Abstract
Objective
Pseudostenosis is a magnetic resonance angiography (MRA) artifact that mimics arterial stenosis. The study objective was to compare imaging and clinical aspects of stenosis and pseudostenosis in a cohort of large‐vessel vasculitis (LVV), including giant‐cell arteritis (GCA) and Takayasu's arteritis (TAK).
Methods
Patients with LVV and comparator conditions (healthy or vasculopathies) underwent MRA of the aortic arch vessels. The subclavian and axillary arteries were systematically assessed for presence of stenosis and pseudostenosis by two independent readers. Serial and delayed imaging and clinical assessments were used to confirm suspected pseudostenoses. Multivariable regression analyses were used to identify associations between angiographic pathology and clinical findings.
Results
One hundred eighty‐four MRA scans were analyzed from patients with GCA (n = 36), TAK (n = 47), and comparators (n = 25). Pseudostenoses were frequently observed (48 of 184 scans, 26%) in the distal subclavian artery only on the side of injection and were shorter in length compared with true stenoses (25 mm vs 78 mm, P < 0.01). There was no difference in prevalence of pseudostenosis by diagnosis (GCA = 33%, TAK = 23%, comparator = 20%, P = 0.44), disease activity status (P = 0.31), or treatment status (P = 1.00). Percent and length of true stenosis were independently associated with pulse and blood pressure abnormalities in the upper extremity. Adjusting for length and stenosis degree, absence of collateral arteries was associated with arm claudication (odds ratio = 2.37, P = 0.03).
Conclusion
Although a pseudostenosis could be falsely interpreted as an arterial stenosis, radiographic and associated clinical features can help distinguish true disease from arterial susceptibility artifacts. In addition, the peripheral vascular examination can help to confirm a suspected true stenosis, as specific aspects of angiographic pathology are associated with vascular examination abnormalities in LVV.
Keywords: cardiovascular imaging, giant cell arteritis, large‐vessel vasculitis, magnetic resonance angiography, Takayasu's arteritis, vasculitis
Introduction
Periodic monitoring of the large arteries by magnetic resonance angiography (MRA) is common clinical practice in patients with large‐vessel vasculitis (LVV) 1, 2, 3. Arterial pathology in the subclavian and axillary arteries is highly prevalent among patients with Takayasu's arteritis (TAK) and a subset of patients with giant cell arteritis (GCA), the two major forms of LVV 4. Stenoses and occlusions within the arteries of the upper extremities can result in loss of pulse, reduced blood pressure, and arm claudication. Over time, collateral arteries can form, potentially improving circulation to an affected limb. Precise mapping of arterial lesions within the subclavian and axillary arteries may improve understanding about the specific aspects of arterial pathology that result in vascular examination abnormalities and claudication.
Clinical assessment of disease activity can be challenging in LVV. Patients with LVV may develop new stenoses during periods of apparent clinical remission, reinforcing the importance of serial angiography to monitor for arterial damage 5. MR imaging is the preferred modality to monitor arterial disease in LVV because it is less invasive than catheter angiography and does not incur the radiation exposure inherit to computerized tomography. However, there are potential pitfalls in the interpretation of MRA that are due to susceptibility artifacts.
Magnetic susceptibility artifacts refer to distortions of local signal changes due to magnetic field inhomogeneity from a variety of compounds 6. Susceptibility artifacts from implanted metallic objects (eg, stents) are common in MRA 7. Gadolinium, a typical component of MR contrast agents, possesses metallurgical properties and can also cause susceptibility artifacts. When MRA images are obtained immediately after gadolinium‐based contrast is injected into an upper extremity, high concentrations of gadolinium in the ipsilateral subclavian vein can create a susceptibility artifact in the adjacent subclavian artery. The resultant irregularity in the subclavian artery can mimic a stenosis and is termed a pseudostenosis 8. Because angiographic progression of disease can be considered evidence of ongoing active disease in patients with LVV, even in absence of overt clinical symptoms, it is imperative to differentiate a pseudostenosis that is due to a gadolinium‐induced susceptibility artifact from a true stenosis to avoid misinterpretation of disease extent and progression in the subclavian and axillary arteries.
The objectives of this study were to compare the morphologic characteristics of true stenosis with pseudostenosis and to correlate specific aspects of subclavian/axillary artery pathology with peripheral vascular examination findings and symptoms in a cohort of patients with LVV.
