Abstract
Objective
Rheumatoid arthritis (RA) conveys an increased risk of cardiovascular disease (CVD), making it imperative that traditional CVD risk factors are well controlled. This study compared blood pressure (BP) trends over 13 years among patients with seropositive RA and patients without RA who received care within a large health care system in Minnesota.
Methods
This retrospective cohort study compared 774 patients with seropositive RA and 3254 patients without RA who were matched on sex and year of birth (±5 years) and observed between 2005 and 2017. Generalized estimating equation models were used for longitudinal analyses, with adjustment for demographics, body mass index, smoking status, Charlson Comorbidity Index, number of BP measurements, and number of antihypertensive and oral glucocorticoid medications.
Results
Patients both with and without RA had a mean age of 55 and were predominately female (78% with RA; 79% without RA). The mean follow‐up was 6.3 (SD 3.4) years for patients with RA and 7.2 (SD 3.3) years for patients without RA. Overall, systolic BP, diastolic BP, and the number of prescribed antihypertensive medications did not differ between groups. Patients with RA were more likely to be current smokers compared with patients without RA (23% vs 11%; P < 0.01) and were less likely to have serum lipid measurements (75% vs 85%; P < 0.01).
Conclusion
BP was similarly controlled in patients with seropositive RA and patients without RA. However, diastolic BP in patients with RA was trending up in most recent years. Patients with RA were also more likely to smoke compared with controls and were less likely to have serum lipid measurements.
Introduction
Although the most clinically apparent manifestation of rheumatoid arthritis (RA) is synovial joint inflammation, patients with RA are also at an increased risk for cardiovascular disease (CVD) 1, 2, 3, 4. Although there are several factors that contribute to the greater CVD risk in patients with RA, much of the elevated risk is attributed to systemic inflammation 5. Other factors, such as widespread corticosteroid use and physical inactivity, are also involved in this increased risk 6, 7. There is also evidence to indicate that hypertension may be partially responsible for this increased risk of CVD in patients with RA. A meta‐analysis found that hypertension produced a relative risk of cardiovascular morbidity of 2.2 in patients with RA, which was greater than that for type 2 diabetes mellitus, smoking, hyperlipidemia, and obesity 8. Furthermore, other research indicated that hypertension is the major determinant of target organ damage in patients with RA 9.
At the same time, evidence is conflicting as to whether blood pressure (BP) is managed similarly between patients with and without RA. One cohort study indicated that rheumatologists are less likely to screen for cardiovascular risk factors, including hypertension, than primary care physicians and that primary care physicians less frequently manage BP in patients with RA 10. Another study found that hypertension was both underdiagnosed and undertreated in patients with RA relative to the general population 11. However, more recent studies suggest that management of BP in patients with RA is similar to that of the general population. A retrospective cohort study involving 24 859 patients with RA with an average follow‐up of more than 5 years in the United Kingdom between 1987 and 2010 matched patients with and without based on CVD risks and found no difference in BP management between patients with RA and controls 12. A US‐based managed‐care cohort study of 9440 patients with RA and 31 009 general controls found that patients with RA were more likely to achieve target BP values but also were more likely to receive an antihypertensive medication than controls 13.
Given the elevated cardiovascular risk for patients with RA, it is imperative that traditional CVD risk factors are well controlled. The potentially important role hypertension holds in the elevated cardiovascular risk for patients with RA and conflicting studies investigating BP management highlight the need for research into how well BP is managed in patients with RA. The objective of the present study was to compare average BP values in patients with seropositive RA over an extended (13‐year) period with those of frequency‐matched patients without RA while accounting for other cardiovascular risk factors.
Patients and methods
Data source
A retrospective cohort of patients from Fairview Health Services was identified using electronic health record data captured in Epic (Epic Systems Corporation) for the period of January 1, 2005, through December 31, 2017 (Figure 1). Fairview Health Services is a nonprofit integrated health system based in Minneapolis, Minnesota, that includes 54 primary care clinics and 12 hospitals, including the University of Minnesota Medical Center. The study was approved by the University of Minnesota Institutional Review Board.
Figure 1.
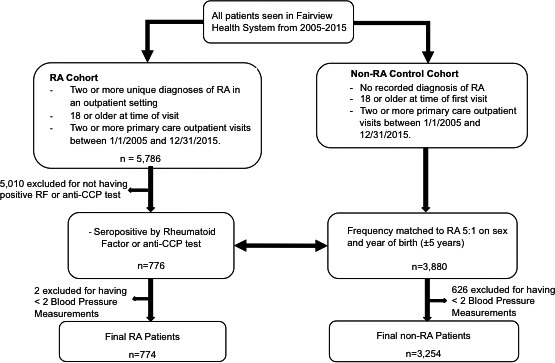
Flow diagram showing patient selection. CCP, cyclic citrullinated peptide; RA, rheumatoid arthritis; RF, rheumatoid factor.
