Abstract
Objective
The objective of this study was to measure cumulative incidence and incidence rate and identify factors associated with new musculoskeletal (MSK) symptomatic areas after total knee replacement (TKR) for osteoarthritis (OA).
Methods
Using data from a randomized controlled trial of patients undergoing elective TKR for OA, we assessed for MSK symptomatic areas by region (neck, hands/wrists/arms/shoulders, back, hips, nonindex knee, and ankles/feet) at baseline (pre‐TKR), and at 3, 6, 12, 24, 36, and 48 months post‐TKR. Cumulative incidence and incidence rates were calculated for each region. Factors associated with incident MSK symptomatic areas were identified using generalized linear mixed models. Time to incident symptomatic area was assessed using Cox proportional hazards regression.
Results
Among 293 subjects, the cumulative incidence of any new MSK symptomatic area over 4 years was 45%; the incidence rate was 19.2 per 100 person‐years. Body site–specific cumulative incidence and incidence rates were highest for nonindex knee and back. Predictors of incident MSK symptomatic areas included female sex (relative risk [RR] 1.64; 95% confidence interval [CI] 1.15‐2.34), body mass index of 35 or higher (RR 1.27; 95% CI 0.88‐1.85), Charlson Comorbidity Index 2 or more (RR 1.28; 95% CI 0.92‐1.78), baseline index knee Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score greater than 40 (RR 1.39; 95% CI 0.99‐1.95), and anxiety/depression (measured by the five‐item Mental Health Index) (RR 1.70; 95% CI 1.20‐2.40).
Conclusion
Incident MSK symptomatic areas occurred in roughly half of recipients of TKR in the 4 years after the operation. Further study is needed to examine the long‐term impact of MSK symptomatic areas on postoperative pain, function, and quality of life.
Introduction
With roughly 723 000 primary total knee replacements (TKRs) performed annually in the United States, postoperative outcomes have been a major focus of research in the field of knee osteoarthritis (OA) 1. A number of preoperative factors have already been implicated as predictors of adverse postoperative outcomes, including age, sex, preoperative pain and function, socioeconomic status, anxiety, and depression 2, 3, 4. Recognition of these predictors has enabled health care providers to better identify candidates for TKR and tailor preoperative counseling according to the needs of the patient.
One risk factor of recent interest has been the presence of concomitant musculoskeletal (MSK) complaints 5, 6, 7. To date, literature on the effects of concomitant MSK complaints on TKR outcomes has been focused on preoperative MSK complaints. For example, Kahn et al 5 reported that preoperative Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, disability, and stiffness scores in the contralateral knee were correlated, although moderately, with worse post‐TKR WOMAC total scores in the index knee (r = 0.34; P < 0.001).
Similarly, Perruccio et al 6 showed that patients with preexisting neck, ankle, feet, or toe pain had statistically significant worse postoperative WOMAC pain scores compared with those without preexisting pain. Those with preoperative total painful joint counts of 4 or greater reported worse pain outcome scores postoperatively 6. Subjects with higher baseline WOMAC pain scores or with anxiety and depression were more likely to have these preoperative MSK complaints 6. Finally, Collados‐Maestre et al 7 demonstrated that patients with concomitant presurgical lower back pain had worse postsurgical WOMAC, Short‐Form 12, visual analogue scale for satisfaction, and functional Knee Society scores. These studies suggest that preoperative MSK symptomatic areas are associated with worse self‐reported pain and self‐reported functional outcomes after TKR.
Beyond preoperative joint complaints, however, no studies have examined the development of new MSK symptomatic areas occurring after TKR. If present, these symptomatic areas could contribute to the overall level of pain and functional limitation post‐TKR, ultimately creating new barriers to optimal recovery and delaying proper rehabilitation. Such limitations could also lead to greater postoperative patient dissatisfaction. By understanding the risk factors for incident MSK symptomatic areas, health care providers can adapt their preoperative conversations to offer realistic patient expectations.
In this report, we aimed to document the cumulative incidence and incidence rate of self‐reported MSK symptomatic areas during 4 years of follow‐up after unilateral TKR for OA. We also identified preoperative factors associated with the development of postoperative MSK symptomatic areas. We hypothesized that self‐reported MSK symptomatic areas would occur frequently after unilateral TKR and that risk factors for these incident symptomatic areas would include higher baseline WOMAC pain scores and feelings of anxiety and depression.
Methods
Study sample
We utilized data from the Adding Value in Knee Arthroplasty (AViKA) Postoperative Care Navigation Trial, a randomized controlled trial that examined the efficacy of a motivational interviewing–based postoperative care navigator, compared with usual care, to improve functional status after TKR for knee OA 8, 9. The active intervention for this trial was conducted at Brigham and Women's Hospital (BWH), a tertiary care medical center in Boston, MA, from 2010 to 2013, with the 4‐year follow‐up data collected through December 2017. We included both the active and control arms of the trial in this analysis because both groups had similar profiles of pain relief 9. At enrollment, all participants spoke English, were 40 years or older at the date of the TKR, and underwent primary TKR for knee OA. Exclusions for AViKA included psychological issues precluding surgery, dementia, nursing home residency, and implantation of unicompartmental knee arthroplasty or bilateral TKR (simultaneous, staged, or planned within 6 months). The AViKA Navigator Trial was registered at ClinicalTrials.gov (identifier: NCT01540851) and was approved by the BWH Institutional Review Board. From the original trial cohort (N = 308), 15 subjects did not respond to any follow‐up questionnaires, leaving 293 participants for this analysis. Close to 90% of subjects returned at least four questionnaires, with the majority of subjects (n = 183) returning all six follow‐up questionnaires.