Patients and Methods
Study population and clinical assessment
Patients 5 years of age or older with known or suspected vasculitis were recruited into a prospective, observational cohort at the National Institutes of Health (NIH) in Bethesda, Maryland (ClinicalTrials.gov Identifier: NCT02257866). All patients with LVV included in this study fulfilled either the 1990 American College of Rheumatology (ACR) Classification Criteria for TAK or the modified 1990 ACR Criteria for GCA 4, 9, 10. Patients could be enrolled at any point during the disease course. To determine if patients with LVV were at an increased risk for pseudostenosis, a comparator group was recruited under the same observational protocol consisting of patients who were evaluated for suspected LVV but were determined to have a vasculopathy of the aorta or branch vessels (eg, fibromuscular dysplasia) and healthy volunteers. All study participants underwent peripheral vascular assessment and physical examination focused upon the upper extremities within 24 hours prior to imaging. Clinical assessment was performed blinded to angiographic data. Radial pulse (absent/barely palpable versus present), blood pressure in all four extremities, and symptoms of limb claudication were recorded. Disease duration was calculated from time of initial symptom onset rather than diagnosis date.
MRA protocol
From 2014‐2019, patients underwent MR imaging on the same Siemens Biograph 3.0 T mMR machine (Siemens Medical Solutions) per a standardized imaging protocol (single breath hold, coronal, 2.68 ms repetition time, 1.06 ms echo time, one average, minimum repetition time and echo time, final resolution 1.2 × 1.1 × 1.0 mm). Patients with LVV underwent MRA imaging at 6‐month intervals, and the comparator group of patients underwent imaging only at a baseline visit. Full details of the MR imaging protocol have been previously reported 11. Pertinent to this study, all patients underwent T1‐VIBE imaging of the entire aorta and branch vessels during the venous phase with image acquisition at a slice thickness of 3 mm per routine clinical practice. At this slice thickness, direct visualization of the distal subclavian arteries was determined to be limited because of image resolution. Therefore, from January 2016 onward, in addition to the three‐station MRA, all patients underwent a near isotropic venous phase three‐dimensional VIBE (1‐mm slice thickness) focused only on the subclavian artery region. The purpose of delayed phase imaging with this additional sequence was to better visualize the subclavian artery region to demonstrate that a pseudostenosis identified during early arterial phase imaging was no longer present during the venous phase 12. Maximum intensity projections from early arterial phase images were formulated with Syngo MR v.B20P (Siemens Medical Solutions). All images were available for review with a Carestream workstation v.12.1.6 (Carestream Health Inc).
Unless there was known severe vascular pathology in a particular limb, injection site was determined at random. A single dose of 0.1 mmol/kg gadolinium (gadobenate dimeglumine), diluted with equal amounts of normal saline, was administered via injection pump at a rate of 2 ml/s followed by 20 ml of normal saline flush. The purpose of the gadolinium dilution was to reduce the appearance of pseudostenosis on first‐pass angiography per common clinical practice 13.
Identification of stenosis and pseudostenosis on early arterial phase images
The arch vessels were independently assessed by two experienced readers (A.A.M., a vascular radiologist, and M.A.A, a nuclear medicine physician, each with more than 10 years of experience interpreting MRA for clinical and research purposes). Readers were blinded to clinical data and injection site. Stenoses and pseudostenoses were defined by reader agreement based upon visual inspection of early arterial phase images, without consideration of any of the delayed phase imaging data, and all discrepancies were resolved by consensus. Early arterial phase images were specifically inspected for arterial narrowing and signal void in the surrounding tissue, suggestive of a pseudostenosis. To determine whether the pseudostenosis characteristics described in this study improve MRA interpretability by less trained readers, a third reader with no prior experience interpreting MRAs (K.C.M.) was instructed on how to identify a pseudostenosis and subsequently evaluated all studies in the cohort.
