Abstract
Objective
Venous thromboembolism (VTE) is a major cause of mortality and morbidity in hospitalized patients, particularly those with autoimmune disorders. The Nationwide Inpatient Sample (NIS) database was analyzed to determine trends in the rate of hospitalization, mortality from VTE, epidemiology, and outcomes in hospitalized patients with systemic lupus erythematosus (SLE) to assess its impact.
Methods
The 2003‐2011 NIS database of the Healthcare Cost and Utilization Project was queried to identify all adults (age 18 years and older) hospitalized with SLE and VTE. Demographic characteristics and in‐hospital outcomes of this population were compared with those of patients with SLE without a VTE diagnosis. A multivariate logistic regression analysis was used to obtain the adjusted odds ratio (OR).
Results
The total number of hospitalized patients with SLE was 299 595, of whom 9175 (3.06%) had VTE. After adjusting for potential confounders, compared with those without VTE, patients with SLE and VTE had significantly higher inpatient mortality (5% vs. 2.0%; OR 2.35 [95% confidence interval (CI) 2.10‐2.62]; P < 0.001), greater disability at discharge (34% vs. 26%; OR 1.53 [95% CI 1.46‐1.62]; P < 0.001), a longer length of stay (LOS) by 3.57 days, and higher cost of hospitalization by $25 400. In this database, patients with SLE and VTE were younger and of male sex. Also, African American race and a higher number of comorbidities were associated with an increased risk of VTE in patients with SLE.
Conclusion
VTE in hospitalized patients with SLE is associated with significantly higher inpatient mortality, greater disability at discharge, an increased LOS, and higher cost of hospitalization. This cross‐sectional study helps with quantifying the risk of VTE in hospitalized patients with SLE and provides information on the immense human and material cost this complication leads to. These data can be very useful in the development and implementation of appropriate prophylactic strategies in the high‐risk population with SLE.
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are manifestations of potentially lethal venous thromboembolism (VTE). Among hospitalized patients, VTE is a major cause of mortality and morbidity and remains one of the major reasons for poor outcomes and increased cost in these patients. The risk of VTE is increased further in these patients by comorbid conditions, such as diabetes, cerebrovascular accident, malignancy, heart failure, and, in particular, autoimmune disorders. Among the autoimmune disorders, it is well proven that there is a high risk of VTE with systemic lupus erythematosus (SLE), especially when associated with antiphospholipid antibodies (APLAs). Yet this increased risk and in‐hospital outcomes have not been well characterized in hospitalized patients with SLE.
VTE is caused by one of three factors according to the Virchow triad: stasis, vessel wall abnormalities, including endothelial dysfunction, and/or a prothrombotic/altered coagulation state 1, 2. There are a number of inherited and acquired causes of altered coagulation states and endothelial dysfunction that include age, APLA syndrome, Factor V deficiency, trauma, surgery, malignancy, heart failure, and systemic inflammatory disorders, such as SLE 1, 2. Among acquired risk factors, advancing age, prolonged immobilization, heart failure, lower‐extremity fracture or surgery, and cancer have most frequently been associated with VTE 3, 4, 5, 6. Among the systemic inflammatory diseases, SLE, inflammatory bowel disease, rheumatoid arthritis, and vasculitides, especially granulomatosis with polyangiitis, are associated with an increased risk of VTE 7, 8, 9.
Furthermore, it is also felt that VTE is a major cause of mortality and morbidity in hospitalized patients with these disorders. Multiple cohort studies in patients with SLE have shown that they are at a higher risk of VTE, especially when associated with APLAs. Notably, Mok et al 10 found a 11.9‐fold higher risk of VTE in patients with SLE than in the general population, and this association has been replicated in other studies 10, 11, 12, 13, 14. Patients with SLE have a higher risk for VTE in the first year after diagnosis 15. Risk factors for arterial thromboembolism and VTE in patients with SLE have also been well defined 16. However, there is paucity of data on the in‐hospital outcomes of these patients. In this study, the Nationwide Inpatient Sample (NIS) database was analyzed to determine trends in the rate of hospitalization and in mortality from VTE in hospitalized patients SLE and to assess its impact on length and cost of hospitalization.
