Abstract
Objective
To assess the safety of abatacept treatment in rheumatoid arthritis (RA) using integrated data from multiple clinical trials.
Methods
Data from nine double‐blind, placebo‐controlled studies of abatacept treatment (seven intravenous, two subcutaneous) in patients with RA were pooled, focusing on safety events in the double‐blind treatment period of each study. Incidence rates (IRs) of adverse events (AEs) per 100 patient‐years of exposure were calculated for abatacept‐ and placebo‐treated patients. AEs in abatacept‐treated patients were combined regardless of dose and formulation.
Results
In total, 2653 patients received abatacept and 1485 received placebo, with 2357 and 1254 patient‐years of exposure, respectively. The mean (SD) durations of exposure in the abatacept and placebo groups were 10.8 (3.3) and 10.3 (3.5) months, respectively. The IRs (95% confidence interval [CI]) for serious AEs were 14.8 (13.3, 16.5) and 14.6 (12.5, 17.0) in the abatacept and placebo groups, respectively. Death occurred in 12 (0.5%) and 12 (0.8%) patients in the abatacept and placebo groups, respectively, and was most commonly caused by cardiac disorders. Malignancies were observed in 31 patients (1.2%) treated with abatacept (IR: 1.32 [95% CI: 0.90, 1.87]) versus 14 (0.9%; IR: 1.12 [0.61, 1.88]) who received placebo. Solid organ tumor was the most frequent malignancy reported in both groups (abatacept: 1.0%; IR: 1.11 [95% CI: 0.72, 1.62]; placebo: 0.8%; 0.96 [0.50, 1.67]).
Conclusion
In this integrated analysis, the IRs of safety events in the abatacept and placebo groups were similar with no new safety concerns identified.
Introduction
Abatacept, a fusion protein composed of the extracellular domain of cytotoxic T‐lymphocyte–associated antigen‐4 and the fragment crystallizable region of human immunoglobulin G1, is a selective modulator of T‐cell co‐stimulation. Abatacept is approved for the treatment of rheumatoid arthritis (RA), juvenile idiopathic arthritis, and psoriatic arthritis in the United States and in Europe, and it has an established efficacy and safety profile ever since its initial approval in 2005 1. Abatacept has consistently demonstrated efficacy benefits over placebo for clinical response, including reduced disease activity measures and improvements in physical function and health‐related quality of life 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. Clinical trials have also shown that abatacept is safe and well tolerated, with similar rates of adverse events (AEs) compared with placebo, demonstrating a favorable benefit‐risk profile 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14.
An increased risk of specific AEs has been observed in patients with RA who are treated with biologic disease‐modifying antirheumatic drugs (DMARDs), including tuberculosis, herpes zoster, and other infections 15, 16, autoimmune diseases 17, and malignancies 18. In addition, the development of anti‐drug antibodies with biologic treatments may lead to a loss of efficacy 19, 20, 21. Subcutaneous (SC) administration of biologic agents also presents the risk of injection‐site reactions 21. Hence, it is of interest to comprehensively evaluate the safety profile of abatacept, a biologic treatment, across multiple clinical studies in patients with RA.
The safety of abatacept treatment has been studied extensively using both SC and intravenous (IV) formulations 22, 23, 24. Previously, an integrated safety database was used to separately analyze data from fives phase II and phase III trials of the SC formulation 22 and eight phase II and phase III trials of the IV formulation 23 (including placebo‐controlled and open‐label trials). Because SC and IV administration has shown similar safety and efficacy profiles 24 and serum concentrations over time at doses of 125 mg SC weekly versus 10 mg/kg IV monthly 25, we pooled data from nine placebo‐controlled, double‐blind trials for this analysis. Data from single randomized, controlled trials can identify strong safety signals, whereas pooling data from multiple trials yields greater patient numbers and patient‐years (py) of exposure, which enables differences in incidence rates (IRs) between study drug and placebo to be estimated with higher precision than with individual trials.
Here, we present a comprehensive analysis of the overall safety profile of abatacept in RA, regardless of route of administration. This analysis represents the largest safety study of abatacept treatment to date and consists of integrated data from nine selected clinical trials.