Outcome measures
All participants received mailed questionnaires at baseline (within 6 weeks prior to TKR), and then at 3‐, 6‐, 12‐, 24‐, 36‐, and 48‐months post‐TKR. We ascertained MSK symptomatic areas at baseline and at each follow‐up questionnaire. Participants were asked, “In the past four weeks, to what extent did problems in the following areas limit your activities?” In response, participants could specify the degree of limitation (“none,” “a little,” or “a lot”) for each of the following regions: neck, hands/wrists/arms/shoulders, back, hips, right knee, left knee, and ankles/feet. We did not include the index knee in our assessment of MSK symptomatic areas. To focus on more clinically relevant levels of functional limitation, we categorized responses of “none” or “a little” limitation as absence of an MSK symptomatic area and “a lot” as presence of an MSK symptomatic area. This categorization ensured more stringent criteria for the development of a new MSK symptomatic area.
The primary outcome was development of a new MSK symptomatic area in any region after TKR, dichotomized as yes or no. To qualify as a new MSK symptomatic area, the area had to score “none” or “a little” on the baseline questionnaire and “a lot” on at least one follow‐up questionnaire. The secondary outcome was first symptomatic area by location (neck, hands/wrists/arms/shoulders, back, hips, nonindex knee, back, and ankles/feet).
Covariates
Covariates of interest were assessed at baseline and separated into four major categories: general demographics, medical comorbidities, MSK comorbidities, and mental health comorbidities. General demographics included age, sex, and body mass index (BMI) (kg/m2). We transformed several covariates from continuous to categorical for ease of clinical interpretation. Age was categorized as younger than 65 years, 65‐75 years, and older than 75 years because the median age of the cohort was 66 years; we wished to examine if an age older than 75 years conferred particularly high risk, and this breakdown was the closest to creating roughly equal tertiles. BMI was categorized as less than 35 and 35 or more because this cutoff is used to differentiate between low risk (class I) versus moderate (class II) to high risk (class III) obesity by the World Health Organization 10.
Medical comorbidities were extracted through an electronic medical record review via clinic‐visit notes and chart problem lists. We calculated the Charlson Comorbidity Index score for each individual participant, which we categorized as 0, 1, and 2 or more 11. We measured lower‐extremity pain and functional limitations using the WOMAC, a validated questionnaire for hip and knee OA that includes a 5‐item pain subscale and a 17‐item functional limitation subscale 12. We transformed WOMAC scores to a 0‐ to 100‐point scale, with 100 indicating the worst score. We dichotomized WOMAC scores at 40 or less or more than 40 to make the scale more interpretable because a score of 40 meant that a respondent endorsed “moderate” to each question about pain or function level (with possible responses being “none,” “mild,” “moderate,” “severe,” or “extreme”). We also chose 40 as the cutoff because it was the median baseline score for both WOMAC pain and function subscales.
Finally, we assessed mental health status using the five‐item Mental Health Inventory (MHI‐5), a validated five‐item screening tool for anxiety and depression 13, 14, 15. Scores were summed and transformed to a linear 0‐ to 100‐point scale, with 0 indicating the worst mental health status. This variable was dichotomized to less than 68 and 68 or higher based on established screening cutoff criteria for identifying persons likely to have depressive symptoms 13, 14, 15. To evaluate participants’ negative or exaggerated attitudes toward pain, we used the Pain Catastrophizing Scale (PCS), a 13‐item score 16, 17. Because there is no established cutoff for high pain catastrophizing, we used a PCS score of 16 because this corresponded to the 75th percentile of PCS scores in our cohort. This method was used by the creators of the PCS instrument for creating cutoff scores 16. Also, a previous study analyzing preoperative pain catastrophizing and post‐TKR outcomes used a similar cutoff score 17.
Statistical analysis
Descriptive statistics for the overall cohort were presented as numbers and percentages for all variables. We compared participants who were included with those excluded from the analysis using χ2 and Fisher's exact tests for proportions. There were no continuous variables included in the descriptive statistics.
Cumulative incidence and incidence rate
We defined the cumulative incidence of an MSK symptomatic area after TKR as the following: (number of individuals who developed a new symptomatic area in a particular body region)/(number of individuals without baseline symptoms in that particular body region). We calculated cumulative incidence for each area separately and for at least one involved area. For the cumulative incidence of at least one involved area, the following equation was used: (number of individuals who developed a new symptomatic area in any body region)/(number of individuals with at least one asymptomatic area at baseline). All subjects had the potential of developing another new MSK symptomatic area for any region that had not been previously reported as painful. At baseline, if subjects did not endorse whether a particular MSK‐related body site was painful, we coded it as not painful. Using follow‐up data, all time points were taken into consideration in determining incidence. In the rare instance in which a subject did not respond to questions about individual body sites at every time point, these sites were assumed to be not painful.