Contralateral limb injection and delayed phase imaging
An arterial pseudostenosis is an artifact present only during early arterial phase imaging when there is persistent gadolinium in the venous system ipsilateral to the site of contrast injection. For patients who underwent serial imaging studies, we investigated whether an identified pseudostenosis was no longer present if contrast injection was performed on the contralateral arm during a prior or subsequent imaging study. In addition, a suspected pseudostenosis should not be visualized on delayed phase imaging, as gadolinium is no longer concentrated in the veins adjacent to the upper extremity arteries. Therefore, for patients studied after January 2016 who underwent venous phase T1 VIBE with 1‐mm slice thickness dedicated images of the subclavian arteries as described above, we investigated whether a pseudostenosis identified on early arterial phase angiography was visualized on dedicated delayed imaging of the subclavian arteries. If radiographic confirmation of a pseudostenosis by serial or delayed phase imaging studies was not available, clinical data were used to confirm a suspected pseudostenosis when there was normal pulse, blood pressure, and absence of claudication symptoms in the corresponding arm. A flow chart of radiographic assessment is provided in Figure 1.
Figure 1.
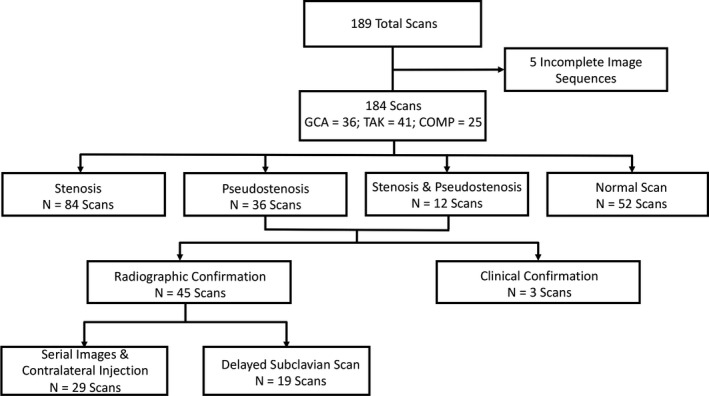
Flow chart of radiographic cohort. 184 MRA studies were analyzed for stenotic lesions throughout the subclavian and axillary arteries. COMP = comparator group consisting of healthy controls and patients with other vasculopathies; GCA = giant cell arteritis; N = the number of scans analyzed; TAK = Takayasu's arteritis.
Characterization of stenosis and pseudostenosis
The left and right subclavian/axillary arteries were divided into three segments to localize pathology. The left upper extremity arterial supply was segmented into the proximal subclavian (origin to bend at level of the clavicle), distal subclavian (linear segment extending to the distal end of the first rib), and axillary artery. The right upper extremity arterial supply was segmented into the brachiocephalic (origin to takeoff of right common carotid artery), subclavian (extending to end of first rib), and axillary artery. On maximum intensity projection (MIP) reconstructions, stenoses and pseudostenoses were characterized by length, degree of stenosis, and location. Degree of stenosis was defined as the percentage of narrowing at the site of the lesion relative to the width of the nearest proximal unaffected segment of the native vessel. Presence or absence of collateral artery formation was assessed by visual inspection in each limb. Appearance of stenosis and pseudostenosis on serial imaging was investigated.
Statistical analysis
Cohen's κ was used to assess inter‐rater reliability (0‐0.20 poor; 0.21‐0.40 fair; 0.41‐0.60 moderate; 0.61‐0.80 good; greater than 0.81 excellent). Differences between distributions of continuous data were compared using a two‐tailed Mann‐Whitney U test. Fisher's exact test was used to assess differences in categorical data. Multivariable mixed effects logistic regression was performed using only the baseline visit data to study the associations between three outcome measures—radial pulse (absent/present), systolic blood pressure (below or above 90 mmHg), and upper extremity claudication (absent/present)—and the following predictor variables: length of stenosis, degree of stenosis, and the absence of collaterals. Within patient‐data was modeled as a random effect. A P value of less than 0.05 defined statistical significance. All statistical analyses were performed on SAS v.9.3 (SAS Institute).
Ethics and informed consent
All patients provided written informed consent. An institutional review board at the NIH approved the research (NCT02257866).