Methods
Data source
We used the NIS database for years 2003‐2011. The NIS contains data on inpatient hospitalization stay from states participating in the Healthcare Cost and Utilization Project (N = 46 in 2011). The NIS is the largest publicly available database in the United States and is designed to approximate a 20% stratified sample of discharges from US community hospitals, defined as “all non‐federal, short‐term, general, and other specialty hospitals excluding rehabilitation and long‐term acute care hospitals.” Discharge weights provided by the NIS allow extrapolation to calculate expected national hospitalization rates. Criteria used for stratified sampling of hospitals include ownership, bed size, teaching status, urban or rural location, and US region. All discharges from sampled hospitals are included in the NIS database. The NIS is an all‐payer database that covers all patients, including those covered by Medicare, Medicaid, and private insurance and those who are uninsured. Inpatient stay records in the NIS include one primary and up to 24 secondary discharge diagnoses together with demographic and patient disposition. An overview of the NIS database is available online (Healthcare Cost and Utilization Project; online at http://www.hcup-us.ahrq.gov/nisoverview.jsp). In accordance with institutional policy, this study used publicly available data and was exempted from institutional review board approval.
Study population
Our study included all patients 18 years of age and older with a diagnosis of SLE who were identified from the NIS database between 2003 and 2011 using the Clinical Classification Software (CCS) codes. The CCS is a categorization scheme that groups the International Classification of Diseases, Ninth Revision (ICD‐9) codes into mutually exclusive categories. CCS code 710.0 represents all diagnoses of SLE. From this cohort, we identified patients with a diagnosis of VTE using ICD‐9 codes for DVT (453.2, 453.3, 453.40, 453.41, 453.42, 453.82‐453.89, 453.9, 453.0, 325, and 452), PE (415.11, 415.12, 415.13, and 415.19), and phlebitis and thrombophlebitis (451.11, 451.19, 451.2, 451.83, 451.81, 451.84, and 451.89). Patients with superficial upper‐ and lower‐extremity VTE were excluded. Only patients with VTE as one of the top three discharge diagnoses were included in the cohort. The comparison group included hospitalized patients with SLE who did not have a diagnosis of VTE.
Patient and hospital characteristics
NIS‐defined patient demographic and clinical characteristics used for our study included age, race, sex, insurance type, comorbidities (diabetes, hypertension, chronic heart failure, chronic renal disease, chronic liver disease, connective tissue disease, chronic lung disease, and valvular heart disease), length of stay (LOS), inpatient mortality, discharge status, and total charges. We also used NIS‐defined hospital characteristics, such as location (rural vs. urban) and teaching status (teaching vs. nonteaching). Discharge status is reported in the NIS database as routine home discharge, home health care, short‐term hospital or other hospital facility (including intermediate care and skilled nursing home), or death. We classified routine discharge as none to minimal disability, and we classified all other discharges as moderate to severe disability, which has previously been described 17, 18, 19.
Outcomes
The primary outcomes of this study were inpatient mortality, trends of hospitalization, and the inpatient mortality rate over the study period (2003‐2011). Secondary outcomes were LOS, cost of hospitalization, and disability at discharge.
Statistical analysis
A bivariate comparison between patients with SLE with and without VTE was done using Mann‐Whitney U tests with a 2‐tailed hypothesis for continuous variables and Pearson's χ2 test for categorical variables to detect any significant univariate associations. Binary outcomes (in‐patient mortality and disability at discharge) were modeled using binomial families with logit links, with results being reported as probabilities and odds ratios (ORs). Likewise, LOS and total charges were modeled with gamma family and identity links, with results being reported on the natural scale. Trend plots were constructed using fully adjusted models that showed the marginal outcomes, and the trend analysis was conducted using year as a continuous variable and interacting with VTE, when appropriate. All models were adjusted for age, sex, race, teaching status of the hospital, hospital location, and presence of comorbid conditions. Adjustments were made (because of NIS sample schemes) using NIS guidelines. Namely, all results were probability weighted and stratified on established NIS weighting and stratification variables. All analyses were performed with Stata version 15.1 (StataCorp LLC).
