Abstract
Objective
To assess methotrexate (MTX) adherence using the Medication Event Monitoring System (MEMS) and characterize associations with adherence in patients with rheumatoid arthritis (RA).
Methods
Eligible patients participated in Forward, the National Databank for Rheumatic Diseases, and recently (12 months or sooner) initiated oral MTX. MEMS was used to compile MTX weekly dosing over 24 weeks. The Beliefs about Medicines Questionnaire (BMQ) was completed, and baseline demographics and disease characteristics obtained. MTX adherence (percentage of weeks dose taken correctly), implementation (percentage of weeks dose taken correctly from initiation until last dose), and persistence (duration from initiation to last dose) were calculated. Analyses measured associations between patient characteristics and adherence, modeled using logistic generalized estimating equations and censored Poisson regression, and persistence modeled using Cox regression.
Results
Overall, 60 of 119 eligible patients were included in the analysis. MTX adherence, implementation, and persistence were 75%, 80%, and 83%, respectively, at 24 weeks. Demographics and disease characteristics were generally similar between patients with 1 week or less and 2 weeks or more of missed MTX. Unemployment, less disability, higher Patient Global scores, and no prior disease‐modifying antirheumatic drug (DMARD) use were associated with correct dosing. No significant differences in adherence were observed between patients receiving concomitant MTX versus MTX monotherapy, and biologic DMARD‐experienced versus biologic DMARD‐naïve patients. Higher scores in BMQ Specific Necessity (indicating a greater belief in the necessity of the medication) was associated with a decreased likelihood of dosing at an interval shorter than prescribed (odds ratio 0.89).
Conclusion
Even in a participatory group over a short period, MTX adherence was suboptimal and associated with certain demographics, medication experience, and beliefs about medicines. This suggests a need for screening and alternative treatment opportunities in nonadherent MTX patients with RA.
Introduction
The conventional synthetic disease‐modifying antirheumatic drug (csDMARD) methotrexate (MTX) is the gold standard and primary initial treatment for rheumatoid arthritis (RA) and is administered once weekly typically in doses of between 7.5 and 25 mg 1, 2, 3. The current treatment guidelines for RA also recommend the concomitant administration of MTX with biologic DMARDs (bDMARDs) 1, 3, as prior evidence has shown that the efficacy and drug survival of bDMARDs are increased when given in combination with MTX vs monotherapy 4, 5. Although MTX use is common, patients may experience adverse events (up to 45%) or inadequate efficacy, which may influence treatment adherence 1, 2, 6, 7, 8.
As treatment strategies for patients with RA promote more aggressive dose alterations and switching of therapies to achieve lower disease activity, the importance of measuring adherence and understanding its impact remains critical 1, 3, 9. Adherence to medications is a blanket term defined as the process by which patients take their medications as prescribed. Medication adherence has three components: initiation, implementation, and discontinuation. Initiation is when the first dose is taken; implementation is the extent to which a patient's actual dosing matches the prescribed regimen from initiation until the last dose; and discontinuation is when the last dose is taken 10.
In patients with RA, poor adherence, such as stopping medication early or taking a “drug holiday” has been associated with worse outcomes, including reduced improvement in clinical disease activity measures and patient‐reported measures, accelerated disease progression, and overall increased costs 11, 12. Conversely, overcompliance (where the patient has taken more than the prescribed amount of medicine) may lead to more adverse events 13. In addition, a literature review suggests that patient beliefs and understanding of their medications may have a great impact on adherence 14.
Many studies of adherence in rheumatology have relied upon review of clinical records, pharmacy prescription fills, and patient reports 15, 16, 17, all of which have significant limitations affecting the precision and reliability for measuring treatment adherence. The measurement of adherence was advanced by the development of the Medication Event Monitoring System (MEMS), which registers electronically when the cap is removed from a medication bottle (MEMS TrackCap), and has been shown to be a reliable way of determining adherence without being overly intrusive to the patient 18, 19, 20. Two prior studies have used the MEMS with MTX in a rheumatology clinic setting; the first study examined patients with RA, gout, and polymyalgia rheumatica from three outpatient clinics in the Netherlands 21, and the second study followed patients with RA from three clinics in Houston, Texas, over 2 years 22. A study involving a greater number of clinics or direct contact with patients is required to obtain a better sense of MTX adherence in a broad population of patients with RA from the United States.
We used a direct‐to‐patient approach to conduct an observational study assessing 24‐week treatment adherence to oral MTX in patients with RA using the MEMS in a real‐world nonclinical trial setting, where a wide variety of patient characteristics, including beliefs about medication, were available. The objective was to assess MTX treatment adherence (with respect to implementation and persistence) and characterize adherence patterns using electronic medication monitoring in a cohort of patients with RA who were “newly treated” (12 months or shorter initiation before study entry) with oral MTX, as monotherapy or in combination with bDMARDs and csDMARDs, in a real‐world setting.
Patients and Methods
Study design and patients
Eligible patients were aged 18 years or older and were living in the United States, had a physician‐confirmed diagnosis of RA, and had participated in Forward, the National Databank for Rheumatic Diseases: US 23. Patients had to be currently receiving oral MTX that had been initiated 12 months or less prior to study enrollment. Patients could have had exposure to oral or subcutaneous MTX prior to the current course but must not have received MTX during the 12 months prior to the current course. Patients also had to be willing to use the MEMS device (Figure 1). Patients were prospectively followed up for 24 weeks, after which adherence patterns were retrospectively determined and correlated with patient characteristics from data collected using questionnaires.
Figure 1.
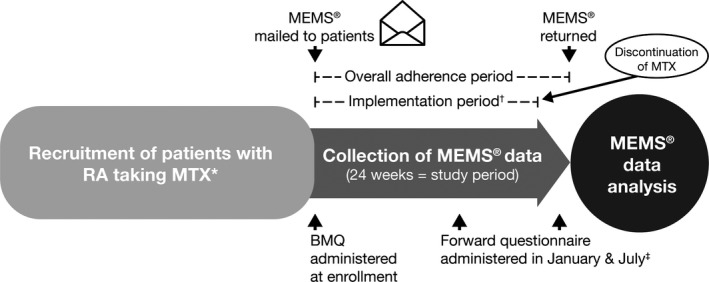
Study design. *MTX must have been initiated ≤12 months prior to study enrollment; †The implementation period varied from patient to patient; for patients who were persistent for the full 24‐week study period, the implementation period was the same as the adherence period. ‡The Forward questionnaire was administered in January and July but answered at any time, typically within 2 months. BMQ, Beliefs about Medicines Questionnaire; MEMS, Medication Event Monitoring System; MTX, methotrexate; RA, rheumatoid arthritis.