Methods
Study design
A total of 16 studies were originally considered for inclusion in this analysis. Of the seven trials that were excluded, four were open label and not placebo‐controlled, two were randomized but not placebo‐controlled, and one was a randomized withdrawal study. Data were pooled from the remaining nine clinical trials of abatacept (IV, SC, or both) treatment in patients with RA that were randomized, placebo‐controlled, had at least one abatacept arm and double‐blinded period of 3 to 12 months to evaluate the safety profile of abatacept versus placebo. All clinical trials sponsored by Bristol‐Myers Squibb prior to June 2016 that met the aforementioned criteria were included in this integrated analysis. Early phase, pharmacokinetic, and country‐specific studies were excluded from consideration as they would not be representative of the global RA population. Background therapy was permitted in all arms. The permitted background therapy, duration of double‐blind treatment, and sample size for each study are outlined in Table 1 3, 4, 7, 9, 12, 14, 26, 27, 28. Other background therapy, excluding glucocorticoids if applicable, was stopped at study entry. Inclusion and exclusion criteria were largely consistent across all included studies (Supplementary Appendix: Inclusion and Exclusion Criteria).The start of the period was defined as the day of the first dose of study medication. All patients who received 1 or more doses of abatacept or placebo in the controlled period (3 to 12 months) were included in the safety analysis. Patients randomized to the infliximab arm in the Abatacept or infliximab versus placebo, a Trial for Tolerability, Efficacy and Safety in Treating RA (ATTEST) trial (n = 165) were excluded from this analysis of the safety of abatacept versus placebo.
Table 1.
Randomized, double‐blind trials of abatacept in patients with rheumatoid arthritis included in the analysis
| Study | Patient Population (All: Adults with Active RA) | Intervention | Duration of Double‐Blinded Period (Months) | Patients (n) | |
|---|---|---|---|---|---|
| Abatacept | Placebo | ||||
| AGREE (NCT00122382) 27 | Early,a erosive, MTX‐naïve | IV ABA or PBO; +bg MTX | 12 | 256 | 253 |
| AVERT (NCT01142726) 26 | Early,a MTX‐ and biologic‐naïve | SC ABA, SC ABA+MTX or PBO+MTX | 12 | ABA: 116; ABA+MTX: 119 | 116 |
| ATTEST (NCT00095147) 12, b | Inadequate response to MTX | IV ABA, IFX or PBO; +bg MTX | 6 | 156 | 110 |
| AIM (NCT00048568) 4 | Inadequate response to MTX | IV ABA or PBO; +bg MTX | 12 | 433 | 219 |
| IM101100 (NCT00162266) 3 | Inadequate response to MTX | IV ABA or PBO; +bg MTX | 12 | 220 | 119 |
| IM101063 (NCT00254293) 28 | With bg DMARD | SC ABA or PBO; +bg DMARD | 3 | 51 | 17 |
| ATTAIN (NCT00048581) 9 | Inadequate response to MTX | IV ABA or PBO; +bg DMARDs | 6 | 258 | 133 |
| ASSURE (NCT00048932) 7 | With bg DMARDs and/or biologics | IV ABA or PBO; +bg RA therapy | 12 | 959 | 482 |
| IM101101 (NCT00162279) 14 | Inadequate response to etanercept | IV ABA or PBO; +bg etanercept | 12 | 85 | 36 |
Abbreviation: ABA, abatacept; AIM, Abatacept in Inadequate responders to Methotrexate; AGREE, Abatacept study to Gauge Remission and joint damage progression in methotrexate‐naïve patients with Early Erosive Rheumatoid Arthritis; ASSURE, Abatacept Study of Safety in Use with other RA therapies; ATTAIN, Abatacept Trial in Treatment of Anti‐TNF Inadequate responders; ATTEST, Abatacept or infliximab versus placebo, a Trial for Tolerability, Efficacy and Safety in Treating RA; AVERT, Assessing Very Early Rheumatoid arthritis Treatment; bg, background; CCP2, cyclic citrullinated peptide‐2; DAS28 (CRP), Disease Activity Score 28 (C‐reactive protein); DMARD, disease‐modifying antirheumatic drug; IFX, infliximab; IV, intravenous; MTX, methotrexate; PBO, placebo; RA, rheumatoid arthritis; SC, subcutaneous.
aEarly RA: disease duration ≤2 years, DAS28 (CRP) ≥3.2 at study entry; anti‐CCP2 positive. bPatients treated with infliximab were not included in this analysis (n = 165).
