Abstract
Introduction
The surviving opioid overdose with naloxone education and resuscitation (SOONER) project uses co-design and trial methods to develop and evaluate a point-of-care overdose education and naloxone distribution (OEND) tool. We plan to conduct a randomised controlled trial to assess the effectiveness of our OEND tool in comparison with best available standard of care by observing participants’ performance as a responder to a simulated overdose. Recruiting and retaining people at risk of or likely to witness opioid overdose raises scientific, logistical and bioethical challenges. A feasibility study is needed to establish the effectiveness of recruitment and retention strategies and acceptability of study procedures prior to launching the full trial.
Methods and analysis
Strategies to enhance recruitment include candidate-driven recruitment, verbal informed consent, and attractive, destigmatising materials. Adults at risk of or likely to witness opioid overdose will be recruited through an urban emergency department, inpatient and ambulatory addiction medicine service, and outpatient family practice settings. Participants randomised to the intervention arm will receive our OEND intervention; those in the control arm will be referred to existing OEND programme. Retention procedures include participant reminders, flexible scheduling, cash and comfort compensation, and strategies to maintain a consistent relationship between individual study staff and participants. Within 2 weeks following recruitment, participants will engage as a responder to a manikin-simulated overdose, and complete overdose knowledge and attitudes questionnaires. The primary outcome is recruitment and retention feasibility, defined as the recruitment of 28 participants within 28 days of recruitment and <50% attrition at the overdose simulation. Staff and participant feedback will also be collected and considered.
Ethics and dissemination
The study has been reviewed by ethics boards at St. Michael’s Hospital, Toronto Public Health and the University of Toronto. Dissemination will occur through peer-reviewed publication and presentations.
Trial registration number
ClinicalTrials.gov registry (NCT03821649).
Keywords: feasibility study, randomised control trial, trial protocol, design for health, recruitment, retention, harm reduction, opioid overdose, overdose education, naloxone distribution, addiction medicine, family medicine, emergency medicine, first aid
Strengths and limitations of this study.
This project’s main strength is the use of a feasibility study to assess and refine a recruitment and retention strategy among people who are at risk of or likely to witness opioid overdose prior to initiating a randomised controlled trial.
The strengths of the proposed recruitment and retentions strategy include cash and comfort compensation for participation, follow-up through multiple communications media, flexible scheduling for follow-up assessments, verbal consent processes and attention to destigmatising language in research processes.
The study’s central limitation is that the proposed recruitment and retention strategy may require adaptation for use in other settings.
Background
Deaths from opioid overdose represent an important and expanding global epidemic.1 Opioid overdose education and naloxone distribution (OEND) programmes train and equip people who are likely to witness overdose to recognise these emergencies and administer essential first aid interventions including naloxone, a widely known and effective competitive opioid antagonist.2–4 Policymakers and practitioners have called for expanded access to OEND programme in clinical settings or ‘point-of-care OEND’. Point-of-care OEND would improve access to this potentially life-saving intervention, and may have a role in emergency departments, family practice, addiction medicine and other inpatient and ambulatory care settings. Although clinicians are willing to provide OEND in principle, the complexity, time requirements for training and current design of naloxone kits remain a barrier to widespread implementation. Effective tools are a prerequisite for widespread OEND implementation in a variety of ambulatory and inpatient care settings.5–8
We plan to conduct a randomised control trial to assess the effectiveness of a point-of-care OEND intervention in comparison to the current standard of care in an emergency department, family medicine and addictions medicine settings, by observing participants’ performance in a simulated overdose emergency.
Conducting trials among people who use drugs or who are likely to witness overdose involves scientific, logistical, sociocultural and bioethical challenges. These challenges contribute to the persistent underevaluation of interventions to enhance the health of this population, and threats to study validity when retention rates are low.9 There is also limited precedent for conducting resuscitation simulations for research participants who are patients or members of the lay public rather than healthcare trainees.10 Most OEND research and programme evaluations involve uncontrolled studies and convenience sampling without active follow-up, with elevated rates of attrition.2 11 The only published simulation-based study of OEND education is an uncontrolled study among 103 people recently released from prison. The study achieved 82.5% retention (85 participants) at a 1-month follow-up simulation.10
Before conducting a full-scale point-of-care OEND trial involving overdose simulations, a feasibility study is needed to establish the effectiveness of our planned recruitment and retention strategies and the acceptability of study procedures in local recruitment sites. A feasibility study will permit the evaluation of randomisation and data collection procedures, and create an opportunity to reconsider study design and analysis.