The Forward databank is a longitudinal and comprehensive US‐based research databank initiated in 1998 23. Forward participants are recruited on a continuing basis from the practices of US and Canadian rheumatologists and are followed up prospectively with comprehensive questionnaires twice per year. Patients from the United States were contacted to request their participation in this study after indicating that they had initiated oral MTX in the 6‐month Forward follow‐up questionnaire between August 2016 and March 2017.
Assessments
At study enrollment, patients were mailed a MEMS bottle with a MEMS TrackCap along with the instructions for its use; patients could contact Forward by telephone to ask questions. Patients used the MEMS, which automatically recorded the date and time when they accessed their MTX treatment. It was assumed that the prescribed dose was taken each time the bottle was opened, excluding the opening of the bottle for refills or for other reasons, which were captured in patient diaries provided at baseline. After 24 weeks, the dosing history data were retrieved from the MEMS, and baseline patient characteristics were summarized overall and by categorical adherence levels. Depending on the number of weeks when the dosing regimen was not followed (interrupted), patients were categorized as having 1‐week or less interruptions or 2‐week or more interruptions. Some of the reasons for nonadherence were captured in patient diaries.
Additionally, at study enrollment, patients were provided with the Beliefs about Medicines Questionnaire (BMQ) 24. The BMQ used in this study included 19 items and 4 components (General‐Harm, General‐Overuse, Specific‐Necessity, and Specific‐Concerns) that assess the patient's beliefs about medicines in general and in relation to RA (Online Supplementary Figure 1). Patients stated how much they agreed or disagreed with each of the statements, with higher BMQ scores indicating stronger beliefs 24. Briefly, higher scores on General‐Harm and General‐Overuse indicate negative attitudes toward medicines. Higher scores on Specific‐Necessity indicate stronger beliefs in the necessity of medicines. Higher scores on Specific‐Concerns indicate stronger concerns about dependency, toxicity, and disruption.
Primary outcomes
Measure of adherence (overall)
Electronically compiled dosing histories using MEMS were used 25 in each individual patient to define a sequence of weekly binary data indicating whether the patient took MTX as planned on a given week (= 1) or not (= 0). This computation resulted in a longitudinal variable of consecutive weekly binary adherence indicators (0/1) over the 24‐week follow‐up period for each patient. The 24‐week individual binary variables were censored earlier if a patient returned the MEMS before the end of 24 weeks.
Measure of initiation
As patients were already treated with MTX prior to inclusion in this study, initiation of MTX therapy was not assessed.
Measure of persistence
Persistence with MTX was defined for each patient as the length of time between study inclusion and the last dose of MTX taken (discontinuation).
Measure of implementation
Implementation of the dosing regimen was measured using the same computation as the measure of adherence described above, but each individual binary variable was truncated at the time of the last dose of MTX taken. Therefore, the extent to which a patient's actual dosing matches the prescribed regimen from initiation until taking the last dose, unaffected by eventual treatment discontinuation, could be assessed.
In addition, more specifically, weekly binary indicators of under‐ and early dosing were defined as equal to 1 when the patient took fewer or more intakes than prescribed and equal to 0 otherwise. All the individual binary indicators of overall adherence or implementation represent distinct dimensions.
The same outcomes were categorized with respect to the total number and percentage of weeks that patients took the required dosing during the time interval from study initiation to the last dose taken (implementation) or over the 24 weeks of the study (overall adherence). Categories were correct dosing (as prescribed), underdosing (fewer doses than prescribed), and early dosing (doses taken at an interval shorter than prescribed) (Online Supplementary Figure 2).
Secondary outcomes
Secondary outcomes included associations between covariables (such as baseline patient characteristics and beliefs about medicines) and correct, under‐, and early dosing during the adherence (overall) and implementation periods. Reasons for discontinuation of MTX were also assessed.
Covariables
Baseline patient characteristics were retrieved from the most recent Forward questionnaire completed prior to use of the MEMS. In addition to the BMQ, Forward variables were also characterized at baseline; these included demographics (eg, age, sex, ethnicity [white versus nonwhite], educational years, and employment status), clinical variables (eg, Rheumatic Disease Comorbidity Index 26, RA duration, and body mass index [BMI]), RA severity outcomes (eg, Health Assessment Questionnaire [HAQ], HAQ‐II, Patient Activity Scale [PAS], PAS‐II, Rheumatoid Arthritis Disease Activity Index, EuroQoL‐5D, and Visual Analog Scales [VAS], including pain, fatigue, and Patient Global Assessment of Disease Activity [PtGA] 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37), prior or concurrent treatments, prior MTX duration and dose, and total number of concomitant medications.
The BMQ was collected at baseline only. All variables collected in the Forward questionnaire were time dependent, except for constants such as sex and ethnicity. The remaining variables were collected from a maximum of three Forward questionnaires and merged with the MEMS adherence data, using the closest prior questionnaire, during the 24‐week study period.
Statistical analysis
In the modeling approaches described below, the baseline demographics listed in the previous section, along with BMI, RA duration, and MTX dose, were included as independent variables. For RA severity variables, only HAQ‐II, PtGA VAS, pain VAS, and fatigue VAS were used in the models. Indicator variables of whether the patient was bDMARD‐naïve versus bDMARD‐experienced, and of whether the patient received MTX as monotherapy or in combination with another csDMARD or bDMARD, were also included as independent variables. For the longitudinal modeling, time (in weeks) was included, as well as a quadratic term for time (weeks2) to assess for any curvature. Independent variables were included as continuous and as main effects (that is, there were no categorical variables and no interaction terms included).
Baseline characteristics and adherence measures were compared for the patients who had 1 or less interrupted week versus 2 or more interrupted weeks. T‐tests and Chi‐squared or Fisher's Exact test were used for continuous and categorical variables, respectively. All tests were two‐sided, with a 5% significance level. The same approach was taken for comparison of bDMARD‐naïve patients and bDMARD‐experienced patients as well as for patients receiving MTX monotherapy and concomitant csDMARD/bDMARD therapy.
Adherence (overall) as measured by the binary sequences of weekly correct dosing over the 24‐week period was analyzed using a logistic generalized estimating equations (GEE) model using a robust covariance matrix estimator (sandwich estimator), and results were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Adherence (overall) was subsequently broken down into implementation and persistence. Implementation, as measured by the binary sequences of weekly correct dosing as well as under‐ and early dosing between the first and the last dose, was also analyzed using logistic GEE models. Persistence was estimated using a Kaplan–Meier curve, and factors associated with persistence of treatment (including patient characteristics, BMQ, and efficacy measures) were assessed using Cox regression models. Results were presented as hazard ratios (HR) with 95% CI.