Study assessments
All studies and their safety evaluations were carried out and reported in accordance with the Declaration of Helsinki and were consistent with International Conference on Harmonisation Good Clinical Practice guidelines 29. All patients were monitored for the occurrence of AEs, serious AEs (SAEs), AEs leading to discontinuation of study treatment, and deaths. Additional details were collected on prespecified AEs of special interest (such as infections, malignancies, and autoimmune diseases). Events were classified using the version of the Medical Dictionary for Regulatory Activities classification (MedDRA) that was current at the time of each study. For the purpose of this research, events were reclassified using the current MedDRA version at the time of this integrated analysis (version 19.0) 30.
Statistics
Baseline characteristics were reported descriptively as either mean (SD) or n (%). Frequencies of AE occurrences were calculated based on the number of patients with one or multiple events (patients with multiple occurrences of the same AE were only counted once for that AE). IRs were calculated for each AE, and pys of exposure were censored at the time of the first event occurrence. The IR of a safety event was calculated as the number of patients with at least one event per 100 py of exposure with 95% confidence interval (CI). AEs were assumed to follow a Poisson distribution. Summary statistics are presented by treatment group (abatacept or placebo) for all abatacept‐treated patients combined, regardless of dose levels or formulation. Duration of exposure to abatacept or placebo was defined as the number of days from the start of therapy to the day of treatment cessation at the end of the double‐blind period (or early discontinuation) plus 56 (or 60 for phase II and phase IV trials) days (approximately 4 half‐lives of abatacept in humans).
Results
In total, 2653 patients received abatacept and 1485 received placebo. The groups were well matched for demographics and disease characteristics at baseline (Table 2). Concomitant medications at day 1 of the treatment periods were also similar between the abatacept and placebo groups, including nonsteroidal anti‐inflammatory drug, methotrexate, oral glucocorticoid, and antitumor necrosis factor use.
Table 2.
Baseline patient demographics and disease characteristics
| Abatacept, n = 2653 | Placebo, n = 1485 | |||
|---|---|---|---|---|
| Value | % of patients with missing data | Value | % of patients with missing data | |
| Patient demographics | ||||
| Age, years | 52 (12) | 0 | 51 (12) | 0 |
| Weight, kg | 74 (19) | 0.1 | 74 (19) | <0.1 |
| Female, n (%) | 2099 (79.1) | 0 | 1184 (79.7) | 0 |
| White, n (%) | 2283 (86.1) | 0.2 | 1285 (86.5) | 0.2 |
| Durations of exposure, mon | 10.8 (3.3) | 0 | 10.3 (3.5) | 0 |
| Disease characteristics | ||||
| Disease duration, yr | 8.1 (8.5) | 2.9 | 7.5 (8.5) | 3.1 |
| hsCRP, mg/L | 26 (30) | 3.2 | 27 (34) | 3.4 |
| Tender joint count (28) | 30 (14) | 39.0 | 30 (14) | 35.4 |
| Swollen joint count (28) | 21 (10) | 39.0 | 21 (10) | 35.4 |
| HAQ‐DI | 1.5 (0.7) | 3.5 | 1.6 (0.7) | 3.9 |
| Patient pain (0‐100 VAS)b | 63 (21) | 16.6 | 63 (21) | 21.0 |
| Concomitant medications | ||||
| NSAIDs, n (%) | 2096 (79.0) | NA | 1182 (79.6) | NA |
| Oral glucocorticoids, n (%) | 1360 (51.3) | NA | 733 (49.4) | NA |
| Oral dose, mg | 7.2 (3.6) | 7.3 (3.3) | ||
| MTX, n (%) | 1800 (67.8) | NA | 936 (63.0) | NA |
| Anti‐TNF, n (%)c | 164 (6.2) | NA | 74 (5.0) | NA |
Abbreviation: HAQ‐DI, health assessment questionnaire–disability index; hsCRP, high‐sensitivity C‐reactive protein; MTX, methotrexate; NA, not available; NSAID, nonsteroidal anti‐inflammatory drug; TNF, tumor necrosis factor; VAS, visual analog scale.
aAll values are mean (SD) unless otherwise stated. bVAS: 0=no pain and 100=worst possible pain. cIn study IM101101 patients in both study arms could receive etanercept concomitantly.
Mean (SD) durations of exposure to abatacept were 10.8 (3.3) months with a total of 2357 py of exposure versus the placebo duration of 10.3 (3.5) months with a total of 1254 py of exposure.