Results
Between 2003 and 2011, 299 595 patients with SLE were hospitalized, of whom 9175 (3.06%) had an accompanying diagnosis of VTE, as defined previously, and were included in the analysis. There were 290 420 patients with SLE without a diagnosis of VTE who served as comparators in the control cohort. Of the study population, 89% were women. The mean age of the study population was 50 years. The mean age was slightly lower in patients who had VTE (48 vs. 51 years; P < 0.001). Overall, a higher proportion of patients were white (54%); however, the VTE rate was higher in African American patients compared with white patients (3.8% vs. 2.7%; P < 0.001). Similarly, the VTE rate was higher in men compared with women (4.3% vs. 2.9%; P < 0.001). The total number of comorbid conditions was higher in patients with VTE (4 vs. 3; P < 0.001). Other baseline characteristics of the patients and hospital characteristics are listed in Table 1.
Table 1.
Weighted descriptive characteristics of patients older than 18 years with systemic lupus erythematosus, NIS (January 2003 through December 2011)
| Variable | All Patients | VTE | No VTE | P |
|---|---|---|---|---|
| N = 299 595 | n = 9175 | n = 290 420 | ||
| Age, mean (SD), y | 50.00 (25.00) | 48.00 (25.00) | 51.00 (25.00) | <0.001 |
| Sex, n (%) | ||||
| Female | 266 969 (89) | 7769 (85) | 259 200 (89) | … |
| Male | 32 626 (11) | 1406 (15) | 31 220 (11) | … |
| Race, n (%) | <0.001 | |||
| White | 132 649 (54) | 3670 (48) | 128 979 (54) | … |
| African American | 71 151 (29) | 2706 (35) | 68 445 (29) | … |
| Hispanic | 29 582 (12) | 948 (12) | 28 634 (12) | … |
| Asian | 5258 (2) | 127 (2) | 5131 (2) | … |
| Native American | 1498 (1) | 46 (1) | 1452 (1) | … |
| Other | 5699 (2) | 187 (2) | 5512 (2) | … |
| No. of comorbidities, mean (SD) | 3.00 (2.00) | 4.00 (3.00) | 3.00 (2.00) | <0.001 |
| Hospital location, n (%) | <.001 | |||
| Urban | 269 880 (91) | 8436 (92) | 261 444 (91) | … |
| Rural | 29 715 (9) | 739 (8) | 28 976 (9) | … |
| Teaching status, n (%) | <.001 | |||
| Nonteaching | 149 853 (50) | 4225 (46) | 145 628 (50) | … |
| Teaching | 149 742 (50) | 4950 (54) | 144 792 (50) | … |
Abbreviation: NIS, Nationwide Inpatient Sample; VTE, venous thromboembolism.
Patients with VTE had worse outcomes in terms of inpatient mortality, LOS, total charges, and disability at discharge compared with patients without VTE, as shown in Table 2. After adjusting for age, primary payer, weekend versus weekday admission, comorbidities, and other potential confounders, compared with patients with SLE without VTE, those with VTE had significantly higher rates of inpatient mortality (5% vs. 2%; adjusted OR 2.35; 95% confidence interval [CI] 2.10‐2.62; P < 0.001), longer expected LOS (9 vs. 6 days; adjusted β 3.57; 95% CI 3.32‐3.83; P < 0.001), higher total expected charges ($67 000 vs. $41 600; adjusted β $25 400; 95% CI $23 000‐$27 900; P < 0.001), and moderate to severe disability at discharge (34% vs. 26%; adjusted OR 1.53; 95% CI 1.46‐1.62; P < 0.001). Table 3 shows the multivariate regression analysis of the study outcomes.
Table 2.
Outcomes of VTE in hospitalized patients with systemic lupus erythematosus
| Variable | All Patients | VTE | No VTE | P |
|---|---|---|---|---|
| Inpatient mortality, n (%) | 6132 (2) | 429 (5) | 5703 (2) | <0.001 |
| LOS, median (IQR), d | 4.00 (5.00) | 6.00 (7.00) | 4.00 (4.00) | <0.001 |
| Total charges, median (IQR), US $10 000 | 2.31 (3.19) | 3.61 (5.81) | 2.28 (3.13) | <0.001 |
| Moderate to severe disability at discharge, n (%) | 77 904 (26) | 3117 (34) | 74 787 (26) | <0.001 |
Abbreviation: IQR, interquartile range; LOS, length of stay; VTE, venous thromboembolism.