Finally, censored Poisson regression models with an offset to account for difference in duration were used to assess the factors that influenced MTX adherence and implementation by examining the number of weeks that patients were receiving correct dosing. Patients were right censored at the total number of weeks they participated in the study or at the time they had their last dose as prescribed. Similar analyses were conducted for under‐ and early dosing. For this analysis, independent variables were all measured at baseline. Results were presented as incidence rate ratios with 95% CI.
For all analyses (logistic GEE, censored Poisson, and Cox regression), the best models were searched using backward selection methods with a 10% significance level at which variables could be removed from the model. The full model began with the following variables: age, sex, white, education, employed, BMI, RA duration, Rheumatic Disease Comorbidity Index, HAQ‐II, VAS scales (pain, PtGA, and fatigue), prior MTX duration, MTX dose, prior use of non‐MTX csDMARDs, prior use of bDMARDs, concomitant treatments, and the BMQ components. At each step, the variable with the largest P value was removed from the model. When all remaining variables met the criterion to remain in the model (P < 10%), the backward elimination process was stopped.
Sensitivity analyses were conducted to provide a comparison with the primary models obtained by backward selection. Adherence (overall), implementation, and persistence were assessed via reduced clinical models (GEE for adherence [overall] and implementation, Cox regression for persistence) that used six variables: age, sex, education, HAQ‐II, prior or concomitant DMARD treatment (csDMARDs or bDMARDs), and time (weeks) in the study. The impact of individual BMQ components was also assessed by adding each as the seventh variable in the clinical model.
All statistical analyses were performed using Stata version 14.2 (StataCorp LP).
The handling of missing data from the Forward questionnaire has been previously reported 23. Missing covariate data on completed questionnaires were handled using multiple imputation by chained equations. Last observation carried forward was performed on one patient who completed a brief questionnaire that omitted variables, such as HAQ, PAS, BMI, etc.
Ethics
The study was conducted in accordance with all legal and regulatory requirements and followed applicable research practices. The study protocol was subject to approval by the investigator's Institutional Review Board/Independent Ethics Committee. All patients provided written, informed consent.
RESULTS
Patients
A total of 182 patients were invited to participate in the study, of which 119 were eligible and 62 were subsequently enrolled. Two patients were excluded from the analysis (one patient was excluded for not returning the MEMS and the other for not answering the follow‐up Forward questionnaire); the final analysis sample therefore consisted of 60 patients. Most patients (85%) each received treatment in a different rheumatology clinic, with the remaining 15% (9 patients) treated across three clinics. No differences regarding demographics, RA severity, or prior treatment were found between eligible patients who did not enroll and patients participating in the study, except for sex, where more women were found in the study group (74% vs 92%). MTX dose was available for 58 patients, only 52 patients had data for PAS and EQ‐5D, 1 patient did not complete the BMQ, and only 1 completed the brief questionnaire. Forward questionnaires were collected on average 2.7 months (SD 2.5) (median 2 [quartile 1‐3: 1‐4] months) before the first use of the MEMS. Patient demographics and baseline disease characteristics are reported in Table 1.
Table 1.
Baseline demographics and disease characteristics stratified by 24‐week adherence
| Baseline Characteristics | All Patients | ≤1 Interrupted Week | ≥2 Interrupted Weeks |
|---|---|---|---|
| Na | 60 | 19 | 41 |
| Age, mean (SD), years | 59.6 (10.8) | 59.2 (9.7) | 59.7 (11.4) |
| Male, % | 8.3 | 10.5 | 7.3 |
| Employed, % | 30.0 | 26.3 | 31.7 |
| Non‐Hispanic Caucasian, % | 88.3 | 94.7 | 85.4 |
| BMI, mean (SD), kg/m2 | 28.8 (7.9) (N = 59) | 29.4 (8.7) | 28.6 (7.6) (N = 40) |
| Duration of education, mean (SD), years | 14.5 (2.5) | 14.4 (2.1) | 14.6 (2.7) |
| Total income (US$), mean (SD) | $67 203.4 (37 637.9) (N = 59) | $66 842.1 (30 102.2) | $67 375.0 (41 090.7) (N = 40) |
| Insurance type, % | |||
| Private | 27.1 (N = 59) | 15.8 | 32.5 (N = 40) |
| HMO | 8.5 (N = 59) | … | 12.5 (N = 40) |
| Medicare | 42.4 (N = 59) | 57.9 | 35.0 (N = 40) |
| PPO | 10.2 (N = 59) | 21.1 | 5.0 (N = 40) |
| Medicaid | 11.9 (N = 59) | 5.3 | 15.0 (N = 40) |
| Duration of disease, mean (SD), years | 17.8 (15.1) | 18.5 (16.7) | 17.5 (14.5) |
| Rheumatic Disease Comorbidity Index (0‐9)b, mean (SD) | 1.5 (1.5) | 1.3 (1.7) | 1.5 (1.4) |
| HAQ (0‐3)c, mean (SD) | 1.2 (0.7) (N = 59) | 1.1 (0.8) | 1.2 (0.7) (N = 40) |
| HAQ‐II (0‐3)c, mean (SD) | 1.0 (0.7) | 0.8 (0.7) | 1.1 (0.7) |
| PAS (0‐10)d, mean (SD) | 3.5 (2.2) (N = 52) | 3.4 (2.7) (N = 17) | 3.6 (2.0) (N = 35) |
| PAS‐II (0‐10)d, mean (SD) | 3.6 (2.1) | 3.5 (2.6) | 3.7 (1.9) |
| EQ‐5D, mean (SD) | 0.8 (0.2) (N = 52) | 0.8 (0.2) (N = 17) | 0.7 (0.1) (N = 35) |
| RADAI (0‐10)d, mean (SD) | 2.8 (1.6) | 2.6 (1.8) | 2.9 (1.4) |
| Pain VAS (0‐10)e, mean (SD) | 3.7 (2.5) | 3.8 (3.0) | 3.7 (2.3) |
| Fatigue VAS (0‐10)e, mean (SD) | 4.9 (3.0) | 5.0 (3.5) | 4.8 (2.8) |
