MTX, we tested the influence of this gene on the response to MTX in JIA patients.
Patients and Methods
Three hundred thirty‐four JIA patients of all subtypes, except systemic JIA, treated with DMARD monotherapy were included in this study. Patients enrolled in the local clinics (Cincinnati Children's Hospital Medical Center or Children's Mercy Hospital) or in multicenter gene expression studies comprised the cohort (Table 1 7. Patients were enrolled in their respective studies from the start of MTX treatment. Written, informed parental consent, and child assent as appropriate, was obtained for each subject. This study complies with the Helsinki Declaration and was approved by the Institutional Review Boards of Cincinnati Children's Hospital Medical Center and Children's Mercy Hospital.
Table 1.
Summary of patient cohort
| JIA Subtype | N |
| Oligoarticular extended | 45 |
| Oligoarticular persistent | 18 |
| Polyarticular (RF‐) | 191 |
| Polyarticular (RF+) | 46 |
| Psoriatic | 7 |
| Undifferentiated | 3 |
| Total | 310 |
| Self‐reported race | N |
| African American | 13 |
| Asian | 4 |
| Caucasian | 293 |
| Self‐reported gender | N |
| Female | 245 |
| Male | 65 |
| Age (years) | Mean (range) |
| 7.3 (0.3‐16.9) | |
| Follow‐up time (months) | Mean (range) |
| 5.6 (1.3‐14) | |
| Dose at MTX start (mg/m 2 ) | Mean (range) |
| 11.6 (5‐26.9) | |
| Active joints at start | Mean (range) |
| 12.5 (2‐61) | |
| Active joints at follow‐up | Mean (range) |
| 6.8 (0‐41) | |
| MTX route | N |
| Subcutaneous | 79 |
| Oral | 125 |
| Unknown | 106 |
| SLCO1B1 alleles a | Frequency |
| *1a (A‐C‐T) | 53.0% |
| *1b (G‐C‐T) | 14.8% |
| *14 (G‐A‐T) | 15.7% |
| *15 (G‐C‐C) | 15.3% |
| *5 (A‐C‐C) | 1.1% |
Abbreviation: JIA, juvenile idiopathic arthritis; N, number; RF, rheumatoid factor; MTX, methotrexate.
The SLCO1B1 alleles are defined by PharmGKB.org at SNPs rs2306283 (A>G), rs11045819 (C>A), and rs4149056 (T>C).
All patients met the International League of Associations for Rheumatology (ILAR) or American College of Rheumatology classification criteria for JIA or juvenile rheumatoid arthritis (JRA). Patients recruited before ILAR criteria were published were originally classified using JRA criteria and subsequently reclassified for this study by ILAR criteria. A patient was considered rheumatoid factor (RF) negative on the basis of a single test. Information about the presence or absence of RF was available for all patients. Of the 334 patients eligible for inclusion in the study, exclusions were made if they had less than two active joints (n = 3) at the baseline visit, (n = 3) had Down Syndrome (n = 3), had less than 1 month to MTX follow‐up (n = 1), failed genotyping (n = 7), or were given a dose of MTX <5 mg/m2 (n = 10). After these exclusions, 310 patients remained in the current analysis. No patients with enthesitis‐related arthritis were included in the study cohort, but patients were not excluded based on this subtype of JIA. Data on the route of administration of MTX was available for 204 patients (Table 1). Genotyping of three single nucleotide polymorphisms (SNPs) in SLCO1B1 (rs4149056, rs2306283, and rs11045819) was performed with TaqMan probes (n = 275) or was available from genome‐wide arrays (n = 248) 8, with complete concordance in genotypes obtained on both platforms. When TaqMan genotyping failed for rs2306283 (n = 20) and rs11045819 (n = 16), the genotype was imputed from a proxy SNP genotyped with a genome‐wide array (rs7973095 and rs11045821, respectively). These proxy SNPs were chosen for the linkage disequilibrium with r 2 = 0.97 for rs2306283 and rs7973095 and r 2 = 0.99 for rs11045819 and rs11045821 in the EUR dataset of the 1000 genomes Phase 1 population (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php) because 33 of the 36 missing genotypes from