Patient selection
Adult patients with seropositive RA (age 18 years and older) were defined as having two or more outpatient diagnoses of RA (International Classification of Diseases, Ninth Revision, Clinical Modification code 714.xx) and a positive cyclic citrullinated peptide or rheumatoid factor test between January 1, 2005, and December 31, 2015.
To serve as a comparison, 3254 patients without non‐RA were randomly selected at a ratio of 5:1 via frequency matching on birth year (±5 years) and sex. The control cohort included adult patients with no recorded diagnosis of RA and at least two outpatient primary care visits within the Fairview System between January 1, 2005, and December 31, 2015. Patients from either group were removed from analysis if they had fewer than two outpatient BP measurements by 2017 or if their follow‐up period was less than 1 year.
The date of the first outpatient RA diagnosis after January 1, 2005, marked the beginning of the follow‐up period for each patient with RA. Patients without RA were assigned start‐of‐follow‐up dates so that the proportion of patients with RA who began follow‐up in any year from 2005 to 2015 was the same as the proportion of patients without RA. This ensured that both groups were observed over similar periods of time.
Candidate confounders to the associations between RA status and CVD risk factors were selected based on extensive evidence for cardiovascular risk factors 5, 8, 14. Selected confounders included age, time‐varying body mass index (BMI), sex, race, median income of zip code, average number of BP measurements per year, smoking status, number of prescribed antihypertensive medications at time of BP measurement, oral glucocorticoid use at BP measurement, and baseline Charlson Comorbidity Index score. The Charlson Comorbidity Index is a well‐established tool to predict 10‐year survival based on the following comorbidities: age, diabetes mellitus, liver disease, malignancy, acquired immunodeficiency syndrome (AIDS), moderate or severe chronic renal disease, heart failure, myocardial infarction, chronic obstructive pulmonary disease, peripheral vascular disease, cerebral vascular attack/transient ischemic attack, dementia, hemiplegia, connective tissue disease, and peptic ulcer disease 15. A list of median household income values for each zip code was acquired from the University of Michigan Population Studies Center, and values were derived from the 2006‐2010 American Community Survey 16. Data on deaths were drawn from both the Fairview Health System as well as the Minnesota Death Index.
All outpatient BP measurements taken on or after the start‐of‐follow‐up date until December 31, 2017, and accompanying medication lists were retrieved from patients’ medical records. BP measurements taken at the emergency department, urgent care, dialysis, infusion, and outpatient surgery were excluded. In addition, laboratory test results available during the follow‐up period were retrieved, including the lipid profile (total cholesterol, high‐density lipoprotein [HDL], and low‐density lipoprotein [LDL]), and hemoglobin A1C levels.
Statistical analysis
Missing BMI values for an outpatient visit were assigned as the patient's recorded BMI at the last visit. No other missing data were filled in based on existing values. Baseline smoking status was defined as never, former, or current smoker based on the first recorded smoking status during a clinical encounter in the year before or after follow‐up began.
Baseline demographic information of patients with and without was compared using χ2 tests for categorical variables, Mann‐Whitney‐Wilcoxon or t tests for continuous variables (depending on their distribution), or Kaplan‐Meier log rank tests for length of follow‐up. Generalized estimating equation (GEE) models were used to compare unadjusted and adjusted differences between patients with and without. All analysis was conducted with SAS software version 9.4 (SAS Institute, Inc).
Results
Seven hundred seventy‐four patients with seropositive RA were identified and frequency‐matched to 3254 patients without RA. Table 1 shows baseline characteristics for the two groups. The mean age was 55 in both groups, and the majority of patients were women (78% of patients with RA and 79% of patients without non‐RA). Patients with RA had a shorter follow‐up time (mean: 6.3 years for patients with RA and 7.2 years for patients without RA) as well as higher mortality over the study period (18% [95% confidence interval (CI): 14%‐22%] for patients with RA and 10% [95% CI: 9%‐11%] for patients without RA at 10 years after study inclusion; log rank test P < 0.01). Both groups were largely white (82% with RA and 84% without RA) and had overweight or obesity at baseline (baseline BMI of 25 kg/m2 or greater; 68% with RA and 71% without RA). Patients with RA, on average, lived in zip codes with a lower median income ($65 100 vs $67 800; P < 0.01) and had a higher median number of BP measurements per year (RA: 6.0; non‐RA: 2.6; P < 0.01).