| PtGA VAS (0‐10)e, mean (SD) | 3.8 (2.5) | 4.0 (3.1) | 3.7 (2.2) |
| Duration of prior MTX use, mean (SD), monthsf | 31.5 (44.8) (N = 59) | 20.8 (36.6) | 36.5 (47.8) (N = 40) |
| Prior MTX use, %g | 66.7 | 63.2 | 68.3 |
| MTX dose, mean (SD)h | 14.1 (5.5) (N = 58) | 15.0 (5.8) (N = 18) | 13.6 (5.3) (N = 40) |
| Prior bDMARDs,% | 70.0 | 68.4 | 70.7 |
| Prior non‐MTX csDMARDs,% | 66.7 | 63.2 | 68.3 |
| Concomitant RA treatment, % | 45.0 | 36.8 | 48.8 |
| bDMARDs | 40.0 | 31.6 | 43.9 |
| csDMARDs | 5.0 | 5.3 | 4.9 |
| BMQ, mean (SD) | |||
| General Harm (4‐20) | 8.1 (2.8) (N = 59) | 8.4 (3.1) | 8.0 (2.7) (N = 40) |
| General Overuse (4‐20) | 11.0 (3.3) (N = 59) | 11.4 (3.6) | 10.8 (3.1) (N = 40) |
| Specific Necessity (5‐25) | 20.4 (3.7) (N = 59) | 20.8 (3.8) | 20.1 (3.7) (N = 40) |
| Specific Concerns (6‐30) | 17.3 (4.6) (N = 59) | 18.0 (4.9) | 17.0 (4.4) (N = 40) |
| Total number of medications | 11.0 (6.9) (N = 58) | 10.5 (5.7) (N = 18) | 11.3 (7.4) (N = 40) |
Abbreviation: bDMARD, biologic DMARD; BMI, body mass index; BMQ, Beliefs about Medicines Questionnaire; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; EQ‐5D, EuroQoL‐5D; HAQ, Health Assessment Questionnaire; HMO, Health Maintenance Organization; MTX, methotrexate; PAS, Patient Activity Scale; PPO, Preferred Provider Organization; PtGA, Patient Global Assessment of Disease Activity; RADAI, Rheumatoid Arthritis Disease Activity Index; SD, standard deviation; VAS, Visual Analogue Scale.
aExcept where indicated. bOut of 11 comorbidities; higher score indicates more comorbidities. cHigher score indicates worse function and greater disability. dHigher score indicates higher levels of disease activity. eHigher score indicates greater intensity/severity. fAlthough 31 (52.5%) patients had prior use of MTX (duration ≥12 months), these patients stopped MTX for 12 months before starting their current MTX course. gOf which 9 patients (15%) had prior exposure to subcutaneous MTX. hAt most recent questionnaire. iHigher scores on General Harm and General Overuse indicate negative attitudes toward medicines. Higher scores on Specific‐Necessity indicate stronger beliefs in the necessity of medicines. Higher scores on Specific‐Concerns indicate stronger concerns about dependency, toxicity, and disruption.
MTX dosing
In the 24‐week adherence (overall) and implementation periods, respectively, mean correct MTX dosing was 75% and 80% per patient, mean underdosing was 21% and 14% per patient, and mean early dosing was 4% and 6% per patient (Table 2).
Table 2.
Adherence (overall) and implementation measures
| Adherence (Overall)a | Implementationb | |||
|---|---|---|---|---|
| Mean (SD) | Median (Q1‐Q3) | Mean (SD) | Median (Q1‐Q3) | |
| Correct dosing (%) | 74.8 (27.6) | 83.3 (65.2‐95.8) | 79.8 (21.7) | 85.4 (68.3‐95.8) |
| Underdosing (%) | 21.2 (27.0) | 11.3 (4.2‐26.1) | 14.2 (16.2) | 8.3 (0.0‐19.1) |
| Early dosing (%) | 4.0 (5.2) | 4.2 (0.0‐5.3) | 6.1 (13.6) | 4.2 (0.0‐8.3) |
Abbreviation: Q, quartile.
aAdherence (overall) is measured as the average percentage of weekly correct dosing, and under‐ and early dosing per patient over the 24‐week period. bImplementation is measured as the average percentage of correct dosing, and under‐ and early dosing per patient between the first and the last dose.
During the overall adherence period, 11 (18.3%) patients had perfect (100%) adherence, 8 (13.3%) had 1 interrupted week, and 41 (68.3%) had 2 or more interrupted weeks, including treatment discontinuation. Online Supplementary Figure 3 shows individual MTX dosing data for all patients using color‐coded cells for each week, illustrating trajectories for persistent and nonpersistent patients.
Association of demographic variables and disease characteristics with MTX dosing using longitudinal models
The GEE model of adherence over time had to be adjusted for a quadratic function of time, indicating that the average percentage of patients adherent to MTX treatment decreased over time. This appeared to be mainly due to nonpersistence, as the GEE analysis of implementation over time showed that the average percentages of patients correctly implementing, under‐, or early dosing MTX treatment were stable over time, and were estimated to be 83%, 12%, and 4%, respectively, considering a univariate GEE model only (Online Supplementary Figure 4).
Table 3 presents the best models selected by backward selection. GEE modeling for the adherence (overall) period showed that patients with worse PtGA but better HAQ‐II disability were more likely to be adherent to MTX at correct dosing levels (OR 1.21 for 1 unit of PtGA, and 0.47 for 1 unit of HAQ‐II disability) and less likely to underdose (Table 3). Patients with prior bDMARDs were 2.4‐fold more likely not to be underdosing. In addition, lower levels of education, Caucasian ethnicity, higher disability but lower levels of pain, and stronger beliefs of specific necessity (BMQ) were associated with increased likelihood of not anticipating dosing. Patients became less adherent over time, but this relationship was attenuated in the implementation period compared with the adherence period (Online Supplementary Table 1). In the implementation period, similar models were found except for patients who underdosed, where prior bDMARD use and BMQ‐General Harm did not remain in the model, and for patients who dosed early, where prior csDMARD use remained but not education level or being employed. Older age and employment were associated with a greater likelihood of treatment discontinuation.
Table 3.