The overall AE profiles, including AEs, SAEs, and deaths, were comparable between groups (Table 3). In the abatacept group, 2334 (88%) patients reported at least one AE (IR: 341 [95% CI: 328, 356] per 100 py), compared with 1258 (85%) patients in the placebo group (IR: 326 [95% CI: 309, 345] per 100 py). Most AEs with abatacept were mild (27%) or moderate (46%) in intensity, similar to the observations in the placebo group (mild, 25%; moderate, 46%). The most common AEs in both treatment groups were headache, upper respiratory tract infection, nasopharyngitis, and nausea (Figure 1). Autoimmune diseases and chronic obstructive pulmonary disease occurred at low and comparable frequencies in both abatacept and placebo groups (Table 3).
Table 3.
Summary of all adverse events (AEs) during the double‐blind, placebo‐controlled perioda
| Outcome | Abatacept, n = 2653 | Placebo, n = 1485 | ||
|---|---|---|---|---|
| n (%) | IR/100 py (95% CI) | n (%) | IR/100 py (95% CI) | |
| AEs | 2334 (88.0) | 341.3 (327.6, 355.5) | 1258 (84.7) | 326.3 (308.5, 344.9) |
| SAEsb | 331 (12.5) | 14.8 (13.3, 16.5) | 174 (11.7) | 14.6 (12.5, 17.0) |
| Deathsc | 12 (0.4) | 0.5 (0.3, 0.9) | 12 (0.8) | 1.0 (0.5, 1.7) |
| Malignancies | 31 (1.2) | 1.3 (0.9, 1.9) | 14 (0.9) | 1.1 (0.6, 1.9) |
| Infections | 1440 (54.3) | 93.2 (88.5, 98.2) | 767 (51.6) | 93.1 (86.6, 99.9) |
| Serious infections and infestations | 70 (2.6) | 3.0 (2.3, 3.8) | 28 (1.9) | 2.3 (1.5, 3.3) |
| Opportunistic infections | 4 (0.2) | 0.2 (0.1, 0.4) | 7 (0.5) | 0.6 (0.2, 1.2) |
| Autoimmune diseases | 198 (7.5) | 8.8 (7.6, 10.1) | 115 (7.7) | 9.6 (7.9, 11.5) |
| COPD | 13 (0.5) | 0.6 (0.3, 1.0) | 2 (0.1) | 0.2 (0.0, 0.6) |
Abbreviation: AE, adverse event; CI, confidence interval; COPD, chronic obstructive pulmonary disease; IR, incidence rate; py, patient‐years; SAE, serious adverse event.
aIncludes data up to 56 (phase III) or 60 (phase II, IV) days after the last dose in the short‐term, double‐blind period or up to the start of the open‐label period, whichever occurred first. Patients may experience more than 1 type of event. bIncludes hospitalizations for elective surgical procedures. cPatients could have more than 1 AE that resulted in death.
Figure 1.
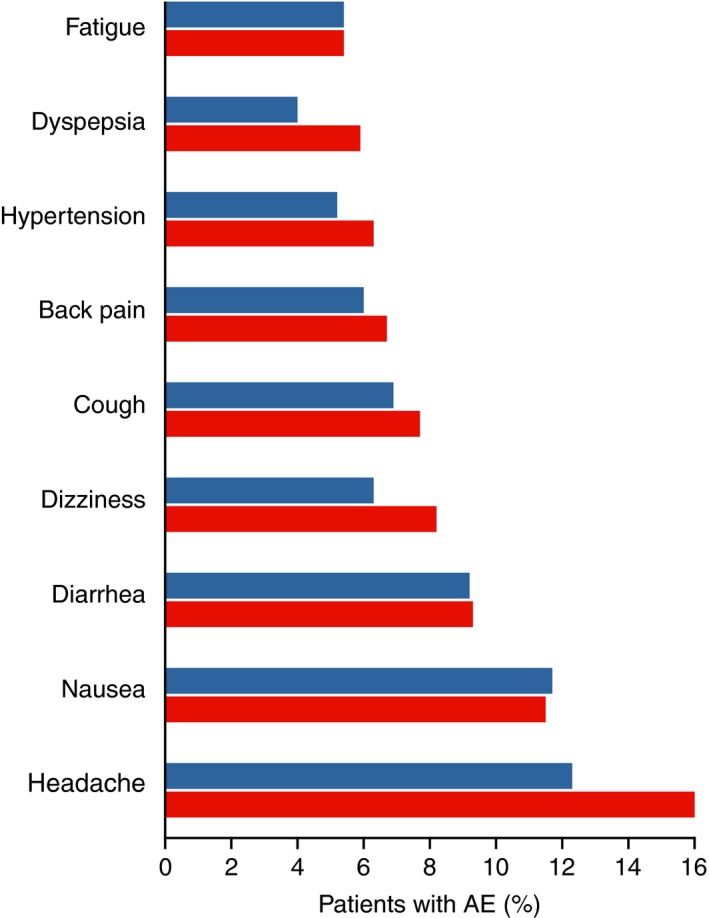
Most frequent adverse events (AEs) (in at least 5% of patients in any treatment group, excluding infections). Red bars represent the abatacept group and blue bars represent the placebo group. Includes data up to 56 (phase III) or 60 (phase II, IV) days after the last dose in the short‐term, double‐blind period or up to the start of the open‐label period, whichever occurred first. Patients may experience more than one type of event.