Table 1.
Baseline characteristics of patients with and without seropositive rheumatoid arthritis
| Rheumatoid Arthritis (n = 774) | Non–Rheumatoid Arthritis (n = 3254) | P a | |
|---|---|---|---|
| Female sex, n (%) | 611 (78.9) | 2578 (79.2) | 0.9 |
| Age, mean ± SD, y | 55.9 ± 14.5 | 55.3 ± 14.7 | 0.3 |
| Race, n (%) | 0.1 | ||
| Non‐white | 82 (10.6) | 328 (10.1) | |
| White | 637 (82.3) | 2751 (84.5) | |
| Missing | 55 (7.1) | 175 (5.4) | |
| Baseline BMI, n (%), kg/m2 | 0.4 | ||
| <20 | 43 (5.6) | 138 (4.2) | |
| 20‐24.9 | 195 (25.2) | 770 (23.7) | |
| 25‐29.9 | 232 (30.0) | 1020 (31.4) | |
| 30+ | 298 (38.5) | 1300 (40.0) | |
| Missing | 6 (0.8) | 26 (0.8) | |
| Median income of zip code, mean ± SD, USD (thousands) | 65.1 ± 16.5 | 67.8 ± 16.7 | <0.01 |
| Length of follow‐up, mean ± SD, y | 6.3 ± 3.4 | 7.2 ± 3.3 | <0.01 |
| Blood pressure measurements per patient, median (IQR) | 35 (15‐62) | 16 (8‐32) | <0.01 |
| Charlson Comorbidity Index, n (%) | 0.3 | ||
| 0 | 640 (82.7) | 2630 (80.8) | |
| 1 | 83 (10.7) | 359 (11.0) | |
| 2+ | 51 (6.6) | 265 (8.1) | |
| Comorbidities, n (%) | |||
| Chronic pulmonary disease | 46 (5.9) | 190 (5.8) | 0.9 |
| Diabetes | 31 (4.0) | 245 (7.5) | <0.01 |
| Cardiovascular disease | 19 (2.5) | 102 (3.1) | 0.3 |
| Moderate or severe renal disease | 19 (2.5) | 74 (2.3) | 0.8 |
| Cancer | 11 (1.4) | 115 (3.5) | <0.01 |
| Baseline smoking status, n (%) | <0.01 | ||
| Current | 183 (23.6) | 365 (11.2) | |
| Former | 219 (28.3) | 883 (27.1) | |
| Never | 369 (47.7) | 1858 (57.1) | |
| Missing | 3 (0.4) | 146 (4.6) | |
| Baseline No. of antihypertensive medications, n (%) | 0.08 | ||
| 0 | 427 (55.2) | 1793 (55.1) | |
| 1 | 157 (20.3) | 563 (17.3) | |
| 2 | 115 (14.8) | 499 (15.3) | |
| 3+ | 75 (9.7) | 399 (12.3) | |
| Baseline oral glucocorticoid use, n (%) | 344 (44.4) | 244 (7.5) | <0.01 |
Abbreviation: BMI, body mass index; IQR, interquartile range; USD, US dollar.
P values are presented for χ2 tests (binary variables), t tests (continuous), and Kaplan‐Meier log rank tests (length of follow‐up).
By using the Charlson Comorbidity Index, patients with and without RA had similar severity of comorbidities (excluding connective tissue disease). In addition, the number of antihypertensive medications at the start of follow‐up did not differ between groups, although more patients with RA were on oral glucocorticoids at the start of follow‐up (44.4% vs 7.5%; P < 0.01). Lastly, patients with RA had a greater proportion of current smokers at baseline (23% vs 11%; P < 0.01). Concerning missing data, 0.4% of patients with RA and 4.6% of patients without RA were missing baseline smoking status data, 0.8% of patients with and without RA were missing any BMI measurements, and 7% of patients with RA and 5% of patients without RA were missing data on race.