Results
Study population
A total of 189 MRA studies were performed. Five studies contained incomplete imaging sequences and were excluded, leaving 184 studies for analysis. One hundred eight patients (GCA = 36, TAK = 47, comparator = 25) contributed at least one imaging study. The comparator population included traumatic subclavian stenosis (n = 1), connective tissue disorders such as fibromuscular dysplasia (n = 4), other forms of inflammatory disease other than LVV (n = 13), and healthy controls (n = 7). Among patients with LVV, 31 out of 84 patients (37%) contributed more than one MRA at different time points in the disease course (GCA = 86 studies; TAK = 73 studies). Out of the 184 analyzed MRA scans, 84 showed stenoses, 36 showed pseudostenoses, 12 showed both stenosis and pseudostenosis, and 52 were normal. A flow chart of vascular lesions found in these scans is presented in Figure 1.
Radiographic analysis of stenosis versus pseudostenosis
Pseudostenoses were identified by visual inspection in 48 of the 184 MRA studies (26.1%) representing 34 (31%) of the study population. There was agreement for 44 pseudostenoses by the experienced readers, and an additional 4 pseudostenoses were identified by consensus review. Agreement between the expert readers was excellent (Cohen's κ = 0.94, SE = 0.03). Agreement between the inexperienced reader and the expert readers was good (Cohen's κ = 0.75, SE = 0.08).
Presence of pseudostenosis was always unilateral, confined to a single segment in the distal subclavian artery, and always occurred on the same side as the upper extremity injection site. Contrast injection into the left versus right arm did not result in increased prevalence of pseudostenosis (left‐sided = 29%; right‐sided = 23%; P = 0.50). There was no difference in the prevalence of pseudostenosis by diagnosis (GCA = 33%, TAK = 23%, comparator = 20%, P = 0.44), disease activity status (active = 21% versus remission = 30%, P = 0.31), or treatment status (on treatment = 34%, off treatment = 32%, P = 1.00).
A true stenosis in either the brachiocephalic, subclavian, or axillary arteries was identified in 42 (51%) patients with LVV. Bilateral disease was seen in 27 (64%) of these patients. Out of the 166 identified stenoses from all available MRA studies, 49 lesions were single segment and 117 were multisegment. Multisegment stenoses typically spanned all segments of both the left and right subclavian/axillary arteries. Single‐segment stenoses in the left upper extremity tended to occur in the proximal left subclavian segment (18 of 23, 78%), whereas single‐segment lesions in the right upper extremity tended to occur in the subclavian segment (25 of 26, 96%) rather than the brachiocephalic artery.
A summary of the differences between stenoses and pseudostenoses are listed in Table 1. In contrast to stenoses, every pseudostenosis identified in this study was single segment and located in the most distal part of the subclavian artery just prior to the start of the axillary artery. The average length of a stenosis was significantly longer than a pseudostenosis (77.9 mm versus 25.2 mm, P < 0.01). The percent narrowing was significantly greater for a stenosis compared with a pseudostenosis (59% versus 34%, P < 0.01). Additionally, stenoses were significantly associated with vascular examination findings in the corresponding upper extremity (limb claudication, systolic blood pressure <90 mmHg, absent pulse) compared with pseudostenoses. Representative images of stenoses and pseudostenoses can be found in Figure 2 and the Supplementary Figure .
Table 1.
Radiological and clinical features of stenosis and pseudostenosis
| Feature | Unit of Measurement | Stenosis (n = 166 limbs) | Pseudostenosis (n = 48 limbs) | P value |
|---|---|---|---|---|
| Location | ||||
| Single segment | N (%) | 49 (29.5) | 48 (100) | <0.01 |
| Multisegment | 117 (70.5) | 0 (0) | <0.01 | |
| Length | mm (SD) | 77.9 (41.7) | 25.2 (6.5) | <0.01 |
| Width of stenosis | % (SD) | 58.9 (23.6) | 34.3 (18.1) | <0.01 |
| Systolic blood pressure <90 | N (%) | 29 (17.5) | 0 (0) | <0.01 |
| Absent pulse | N (%) | 52 (31.3) | 1 (2.1)a | <0.01 |
| Limb claudication | N (%) | 59 (35.5) | 1 (2.1)a | <0.01 |
| Serial imaging | … | Never completely resolved | Completely resolved | … |
Patient had both a stenosis and a pseudostenosis in the same limb.
Figure 2.