Table 3.
Multivariate analysis predicting individual outcomes and adjusting for other variables[Link]
| Variable | Adjusted Estimate (95% CI) | P |
|---|---|---|
| Inpatient mortality for VTE vs. no VTE | OR = 2.35 (2.10‐2.62) | <0.001 |
| Expected additional LOS for VTE vs. no VTE, d | 3.57 (3.32‐3.83) | <0.001 |
| Expected additional charges for VTE vs. no VTE, $ | 25 400 (23 000‐27 900) | <0.001 |
| Moderate to severe disability at discharge for VTE vs. no VTE | OR = 1.53 (1.46‐1.62) | <0.001 |
Abbreviation: CI, confidence interval; LOS, length of stay; OR, odds ratio; VTE, venous thromboembolism.
Adjustment was made for age, sex, primary expected payer, teaching status of the hospital, hospital location, and presence of comorbid conditions.
A sudden spike in VTE rates was observed from 2004 to 2005 (OR 1.64; P < 0.001) (Figure 1); however, trends both before and after this period were stable (OR 0.98 [P = 0.812] and OR 0.99 [P = 0.401], respectively) (Figure 1). A trending decrease in year mortality rates was observed for patients both with and without VTE was also observed (OR 0.90 per year; P < 0.001) (Figure 2). There was no difference in yearly mortality trends between patients with and without VTE (P = 0.348).
Figure 1.
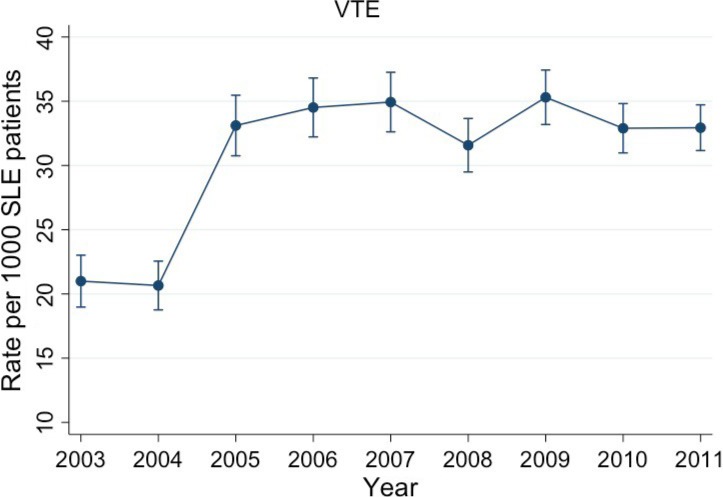
Annual rate of venous thromboembolism (VTE) in hospitalized patients with systemic lupus erythematosus (SLE).
Figure 2.
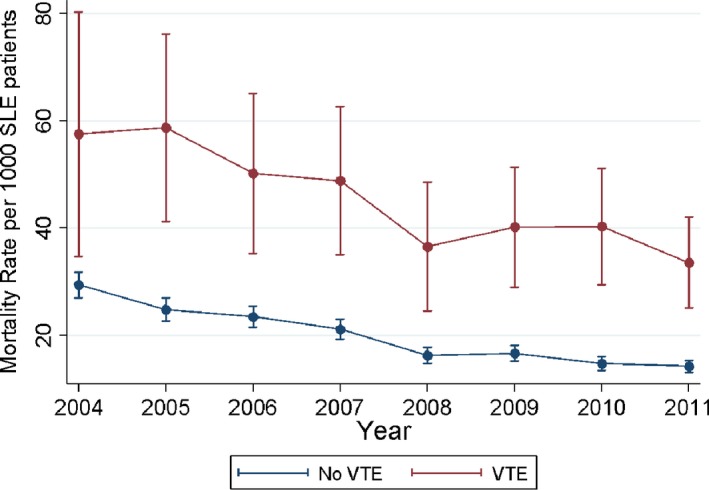
Mortality rate of hospitalized patients with systemic lupus erythematosus (SLE) with and without venous thromboembolism (VTE).
These results highlight that the risk of VTE is significantly increased in hospitalized patients with SLE. Furthermore, VTE in hospitalized patients with SLE is associated with adverse outcomes and leads to a higher morbidity and mortality as well as a prolonged LOS and higher cost of admission.