TaqMan genotyping were in Caucasian patients. The observed genotype frequencies were consistent with Hardy‐Weinberg equilibrium. A patient's SLCO1B1 alleles were determined by these three SNPs that make up the *1a (wild‐type), *1b (rs2306283), *4 (rs11045819), *5 (rs4149056), *14 (rs2306283 + rs11045819), and *15 (rs2306283 + rs4149056) alleles. The variant that distinguishes the *17 allele from the *15 allele was not genotyped (rs4149015). The Dandelion program within the SNPLASH suite was used to implement the Expectation‐Maximization algorithm to calculate haplotype frequency maximum likelihood estimates (https://github.com/guyrt/WFUBMC/). Given an individual's genotype at each SNP, all possible haplotype pairs were exported, along with their probabilities. Each possible haplogenotype for an individual is included in the analysis, weighted by the within‐person haplogenotype probability. The patients identified as carrying the *15 allele could have either the *15 or the *17 allele because the rs4149015 genotype was not obtained. Frequency of the alleles in our cohort were similar to the expected population frequency 9 and previous multiethnic cohort studies 4. The *4 allele was not present in the patient samples, and the *5 allele was too rare to test for association with MTX response (Table 1). The *1b allele is considered to confer normal function and was not tested. To test for an association between *14 and *15 allele dosage (additive model) with active joint count (at the visit closest to 6 months of treatment and prior to starting tumor necrosis factor inhibitor), a negative binomial regression model was computed to where the outcome is the active joint count at follow‐up and the active joint count at baseline was a covariate. The active joint count at follow‐up was chosen for the outcome variable with adjustment in the regression model for baseline active joint count because the magnitude of change depends on the number of joints affected at baseline (for example, a reduction in two joints is different in patients who start with three joints affected versus those who start with 20 joints affected). The sample size was not large enough to control for each subtype of JIA and, importantly, there has been recent recognition in the limitation of the JIA ILAR classification system, which is based primarily on clinical phenotype. Future revisions in the JIA classification terminology based on pathophysiological and genetic features are likely 10, 11. The negative binomial regression is a generalized linear model that is designed for count data with overdispersion. Because two tests were computed (*14 and *15 alleles), P values of <0.025 were considered statistically significant.
Results
The SLCO1B1*14 allele was associated with less response to MTX (less reduction of active joint count, P = 0.024, Figure 1). The median improvement during MTX monotherapy was 62.5% for patients carrying no *14 alleles versus 50% for those carrying one *14 allele, and for those carrying two *14 alleles, improvement was 42.9%. There was no association between response to MTX and the *15 allele (P = 0.392). When the model included additional covariates that may be related to response (age, gender, and follow‐up time; Table 2), the association with the SLCO1B1*14 allele remained. When only Caucasian patients (n = 293) were included in the model, the direction and magnitude of the effect of the SLCO1B1*14 allele were similar (P = 0.043).
Figure 1.
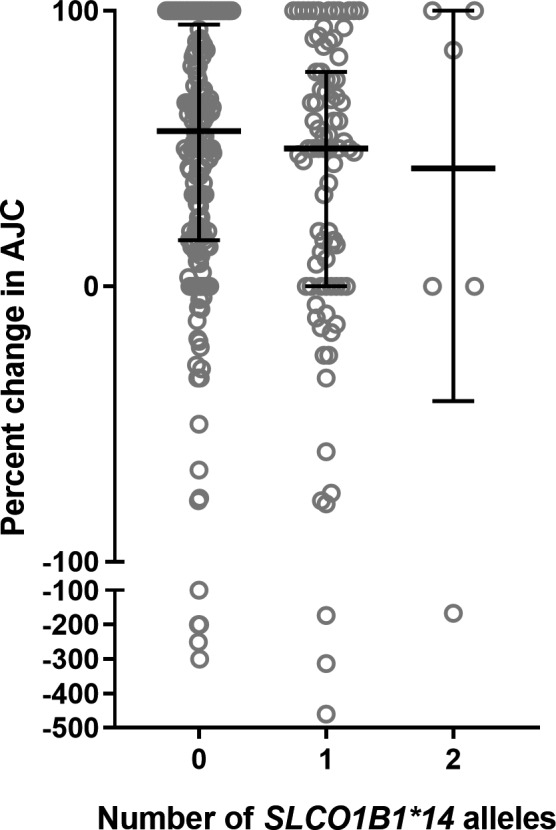
Response to methotrexate (MTX) is associated with the number of SLCO1B1*14 alleles. The percent change in active joint count versus number of SLCO1B1 alleles is plotted for patients treated with MTX. Bars indicate median, whiskers indicate interquartile range, and circles indicate each patient.
Table 2.