To find the average systolic and diastolic BP, 117 470 unique systolic BP measurements (RA: 36 475; non‐RA: 80 995) and 117 396 unique diastolic BP measurements (RA: 36 459; non‐RA: 80 937) were averaged. Both groups had a mean systolic BP of 125 mm Hg and no difference in the mean diastolic BP (RA: 74 mm Hg; non‐RA: 73 mm Hg; P = 0.1) (Table 2). There was also no difference, on average, between patients with RA and patients without RA in serum lipid and hemoglobin A1C values. The mean values and SEs for HDL, LDL, total cholesterol, and hemoglobin A1C were 55 ± 1, 102 ± 2, 186 ± 2, and 6.7 ± 0.1 mg/dl, respectively, for patients with RA and 54 ± 0.4, 101 ± 1, 183 ± 1, and 6.9 ± 0.05 mg/dl, respectively, for patients without RA (Table 2). However, after we adjusted for length of follow‐up, patients with RA were half as likely as patients without RA to have had serum lipids measured (P < 0.01 for all lipid measurements). Of patients with and without RA, 25% and 15% were lacking any HDL cholesterol or total cholesterol measurement, 26% and 16% were lacking any LDL cholesterol measurement, and 61% and 59% were lacking any hemoglobin A1C measurement, respectively.
Table 2.
Average unadjusted mean levels of blood pressure, serum lipids, and hemoglobin A1C measured from 2005 to 2017
| Rheumatoid Arthritis (n = 774) | Non–Rheumatoid Arthritis (n = 3254) | P a | |
|---|---|---|---|
| Systolic blood pressure, mean (95% CI), mm Hg | 125.8 (124.9‐126.8) | 125.9 (125.4‐126.5) | 0.9 |
| Diastolic blood pressure, mean (95% CI), mm Hg | 74.1 (73.5‐74.8) | 73.4 (73.1‐73.8) | 0.1 |
| HDL cholesterol, mean (95% CI), mg/dl | 55.5 (53.6‐57.3) | 54.1 (53.4‐54.9) | 0.2 |
| Missing any value, % | 25 | 15 | <0.01 |
| LDL cholesterol, mean (95% CI), mg/dl | 102.0 (98.9‐105.3) | 101.1 (99.8‐102.5) | 0.6 |
| Missing any value, % | 26 | 16 | <0.01 |
| Total cholesterol, mean (95% CI), mg/dl | 186.2 (182.3‐190.1) | 183.0 (181.4‐184.6) | 0.1 |
| Missing any value, % | 25 | 15 | <0.01 |
| Hemoglobin A1C, mean (95% CI), % | 6.7 (6.5‐7.0) | 6.9 (6.8‐7.0) | 0.1 |
| Missing any value, % | 61 | 59 | 0.4 |
Abbreviation: CI, confidence interval; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
P values are presented for generalized estimating equations (blood pressure, serum lipid levels, and hemoglobin A1C levels) and for logistic regression adjusted for length of follow‐up (percentage missing any value).
To examine whether the lack of a difference in the average BP between patients with and without RA was consistent across the study period, the average age‐adjusted systolic and diastolic BP was calculated for each year for patients both with and without RA. There was no difference in the average systolic BP over the studied period (Figure 2A). For diastolic BP, patients with RA had a significantly higher average diastolic BP during the years 2006, 2015, 2016, and 2017, but no difference between patients with and without RA was observed in the remaining years (Figure 2B). To examine whether the prescribing of antihypertensive medications differed between patients with and without RA, the maximum number of antihypertensive medications listed on a patient's medicine list at any one visit was found for each year. There was no difference between patients with and without RA in the frequencies of the maximum number of medications listed in any year (Figure 2C).
Figure 2.
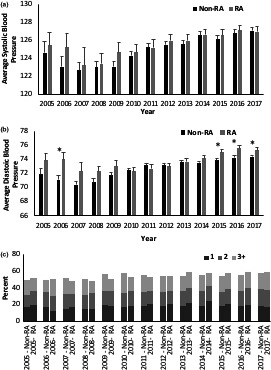
Systolic blood pressure (A), diastolic blood pressure (B), and antihypertensive medication use (C) trends among patients with seropositive rheumatoid arthritis (RA) and patients without RA, 2005‐2017.
To assess whether the lack of a difference in the average systolic and diastolic BP between patients with and without RA was due to confounding, a GEE model was run with minimal adjustment and full adjustment (Table 3). In the minimally adjusted model, RA was not associated with a significant difference in systolic BP (P = 0.5) or diastolic BP (P = 0.07). In the fully adjusted model, RA was still not associated with a significant difference in systolic BP (P = 0.5) or diastolic BP (P = 0.2).
Table 3.