In the abatacept group, 331 (13%) patients reported SAEs (IR: 15 [95% CI: 13, 17] per 100 py), compared with 174 (12%; 15 [13, 17] per 100 py) in the placebo group. The most frequently reported SAEs (0.4% or greater) in the abatacept group were pneumonia (n = 16 [0.6%]), basal cell carcinoma (n = 12 [0.5%]), and chest pain (n = 11 [0.4%]). In the placebo group, these were pneumonia (n = 9 [0.6%]), osteoarthritis (n = 8 [0.5%]), and congestive cardiac failure (n = 6 [0.4%]). A total of 138 (5.2%) abatacept versus 55 (3.7%) placebo patients discontinued because of AEs; of these, 68 (2.6%) versus 22 (1.5%) were due to SAEs, respectively. All individual types of AE resulting in discontinuation occurred at frequencies at or less than 0.2% in the abatacept and placebo groups.
The abatacept group reported 31 (1.2%) patients with at least one occurrence of malignancy (incidence rate [IR]: 1.32 [95% CI: 0.90, 1.87] per 100 py) compared with 14 (0.9%; IR: 1.12 [95% CI: 0.61, 1.88] per 100 py) in the placebo group (Supplementary Table 2). Solid organ tumor was the most frequently observed malignancy in both groups followed by nonmelanoma skin cancer.
A total of 1440 (54%) patients in the abatacept group reported infections and infestations (IR: 93 [95% CI: 88, 98] per 100 py) compared with 767 (52%; IR: 93 [95% CI: 87, 100] per 100 py) in the placebo group. The most frequent infections in both groups were upper respiratory tract infection, nasopharyngitis, bronchitis, urinary tract infection, sinusitis, and influenza (Table 4).
Table 4.
Incidence rates of the most frequent infections with IR ≥5.0/100 pya
| Infections and Infestations | Abatacept, n = 2653 | Placebo: n = 1485 | ||
|---|---|---|---|---|
| n (%) | IR/100 py (95% CI) | n (%) | IR/100 py (95% CI) | |
| Upper respiratory tract infection | 316.0 (11.9) | 14.3 (12.8, 16.0) | 180.0 (12.1) | 15.4 (13.22, 17.81) |
| Nasopharyngitis | 313.0 (11.8) | 14.3 (12.8, 16.0) | 149.0 (10.0) | 12.7 (10.7, 14.9) |
| Bronchitis | 173.0 (6.5) | 7.6 (6.5, 8.8) | 86.0 (5.8) | 7.1 (5.7, 8.8) |
| Urinary tract infection | 171.0 (6.4) | 7.5 (6.5, 8.8) | 91.0 (6.1) | 7.5 (6.1, 9.2) |
| Sinusitis | 159.0 (6.0) | 7.0 (5.9, 8.1) | 88.0 (5.9) | 7.2 (5.8, 8.9) |
| Influenza | 154.0 (5.8) | 6.8 (5.7, 7.9) | 87.0 (5.9) | 7.2 (5.8, 8.9) |
Abbreviation: CI, confidence interval; IR, incidence rate; py, patient‐years.
Includes data up to 56 (phase III) or 60 (phase II, IV) days after the last dose in the short term, double‐blind period or up to the start of the open‐label period, whichever occurred first. Patients could have more than one infection.