Association between number of weeks of correct, under‐, and early dosing of MTX and baseline characteristics, showing odds ratios (95% CI) from longitudinal logistic GEE model and hazard ratios (95% CI) from Cox regression model for persistence, using backward selection from the full set of variables considereda
| Adherence (Overall) | Implementation | Persistence | |||||
|---|---|---|---|---|---|---|---|
| Correct dosing | Underdosing | Early dosing | Correct dosing | Underdosing | Early dosing | ||
| Age (years) | 1.10b (1.02‐1.20) | ||||||
| White ethnicity (1 white, 0 nonwhite) | 0.19b (0.10‐0.37) | 0.16b (0.07‐0.33) | |||||
| Education level (years) | 1.19b (1.00‐1.41) | ||||||
| Employed | 0.47 (0.22‐1.03) | 12.03b (2.45‐59.18) | |||||
| BMI (kg/m2) | 1.04 (0.99‐1.09) | 1.04 (1.00‐1.08) | |||||
| HAQ‐II (0‐3) | 0.47b (0.24‐0.94) | 2.63b (1.27‐5.44) | 0.37b (0.19‐0.73) | 0.50b (0.28‐0.89) | 2.48b (1.35‐4.57) | 0.34b (0.17‐0.69) | |
| Pain VAS (0‐10) | 1.24b (1.04‐1.47) | 1.21b (1.02‐1.43) | |||||
| PtGA VAS (0‐10) | 1.21b (1.03‐1.43) | 0.80b (0.68‐0.95) | 0.85b (0.73‐0.99) | 1.28b (1.10‐1.49) | 0.75b (0.63‐0.89) | 0.85 (0.71‐1.00) | |
| Prior csDMARD use (not MTX) | 2.01 (0.95‐4.25) | ||||||
| Prior bDMARD use | 0.42b (0.18‐0.98) | ||||||
| BMQ general harmc | 0.87 (0.74‐1.02) | ||||||
| BMQ‐specific necessityd | 0.89b (0.82‐0.95) | 0.85b (0.77‐0.93) | |||||
| Time (weeks) | 0.88b (0.79‐0.97) | 1.22b (1.10‐1.35) | 0.88b (0.78‐0.99) | 1.27b (1.18‐1.43) | |||
| Time2 (weeks2) | 1.00b (1.00‐1.01) | 0.99b (0.99‐1.00) | 1.00b (1.00‐1.01) | 0.99b (0.99‐1.00) | |||
Abbreviation: bDMARD, biologic DMARD; BMI, body mass index; BMQ, Beliefs about Medicines Questionnaire; CI, confidence interval; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; GEE, Generalized Estimating Equations; HAQ, Health Assessment Questionnaire; MTX, methotrexate; PtGA, Patient Global Assessment of Disease Activity; VAS, visual analog scale.
aEach model represents a dimension. Correct dosing cannot be directly compared with under‐ or early dosing. See Online Supplementary Table 1 for correct dosing predictions. b P < 0.05. cBMQ general harm: higher score equals greater belief that medicines are harmful, addictive, poisons that should not be taken continuously. dBMQ‐specific necessity: higher score indicates greater belief in the necessity of the medication.
Clinical models (Online Supplementary Table 2) that used only six variables as a sensitivity analysis aligned with the primary backward selection models: variables such as HAQ‐II disability, time, prior DMARD treatment, and BMQ (as a seventh variable), had similar interpretations, although with different OR estimates. Predictions from the GEE model of correct adherence (overall) and implementation, per both the primary backward selection and clinical models, are presented in Online Supplementary Table 1 for several values of some variables, keeping the remaining variables in the model fixed. For both backward selection and clinical models, there is considerable variation in correct adherence (overall) or implementation by increased HAQ‐II disability.
Association of demographic variables and baseline disease characteristics with MTX dosing using Poisson regression models
No differences were seen in patient demographics or baseline disease characteristics between those patients with 1 or fewer and 2 or more interrupted weeks of MTX use (Table 1).
Best censored Poisson regression models selected by the backward selection are presented in Table 4. The models showed that, during the adherence (overall) period, patients who were unemployed, had less disability, had higher PtGA scores, and had not previously used other non‐MTX csDMARDs were more likely to have more weeks of correct MTX dosing. When the analysis was restricted to the implementation period, MTX dose was the only important factor, ie those who had been on a higher MTX dose were more likely to have more weeks of correct dosing (Table 4).
Table 4.
Association between number of weeks of correct, under‐, and early dosing and baseline characteristics, showing incidence rate ratios (95% CI) from censored Poisson regression models using backward selection from the full set of variables considered
| Adherence (Overall) | Implementation | |||||
|---|---|---|---|---|---|---|
| Correct dosing | Underdosing | Early dosing | Correct dosing | Underdosing | Early dosing | |
| Age (years) | 1.03a (1.01‐1.04) | |||||
| White ethnicity (1 white, 0 nonwhite) | 0.34a (0.12‐0.96) | |||||
| Employed | 0.82a (0.71‐0.94) | 2.33a (1.75‐3.11) | ||||
| HAQ‐II (0‐3) | 0.77a (0.68‐0.87) | 1.99a (1.57‐2.52) | ||||
| Pain VAS (0‐10) | 0.88a (0.82‐0.94) | |||||
| PtGA VAS (0‐10) | 1.05a (1.01‐1.08) | 0.69a (0.58‐0.83) | 0.74a (0.62‐0.87) | |||
| Fatigue VAS (0‐10) | 1.27a (1.10‐1.47) | 1.21a (1.06‐1.38) | ||||
| Monthly duration on MTX | 1.00a (0.99‐1.00) | 1.01a (1.00‐1.01) | 1.01a (1.00‐1.01) | 1.01a (1.00‐1.02) | ||
| Average weekly dose of any MTX | 1.01a (1.00‐1.03) | 0.90a (0.87‐0.93) | ||||
| Prior csDMARD use (not MTX) | 0.84a (0.73‐0.96) | 1.50a (1.11‐2.03) | 0.69a (0.49‐0.99) | |||
| Prior bDMARD use | 0.62a (0.41‐0.92) | |||||
| BMQ general overuseb | 1.11a (1.01‐1.21) | 1.12a (1.02‐1.23) | ||||
| BMQ‐specific necessityc | 0.85a (0.76‐0.94) | 0.89a (0.81‐0.97) | ||||
| BMQ‐specific concernsd | 1.07a (1.02‐1.11) | |||||
| Time (weeks) | 11.87a (1.43‐98.16) | 1.11a (1.08‐1.13) | 1.88a (1.29‐2.74) | |||
| Time2 (weeks2) | 0.95a (0.90‐0.99) | 0.98a (0.97‐0.99) | ||||
Abbreviation: bDMARD, biologic DMARD; BMQ, Beliefs about Medicines Questionnaire; CI, confidence interval; csDMARD, conventional synthetic DMARD; DMARD, disease‐modifying antirheumatic drug; HAQ, Health Assessment Questionnaire; MTX, methotrexate; PtGA, Patient Global Assessment of Disease Activity; VAS, Visual Analogue Scale.
a P < 0.05. bBMQgGeneral overuse: higher score indicates greater belief that medicines are overused by doctors. cBMQ‐specific necessity: higher score indicates greater belief in the necessity of the medication. dBMQ‐specific concerns: higher score indicates greater belief in the danger of dependence, long‐term toxicity, and disruptive effect of the medication.