Minimally adjusted and fully adjusted longitudinal analyses of systolic and diastolic blood pressure estimates in patients with seropositive RA and the control cohort
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% Confidence Interval | P a | Estimate | 95% Confidence Interval | P a | |
| Minimally adjusted GEE model | ||||||
| Patient with RA | 0.35 | (−0.70 to 1.41) | 0.5 | 0.64 | (−0.04 to 1.32) | 0.07 |
| Female sex | −1.12 | (−2.40 to 0.16) | 0.09 | −1.5 | (−2.31 to −0.69) | <0.01 |
| Non‐white race | −0.41 | (−1.71 to 0.90) | 0.5 | 0.7 | (−0.34 to 1.73) | 0.2 |
| BMI, kg/m2 | 0.35 | (0.29 to 0.41) | <0.01 | 0.15 | (0.12 to 0.19) | <0.01 |
| Age, y | 0.28 | (0.25 to 0.31) | <0.01 | −0.12 | (−0.14 to −0.10) | <0.01 |
| Fully adjusted GEE model | ||||||
| Patient with RA | 0.06 | (−1.05 to 1.18) | 0.9 | 0.46 | (−0.25 to 1.16) | 0.2 |
| Female sex | −0.82 | (−2.10 to 0.45) | 0.2 | −1.43 | (−2.19 to −0.67) | <0.01 |
| Non‐white race | −0.80 | (−2.08 to 0.48) | 0.2 | 0.63 | (−0.40 to 1.66) | 0.2 |
| Age, y | 0.23 | (0.20 to 0.27) | <0.01 | −0.11 | (−0.13 to −0.09) | <0.01 |
| Median income of zip code, USD (thousands) | −0.02 | (−0.05 to 0.01) | 0.2 | −0.01 | (−0.03 to 0.01) | 0.4 |
| BMI, kg/m2 | 0.31 | (0.25 to 0.37) | <0.01 | 0.17 | (0.14 to 0.21) | <0.01 |
| Smoking status | 0.7 | 0.01 | ||||
| Current | 0.35 | (−1.04 to 1.74) | 0.6 | 1.31 | (0.47 to 2.15) | <0.01 |
| Former | −0.29 | (−1.26 to 0.67) | 0.6 | 0.18 | (−0.46 to 0.83) | −0.6 |
| Never | Reference | … | … | Reference | … | … |
| Charlson Comorbidity Index | 0.01 | <0.01 | ||||
| 0 | Reference | … | … | Reference | … | … |
| 1 | 0.50 | (−0.81 to 1.80) | 0.5 | −0.44 | (−1.46 to 0.59) | 0.4 |
| 2+ | −2.61 | (−4.38 to −0.84) | <0.01 | −1.90 | (−2.98 to −0.82) | <0.01 |
| Antihypertensive medications, count | <0.01 | <0.01 | ||||
| 0 | Reference | … | … | Reference | … | … |
| 1 | 3.90 | (3.06 to 4.74) | <0.01 | 1.76 | (1.21 to 2.31) | <0.01a |
| 2 | 3.79 | (2.76 to 4.82) | <0.01 | 0.95 | (0.34 to 1.56) | <0.01a |
| 3+ | 3.84 | (2.60 to 5.09) | <0.01 | −0.71 | (−1.52 to 0.09) | −0.1a |
| Oral glucocorticoids, yes/no | 1.09 | (0.14 to 2.04) | 0.03 | 0.86 | (0.28 to 1.44) | <0.01 |
| Blood pressure measurements per year, count | −0.05 | (−0.15 to 0.04) | 0.3 | −0.08 | (−0.13 to −0.02) | <0.01 |
Abbreviation: BMI, body mass index; GEE, generalized estimating equation; RA, rheumatoid arthritis; USD, US dollar.
P values are presented for GEEs.
P values are for levels of categorical variables.
In both the minimally and fully adjusted models, age and BMI were significant independent predictors of systolic and diastolic BP, although age was positive for systolic BP and negative for diastolic BP, whereas BMI was positive for both. Similarly, the number of prescribed antihypertensive medications, whether the patient was on oral glucocorticoids, and the baseline Charlson Comorbidity Index score were significant for both systolic and diastolic BP in the fully adjusted model. Sex, smoking status, and number of BP medications were significant predictors of diastolic BP but not systolic BP. Race and median income of zip code were not significant predictors for systolic or diastolic BP.
In summary, average systolic BP and diastolic BP were within normal limits in patients with seropositive RA and controls. Systolic BP and diastolic BP did not differ between cohorts in fully adjusted GEE models. Patients with RA were more likely to smoke than patients without RA and were less likely to have serum lipid measurements.
Discussion
This retrospective cohort study investigated whether patients with seropositive RA had similar control of BP relative to patients without RA. On average, there was no difference between these two populations in systolic BP, in diastolic BP, or in the number of prescribed antihypertensive medications.