Discussion
VTE is a major health care burden in the US population, with an annual incidence of one to two cases per 1000 people and increasing prevalence 20. Studies have shown an increased risk of thromboembolism in patients with SLE as well as an increased mortality associated with VTE in these patients 21, 22. Risk of PE during the first year after admission for SLE in a Swedish registry was significantly high 21. Similar results were reported in a Chinese and a Canadian cohort with SLE, with an increased risk of developing DVT or a PE in the patient group with SLE 23, 24. In a 10‐year prospective cohort study of patients, thrombosis was one of the leading causes (26.5%) of deaths, with thrombosis dominating the second 5‐year period of follow‐up 22.
However, there continues to remain an understanding gap between well‐defined overall association of VTE in the general population with SLE and information on the hospitalized patients with SLE. This analysis focuses on the hospitalized patients with SLE and provides significant insight into this association for these patients. Results from our study showed a significantly high risk of mortality in patients admitted with VTE in SLE (OR 2.35), thereby corroborating the association between the two disease processes. We also found that in this subset of hospitalized patients with SLE, prevalence of VTE is increased over the period of follow‐up, almost doubling from 2003‐2004 to the period thereafter and then remaining stable from 2005 to 2011. This finding is similar to results of previous studies in the general population 20.
There are a number of possible explanations for this association of a higher prevalence of VTE in hospitalized patients with SLE. The biggest contributing factor is likely the presence of APLAs 8, 9, 10, 16. However, there are other factors, such as endothelial dysfunction from inflammatory processes in SLE that causes microvascular thrombosis and alteration in cytokines that promotes procoagulant activity, downregulation of anticoagulants, and suppression of fibrinolysis 10. There is a high prevalence of corticosteroid use in these patients, which increases the risk of hemostasis and could contribute to risk of VTE in SLE 25. There is also increased prevalence of hypertension, diabetes mellitus, and dyslipidemia patients with SLE, and all of these factors have been associated with thrombosis 26.
Another highlight of the analysis was the decrease in mortality of hospitalized patients with SLE both with and without VTE, giving a reassuring insight into the advances in the care of these patients. The results also show that increased risk is significantly affected by both age and race. The patients with VTE and SLE were younger than the ones who did not develop VTE. This may reflect the severity of disease in these patients. Also, the percentage of VTE in hospitalized patients with SLE was higher for African American patients, and white patients had a lower likelihood of developing VTE (35% vs. 29% for African American patients and 48% vs. 54% for white patients). In addition, the VTE rate was significantly higher in men compared with women (4.3% vs. 2.9%) possibly because of more severe disease. The presence of comorbid conditions significantly raised the risk of VTE in these patients. Those with VTE had a higher number of comorbidities than those without VTE (4 vs. 3). There also were minor differences in the risk based on hospital setting (rural vs. urban and teaching vs. nonteaching hospital). As mentioned previously, this analysis provides major insight into the epidemiology of VTE in hospitalized patients with SLE.
Furthermore, the analysis quantifies and sheds light on the huge morbidity and mortality as well as cost to the health system that occurrence of VTE in these patients leads to. The patients with VTE were 2.5 times more likely to die and had a significant increase in moderate to severe disability at discharge (34% vs. 26%). Development of VTE led to three additional days of hospital stay and increased cost by about $25 400.00 in this analysis.
The strength of this study is a large sample of patients over a span of 9 years. This is one of the largest retrospective analysis of VTE hospitalizations for patients with SLE and provides huge confidence that the results likely reflect a true prevalence. The NIS also collects data from a number of centers across the geographic United States, and hence the analysis is likely to be free of geographic bias and is applicable to the US population with SLE.
Despite a large number of patients available in the NIS database, which gives the analysis a high power, there are significant limitations to our study secondary to the database being based on administrative coding. Accuracy of certain variables may be influenced by hospital coding practices. The NIS is a discharge‐level database; hence, it was impossible to distinguish whether VTE was the primary reason for admission or a hospital‐acquired complication during the patient's stay. We limited our selection of the cohort with VTE to patients with VTE as a top three discharge diagnosis to avoid potential bias. This is a well‐accepted methodology to minimize coding bias. A number less than three is likely to miss a significant number of cases, and a higher number is likely to add miscoded cases, thereby inflating numbers. However, there is no definite way to ensure that the sample is completely accurate. This is a descriptive cross‐sectional study across each hospitalization, and thus we were unable to draw any causal inference.