Censoring occurred at time of outcome, death, date of last completed questionnaire, or the last day of the study. Person‐time was defined as the study start date to the time of censoring. We calculated person‐time for determining the incidence rate, not as an outcome of interest. Time of outcome was defined as the midway point between a positive questionnaire (reporting new symptomatic area) and the immediate preceding questionnaire. For the outcome of at least one involved area, person‐time was truncated at the development of the first new symptomatic area.
The incidence rate of each MSK symptomatic area after TKR was defined as follows: (number of individuals who developed a new symptomatic area in a particular body region)/(total person‐time of the population with no baseline symptoms in that particular body region). Similarly, the incidence rate of at least one involved area was defined as follows: (number of individuals who developed a new symptomatic area in any body region)/(total person‐time of the population with at least one asymptomatic area at baseline). Total person‐time was defined as the study start date to the time of censoring and was summed across all cohort members. Incidence rates with corresponding 95% confidence intervals (CIs) were calculated using the Greenland and Rothman method for each area separately and for the outcome at least one area involved 18.
Primary analysis: Poisson regression model
To identify baseline factors associated with any incident MSK symptomatic area after TKR, we used generalized linear mixed models to account for within‐subject correlations. The first report of a new MSK symptomatic area was coded as incident. If an area became symptomatic, resolved, and then became symptomatic again, the area was still counted once. Those areas that remained painful at multiple time points were also only counted once.
To provide risk ratios, we used a modified Poisson regression approach with robust error variance 19. Risk‐factor modeling involved the identification of potential covariates via screening based on a two‐sided P value less than 0.05, relative risk (RR) greater than 1.25, or RR less than 0.8, as determined in the bivariate analyses. Covariates that passed the bivariate screen were incorporated simultaneously into the full multivariate Poisson model. To build the parsimonious model, we removed factors one at a time based on least significant P values until all remaining covariates either demonstrated statistical significance by P value or had an RR greater than 1.25 or less than 0.8. Once all covariates met the P value or RR threshold criteria, we considered the parsimonious model complete. Of note, we collapsed the covariate “number of symptomatic areas at baseline” into two categories (0‐1 and 2 or more) because univariate analyses suggested a threshold effect at 2.
Secondary analysis: survival analysis
We performed an analysis for time to first incident MSK symptomatic area using Cox proportional hazard regression modeling. Subjects were censored at time of first event or at date of last contact for those not experiencing an incident MSK symptomatic area. Covariate selection for the multivariable Cox regression model was performed by stepwise selection (the entry and exit criterion was P < 0.15). Because WOMAC pain and function scales are moderately correlated with one another, we also examined the effect of substituting one with the other on the hazard ratios (HRs) associated with other covariates. We summarized the results as HRs and 95% CIs. Proportional hazards assumptions were evaluated and satisfied for the outcome.
Analyses were conducted using SAS version 9.4 (SAS Institute, Inc). Reported P values are two‐sided, and P < 0.05 was considered statistically significant.
Results
Patient characteristics
Baseline characteristics of the cohort are summarized in Table 1. Among the 308 eligible and enrolled participants, 293 (95%) completed at least 1 follow‐up questionnaire and were included in this analysis. The subjects excluded from the analysis (n = 15), compared with the analytic cohort, had similar baseline distributions in age, comorbid conditions, WOMAC function scores, and measures of psychosocial conditions. Excluded subjects were significantly more likely to report baseline WOMAC pain scores over 40 (77% vs 44%; P = 0.02) and baseline MHI‐5 scores less than 68 (54% vs 20%; P = 0.008) compared with subjects in the analytic cohort.
Table 1.
Baseline characteristics of the cohort (N = 293)a
| n (%) | |
|---|---|
| Demographics | |
| Age, y | |
| <65 | 123 (42) |
| 65‐75 | 130 (44) |
| >75 | 40 (14) |
| Sex | |
| Male | 115 (39) |
| Female | 178 (61) |
| Body mass index, kg/m2 | |
| <35 | 224 (78) |
| ≥35 | 63 (22) |
| Medical comorbidities: Charlson Comorbidity Index | |
| 0 | 125 (46) |
| 1 | 48 (18) |
| ≥2 | 100 (37) |
| Orthopedic comorbidities | |
| No. of musculoskeletal symptomatic areas at baselineb | |
| 0 | 219 (75) |
| 1 | 48 (16) |
| ≥2 | 26 (9) |
| Baseline WOMAC score (pain)c | |
| ≤40 | 161 (56) |
| >40 | 127 (44) |
| Baseline WOMAC score (function) | |
| ≤40 | 145 (50) |
| >40 | 147 (50) |
| Mental health comorbidities | |
| MHI‐5 scored | |
| <68 | 58 (20) |
| ≥68 | 235 (80) |
| PCS scoree | |
| <16 | 219 (76) |
| ≥16 | 70 (24) |
Abbreviation: MHI‐5, five‐item Mental Health Inventory; PCS, Pain Catastrophizing Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The percentage of individuals with missing data ranged from 0% to 6.8%.
Presence of baseline musculoskeletal symptomatic area was defined as a response of “a lot of limitation” for at least one region on the baseline questionnaire.
Scores were transformed to a 0‐ to 100‐point scale (100 indicating the worst score).
Scores were transformed to a 0‐ to 100‐point scale (0 indicating the worst score).
A score ≥16 was considered high pain catastrophizing.