Older age, being in employment, greater disability, less pain, greater prior use of csDMARDs, and longer time on MTX were factors associated with a higher percentage of weeks of underdosing. Restricting the analysis to the implementation period, longer duration on MTX, lower MTX dose, less exposure to other csDMARDs or bDMARDs, and having specific concerns about the medicine were factors associated with more weeks of underdosing while continuing MTX treatment. In both the adherence and implementation periods, higher levels of fatigue, longer treatment duration on MTX, and greater patient beliefs that drugs were overused by physicians were associated with more weeks of dosing early. Weaker beliefs of the specific necessity of the drug (BMQ) was also found to be associated with early dosing in both periods. Non‐Caucasian patients were also more likely than Caucasian patients to have a high percentage of early dosing in the adherence period (Table 4).
MTX persistence
Of the 60 patients included in this analysis, 10 (17%) discontinued MTX prior to the end of the study. This represented an average of 21.7 weeks of follow up in a total of 1303 patient‐weeks. At 12 weeks, 92% of patients remained on MTX and at 24 weeks, 83% of patients remained on MTX (Figure 2). As previously mentioned, being older and employed were associated with discontinuation of treatment (Table 3).
Figure 2.
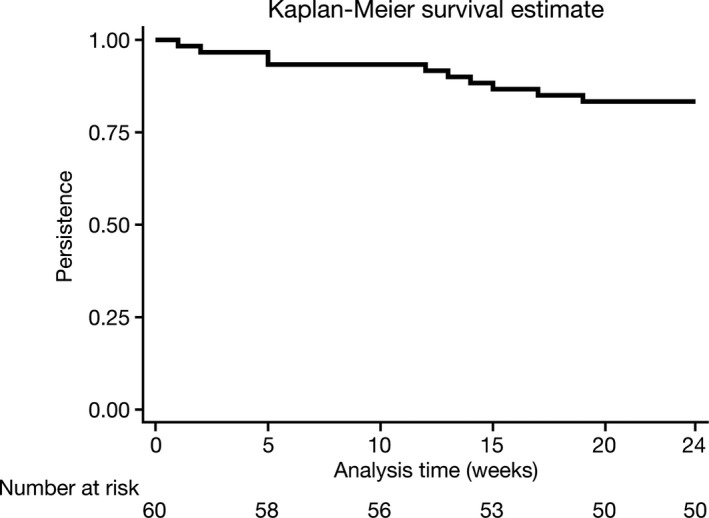
Persistence estimated using the Kaplan–Meier curve.
Association of prior bDMARD use with baseline characteristics, MTX adherence, and MTX implementation
Overall, 42 (70%) patients had prior exposure to bDMARDs. Patients with prior bDMARD experience had longer disease duration, longer MTX duration, and tended to have weaker association with specific concerns or general harm beliefs (BMQ components) compared with those without previous bDMARD use. No differences were found in RA severity between patients with and without prior bDMARD use (Online Supplementary Table 3).
No differences were seen in adherence measures between patients who previously received bDMARDs and those with no prior bDMARD use (Online Supplementary Table 4). Similarly, they did not differ in terms of persistence (log‐rank P = 0.59). At the end of the study (24 weeks), persistence was estimated and presented as percentages: 81% (95% CI 66% to 90%) for those with prior bDMARD use and 89% (95% CI 62% to 97%) for those with no prior bDMARD use (Online Supplementary Figure 5).
Association of concomitant DMARD therapy with baseline characteristics, MTX adherence, and MTX implementation
Overall 26 (43%) patients were receiving other DMARDs—mostly bDMARDs—concomitantly with MTX. Patients receiving MTX as monotherapy had shorter disease duration and less use of prior bDMARDs, but no differences were found in RA severity based on concomitant csDMARD or bDMARD use (Online Supplementary Table 5). Taking MTX as monotherapy was associated with a numerically higher rate of correct dosing versus concomitant DMARD therapy, but this difference was not statistically significant (Online Supplementary Table 4). No differences were found in persistence (log‐rank P = 0.47), although patients on MTX monotherapy tended to be less likely to discontinue treatment than those receiving MTX with concomitant csDMARDs or bDMARDs. Persistence estimates by the Kaplan–Meier estimator at week 24 were 86% (95% CI 66% to 94%) for monotherapy and 79% (95% CI 58% to 90%) for concomitant therapy (Online Supplementary Figure 6).
Reasons for discontinuing MTX
Overall, 12 (20%) patients temporarily discontinued MTX (for 3 or more consecutive weeks and then restarted), and 10 (17%) patients discontinued MTX without restarting during the 24‐week period, one at the suggestion of the treating physician. Reasons for discontinuation without restarting MTX obtained from patient diaries indicated side effects as the primary reason in 3 of 10 (30%) patients. Other reasons recorded in the diaries were surgical procedure, illness, and holiday.
Discussion
To our knowledge this is one of the first studies assessing MTX adherence using the MEMS in a real‐world, nonclinical setting. Furthermore, in this study, adherence to medication was analyzed as implementation (defined as the extent to which a patient's actual dosing matched the prescribed regimen from initiation of the first dose until the last dose taken) and persistence (defined as the length of time between study inclusion and the last dose of MTX taken, ie, discontinuation) 10; we believe this to be the first report of a study in RA using this approach.
Our results showed that MTX adherence gradually decreased over time, even though the majority of patients took it correctly during the overall adherence period (75%) and during the implementation period (80%). Rates of MTX adherence vary across studies depending on a variety of factors, including the definition of adherence and the method of measurement used 7. For example, in claims‐based studies of real‐world data, adherence varied from 80% (MTX monotherapy) and 64% to 72% (MTX combination therapy) when measured by medication possession ratio 16 and from 36% to 50% (csDMARDs, including MTX, prescribed with bDMARDs) when measured by prescriptions of csDMARDs filled 38. Our findings are comparable with the findings of de Klerk et al, who reported that MTX was taken correctly 81% of the time 21. In both studies, MTX adherence was assessed using the MEMS for a period of approximately 6 months and correct dosing was reported. However, although our study enrolled patients from a research databank, the de Klerk study recruited patients from clinics.