Parameters included in the analysis of active joint count at follow‐up using a maximum likelihood model
| Parameter | Estimate | SE | P Value |
|---|---|---|---|
| Baseline AJC | 0.065 | 0.007 | <0.0001 |
| Age | −0.018 | 0.015 | 0.22 |
| Follow‐up time | −0.0007 | 0.041 | 0.87 |
| Gender | −0.180 | 0.160 | 0.26 |
| SLCO1B1*14 | 0.267 | 0.131 | 0.04 |
Abbreviation: AJC, active joint count.
Discussion
The SLCO1B1*14 allele may be associated with poor response to weekly low‐dose MTX for JIA patients. This association remained significant after including other parameters in the model that may be related to response (age, gender, follow‐up time) and restriction to Caucasian patients. In patients with leukemia receiving much higher doses of MTX (2‐5 g/m2), the *14 allele has been associated with fast clearance and low exposure 4. The *14 allele increases expression of the transporter 12, which would likely result in more effective drug transport into the liver to be excreted, thus lower blood concentrations, less MTX in the target tissues, and less efficacy. The G allele of rs2306283 was associated with poor response to MTX in Slovenian rheumatoid arthritis patients 13, and this allele is included in the *1b, *14, and *15 haplotypes. The *1b allele was not tested for association with MTX response in our study because it is defined by the Clinical Pharmacogenetics Implementation Consortium as conferring normal activity of the transporter 6. The *15 allele was tested for association with MTX response because it was associated with slow clearance and high exposure after high‐dose MTX in leukemia patients 4, but the hypothesized association with a good response was not observed in JIA patients. Interestingly, the SLCO1B1 gene was not identified as a region of interest in the only published genome‐wide association study of MTX response in JIA patients 14, but this study did not test for individual SLCO1B1 alleles.
There are some limitations to this study. Although the majority of patients were enrolled over the last 10 years, DNA samples and clinical data were combined from collections occurring over a 28‐year period, leading to missing data for MTX route, concomitant steroid usage, and/or the complete clinical data required to calculate composite scores, such as ACR response or juvenile arthritis disease activity score (JADAS). We utilized the active joint count as the outcome measure because it was available for all patients and remains a clinically meaningful outcome as well as a large driver for the ACR and JADAS composite scores. An analysis of how the SLCO1B1*14 allele affects MTX response as assessed by ACR or JADAS composite scores in a validation cohort is needed. Further investigation of pharmacodynamic measurements, such as red blood cell MTX polyglutamate concentrations in relation to SLCO1B1*14 allele status, may lend additional support the hypothesis of faster clearance. Other variables that have been previously associated with response to MTX that were not evaluated in this study, such as baseline folate polyglutamate profile 15, and gene expression 7 or protein levels 16, 17, could account for some of the unexplained variability in response. Nonetheless, the SLCO1B1*14 allele showed a significant association with response to MTX. Thus, the SLCO1B1 gene may be informative for precision dosing of MTX in JIA patients. Patients carrying this allele may require a higher dose than noncarriers to achieve a similar response to MTX, which could be tested in future clinical trials of allele‐guided dosing of MTX.
Acknowledgements
All the authors thank the patients, families, and staff who contributed to this study. The authors gratefully acknowledge the contributions of Tracey A. Weiler and Lauren E. Thompson.
DNA samples for this study were collected in other studies, including The Gene Expression in Pediatric Arthritis Study. The investigators for this study include:
Cincinnati Children's Hospital Medical Center, Cincinnati, OH: Susan Thompson PhD; Halima Moncrieffe PhD; Daniel J. Lovell, MD, MPH; Hermine I. Brunner, MD, MBA, MSc; Jennifer L. Huggins, MD; Alexei A. Grom MD; Janalee Taylor, MSN; Tracy V. Ting MD; Michael Henrickson MD; Esi Morgan MD; Lorie K. Luyrink; Monica Tsoras; Anne L. Johnson CCRP; Kimberly Solomon; Allen Watts.
Emory University School of Medicine, Atlanta, GA: Sampath Prahalad, MD; Larry Vogler, MD; Sheila Angeles‐Han, MD, MSc; Patricia Vega‐Hernandez; Kelly Rouster Stevens, MD; Elaine Ramsay; Christine Kennedy, CPNP; Lori Ponder.
Children's Corporate Center, Wauwatosa, WI: Judy Olson, MD; Calvin Williams, MD, PHD; James Nocton, MD; James Verbsky, MD; Dominic Co, MD; Elizabeth Roth – Wojcicki, RN, MSN, CPNP; Study Coordinator: Marsha Malloy, RN, MBA, CCRC.
Levine Children's Hospital, Charlotte, NC: Thomas Griffin MD, PHD; Sheetal Vora.