Because the NIS does not collect medication data, our study was not able to ascertain whether this large number of at‐risk patients did indeed receive VTE treatment or prophylaxis, thus limiting insight or inference on inpatient practice in this population. Despite clear practice guidelines, many eligible patients either receive no or suboptimal prophylaxis 27, 28. In addition, there is no information on the severity of SLE, major organ involvement (especially lupus nephritis and APLA serology), all known risk factors for VTE as well as prognosis of patients with SLE, which are potential confounders. To overcome this limitation, the multivariate analysis included adjustments for coagulopathies and chronic kidney disease. VTE events were not further adjudicated in this analysis because of the potential of introducing coding errors in the analysis and the potential of decreasing the power of the analysis to obtain relevant results. By repeating this analysis using International Classification of Diseases, 10th Revision (ICD‐10) data, the study may be able to overcome this limitation.
These results, however, call for increased awareness about the prevalence of VTE in SLE. Further longitudinal studies that evaluate the role of known risk factors, such as APLAs and their routine evaluation in hospitalized patients with SLE, will be very useful. Increased vigilance to detect VTE and risk factors for VTE in SLE and implementation of preventive intervention in such patients can limit mortality associated with VTE. Despite these limitations, our findings are very likely reflective of true trends and outcomes of VTE in hospitalized patients with SLE.
In conclusion, this analysis suggests that the risk of VTE is significantly increased in hospitalized patients with SLE. Furthermore, VTE in hospitalized patients with SLE is associated with adverse outcomes and leads to a higher morbidity and mortality as well as prolonged LOS and higher cost of admission. Based on these results, we recommend that hospitalized patients with SLE be considered for VTE treatment with prophylaxis as appropriate and be monitored for development of this serious complication. We also recommend additional analytic studies in other databases to confirm these findings, to further quantify the risks, and to also develop an appropriate monitoring and prophylactic strategy in the hospitalized population with SLE.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Study conception and design
Kishore, Mittal, Majithia.
Acquisition of data
Kishore, Lirette, Mittal, Majithia.
Analysis and interpretation of data
Kishore, Jatwani, Malhotra, Lirette, Majithia.
No potential conflicts of interest relevant to this article were reported.
There was no financial support/funding for this project/manuscript.
References
- 1. Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev 2009;23:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reitsma PH, Versteeg HH, Middeldorp S. Mechanistic view of risk factors for venous thromboembolism. Arterioscler Thromb Vasc Biol 2012;32:563–8. [DOI] [PubMed] [Google Scholar]
- 3. Dean SM, Abraham W. Venous thromboembolic disease in congestive heart failure. Congest Heart Fail 2010;16:164–9. [DOI] [PubMed] [Google Scholar]
- 4. Falck‐Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141 Suppl:e278S–325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol 2005;6:401–10. [DOI] [PubMed] [Google Scholar]
- 6. Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis – current understanding from an epidemiological point of view. Br J Haematol 2010;149:824–33. [DOI] [PubMed] [Google Scholar]
- 7. Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol 2011;106:713–8. [DOI] [PubMed] [Google Scholar]
- 8. Choi HK, Rho YH, Zhu Y, Cea‐Soriano L, Avina‐Zubieta JA, Zhang Y. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population‐based outpatient cohort study. Ann Rheum Dis 2013;72:1182–7. [DOI] [PubMed] [Google Scholar]
- 9. Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5–6, 8‐9. [PubMed] [Google Scholar]