Our study showed that the decrease in adherence observed over time was mainly due to nonpersistence (ie, discontinuation of MTX), as implementation was stable. Although correct dosing may indicate a patient's desire to take the medication as prescribed, it does not reflect continuation of the treatment (persistence). Persistence by week 24 had decreased to 83%. Older age and being employed were associated with discontinuation. Similar persistence levels were reported in a previous observational study of MTX‐naïve patients with RA 39, with discontinuations mostly due to adverse events (47%) and lack of efficacy (30%). Our study also showed that one‐third of the patients who discontinued permanently indicated side effects as the primary reason.
Of the RA severity measures, HAQ‐II and PtGA scores were associated with correct dosing. Patients with worse disability were less likely to be at a correct dose (predictions of the probability of being at a correct dose were 0.83 vs 0.62 for HAQ‐II values of 0.5 vs 2, respectively) and more likely to be underdosing (OR of underdosing was 2.63 for 1 unit of HAQ‐II); this might be because they have difficulty functioning and taking their medication. Patients with worse PtGA scores were more likely to be correctly adherent, with a stronger effect while they were taking their medication (OR of correct dosing: 1.21 vs 1.28 for 1 unit of PtGA, in the overall adherence vs implementation period). Pain scores were only significantly associated with early dosing, with higher scores linked to a higher likelihood of early dosing.
The use of general and RA‐specific BMQs together with the MEMS data was also a unique aspect of this study. Weaker beliefs in the necessity of prescribed medications were associated with the likelihood of taking MTX too early; stronger beliefs in the overuse of medicine were also associated with more weeks of early dosing. However, most of the results using the BMQ were not significant. It is possible that most patients who were recruited had initiated MTX prior to entering the study; this might have influenced these findings as they had some experience with the treatment. Ideally, the BMQ should be administered at treatment initiation so that patients' concerns and beliefs can be captured at the start of treatment and appropriate interventions can be considered at treatment initiation.
We observed a higher percentage of correct dosing within patients in the MTX monotherapy group compared with patients in the MTX combination group; however, the difference was not statistically significant. Nonadherence to MTX is more common in patients receiving combination therapy, for reasons such as treatment burden and safety concerns 38, 40, 41. There is also a probability that patients receiving MTX combination therapy may assume that they are adequately treated via the new, more advanced therapy and that there is less need to take MTX, or that patients received combination therapy because they were nonadherent to MTX as monotherapy and another DMARD was added due to poor response. These patients on concomitant therapy also had more experience with various bDMARDs, and prior bDMARD use was not associated with correct dosing.
In an earlier study that utilized MEMS to assess MTX adherence over 2 years in 76 patients with RA from three US clinics, 63% of doses were taken as prescribed 22, compared with 75% during the adherence period in our study. Underdosing was comparable in the two studies, but overdosing in the previous study was 14%, compared with 4% of early dosing in our study. A higher proportion of patients in our study were white, had higher household income, and a higher level of health insurance coverage compared with the previous study. Furthermore, as the patients in our study were recruited from all over the United States, we believe that our results may be more generalizable to the wider US population.
Our study has some limitations. Firstly, it is specific to the oral formulation of MTX, thus findings cannot be extrapolated to subcutaneously administered MTX. We did not measure initiation, an important phase of adherence 10, because all patients had already started MTX before enrollment and we were unable to capture initiation history. In addition, most of the patients who entered the study already had prior experience with MTX, which may explain why adherence was higher in these patients than reported elsewhere, as the most common reasons for nonadherence are more often seen early in treatment. Furthermore, patients were analyzed per the definition of implementation, irrespective of the reasons for temporary discontinuation (ie, whether it was the patient's decision to discontinue or if it was instructed by the health care provider). The relatively short study duration and small sample size resulted in limited statistical power and reduced ability to make generalizations but were adequate for generating signals from the wide array of covariables and were suitable for this study, the first of its kind, with patients participating from around the United States.
Although the MEMS is considered to be a no‐invasive gold standard for measuring adherence 42, it has some limitations. The MEMS does not measure the number of pills taken from the bottle; we compensated for this by capturing details in a patient diary. Also, we were not able to time the administration of the BMQ with treatment initiation; this may have led to some of the nonsignificant results seen in our study. Lastly, the number of nonadherent patients was relatively small, and adherence may have been artificially elevated because patients knew they were being monitored electronically; however, a randomized controlled trial assessing the reactive effect of electronic monitoring in type 2 diabetic patients found that the increase in adherence observed with electronic monitoring was nonsignificant compared with patients who were not monitored 43.
In conclusion, this study demonstrated the feasibility of using remote electronic medication monitoring in a participatory US‐wide RA population. It also demonstrated that having less disability and higher PtGA scores was associated with correct MTX dosing in the overall adherence period and that unemployment and having no prior csDMARD experience were contributing factors to the number of weeks of correct MTX dosing. Our results should help inform physicians of the different stages of adherence, and as different factors may affect adherence during these stages, this study should provide insight into how a thoughtful intervention at each stage could enhance adherence. Physicians could make use of tools, such as the BMQ, to understand their patients' concerns and beliefs about medications prior to treatment initiation, and follow up with patients if they observe that MTX has not been refilled on time. Further research to advance understanding in this area will be helpful to identify and address barriers to optimal adherence. An important next step is to identify patients with suboptimal adherence to MTX in combination with an advanced therapy, which might help identify patients who can benefit from advanced RA treatments at an earlier time. Thus, further work, possibly with longer follow‐up periods, is needed to gain a better understanding of MTX initiation and suboptimal MTX adherence and to assess adherence for other csDMARDs and bDMARDs in patients with RA.
Author Contributions
All authors contributed to the analysis of the data, critical revision of the article, and final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Study conception and design
Michaud, Vrijens, Dasic, Chen, Agarwal, Suarez‐Almazor.
Acquisition of data
Michaud, Schumacher.
Analysis and Interpretation of data
Michaud, Vrijens, Tousset, Pedro, Dasic, Chen, Agarwal, Suarez‐Almazor.
Supporting information
Acknowledgments
The authors thank the patients who participated in this study
This analysis was funded by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Karen Irving, PhD, and Christina Viegelmann, PhD, of CMC Connect, a division of McCann Health Medical Communications Ltd., Glasgow, UK, and Carole Evans, PhD, on behalf of CMC Connect and funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461‐464).
Dr. Michaud has previously received research grant funding from Pfizer Inc. and the Rheumatology Research Foundation. Drs. Vrijens and Tousset are employees of the AARDEX Group. Drs. Dasic and Chen are shareholders of Pfizer Inc. Ms. Agarwal is a consultant for Pfizer Inc. Dr. Suarez‐Almazor has been a consultant for Bristol‐Myers Squibb, Eli Lilly, and Pfizer Inc. No other disclosures relevant to this article were reported.