Opportunistic infections were reported in four patients in the abatacept group (IR: 0.17 [95% CI: 0.05, 0.43] per 100 py) compared with seven patients in the placebo group (IR: 0.56 [95% CI: 0.22, 1.15] per 100 py). The opportunistic infections in the abatacept group included bronchopulmonary aspergillosis, fungal eye infection, pseudomonal pneumonia, and tuberculosis (all n = 1; IR: 0.04 [95% CI: 0.00, 0.24] per 100 py) and those in the placebo group included fungal esophagitis, gastrointestinal candidiasis, cryptococcal meningitis, esophageal candidiasis, Pneumocystis jirovecii pneumonia, respiratory moniliasis, and tuberculosis (all n = 1; IR: 0.08 [95% CI: 0.00, 0.44] per 100 py). Herpes zoster was reported in 40 patients (1.5%; IR: 1.71 [95% CI: 1.22, 2.33] per 100 py) in the abatacept group compared with 21 (1.4%; IR: 1.69 [95% CI: 1.05, 2.58] per 100 py) patients in the placebo group.
Both groups reported 12 deaths (0.4% and 0.8% for abatacept and placebo, respectively). The most common cause of death in both groups (Supplementary Table 3) was cardiac disorders.
Discussion
In this integrated analysis of nine placebo‐controlled clinical trials in 2653 patients who received abatacept (2357 py of exposure) and 1485 who received placebo (1254 py of exposure), abatacept was well tolerated with no new safety signals identified. Despite the large amount of real‐world observational data available, randomized, controlled trials remain the gold standard for investigating the safety and efficacy of drugs, and as such, it is important to monitor and communicate safety information generated from clinical trials. Although previous integrated analyses have reported the safety of abatacept separately from IV and SC trials 22, 23, this is the first study to pool data from both IV and SC placebo‐controlled trials as well as to include data from the active comparator‐controlled Abatacept study to Gauge Remission and joint damage progression in methotrexate‐naïve patients with Early Erosive rheumatoid arthritis (AGREE) and Assessing Very Early Rheumatoid arthritis Treatment (AVERT) trials 26, 27.
The increased risk of comorbidities among patients with RA, particularly in cardiac and lung disorders, is of interest because of the autoimmune pathogenesis of RA 31, 32. Previous reports have suggested that the use of biologic agents for the treatment of RA can increase the risk of infections, malignancy, and autoimmune events 33. However, results from this pooled analysis of abatacept treatment are consistent with previous integrated safety analyses of IV and SC abatacept studies, which show no such increases compared with placebo 23.
Furthermore, both deaths and discontinuation were similar between abatacept and placebo. The IRs for the causes of death in abatacept‐treated patients are consistent with previous safety analyses of both IV and SC abatacept treatment 34, 35. The most common causes of death in this analysis were cardiac disorders, followed by infections and infestations. Discontinuation of therapy occurred at low frequency with no identified common reason. Of note, the IRs for both death and discontinuation were numerically higher in the placebo group.
Patients with RA have an increased risk of developing cardiac disorders (including heart failure and coronary artery disease) compared with the general population 32, 36. This analysis confirms findings from previous individual trials, which determined that abatacept does not exacerbate the risk of cardiac disorders in patients with RA who are on a variety of background therapies 4, 7, 14.
Previous reports from meta‐analyses, registry, and database studies also show an increased risk of malignancy in patients with RA compared with the overall population 31, 37, 38, 39. In this analysis, the most frequently reported malignancies were solid organ tumor and nonmelanoma skin cancer in both the abatacept and placebo groups, with similar low IRs (1.11% or less) in the two groups. A higher prevalence of nonmelanoma skin cancers in patients with RA compared with the overall population has been previously reported 39. Although reassuring, these findings should be considered with limitations of the short‐term study period (double‐blind period of 3 to 12 months) and the long latency of cancer.
Increased risk of serious infection has been reported in patients with RA compared with the general population and with the use of standard‐dose biologics 16, 40, 41. Historically, however, abatacept has been associated with a lower rate of infection compared with other biologic agents 42. In this analysis, the IR of infections was comparable between the abatacept and placebo groups. This is consistent with the findings of a meta‐analysis of serious infections in patients with RA, which demonstrated that abatacept did not increase the risk of serious infections 43.
In this analysis, the IRs for tuberculosis and herpes zoster were very low and comparable in both the abatacept and placebo groups. This is consistent with the low risk of tuberculosis in patients with RA overall, and illustrates that increased tuberculosis risk has not been observed with abatacept treatment 44. Similarly, IRs of herpes zoster in the abatacept and placebo groups (1.71/100 py and 1.69/100 py, respectively) are consistent with findings from a retrospective analysis of a large cohort of patients with RA in which the IRs of herpes zoster were 1.61/100 py‐2.45/100 py with no statistically significant difference among patients who received biologic agents with different mechanisms of action, including abatacept 45. Taken together, the findings support that abatacept does not pose any further increased risk of tuberculosis or herpes zoster compared with placebo.