Arkansas Children's Hospital Research Institute, Little Rock, AR: Paula Morris MD; Jason Dare, MD; Kathy Hummel, RN.
Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA: Margalit Rosenkranz, MD; Elaine Cassidy, MD; Kathryn Torok, MD; Daniel Kietz, MD; Kimberly Francis, MS, MPA.
Children's Mercy Hospital, Kansas City, MO: Mara Becker, MD; Maria Ibarra, MD; Cara Hoffart, DO; Ashley Cooper, MD; Elizabeth Kessler, MD; Julia Harris, MD; Emily Fox, MD; Chelsey Smith; Anna Mueller.
Riley Hospital for Children, Indianapolis IN: Kathleen O'Neil, MD; Stacey Tarvin, MD; Susan Ballinger, MD; Michael Blakley, MD; Thomas Klausmeier, MD; Ellen Go, MD; Andrea Hudgins, CCRP; Amy Rakestraw, MPT, MPH.
Boston Children's Hospital, Boston MA: Peter A. Nigrovic, MD; Edwin Anderson.
This work was financially supported by grants P30 AR070549, R01HD089928, P01 AR048929 from the Rheumatology Research Foundation.
Drs. Ramsey and Moncrieffe contributed equally to this work.
References
- 1. Petty RE, Cassidy JT. Chronic arthritis in childhood In: Cassidy JT, Petty RE, Laxer RM, Lindsey JB, editors. Textbook of Pediatric Rheumatology. 5th ed Philadelphia: WB Saunders; 2011. p. 211–35. [Google Scholar]
- 2. Beukelman T, Patkar NM, Saag KG, Tolleson‐Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 2011;63:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O'Neil KM, Zeft AS, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012;64:2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramsey LB, Bruun GH, Yang W, Trevino LR, Vattathil S, Scheet P, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res 2012;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramsey LB, Panetta JC, Smith C, Yang W, Fan Y, Winick NJ, et al. Genome‐wide study of methotrexate clearance replicates SLCO1B1. Blood 2013;121:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramsey LB, Johnson SG, Caudle KE, Haidar CE, Voora D, Wilke RA, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin‐induced myopathy: 2014 update. Clin Pharmacol Ther 2014;96:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moncrieffe H, Bennett MF, Tsoras M, Luyrink LK, Johnson AL, Xu H, et al. Transcriptional profiles of JIA patient blood with subsequent poor response to methotrexate. Rheumatology (Oxford) 2017;56:1542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McIntosh LA, Marion MC, Sudman M, Comeau ME, Becker ML, Bohnsack JF, et al. Genome‐wide association meta‐analysis reveals novel juvenile Idiopathic arthritis susceptibility lsoci. Arthritis Rheumatol 2017;69:2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasanen MK, Neuvonen PJ, Niemi M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics 2008;9:19–33. [DOI] [PubMed] [Google Scholar]
- 10. Martini A, Ravelli A, Avcin T, Beresford MW, Burgos‐Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol 2018. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11. Nigrovic PA, Raychaudhuri S, Thompson SD. Review: genetics and the classification of arthritis in adults and children. Arthritis Rheumatol 2018;70:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nies AT, Niemi M, Burk O, Winter S, Zanger UM, Stieger B, et al. Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. Genome Med 2013;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jenko B, Tomsic M, Jekic B, Milic V, Dolzan V, Praprotnik S. Clinical pharmacogenetic models of treatment response to methotrexate monotherapy in Slovenian and Serbian rheumatoid arthritis patients: differences in patient's management may preclude generalization of the models. Front Pharmacol 2018;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cobb J, Cule E, Moncrieffe H, Hinks A, Ursu S, Patrick F, et al. Genome‐wide data reveal novel genes for methotrexate response in a large cohort of juvenile idiopathic arthritis cases. The Pharmacogenomics J 2014;14:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Funk RS, van Haandel L, Leeder JS, Becker ML. Folate depletion and increased glutamation in juvenile idiopathic arthritis patients treated with methotrexate. Arthritis Rheumatol 2014;66:3476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gohar F, Anink J, Moncrieffe H, Van Suijlekom‐Smit LWA, Prince FHM, van Rossum MAJ, et al. S100A12 is associated with response to therapy in juvenile idiopathic arthritis. J Rheumatol 2018;45:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moncrieffe H, Ursu S, Holzinger D, Patrick F, Kassoumeri L, Wade A, et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 2013;52:1467–76. [DOI] [PubMed] [Google Scholar]