References
- 1. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 2. Bello AE, Perkins EL, Jay R, Efthimiou P. Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol 2017;9:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 4. Nam JL, Ramiro S, Gaujoux‐Viala C, Takase K, Leon‐Garcia M, Emery P, et al. Efficacy of biological disease‐modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2014;73:516–28. [DOI] [PubMed] [Google Scholar]
- 5. Manders SH, Kievit W, Jansen TL, Stolk JN, Visser H, Schilder AM, et al. Effectiveness of tumor necrosis factor inhibitors in combination with various csDMARD in the treatment of rheumatoid arthritis: data from the DREAM registry. J Rheumatol 2016;43:1787–94. [DOI] [PubMed] [Google Scholar]
- 6. Lopez‐Olivo MA, Siddhanamatha HR, Shea B, Tugwell P, Wells GA, Suarez‐Almazor ME. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst Rev 2014;10:CD000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol 2016;43:1997–2009. [DOI] [PubMed] [Google Scholar]
- 8. Curtis JR, Xie F, Mackey D, Gerber N, Bharat A, Beukelman T, et al. Patient's experience with subcutaneous and oral methotrexate for the treatment of rheumatoid arthritis. BMC Musculoskelet Disord 2016;17:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients’ adherence to subcutaneous anti‐TNF therapies and costs. Curr Med Res Opin 2009;25:1365–77. [DOI] [PubMed] [Google Scholar]
- 12. Wabe N, Lee A, Wechalekar M, McWilliams L, Proudman S, Wiese M. Adherence to combination DMARD therapy and treatment outcomes in rheumatoid arthritis: a longitudinal study of new and existing DMARD users. Rheumatol Int 2017;37:897–904. [DOI] [PubMed] [Google Scholar]
- 13. Métry J‐M. Patient compliance: pharmionics, a new discipline In: Edwards LD, Fletcher AJ, Fox AW, Stonier PD, editors. Principles and Practice of Pharmaceutical Medicine. 2nd ed Hoboken (NJ): John Wiley & Sons; 2007. p. 355–373. [Google Scholar]
- 14. Wong PK. Medication adherence in patients with rheumatoid arthritis: why do patients not take what we prescribe? [review]. Rheumatol Int 2016;36:1535–42. [DOI] [PubMed] [Google Scholar]
- 15. De Klerk E, van der Heijde D, van der Tempel H, van der Linden S. Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. J Rheumatol 1999;26:2635–41. [PubMed] [Google Scholar]
- 16. Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF Jr, Griffin MR. Assessment of adherence to and persistence on disease‐modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care 2007;45 Suppl 2:S66–76. [DOI] [PubMed] [Google Scholar]
- 17. Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care 2003;9:S136–43. [PubMed] [Google Scholar]
- 18. Feinstein AR. On white‐coat effects and the electronic monitoring of compliance. Arch Intern Med 1990;150:1377–8. [PubMed] [Google Scholar]
- 19. Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet 1997;32:345–56. [DOI] [PubMed] [Google Scholar]
- 20. Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique [published erratum appears in JAMA 1989; 262:1472]. JAMA 1989;261:3273–7. [PubMed] [Google Scholar]
- 21. De Klerk E, van der Heijde D, Landewé R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout [published erratum appears in J Rheumaol 2003;30:423]. J Rheumatol 2003;30:44–54. [PubMed] [Google Scholar]
- 22. Waimann CA, Marengo MF, de Achaval S, Cox VL, Garcia‐Gonzalez A, Reveille JD, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum 2013;65:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolfe F, Michaud K. The National Data Bank for rheumatic diseases: a multi‐registry rheumatic disease data bank. Rheumatology (Oxford) 2011;50:16–24. [DOI] [PubMed] [Google Scholar]
- 24. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. [Google Scholar]
- 25. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken) 2015;67:865–72. [DOI] [PubMed] [Google Scholar]
- 27. Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789–93. [PubMed] [Google Scholar]
- 28. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 29. Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 1996;23:1407–17. [PubMed] [Google Scholar]
- 30. Wolfe F, Michaud K, Pincus T. A composite disease activity scale for clinical practice, observational studies, and clinical trials: the patient activity scale (PAS/PAS‐II). J Rheumatol 2005;32:2410–5. [PubMed] [Google Scholar]
- 31. Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28‐Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score‐II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index‐5 (RADAI‐5), Chronic Arthritis Systemic Index (CASI), Patient‐Based Disease Activity Score With ESR (PDAS1) and Patient‐Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI‐RA). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S14–36. [DOI] [PubMed] [Google Scholar]
- 33. Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health‐related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ‐5D). Br J Rheumatol 1997;36:551–9. [DOI] [PubMed] [Google Scholar]
- 34. Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1993;36:729–40. [DOI] [PubMed] [Google Scholar]
- 35. Hewlett S, Hehir M, Kirwan JR. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum 2007;57:429–39. [DOI] [PubMed] [Google Scholar]
- 36. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63:S240–52. [DOI] [PubMed] [Google Scholar]
- 37. Maska L, Anderson J, Michaud K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ‐II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S4–13. [DOI] [PubMed] [Google Scholar]
- 38. Harnett J, Wiederkehr D, Gerber R, Gruben D, Bourret J, Koenig A. Primary nonadherence, associated clinical outcomes and health care resource use among patients with rheumatoid arthritis prescribed treatment with injectable biologic disease‐modifying antirheumatic drugs. J Manag Care and Spec Pharm 2016;22:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lie E, van der Heijde D, Uhlig T, Heiberg MS, Koldingsnes W, Rødevand E, et al. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate‐treated patients with rheumatoid arthritis. Ann Rheum Dis 2010;69:671–6. [DOI] [PubMed] [Google Scholar]
- 40. Contreras‐Yáñez I, Ponce De León S, Cabiedes J, Rull‐Gabayet M, Pascual‐Ramos V. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease‐modifying antirheumatic drugs. Am J Med Sci 2010;340:282–90. [DOI] [PubMed] [Google Scholar]
- 41. DiBenedetti DB, Zhou X, Reynolds M, Ogale S, Best JH. Assessing methotrexate adherence in rheumatoid arthritis: a cross‐sectional survey. Rheumatol Ther 2015;2:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs 1995;49:321–7. [DOI] [PubMed] [Google Scholar]
- 43. Sutton S, Kinmonth AL, Hardeman W, Hughes D, Boase S, Prevost AT, et al. Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med 2014;48:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