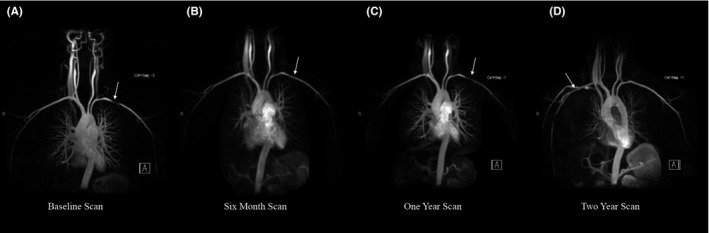
Appearance and disappearance of pseudostenosis on serial imaging. Serial images from the same patient demonstrate prominent pseudostenosis in the left subclavian artery (A) that is barely visible on repeat imaging 6 months later (B) and is again prominent on imaging 1 year later (C). Contrast was injected into the left arm for for each of these studies. When contrast was injected into the right arm during the two‐year scan, the left subclavian artery now appears completely normal with appearance of a new right‐sided pseudostenosis (D).
Radiographic confirmation of a pseudostenosis was demonstrated for 45 out of 48 pseudostenoses identified by the readers. For 29 of the 48 pseudostenoses, imaging data were available from the same patient at a different study date where contrast was injected into the contralateral arm. In each instance, the identified pseudostenosis was no longer visualized. For 19 pseudostenoses, delayed phase imaging of the subclavian arteries at 1‐mm slice thickness was available and confirmed nonvisualization of the pseudostenosis that had been identified on early arterial phase imaging (Figure 3). For three pseudostenoses, serial imaging or dedicated venous phase imaging of the subclavian arteries was not available. In these cases, no associated clinical findings on vascular examination of the upper extremity were observed for any of these patients. For 31 patients with LVV who underwent longitudinal angiography and had true arterial stenoses within the subclavian and axillary arteries, complete resolution of a stenosis on serial angiography was never observed. Delayed phase imaging was available for 25 patients with LVV with an identified true stenosis, and in each case, the arterial lesion was still present on delayed imaging.
Figure 3.
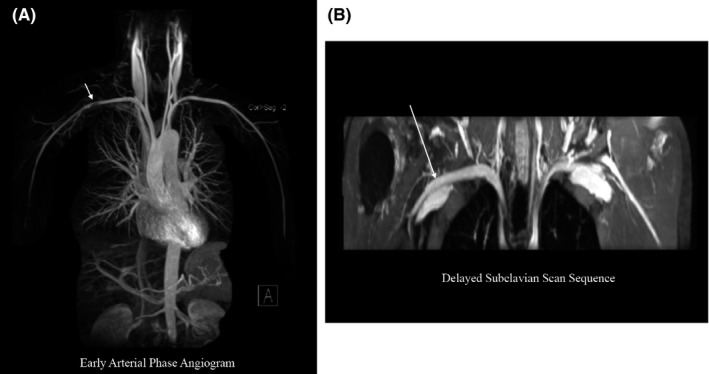
Early arterial phase angiography versus venous phase imaging. A, Maximum intensity projection (MIP) reconstruction of an early arterial phase angiogram shows a pseudostenosis in the right subclavian artery in a healthy subject. B, A delayed phase dedicated subclavian scan shows that the suspected pseudostenosis seen during the early arterial phase is no longer visualized when imaging the same subject during the venous phase.
Association of vascular examination findings and characteristics of arterial lesions
Of the 166 limbs from 83 patients with LVV imaged during the baseline study visit, absent radial pulse was observed 37 times (22%), systolic blood pressure lower than 90 mmHg was measured 17 times (10%), and claudication was reported in 44 limbs (27%). In univariate regression analyses, length and degree of stenosis were significantly associated with absence of radial pulse, systolic blood pressure lower than 90 mmHg, and arm claudication (P < 0.01) (Table 2). For every 1‐cm increase in length of stenosis, there was 23% and 22% increased odds for absence of radial pulse or reduction of systolic blood pressure, respectively. For every 10% increase in degree of stenosis, there was 38% and 66% increased odds for an absent radial pulse or reduction of systolic blood pressure, respectively. In multivariable analyses, length and degree of stenosis were independently associated with radial pulse and blood pressure findings. Presence or absence of collateral artery formation was not associated with pulse abnormalities, reduction in blood pressure, or arm claudication in univariate analyses. However, in multivariable analysis, arm claudication was associated with degree of stenosis (Odds Ratio [OR] =1.30, 95% confidence interval [CI] 1.16‐1.44, P < 0.01) and absence of collateral artery formation (OR = 2.37, 95% CI 1.10‐5.11, P = 0.03), but was not associated with length of stenosis (OR = 1.08, 95% CI 1.00‐1.17, P = 0.06). Adjusting for length and degree of stenosis, patients who had not developed collateral artery circulation to the affected upper limb had a 237% increased odds to report arm claudication compared with patients who had developed collateral circulation.