Of the 293 subjects in the analysis sample, the mean (SD) age was 66 (8.2) years, roughly 44% were between the ages of 65 and 75 years old, 61% were women, and 78% had a BMI of less than 35. Nearly two‐thirds of the subjects (64%) had a Charlson Comorbidity Index of 0 or 1. Roughly 75% of subjects had no MSK symptomatic areas (besides the index knee) at baseline, whereas 16% had 1 area and 9% had 2 or more areas at baseline. The median baseline WOMAC score for both pain and function was 40. Twenty percent of participants reported baseline symptoms of anxiety and depression, with an MHI‐5 score of less than 68. One‐quarter of patients demonstrated high pain catastrophizing at baseline, with a PCS 16 or more.
Baseline and incident MSK symptomatic areas by region
Table 2 summarizes baseline and incident MSK symptomatic areas by region for the analytic cohort. Baseline MSK symptomatic areas were most commonly reported in the nonindex knee (10%), back (10%), and ankles/feet (9%), followed by hands/wrists/arms/shoulders (7%).
Table 2.
Baseline and incident musculoskeletal symptomatic areas by region at 4 ya
| Area | Presence of Baseline Symptomatic Areas, n (%)b | No. Who Could Develop New Symptomatic Areas, n (%)c | Time at Risk, PYd | No. With New Symptomatic Arease | Cumulative Incidence, %f | Incidence Rate per 100 PY (95% CI)g |
|---|---|---|---|---|---|---|
| Neck | 6 (2) | 287 (98) | 913.89 | 24 | 8 | 2.6 (1.7‐4.0) |
| Hands, wrists, arms, or shoulders | 19 (7) | 274 (94) | 857.93 | 36 | 13 | 4.2 (3.0‐6.0) |
| Back | 27 (10) | 266 (91) | 772.47 | 41 | 15 | 5.3 (3.8‐7.4) |
| Hips | 13 (5) | 280 (96) | 843.13 | 37 | 13 | 4.4 (3.1‐6.2) |
| Nonindex knee | 29 (10) | 264 (90) | 866.62 | 60 | 23 | 6.9 (5.2‐9.2) |
| Ankles or feet | 27 (9) | 266 (91) | 851.34 | 28 | 11 | 3.3 (2.2‐4.9) |
| ≥1 area involved | 74 (25) | 293 (100) | 681.59 | 131 | 45 | 19.2 (15.3‐24.2) |
Abbreviation: CI, confidence interval; PY, person‐years.
For participants with missing data, baseline and/or incident symptomatic areas were presumed to be absent.
Presence of baseline musculoskeletal symptomatic area was defined as a response of “a lot of limitation” for ≥1 baseline questionnaire; Percentages = (number of individuals with a baseline symptomatic area)/293.
Number who could develop new symptomatic area was defined as those having “none” or “a little” limitation for a specified symptomatic area on the baseline questionnaire; N = 293 (number with baseline symptomatic area); Percentages = (number of individuals who could develop new symptomatic areas)/293.
Time at risk for each symptomatic area was measured from baseline to the time of incident symptomatic area, defined as the midway point between a “positive” questionnaire and the preceding “negative” questionnaire. Times were summed across all individuals to obtain the total time at risk for each symptomatic area.
A new symptomatic area was defined as a response of “a lot of limitation” for a specified symptomatic area on ≥1 follow‐up questionnaire. The percentage was excluded because of differential follow‐up.
Cumulative incidence over 4 y = (number of individuals who developed a new symptomatic area)/(number of individuals without baseline symptoms in that particular body region) × 100.
Incidence rate = (number of individuals who developed a new symptomatic area)/(time at risk).
The cumulative incidence of MSK symptomatic areas after TKR by location was highest for the nonindex knee (23%), followed by the back (15%), hands/wrists/arms/shoulders (13%), and hips (13%). Corresponding incidence rates were 6.9 per 100 person‐years for the nonindex knee (95% CI 5.2‐9.2 per 100 person‐years), 5.3 per 100 person‐years for the back (95% CI 3.8‐7.4 per 100 person‐years), 4.2 per 100 person‐years for hands/wrists/arms/shoulders (95% CI 3.0‐6.0 per 100 person‐years), and 4.4 per 100 person‐years for hips (95% CI 3.1‐6.2 per 100 person‐years). Cumulative incidence for any MSK symptomatic area was 45%, whereas the corresponding incidence rate was 19.2 per 100 person‐years (95% CI 15.3‐24.2 per 100 person‐years). Of note, although 74 individuals had at least 1 symptomatic MSK area at baseline, no one had symptoms in all 6 regions at baseline. Thus, all 293 subjects were at risk for developing an incident symptomatic area.
Primary analysis: factors associated with incident MSK symptomatic area
Based on the final multivariate Poisson regression model (Table 3), preoperative factors associated with any incident MSK symptomatic areas after TKR included female sex (RR 1.64; 95% CI 1.15‐2.34), BMI of 35 or higher (RR 1.27; 95% CI 0.88‐1.85), Charlson Comorbidity Index of 2 or more (RR 1.28; 95% CI 0.92‐1.78), baseline index knee WOMAC pain score greater than 40 (RR 1.39; 95% CI 0.99‐1.95), and symptoms consistent with anxiety/depression (MHI‐5 less than 68) (RR 1.70; 95% CI 1.20‐2.40).