In conclusion, in this large integrated database of nine placebo‐controlled abatacept clinical trials in RA, safety events, including deaths, SAEs, infections, opportunistic infections, malignancies, and autoimmune diseases, occurred at similar frequencies and rates in the abatacept and placebo groups. No new or unexpected safety concerns were identified. The findings from this integrated pooled analysis of IV and SC abatacept add robust confirmation of the safety profile of abatacept.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Teresa A. Simon and Maarten Boers had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Hochberg, Torbeyns, Banerjee.
Acquisition of data. Soule.
Analysis and interpretation of data. all authors.
Supporting information
Acknowledgments
Professional medical writing and editorial assistance was provided by Natalie Griffiths, PhD, and Bu Reinen, PhD, at Caudex. Bristol‐Myers Squibb's policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html
The work herein was supported by Bristol‐Myers Squibb.
Teresa A. Simon, MPH, Benjamin P. Soule, MD, Douglas Fleming MD, Subhashis Banerjee, MD: Bristol‐Myers Squibb, Princeton, New Jersey; 2Marc Hochberg, MD, MPH: University of Maryland, Baltimore; 3Anne Torbeyns, PhD: Bristol‐Myers Squibb, Braine‐l'Alleud, Belgium; 4Maarten Boers, MD, PhD: Amsterdam University Medical Centers, Vrije Universiteit, Amsterdam, Netherlands
Teresa A. Simon is a former employee of Bristol‐Myers Squibb. Benjamin Soule, Douglas Fleming, Anne Torbeyns, and Subhashis Banerjee are shareholders and employees of Bristol‐Myers Squibb. Marc Hochberg is a shareholder of Theralogix LLC, has received grant/research support (<$10,000) from NIH, and is a consultant for Bristol‐Myers Squibb, EMD Serono, Genentech/Roche, Novartis, Pfizer, and UCB. Maarten Boers is a consultant for Bristol‐Myers Squibb and has received consulting fees (<$10,000) from Pfizer, UCB, and Teva in the past 2 years. No other disclosures relevant to this article were reported.
References
- 1. Bristol‐Myers Squibb . Orencia (abatacept) prescribing information. 2017. URL: http://packageinserts.bms.com/pi/pi_orencia.pdf
- 2. Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. N Engl J Med 2003;349:1907–15. [DOI] [PubMed] [Google Scholar]
- 3. Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve‐month results of a phase IIb, double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005;52:2263–71. [DOI] [PubMed] [Google Scholar]
- 4. Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006;144:865–76. [DOI] [PubMed] [Google Scholar]
- 5. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 6. Ruperto N, Lovell DJ, Tzaribachez N, Vega‐Cornejo G, Louw I, Berman A, et al. Subcutaneous abatacept in patients with polyarticular juvenile idiopathic arthritis and inadequate response to biologic or non‐biologic disease‐modifying antirheumatic drugs: pharmacokinetics, efficacy and safety. Ann Rheum Dis 2016;75:138. [Google Scholar]
- 7. Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease‐modifying antirheumatic drugs: a one‐year randomized, placebo‐controlled study. Arthritis Rheum 2006;54:2807–16. [DOI] [PubMed] [Google Scholar]
- 8. Westhovens R, Kremer JM, Emery P, Russell AS, Alten R, Barré E, et al. Long‐term safety and efficacy of abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: a 7‐year extended study. Clin Exp Rheumatol 2014;32:553–62. [PubMed] [Google Scholar]
- 9. Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med 2005;353:1114–23. [DOI] [PubMed] [Google Scholar]
- 10. Genovese MC, Schiff M, Luggen M, Le Bars M, Aranda R, Elegbe A, et al. Longterm safety and efficacy of abatacept through 5 years of treatment in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor inhibitor therapy. J Rheumatol 2012;39:1546–54. [DOI] [PubMed] [Google Scholar]
- 11. Kremer JM, Russell AS, Emery P, Abud‐Mendoza C, Szechinski J, Westhovens R, et al. Long‐term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3‐year results from the AIM trial. Ann Rheum Dis 2011;70:1826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2008;67:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiff M, Pritchard C, Huffstutter JE, Rodriguez‐Valverde V, Durez P, Zhou X, et al. The 6‐month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti‐tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis 2009;68:1708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis 2007;66:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004;38:1261–5. [DOI] [PubMed] [Google Scholar]
- 16. Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 2008;35:387–93. [PubMed] [Google Scholar]
- 17. Ramos‐Casals M, Brito‐Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF‐targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86:242–51. [DOI] [PubMed] [Google Scholar]
- 18. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85. [DOI] [PubMed] [Google Scholar]