Table 2.
Association of vascular examination and radiographic findings
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Absent radial pulse | ||||
|
Stenosis length per 1 cm increase |
1.23 (1.16‐1.30) |
<0.01 |
1.13 (1.04‐1.22) |
0.05 |
|
Degree of stenosis per 10% increase |
1.38 (1.26‐1.49) |
<0.01 |
1.26 (1.12‐1.41) |
<0.01 |
|
Absent collaterals |
1.08 (0.98‐2.32) |
0.32 |
1.38 (0.62‐3.04) |
0.43 |
| Systolic blood pressure <90 mmHg | ||||
|
Stenosis length per 1 cm increase |
1.22 (1.13‐1.30) |
<0.01 |
1.05 (0.99‐1.20) |
0.34 |
|
Degree of stenosis per 10% increase |
1.66 (1.41‐1.91) |
<0.01 |
1.64 (1.37‐1.92) |
<0.01 |
| Absent collaterals |
0.35 (0.10‐1.08) |
0.08 |
1.30 (0.40‐4.16) |
0.66 |
| Arm claudication | ||||
|
Stenosis length per 1 cm increase |
1.19 (1.13‐1.25) |
<0.01 |
1.08 (1.00‐1.17) |
0.06 |
|
Degree of stenosis per 10% increase |
1.34 (1.24‐1.44) |
<0.01 |
1.30 (1.16‐1.44) |
<0.01 |
| Absent collaterals |
0.64 (0.36‐1.10) |
0.10 |
2.37 (1.10‐5.11) |
0.03 |
Abbreviation: CI, confidence interval; OR, odds ratio.
Discussion
This study demonstrates that true arterial stenoses as well as arterial pseudostenoses from MR susceptibility artifacts are common within the upper extremity arteries in patients with LVV. Without careful assessment, a pseudostenosis could be incorrectly interpreted as a true arterial stenosis in a patient with LVV. However, a pseudostenosis that is due to a gadolinium‐induced susceptibility artifact has unique radiographic characteristics that can readily be distinguished from true arterial pathology with proper assessment. Pseudostenoses of the upper extremity arteries always occur on the same limb as the contrast injection site, are focal, and are typically located in the most distal segment of the subclavian artery immediately prior to the axillary artery.
Pseudostenoses occur in the upper extremities during early arterial phase angiography when a high concentration of gadolinium remains in the subclavian vein immediately following contrast injection, leading to a susceptibility artifact in the adjacent subclavian artery that can resemble a true stenosis. As such, repeat imaging with injection of contrast into the contralateral limb or dedicated, high‐resolution delayed imaging of the subclavian arteries can confirm the pseudostenosis is indeed an artifact. Anecdotally, it has been suggested that a higher frequency of pseudostenoses occur in the left upper extremity compared with the right upper extremity, presumably because of the longer course of venous return 8. In this study, the largest of its kind to assess prevalence of pseudostenosis in a cohort of patients using a standardized imaging protocol, an equal prevalence of pseudostenosis was found in the right and left upper extremities. As such, preferential injection of one arm to reduce the frequency of pseudostenosis is not advised. Pseudostenosis in the carotid and vertebral arteries has been rarely reported; however, in this study, pseudostenoses were only identified in the distal subclavian artery 14. Pseudostenoses are expected to be uncommon in vascular territories other than the subclavian arteries. Gadolinium concentration is highest in the subclavian veins shortly following contrast injection with subsequent blood pool dilution principally with nonenhanced blood from the inferior vena cava, which effectively removes the artifact.