Table 3.
Factors associated with incident MSK symptomatic areas by Poisson regression
| Univariate Analysis | Multivariate Analysis (Full) | Multivariate Analysis (Parsimonious) | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | |
| Demographics | ||||||
| Age | 0.69 | |||||
| <65 y | 1.0 (reference) | – | – | – | – | – |
| 65‐75 y | 0.86 (0.60‐1.24) | – | – | – | – | – |
| >75 y | 1.00 (0.60‐1.69) | – | – | – | – | – |
| Sex | 0.0008 | 0.007 | 0.007 | |||
| Male | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| Female | 1.88 (1.30‐2.73) | – | 1.66 (1.15‐2.40) | – | 1.64 (1.15‐2.34) | – |
| BMI (kg/m2) | 0.06 | 0.19 | 0.20 | |||
| <35 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| ≥35 | 1.42 (0.99‐2.04) | – | 1.28 (0.89‐1.86) | – | 1.27 (0.88‐1.85) | – |
| Medical comorbidities | ||||||
| CCI | 0.22 | 0.11 | 0.14 | |||
| 0‐1 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| ≥2 | 1.25 (0.87‐1.77) | – | 1.31 (0.94‐1.82) | – | 1.28 (0.92‐1.78) | – |
| Orthopedic comorbidities | ||||||
| No. of symptomatic areas at baseline | 0.47 | |||||
| 0‐1 | 1.0 (reference) | – | – | – | – | – |
| ≥2 | 0.78 (0.41‐1.51) | – | – | – | – | – |
| Baseline WOMAC pain | 0.005 | 0.21 | 0.06 | |||
| ≤40 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| >40 | 1.59 (1.15‐2.21) | – | 1.28 (0.87‐1.89) | – | 1.39 (0.99‐1.95) | – |
| Baseline WOMAC function | 0.007 | 0.79 | ||||
| ≤40 | 1.0 (reference) | – | 1.0 (reference) | – | – | – |
| >40 | 1.58 (1.13‐2.20) | – | 1.06 (0.70‐1.59) | – | – | – |
| Mental health comorbidities | ||||||
| MHI‐5 score | 0.02 | 0.01 | 0.003 | |||
| <68 | 1.59 (1.09‐2.34) | – | 1.57 (1.09‐2.26) | – | 1.70 (1.20‐2.40) | – |
| ≥68 | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| PCS score | 0.02 | 0.26 | ||||
| <16 | 1.0 (reference) | – | 1.0 (reference) | – | – | – |
| ≥16 | 1.53 (1.08‐2.17) | – | 1.22 (0.86‐1.74) | – | – | – |
Abbreviation: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; MHI‐5, five‐item Mental Health Inventory; MSK, musculoskeletal; PCS, Pain Catastrophizing Scale; RR, relative risk; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Secondary analysis: survival analysis
Based on the multivariable Cox regression model (Figure 1), preoperative factors associated with shorter time to incident MSK symptomatic area included female sex (HR 1.86; 95% CI 1.23‐2.82), BMI of 35 or higher (HR 1.69; 95% CI 1.11‐2.55), Charlson Comorbidity Index of 2 or more (HR 1.44; 95% CI 0.99‐2.09), baseline index knee WOMAC function score greater than 40 (HR 1.75; 95% CI 1.19‐2.57), and symptoms consistent with anxiety/depression (MHI‐5 less than 68) (HR 1.92; 95% CI 1.26‐2.91). These results were consistent with the covariates selected by the final Poisson regression model, except for the WOMAC function score, which replaced the WOMAC pain score. When we reran the Cox regression model and inserted WOMAC pain into the model instead of WOMAC function, we obtained similar HRs for female sex, BMI of 35 or higher, Charlson Comorbidity Index, and MHI‐5 less than 68; the HR for a WOMAC pain score greater than 40 was 1.63 (95% CI 1.12‐2.37).
Figure 1.
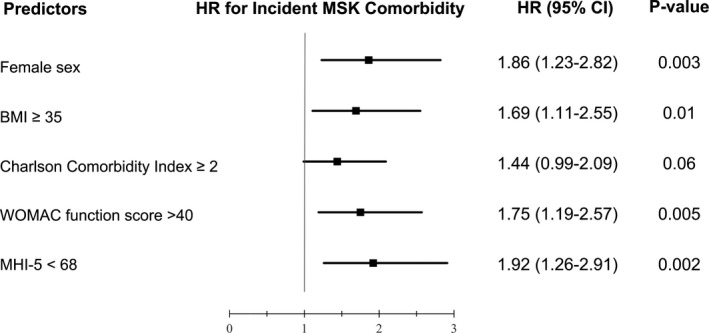
Multivariable Cox regression model: factors associated with time to incident musculoskeletal (MSK) symptomatic area. BMI, body mass index, CI, confidence interval; HR, hazard ratio; MHI‐5, five‐item Mental Health Inventory; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
In this study of 293 patients undergoing elective TKR for knee OA, nearly half of participants developed a new MSK symptomatic area after surgery. Female participants and participants who had a baseline BMI of 35 or higher, Charlson Comorbidity Index of 2 or more, baseline index knee WOMAC pain score greater than 40, or preoperative symptoms consistent with anxiety/depression (MHI‐5 less than 68) were at higher risk of developing incident MSK symptomatic areas. These findings highlight the susceptibility of patients to new MSK symptomatic areas after knee replacement. Moreover, identification of these risk factors for incident MSK symptomatic areas ensures that health care providers can focus on vulnerable populations and offer appropriate counseling (ranging from weight to mental health) to best prepare patients for TKR.