- 10. Mok CC, Ho LY, Yu KL, To CH. Venous thromboembolism in southern Chinese patients with systemic lupus erythematosus. Clin Rheumatol 2010;29:599–604. [DOI] [PubMed] [Google Scholar]
- 11. Weng CT, Chung TJ, Liu MF, Weng MY, Lee CH, Chen JY, et al. A retrospective study of pulmonary infarction in patients with systemic lupus erythematosus from southern Taiwan. Lupus 2011;20:876–85. [DOI] [PubMed] [Google Scholar]
- 12. Aviña‐Zubieta JA, Lacaille D, Sayre EC, Kopec J, Choi HK, Esdaile JM. Risk of pulmonary embolism and deep vein thrombosis in systemic lupus erythematosus: a population‐based cohort study. Arthritis Res Ther 2012;14 Suppl 3:A53. [Google Scholar]
- 13. Ahlehoff O, Wu JJ, Raunsø J, Kristensen SL, Khalid U, Kofoed K, et al. Cutaneous lupus erythematosus and the risk of deep venous thrombosis and pulmonary embolism: a Danish nationwide cohort study. Lupus 2017;26:1435–9. [DOI] [PubMed] [Google Scholar]
- 14. Yusuf HR, Hooper WC, Grosse SD, Parker CS, Boulet SL. Ortel TL. Risk of venous thromboembolism occurrence among adults with selected autoimmune diseases: a study among a U.S. cohort of commercial insurance enrollees. Thromb Res 2015;135:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aviña‐Zubieta JA, Vostretsova K, de Vera MA, Sayre EC, Choi HK. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: a general population‐based study. Semin Arthritis Rheum 2015;45:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinojosa‐Azaola A, Romero‐Diaz J, Vargas‐Ruiz AG, Nuñez‐Alvarez CA, Cicero‐Casarrubias A, Ocampo‐Torres MC, et al. Venous and arterial thrombotic events in systemic lupus erythematosus. J Rheumatol 2016;43:576–86. [DOI] [PubMed] [Google Scholar]
- 17. Steuer CE, Behera M, Kim S, Patel N, Chen Z, Pillai R, et al. Predictors and outcomes of venous thromboembolism in hospitalized lung cancer patients: a Nationwide Inpatient Sample database analysis. Lung Cancer 2015;88:80–4. [DOI] [PubMed] [Google Scholar]
- 18. Devani K, Patil N, Simons‐Linares CR, Patel N, Jaiswal P, Patel P, et al. Trends in hospitalization and mortality of venous thromboembolism in hospitalized patients with colon cancer and their outcomes: US perspective. Clin Colorectal Cancer 2017;16:e199–204. [DOI] [PubMed] [Google Scholar]
- 19. Maiser S, Adil MM, Roohani P, Tariq N. Patients with transverse myelitis who developed venous thromboembolism while hospitalized have increased rate for inpatient mortality. J Neuroimmunol 2013;261:120–2. [DOI] [PubMed] [Google Scholar]
- 20. Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011;86:217–20. [DOI] [PubMed] [Google Scholar]
- 21. Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis 2012;2:171–83. [PMC free article] [PubMed] [Google Scholar]
- 22. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10‐year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. [DOI] [PubMed] [Google Scholar]
- 23. Chung WS, Lin CL, Chang SN, Lu CC, Kao CH. Systemic lupus erythematosus increases the risks of deep vein thrombosis and pulmonary embolism: a nationwide cohort study. J Thromb Haemost 2014;12:452–8. [DOI] [PubMed] [Google Scholar]
- 24. Aviña‐Zubieta JA, Bhole VM, Amiri N, Sayre EC, Choi HK. The risk of deep venous thrombosis and pulmonary embolism in giant cell arteritis: a general population‐based study. Ann Rheum Dis 2016;75:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jilma B, Cvitko T, Winter‐Fabry A, Petroczi K, Quehenberger P, Blann AD. High dose dexamethasone increases circulating P‐selectin and von Willebrand factor levels in healthy men. Thromb Haemost 2005;94:797–801. [DOI] [PubMed] [Google Scholar]
- 26. Toloza SM, Uribe AG, McGwin G Jr, Alarcón GS, Fessler BJ, Bastian HM, et al. for the LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum 2004;50:3947–57. [DOI] [PubMed] [Google Scholar]
- 27. Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines [published erratum appears in Chest 2012;141:1369]. Chest 2012;141 Suppl:e227S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tapson VF, Hyers TM, Waldo AL, Ballard DJ, Becker RC, Caprini JA, et al. Antithrombotic therapy practices in US hospitals in an era of practice guidelines. Arch Intern Med 2005;165:1458–64. [DOI] [PubMed] [Google Scholar]