Misinterpretation of a pseudostenosis in a patient with LVV could lead to incorrect assumptions about the extent of arterial disease and the progression of disease over time. The clinical relevance of this potential pitfall is underscored by the clinical practice to consider asymptomatic angiographic progression as evidence of ongoing active disease in LVV. Radiographic characteristics and corresponding clinical assessments are helpful to differentiate stenosis and pseudostenosis. A pseudostenosis is a focal lesion in the distal subclavian artery, whereas focal stenotic lesions in LVV are typically more proximal in the left or right subclavian arteries. No corresponding vascular examination abnormalities or claudicatory symptoms were observed in association with a suspected pseudostenosis, unless a patient has both a pseudostenosis and a true stenosis within the upper extremity arteries. In situations where a pseudostenoses occurs within the location of a concomitant true stenosis, it is challenging to assess progression of disease within the artery.
In cases where there is uncertainty about whether there is a true stenosis or pseudostenosis, repeat imaging with injection of contrast in the contralateral arm or delayed imaging sequences are potential options when radiographic confirmation of a suspected pseudostenosis is clinically necessary. In contrast, complete resolution of a true stenosis by serial angiography or delayed imaging was not observed in this study. A flow chart summarizing how to assess a suspected pseudostenosis versus true stenosis is provided in Figure 4.
Figure 4.
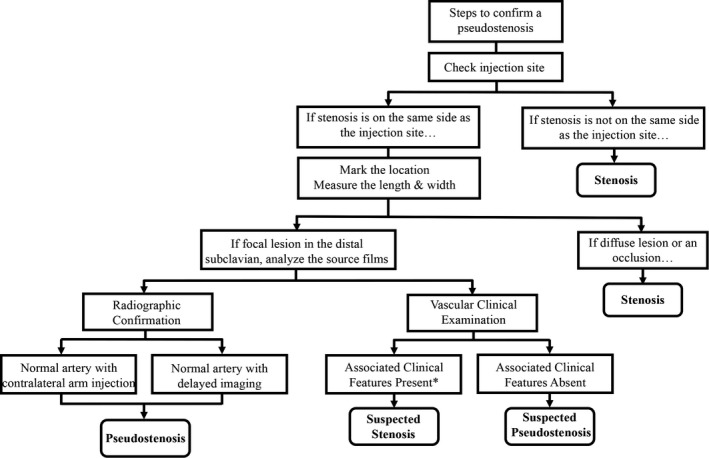
Steps to differentiate arterial stenosis and pseudostenosis. If a pseudostenosis is suspected, check the contrast injection site and examine the length and width of the lesion. Injection of the contralateral arm or delayed sequence imaging with nonvisualization of the suspected pseudostenosis is confirmatory. In absence of radiologic confirmation, no associated vascular examination findings in the corresponding limb can provide clinical support to confirm a suspected pseudostenosis. *Clinical symptoms in a corresponding limb may be present in cases of suspected pseudostenosis when there is a concurrent true stenosis in the same artery.
Although previous studies have examined whether abnormalities on vascular examination are useful to detect angiographic pathology in LVV, this study is the first to examine how specific aspects of angiographic pathology are associated with abnormalities on vascular examination 15. Length and degree of stenosis independently contribute to the presence of vascular examination abnormalities of the arm (eg, loss of radial pulse or reduction of systolic blood pressure). Degree of stenosis is more strongly associated with reduction of systolic blood pressure in an affected limb compared to the length of stenosis. Adjusting for length and degree of stenosis, formation of collateral arteries in an affected limb is not associated with radial pulse or systolic blood pressure abnormalities, underscoring the clinical observation that severe pulse and blood pressure abnormalities are rarely reversible in LVV, even in patients who have developed compensatory collateral circulation. The association of angiographic findings with arm claudication is more complex than the association with vascular examination abnormalities. Adjusting for length and percent of stenosis, development of collateral artery circulation in an affected limb is strongly associated with whether or not a patient reports symptoms of arm claudication. Future studies into how collateral arteries develop and how to promote their formation are warranted and could facilitate novel therapeutic approaches to the management of persistent limb claudication in patients with LVV.
This study has several limitations to consider. This was a single‐center study subject to assessment bias from the participating investigators. However, imaging sequences were performed according to a standardized protocol enabling comparisons across the cohort, and pseudostenoses were identified by two independent readers with excellent agreement. Measurements of stenosis length and percent narrowing on MIP reconstructions may be less precise than similar measurements on source images. Radiographic confirmation of pseudostenosis by injection of the contralateral arm during repeat imaging or delayed sequence images was performed in the majority (94%), but not all, cases of suspected pseudostenosis. Clinical confirmation of a normal upper extremity vascular examination was performed in the three patients who did not have radiographic confirmation of a suspected pseudostenosis, acknowledging that negative physical examination findings do not always rule out a true stenosis 15.