It is unclear whether incident MSK symptomatic areas, particularly those in the lower extremities, arise as a direct consequence of TKR or are unrelated to the surgery and unmasked by the resolution of a formerly painful index knee. Cumulative incidence and incidence rates were highest for the nonindex knee and back. It is conceivable that regional pain in these areas arises from changes in gait and asymmetric loading after TKR 20, 21, 22, 23, 24. Regarding the nonindex knee, multiple studies have noted an association between index and nonindex knee OA. Chitnavis et al 25 reported that among patients who were surveyed shortly after TKR, roughly one‐third had bilateral knee replacement, whereas two‐fifths of those with unilateral TKR had chronic pain in the contralateral knee. Shakoor et al 26 found that among recipients of TKR who underwent a second total joint replacement, 92% of the time, it occurred in the contralateral knee. Although degenerative changes likely existed bilaterally prior to TKR, this association suggests a nonrandom progression of OA in the contralateral knee after arthroplasty TKR 26.
Other causes of nonindex knee pain include new gait abnormalities; postoperatively, recipients favor the nonoperated limb because of decreased quadriceps strength, decreased knee flexion, and decreased hip abduction in the operated limb 24, 27, 28. As for the back, limited knee extension caused by knee OA has been associated with decreased lumbar lordosis and worse lower back pain, a phenomenon termed “knee‐spine syndrome” 22, 23. All of these mechanisms potentially explain why recipients of TKR are susceptible to developing incident nonindex knee and back symptoms postoperatively.
The development of incident upper‐extremity MSK symptomatic areas (ie, neck, hands, wrists, arms, and shoulders) after TKR is more difficult to explain on biomechanical grounds, although upper‐extremity problems could stem from use of assistive devices. It is also possible that the MSK symptomatic areas these subjects reported reflect an underlying predisposition to pain reporting, perhaps related to depression and catastrophizing, as seen in the analysis. Upper‐extremity MSK symptomatic areas, specifically the elbow, were similarly noted by Suri et al 29 in their study of participants with nonsurgical symptomatic knee OA from the Osteoarthritis Initiative. In this study, participants were assessed for knee pain and MSK symptomatic areas at a single time point (time of enrollment). The authors found that ipsilateral elbow pain was associated with higher WOMAC pain subscale scores in the more symptomatic knee. Like our study, this observation of an association between knee OA pain severity and elbow pain is suggestive of a generalized pain syndrome in some individuals with knee OA.
Similarly, multiple studies have demonstrated a positive association between knee OA and pain sensitization 30, 31. More specifically, subjects with lower‐pressure pain thresholds have been found to have greater OA‐associated pain, regardless of disease duration or radiographic severity. This may explain why people undergoing TKR develop incident MSK symptomatic areas elsewhere.
In our study, several baseline covariates were associated with incident MSK symptomatic areas. In the primary analysis, we used a repeated‐measures regression model for time to new MSK symptom, and risk factors included female sex, obesity, Charlson Comorbidity Index of 2 or more, baseline index knee WOMAC pain score greater than 40, and symptoms consistent with clinical anxiety/depression. The secondary Cox survival analysis identified the same set of risk factors (for shorter time to 4‐year incident MSK symptomatic area), except that the repeated measures analysis included baseline WOMAC pain and the Cox model included baseline WOMAC function. These two variables are highly correlated (r = 0.83; P < 0.0001). Our findings of an association between baseline WOMAC pain, anxiety or depression, and symptomatic nonindex joint counts are consistent with results by Perruccio et al 6. Although their study was focused on pre‐TKR MSK symptomatic areas, they similarly noted that higher baseline WOMAC pain and anxiety/depression scores correlated with higher presurgical symptomatic joint counts 6.
Our findings also mirror past studies that show that female sex and depression are risk factors for diminished postoperative pain relief and gain of function 2, 3, 32, 33, 34, 35. Interestingly, obesity, which is associated with surgical complications in general, has not been implicated in poor knee recovery. Patients with obesity, regardless of BMI severity, are able to achieve similar absolute pain and function scores as patients without obesity within 3‐6 months post‐TKR 36, 37, 38. Why patients with obesity were at risk of incident MSK symptomatic areas in our study is unclear. It is possible that weight‐related incident MSK symptomatic areas occur through alternative mechanisms, such as the postoperative asymmetric loading discussed previously 27, 28.
We note several limitations. First, the study was conducted at a single tertiary academic center, which may limit generalizability. However, the baseline demographics of our cohort, including age and sex, are representative of patients undergoing TKR nationally 1.
Second, our questionnaire assessed MSK symptomatic areas by region rather than joint, with multiple joints grouped together in some instances (ie, hands/wrists/arms/shoulders and ankles/feet). This may have led to underreporting of symptomatic joints.
Third, our baseline questionnaires did not assess laterality of symptomatic areas (left vs right); it is possible that incidences and RRs differed based on whether the area was ipsilateral or contralateral to the index knee.