Our findings were overall concordant with two other large retrospective cohort studies, which found BP to be managed similarly in patients with and without RA 12, 13 An et al 13 found that there was no difference in BP between patients with RA and patients without RA at baseline and at 1‐year follow‐up. However, unlike the present study, only patients (patients both with and without RA) with known hypertension were included in the analysis. Alemao et al 12 found no difference in BP between patients with and without RA at baseline and at 1 and 3 years. Of note, the average BP throughout this study for both groups (systolic BP: 136 mm Hg; diastolic BP: 79 mm Hg) was higher than in the present study. In addition, similar to Alemao et al 12, we found that baseline prescription of antihypertensives was similar between patients with and without RA. Although An et al 13 found a difference between patients with and without RA in antihypertensive prescriptions, they investigated whether patients received at least one prescription of an antihypertensive during 1 year of follow‐up and found that 97.5% of patients without RA and 95.8% of patients with RA had been prescribed at least one antihypertensive during that year. The agreement of these three studies provides strong support that BP is managed similarly between patients with and without RA.
In contrast to these studies by An et al 13 and Alemao et al 12, other studies found that CVD risk factors were managed more poorly in patients with RA than in controls without RA. Desai et al 10 found that BP, diabetes mellitus, and dyslipidemia were all less adequately managed in patients with RA compared with patients without RA in a cohort of 251 patients with RA. Chung et al 17 found that patients with RA had higher systolic and diastolic BP than patients without RA in a cohort of 197 patients with RA. Bartels et al 18 found that patients with RA were more likely to have undiagnosed hypertension than controls without RA in a cohort of 201 patients with RA. It is important to note that none of these studies that found a difference in the management of CVD risk factors between patients with and without RA included more than 251 patients without RA, whereas the An et al 13 and Alemao et al 12 studies that did not find a difference in CVD risk management included 9440 and 24 589 patients with RA, respectively. Additionally, the Bartels et al 18 study was cross‐sectional, whereas the other studies were retrospective cohorts.
Practice settings also may have affected the differences in findings between studies. The studies by An et al 13 (Kaiser Permanente Southern California) and Alemao et al 12 (UK clinical practice database) were conducted in community health care settings, whereas the studies by Desai et al 10, Chung et al 17, and Bartels et al 18 were all done at academic centers. It may be that CVD risk factor management is superior in community settings relative to academic settings because of lower complexity of cases and a higher emphasis on preventive care. One possibility for the differences in the results of the studies was the years included in the study period. The two studies that did not find a difference in CVD risk management in patients with RA were both published in 2016 12, 13, whereas the three studies that did find a difference were published in 2012 10, 17 and 2014 18. Because the European League Against Rheumatism (EULAR) guidelines that recommended annual CVD risk assessment in patients with RA were published in early 2010, it could be that studies that did not find a difference in the management of CVD risk factors were based on encounters after the publication of these guidelines. This was not the case because the study periods for An et al 13 and Alemao et al 12 were 2007‐2011 and 1987‐2010, respectively, and therefore the difference in the results of the studies was not due to differences in existing guidelines.
It is concerning, however, that we saw a higher diastolic BP in patients with RA in recent years (2015‐2017). This finding may indicate lower provider vigilance following recent reassuring reports or it may indicate a change in BP guidelines. During the study period, the guidelines for BP management in the general population changed several times. The BP guidelines outlined in the 2003 report of the Seventh Joint National Committee (JNC7) recommended treatment of a systolic BP greater than 140 mm Hg or a diastolic BP greater than 90 mm Hg, with tighter control for patients with diabetes or chronic kidney disease (treatment of a systolic BP greater than 130 mm Hg or a diastolic BP greater than 80 mm Hg) 19. In 2008, the Minnesota Health Reform Law required providers to submit clinical data through the Minnesota Statewide Quality Reporting and Measurement System 20. This was followed by years of the lowest BP values in patients with RA and controls without RA in our study. In 2014, the report of the JNC8 was published, which relaxed guidelines for BP control in patients older than 65 to a systolic BP greater than 150 mm Hg and no longer recommended increased control for diabetes and chronic kidney disease 21. This shortly preceded the trend toward increasing BP in our study.