- 19. Dore RK, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A, et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin Exp Rheumatol 2007;25:40–6. [PubMed] [Google Scholar]
- 20. Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 2009;68:1739–45. [DOI] [PubMed] [Google Scholar]
- 21. Curtis JR, Hobar C, Hansbrough K. Injection‐site burning and stinging in patients with rheumatoid arthritis using injectable biologics. Curr Med Res Opin 2011;27:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alten R, Kaine J, Keystone E, Nash P, Delaet I, Genovese MC. Long‐term safety of subcutaneous abatacept in rheumatoid arthritis: integrated analysis of clinical trial data representing more than four years of treatment. Arthritis Rheumatol 2014;66:1987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weinblatt ME, Moreland LW, Westhovens R, Cohen RB, Kelly SM, Khan N, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol 2013;40:787–97. [DOI] [PubMed] [Google Scholar]
- 24. Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheumatol 2011;63:2854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corbo M, Valencia X, Raymond R, Summerill R, Agrawal S, Shergy W, et al. A subcutaneous administration regimen for abatacept in patients with rheumatoid arthritis: pharmacokinetics, safety and immunogenicity [abstract]. Arthritis Rheum 2008;58:S307. [Google Scholar]
- 26. Emery P, Burmester GR, Bykerk VP, Combe BG, Furst DE, Barré E, et al. Evaluating drug‐free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active‐controlled AVERT study of 24 months, with a 12‐month, double‐blind treatment period. Ann Rheum Dis 2015;74:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate‐naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009;68:1870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corbo M, Valencia X, Raymond R, Summerill R, Agrawal S, Townsend R, et al. Subcutaneous administration of abatacept in patients with rheumatoid arthritis: pharmacokinetics, safety and immunogenicity. Ann Rheum Dis 2009;68:S574. [Google Scholar]
- 29. ICH Harmonised Tripartite Guideline . Guideline for Good Clinical Practice. J Postgrad Med 2001;47:199–203. [PubMed] [Google Scholar]
- 30. The Medical Dictionary for Regulatory Activities (MedDRA) . International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use 2016. URL: https://www.meddra.org/sites/default/files/guidance/file/intguide_19_0_english.pdf
- 31. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta‐analysis. Arthritis Res Ther 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 2006;65:1608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goekoop‐Ruiterman YP, de Vries‐Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 34. Wells AF, Jodat N, Schiff M. A critical evaluation of the role of subcutaneous abatacept in the treatment of rheumatoid arthritis: patient considerations. Biologics 2014;8:41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alten R, Kaine J, Keystone EC, Nash P, Delaet I, Qi K, et al. Safety of subcutaneous abatacept in patients with rheumatoid arthritis (RA): Integrated analysis of five clinical trials up to 4.5 years. Ann Rheum Dis 2011;70 (Suppl 3):617. [Google Scholar]
- 36. Nicola PJ, Maradit‐Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population‐based study over 46 years. Arthritis Rheum 2005;52:412–20. [DOI] [PubMed] [Google Scholar]
- 37. Hemminki K, Li X, Sundquist K, Sundquist J. Cancer risk in hospitalized rheumatoid arthritis patients. Rheumatology (Oxford) 2008;47:698–701. [DOI] [PubMed] [Google Scholar]
- 38. Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta‐analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther 2008;10:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mercer LK, Green AC, Galloway JB, Davies R, Lunt M, Dixon WG, et al. The influence of anti‐TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2012;71:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta‐analysis. Clin Infect Dis 2014;58:1649–57. [DOI] [PubMed] [Google Scholar]
- 41. Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta‐analysis. Lancet 2015;386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yun H, Xie F, Delzell E, Levitan EB, Chen L, Lewis JD, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in medicare. Arthritis Rheumatol 2016;68:56–66. [DOI] [PubMed] [Google Scholar]
- 43. Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta‐analyses of randomised placebo‐controlled trials. Ann Rheum Dis 2009;68:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arkema EV, Jonsson J, Baecklund E, Bruchfeld J, Feltelius N, Askling J. Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatments? Ann Rheum Dis 2015;74:1212–7. [DOI] [PubMed] [Google Scholar]
- 45. Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease‐modifying therapy. Arthritis Care Res (Hoboken) 2015;67:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