Fourth, our definition of new MSK symptomatic area only included subjects with “a lot” of limitation, whereas those with “a little” limitation were excluded. It is possible that this resulted in underestimation of cumulative incidences and incidence rates. Our stringent definition resulted in cumulative incidences as high as 15% for the back and 23% for the nonindex knee. Including participants with “a little” limitation would have led to even higher rates.
Fifth, our study did not differentiate between transient or persistent incident MSK symptomatic areas. The impact of a persistent incident MSK symptomatic area is presumably greater than a transient MSK symptomatic area.
Sixth, we did not analyze the data by individual medical comorbidity but rather by Charlson Comorbidity Index score. As a result, we could not adjust for specific medical comorbidities in our assessment of incident MSK symptomatic areas. It is possible that certain medical conditions led to areas of pain or impairment, independent of TKR.
Seventh, a small number of responses regarding MSK pain areas were left blank on follow‐up questionnaires, creating ambiguity on whether to consider the response as “no pain” or simply missing. We examined the cumulative incidence and incidence rates of new MSK complaints in each area under both assumptions and found the cumulative incidence differed between the two approaches by less than 1% for each anatomic area.
Eighth, we did not collect sufficient follow‐up data on the use of assistive devices, data which may have been helpful in explaining why incident MSK symptoms developed in the upper extremities.
Finally, subjects who were excluded from the study because they had zero follow‐up questionnaires were significantly more likely to report worse baseline WOMAC pain scores (greater than 40). Although we cannot definitively determine their impact, it is likely inclusion of these subjects would have led to even higher cumulative incidence and incidence rates of postoperative MSK symptomatic areas.
Incident MSK symptomatic areas, particularly those in the nonindex knee and back, occur in roughly half of recipients of elective TKR in the 4 years following TKR. Predictors of incident MSK symptomatic areas include sex, obesity, medical comorbidities, worse baseline index knee pain scores, and anxiety/depression. The effects of these incident MSK symptomatic areas on pain, functional status, and quality of life for recipients of TKR have yet to be elucidated. Understanding the prevalence, risk factors, and, ultimately, the impact of incident MSK symptomatic areas may offer guidance for clinicians on how to optimize the post‐TKR rehabilitation process.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Katz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Zhang, Selzer, Losina, Collins, Katz.
Acquisition of data
Selzer, Losina, Collins, Katz.
Analysis and interpretation of data
Zhang, Selzer, Losina, Collins, Katz.
Drs. Zhang and Losina's work and Drs. Selzer, Losina, and Katz's work were supported by grants from the NIH (grants T32‐AR‐055885, K24‐AR‐057827, and P30‐AR‐072577, respectively).
Dr. Losina has received research grants to Brigham and Women's Hospital from Genentech and Samumed and has served as a consultant for Samumed and TissueGene (less than $10 000 each) more than 12 months ago. She serves as The Journal of Bone and Joint Surgery Deputy Editor for Methodology and Statistics. Dr. Katz has received research grants to Brigham and Women's Hospital from Samumed and Flexion Therapeutics. He serves as The Journal of Bone and Joint Surgery Deputy Editor for Methodology and Statistics. No other disclosures relevant to this article were reported.
References
- 1. Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) . Agency for Healthcare Research and Quality. URL: https://hcupnet.ahrq.gov/. [PubMed]
- 2. Mehta SP, Perruccio AV, Palaganas M, Davis AM. Do women have poorer outcomes following total knee replacement? Osteoarthritis Cartilage 2015;23:1476–82. [DOI] [PubMed] [Google Scholar]
- 3. Lopez‐Olivo MA, Landon GC, Siff SJ, Edelstein D, Pak C, Kallen MA, et al. Psychosocial determinants of outcomes in knee replacement. Ann Rheum Dis 2011;70:1775–81. [DOI] [PubMed] [Google Scholar]
- 4. Judge A, Arden NK, Cooper C, Kassim Javaid M, Carr AJ, Field RE, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford) 2012;51:1804–13. [DOI] [PubMed] [Google Scholar]
- 5. Kahn TL, Soheili AC, Schwarzkopf R. Poor WOMAC scores in contralateral knee negatively impact TKA outcomes: data from the osteoarthritis initiative. J Arthroplasty 2014;29:1580–5. [DOI] [PubMed] [Google Scholar]
- 6. Perruccio AV, Power JD, Evans HM, Mahomed SR, Gandhi R, Mahomed NN, et al. Multiple joint involvement in total knee replacement for osteoarthritis: effects on patient‐reported outcomes. Arthritis Care Res (Hoboken) 2012;64:838–46. [DOI] [PubMed] [Google Scholar]
- 7. Collados‐Maestre I, Lizaur‐Utrilla A, Martinez‐Mendez D, Marco‐Gomez L, Lopez‐Prats FA. Concomitant low back pain impairs outcomes after primary total knee arthroplasty in patients over 65 years: a prospective, matched cohort study. Arch Orthop Trauma Surg 2016;136:1767–71. [DOI] [PubMed] [Google Scholar]