Attention to BP control in patients with RA is of high importance. One cross‐sectional study found that 78% of patients with RA with target organ damage (left ventricular hypertrophy, microalbuminuria, and elevated creatinine levels) had either poorly controlled or undiagnosed hypertension 9. Additionally, a single‐center, cross‐sectional study of 400 consecutive patients with RA in an outpatient setting in England found that although hypertension was present in 71% of patients with RA, only 61% of those with hypertension were being treated 11. Similarly, a US health care record–based cohort study found delays in care for hypertension among patients with RA compared with patients without RA 18. The trend toward rising diastolic BP among patients with RA in 2015‐2017 in our study likely reflects a higher incidence of diastolic hypertension and could lead to worse cardiovascular outcomes in the future.
Although our study demonstrated similar BP values in patients with RA and patients without RA, we noted other areas for CVD risk improvement. Fewer patients with RA than without RA had serum lipids measured. This was consistent with a report by Bartels et al 22 that showed that only 45% of eligible patients with RA received lipid screening in a Medicare population sample. In our study, lipid measurement rates were higher (75%) but still inferior to those in matched patients without RA (85%). As lipid level lowering and statin use improve outcomes in patients with RA 23, 24, more universal cholesterol screening may improve care. Additionally, patients with RA in our study were more likely to be current smokers than patients without RA at the start of follow‐up. The 23% of current smokers at baseline in the present study was comparable with that found in some previously published cohort studies (baseline percentages of 24% for current smokers and 43% for former smokers) 1, 25 but was greater than that found in other studies (14% and 12%, respectively) 10, 13. Because smoking not only predisposes patients to RA 26 but also conveys a 50% higher risk of a cardiovascular event among patients with RA 8, smoking cessation is another important area for improvement of care in patients with RA.
A main strength of this study was the ability to track BP and serum lipid levels over several years. This longitudinal approach to measurement better captures BP control than repeat measurements done at a single visit, as in a previous study that found that hypertension was underdiagnosed and undertreated in patients with RA 11. A second strength of this study was the restriction of RA to only seropositive cases because this ensured that we captured patients who truly had RA. Previous cohort studies defined RA by the 1987 American College of Rheumatology criteria for RA 1, 10, 25 or by having two diagnoses of RA and a prescription of a disease‐modifying rheumatic drug 13. However, seropositivity is associated with more severe RA and with increased all‐cause and cardiovascular mortality 27, 28, 29 Thus, our study contributes findings specific to the management of patients with seropositive RA.
Among the limitations of the study was the inability to assess more nuanced trends in BP. Although mean is an efficient way to track longitudinal control, it fails to reveal whether BP is, on average, variably controlled with occasional high measurements or if BP is tightly controlled to a narrow range. Both of these scenarios could result in similar longitudinal averages but require different clinical treatment approaches. In addition, data on medication use were limited to those available in the electronic medical record and may not accurately reflect compliance nor account for possible drug interactions and confounding by indication. We were unable to quantify all potential factors that influence the relationship between RA and BP because of the limitations of a retrospective cohort design and the electronic medical record data source.
Because this study was performed in a large health care system database, it relied on health care utilization and did not capture healthier community members or patients who could not afford health care. Hence, although the results are broadly generalizable to other health care systems in the United States, they are not generalizable to the population at large. The study was also unable to account for lifetime health risks, including incidence of RA and hypertension in both cohorts outside the study period. In addition, our study population was overwhelmingly white; thus, the results may not be applicable to patients of other races. Of interest are studies in disadvantaged patients with RA as well as detailed analyses in subgroups of patients with and without RA with a diagnosis of hypertension who are on antihypertensive medications and those with different patterns of BP control.
In conclusion, BP was controlled similarly in patients with and without RA. This was concordant with previously published retrospective cohort studies, suggesting that patients with and without RA receive similar management of traditional cardiovascular risk factors. However, in our study, diastolic BP in patients with seropositive RA was trending up in most recent years, which calls for increased provider attention to BP management in this population. Patients with RA were also more likely to smoke than patients without RA and were less likely to have serum lipid measurements. These are important areas of practice improvement of CVD risk management in patients with RA.
Author contributions
All authors drafted the article, revised it critically for important intellectual content, approved the final version to be published, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Boersma, McElwee, Shmagel.
Acquisition of data
Boersma, Hashmi, Shmagel.
Analysis and interpretation of data
Boersma, McElwee, Schreiner, Demmer, Shmagel.
No potential conflicts of interest relevant to this article were reported.