In conclusion, pseudostenosis was commonly identified within the subclavian arteries on MRA in a cohort of patients with LVV and comparator conditions. Because disease activity assessment in LVV can be informed based upon angiographic progression of disease, this study demonstrates the importance of the clinician's ability to recognize and differentiate a pseudostenosis that is due to a gadolinium‐induced susceptibility artifact from true arterial pathology to ensure that clinical management decisions are based upon accurate angiographic assessments. Additionally, by comparing specific aspects of arterial pathology with clinical vascular findings, this study demonstrates the value of the vascular physical examination in LVV. Although angiography remains a core component of disease assessment in LVV, there are potential pitfalls to the interpretation of MRA findings that must be considered when assessing arterial pathology in these diseases.
Author Contributions
All authors were involved in data acquisition, contributed to data analysis and interpretation of data, and were involved in drafting the article or revising it critically. All authors approved the final version of the article for submission.
Study conception and design.
Ahlman, Malayeri, Evers, Grayson
Acquisition of data.
Marinelli, Ahlman, Quinn, Malayeri, Evers, Grayson
Analysis and interpretation of data.
Marinelli, Ahlman, Quinn, Malayeri, Evers, Grayson
Supporting information
This work was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
No potential conflicts of interest relevant to this article were reported.
References
- 1. Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009;68:318–23. [DOI] [PubMed] [Google Scholar]
- 2. Barra L, Liang P, Benseler SM, Cabral DA, Fifi‐Mah A, Li Y, et al. Variations in the clinical practice of physicians managing Takayasu arteritis: a nationwide survey. Open Access Rheumatol 2017;9:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–43. [DOI] [PubMed] [Google Scholar]
- 4. Grayson PC, Maksimowicz‐McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, et al. Distribution of arterial lesions in Takayasu's arteritis and giant cell arteritis Ann Rheum Dis. 2012;71:1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med 1994;120:919–29. [DOI] [PubMed] [Google Scholar]
- 6. Huang SY, Seethamraju RT, Patel P, Hahn PF, Kirsch JE, Guimaraes AR. Body MR Imaging: Artifacts, k‐Space, and Solutions. Radiographics 2015;35:1439–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glockner JF. MR angiography interpretation: techniques and pitfalls. Magn Reson Imaging Clin N Am 2005;13:23–40. [DOI] [PubMed] [Google Scholar]
- 8. Lee VS, Martin DJ, Krinsky GA, Rofsky NM. Gadolinium‐enhanced MR angiography: artifacts and pitfalls. AJR Am J Roentgenol 2000;175:197–205. [DOI] [PubMed] [Google Scholar]
- 9. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 10. Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–8. [DOI] [PubMed] [Google Scholar]
- 11. Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS, et al. Comparison of magnetic resonance angiography and (18)F‐fluorodeoxyglucose positron emission tomography in large‐vessel vasculitis. Ann Rheum Dis 2018;17:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maki JH, Prince MR, Londy FJ, Chenevert TL. The effects of time varying intravascular signal intensity and k‐space acquisition order on three‐dimensional MR angiography image quality. J Magn Reson Imaging 1996;6:642–51. [DOI] [PubMed] [Google Scholar]
- 13. Neimatallah MA, Chenevert TL, Carlos RC, Londy FJ, Dong Q, Prince MR, et al. Subclavian MR arteriography: reduction of susceptibility artifact with short echo time and dilute gadopentetate dimeglumine. Radiology 2000;217:581–6. [DOI] [PubMed] [Google Scholar]
- 14. Tirkes AT, Rosen MA, Siegelman ES. Gadolinium susceptibility artifact causing false positive stenosis isolated to the proximal common carotid artery in 3D dynamic contrast medium enhanced MR angiography of the thorax–a brief review of causes and prevention. Int J Cardiovasc Imaging 2003;19:151–5. [DOI] [PubMed] [Google Scholar]
- 15. Grayson PC, Tomasson G, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, et al. Association of vascular physical examination findings and arteriographic lesions in large vessel vasculitis. J Rheumatol 2012;39:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