- 8. Losina E, Collins JE, Daigle ME, Donnell‐Fink LA, Prokopetz JJ, Strnad D, et al. The AViKA (Adding Value in Knee Arthroplasty) postoperative care navigation trial: rationale and design features. BMC Musculoskelet Disord 2013;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Losina E, Collins JE, Wright J, Daigle ME, Donnell‐Fink LA, Strnad D, et al. Postoperative care navigation for total knee arthroplasty patients: a randomized controlled trial. Arthritis Care Res (Hoboken) 2016;68:1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Obesity: preventing and managing the global epidemic: report of a WHO Consultation on Obesity. Geneva: World Health Organziation; 1997. [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 12. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 13. Berwick DM, Murphy JM, Goldman PA, Ware JE Jr, Barsky AJ, Weinstein MC. Performance of a five‐item mental health screening test. Med Care 1991;29:169–76. [DOI] [PubMed] [Google Scholar]
- 14. Yamazaki S, Fukuhara S, Green J. Usefulness of five‐item and three‐item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual Life Outcomes 2005;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rumpf HJ, Meyer C, Hapke U, John U. Screening for mental health: validity of the MHI‐5 using DSM‐IV Axis I psychiatric disorders as gold standard. Psychiatry Res 2001;105:243–53. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- 17. Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010;468:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothman KJ, Greenland S, Lash TL. (eds). Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 19. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 20. Saunders JB, Inman VT, Eberhart HD. The major determinants in normal and pathological gait. J Bone Joint Surg Am 1953;35‐A:543–58. [PubMed] [Google Scholar]
- 21. Burnett DR, Campbell‐Kyureghyan NH, Topp RV, Quesada PM. Biomechanics of lower limbs during walking among candidates for total knee arthroplasty with and without low back pain. Biomed Res Int 2015;2015:142562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuji T, Matsuyama Y, Goto M, Yimin Y, Sato K, Hasegawa Y, et al. Knee‐spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci 2002;7:519–23. [DOI] [PubMed] [Google Scholar]
- 23. Murata Y, Takahashi K, Yamagata M, Hanaoka E, Moriya H. The knee‐spine syndrome. Association between lumbar lordosis and extension of the knee. J Bone Joint Surg Br 2003;85:95–9. [DOI] [PubMed] [Google Scholar]
- 24. Milner CE. Is gait normal after total knee arthroplasty? Systematic review of the literature. J Orthop Sci 2009;14:114–20. [DOI] [PubMed] [Google Scholar]
- 25. Chitnavis J, Sinsheimer JS, Suchard MA, Clipsham K, Carr AJ. End‐stage coxarthrosis and gonarthrosis. Aetiology, clinical patterns and radiological features of idiopathic osteoarthritis. Rheumatology (Oxford) 2000;39:612–9. [DOI] [PubMed] [Google Scholar]
- 26. Shakoor N, Block JA, Shott S, Case JP. Nonrandom evolution of end‐stage osteoarthritis of the lower limbs. Arthritis Rheum 2002;46:3185–9. [DOI] [PubMed] [Google Scholar]
- 27. Pozzi F, Snyder‐Mackler L, Zeni J Jr. Relationship between biomechanical asymmetries during a step up and over task and stair climbing after total knee arthroplasty. Clin Biomech (Bristol, Avon) 2015;30:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alnahdi AH, Zeni JA, Snyder‐Mackler L. Quadriceps strength asymmetry predicts loading asymmetry during sit‐to‐stand task in patients with unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2016;24:2587–94. [DOI] [PubMed] [Google Scholar]
- 29. Suri P, Morgenroth DC, Kwoh CK, Bean JF, Kalichman L, Hunter DJ. Low back pain and other musculoskeletal pain comorbidities in individuals with symptomatic Ooteoarthritis of the knee: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2010;62:1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arendt‐Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81. [DOI] [PubMed] [Google Scholar]
- 31. Neogi T, Frey‐Law L, Scholz J, Niu J, Arendt‐Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis 2015;74:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karlson EW, Daltroy LH, Liang MH, Eaton HE, Katz JN. Gender differences in patient preferences may underlie differential utilization of elective surgery. Am J Med 1997;102:524–30. [DOI] [PubMed] [Google Scholar]
- 33. Vina ER, Cloonan YK, Ibrahim SA, Hannon MJ, Boudreau RM, Kwoh CK. Race, sex, and total knee replacement consideration: role of social support. Arthritis Care Res (Hoboken) 2013;65:1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borkhoff CM, Hawker GA, Kreder HJ, Glazier RH, Mahomed NN, Wright JG. The effect of patients’ sex on physicians’ recommendations for total knee arthroplasty. CMAJ 2008;178:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borkhoff CM, Hawker GA, Kreder HJ, Glazier RH, Mahomed NN, Wright JG. Patients’ gender affected physicians’ clinical decisions when presented with standardized patients but not for matching paper patients. J Clin Epidemiol 2009;62:527–41. [DOI] [PubMed] [Google Scholar]
- 36. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg Am 2017;99:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collins JE, Donnell‐Fink LA, Yang HY, Usiskin IM, Lape EC, Wright J, et al. Effect of obesity on pain and functional recovery following total knee arthroplasty. J Bone Joint Surg Am 2017;99:1812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones CA, Cox V, Jhangri GS, Suarez‐Almazor ME. Delineating the impact of obesity and its relationship on recovery after total joint arthroplasties. Osteoarthritis Cartilage 2012;20:511–8. [DOI] [PubMed] [Google Scholar]