References
- 1. Maradit‐Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population‐based study. Arthritis Rheum 2005;52:722–32. [DOI] [PubMed] [Google Scholar]
- 2. Aviña‐Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Rheum 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 3. Wållberg‐Jonsson S, Öhman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol 1997;24:445–51. [PubMed] [Google Scholar]
- 4. Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 2003;30:36–40. [PubMed] [Google Scholar]
- 5. Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives [published erratum appears in Ann Rheum Dis 2011;70:1520]. Ann Rheum Dis 2011;70:8–14. [DOI] [PubMed] [Google Scholar]
- 6. Panoulas VF, Douglas KM, Stavropoulos‐Kalinoglou A, Metsios GS, Nightingale P, Kita MD, et al. Long‐term exposure to medium‐dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008;47:72–5. [DOI] [PubMed] [Google Scholar]
- 7. Metsios GS, Stavropoulos‐Kalinoglou A, Panoulas VF, Wilson M, Nevill AM, Koutedakis Y, et al. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil 2009;16:188–94. [DOI] [PubMed] [Google Scholar]
- 8. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta‐analysis. PLoS One 2015;10:e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panoulas VF, Toms TE, Metsios GS, Stavropoulos‐Kalinoglou A, Kosovitsas A, Milionis HJ, et al. Target organ damage in patients with rheumatoid arthritis: the role of blood pressure and heart rate. Atherosclerosis 2010;209:255–60. [DOI] [PubMed] [Google Scholar]
- 10. Desai SS, Myles JD, Kaplan MJ. Suboptimal cardiovascular risk factor identification and management in patients with rheumatoid arthritis: a cohort analysis. Arthritis Res Ther 2012;14:R270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos‐Kalinglou A, Nightingale P, Kita MD, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–82. [DOI] [PubMed] [Google Scholar]
- 12. Alemao E, Cawston H, Bourhis F, Al M, Rutten‐van Mölken MP, Liao KP, et al. Cardiovascular risk factor management in patients with RA compared to matched non‐RA patients. Rheumatology (Oxford) 2016;55:809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. An J, Cheetham TC, Reynolds K, Alemao E, Kawabata H, Liao KP, et al. Traditional cardiovascular disease risk factor management in rheumatoid arthritis compared to matched nonrheumatoid arthritis in a US managed care setting. Arthritis Care Res (Hoboken) 2016;68:629–37. [DOI] [PubMed] [Google Scholar]
- 14. Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty‐year period. Arthritis Rheum 2002;46:625–31. [DOI] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 16. University of Michigan Population Studies Center . How to compute characteristics at the zip code level. URL: https://www.psc.isr.umich.edu/dis/census/Features/tract2zip/methods.html.
- 17. Chung CP, Giles JT, Petri M, Szklo M, Post W, Blumenthal RS, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi‐ethnic study of atherosclerosis. Semin Arthritis Rheum 2012;41:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartels CM, Johnson H, Voelker K, Thorpe C, McBride P, Jacobs EA, et al. Impact of rheumatoid arthritis on receiving a diagnosis of hypertension among patients with regular primary care. Arthritis Care Res (Hoboken) 2014;66:1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 20. Minnesota Department of Health . Health care quality measures. URL: https://www.health.state.mn.us/data/hcquality/index.html.
- 21. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 22. Bartels CM, Kind AJ, Everett C, Mell M, McBride P, Smith M. Low frequency of primary lipid screening among Medicare patients with rheumatoid arthritis. Arthritis Rheum 2011;63:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schoenfeld SR, Lu L, Rai SK, Seeger JD, Zhang Y, Choi HK. Statin use and mortality in rheumatoid arthritis: a general population‐based cohort study. Ann Rheum Dis 2016;75:1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. An J, Alemao E, Reynolds K, Kawabata H, Solomon DH, Liao KP, et al. Cardiovascular outcomes associated with lowering low‐density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol 2016;43:1989–96. [DOI] [PubMed] [Google Scholar]
- 25. Del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45. [DOI] [PubMed] [Google Scholar]
- 26. Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta‐analysis of observational studies. Ann Rheum Dis 2010;69:70–81. [DOI] [PubMed] [Google Scholar]
- 27. Van Gaalen FA, van Aken J, Huizinga TW, Schreuder GM, Breedveld FC, Zanelli E, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum 2004;50:2113–21. [DOI] [PubMed] [Google Scholar]
- 28. Tomasson G, Aspelund T, Jonsson T, Valdimarsson H, Felson DT, Gudnason V. Effect of rheumatoid factor on mortality and coronary heart disease. Ann Rheum Dis 2010;69:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez A, Icen M, Kremers HM, Crowson CS, Davis JM III, Therneau TM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol 2008;35:1009–14. [PMC free article] [PubMed] [Google Scholar]