Study objectives
The primary objective of this feasibility study is to identify if an integrated participant recruitment and retention strategy can recruit approximately28 eligible participants within 28 days of recruitment, and maintain <50% attrition at the study’s primary 2-week outcome assessment. This is in the context of a randomised trial on point-of-care OEND and simulated overdose resuscitation performance in urban and inner-city academic family practice, emergency department and addiction medicine settings.
The secondary objectives of this study are to
Assess the rate of participant recruitment in each of the family practice, emergency department and addiction medicine sites at a single academic healthcare centre.
Compare participant retention rates in the study intervention and control arms.
Describe challenges and opportunities for improving study procedures for participants, study staff and site staff with respect to all study processes including participant recruitment, randomisation, implementation of the intervention and control, retention, follow-up, outcome assessment and data collection.
Methods
The SOONER project
This feasibility study is part of the larger Surviving Opioid Overdose with Naloxone Education and Resuscitation (SOONER) Project, which combines co-design, clinical trial and community engagement methods (www.soonerproject.ca). The goal of the SOONER Project is to develop and evaluate an effective point-of-care OEND tool, and to reduce opioid-related stigma and inequity. The SOONER Project consists of three phases: phase I is a service design and participatory co-design initiative, where scientists, design researchers and community members cocreated a point-of-care OEND toolkit12 13 which will be evaluated in subsequent phases. Phase II is the feasibility study presented in this protocol, and phase III is the subsequent randomised trial that will be developed based on the results of this feasibility study.
Patient and public involvement
Drawing on principles of community engagement and participatory research, community agencies and representatives with lived experience of opioid use and overdose are involved in all aspects of the SOONER Project’s development and implementation.14 15 A group of community representatives are also engaged as ad hoc members of the study’s steering committee, to refine the study research questions and measures and assess the appropriateness of study procedures and interventions. A summary of study results will be disseminated to participants, community agencies and representatives and made available through open access publication.
Feasibility trial design
The proposed study is a mixed-methods feasibility study to evaluate the recruitment and retention strategy and study logistics for a randomised trial.16 The underlying randomised trial is a pragmatic, multisite, two-armed, parallel-group, best-available-care controlled, analyst-blinded and outcome assessor-blinded, superiority trial of point-of-care OEND training. The study protocol was developed as a feasibility study using the Standard Protocol Items: Recommendations for Interventional Trials statement and recommendations on standard elements for protocols for interventional trials, adapted where necessary for a feasibility study.17 The protocol is registered prospectively through ClinicalTrials.gov. Recruitment is anticipated to launch in January 2019.
Participants
Participants will be recruited through three study settings:
Emergency department: the St. Michael’s Hospital Emergency Department.
Family practice: both the St. Michael’s Academic Family Health Team and St. Michael’s-affiliated Inner City Family Health Team.
Addiction medicine: the St. Michael’s Hospital Addiction Medicine Service, including both inpatient and ambulatory services.
Primary outcome assessment will occur through a follow-up visit at the St. Michael’s Hospital Allan Waters Family Simulation Centre (SMH Simulation Centre). Although the three recruitment settings are all affiliated with the same hospital, the trial is termed ‘multi-site’ because of the substantial difference between the clinical contexts in the three recruitment settings.
The three recruitment settings provide routine clinical services to people at risk of opioid overdose and likely to witness overdose, but each with widely differing clinical interactions and follow-up procedures. These sites have been selected to strike a balance between study generalisability, pragmatism and feasibility.18 The chosen settings will permit recruitment of study participants representing a diverse urban population, with varied access to and use of emergency services, primary care and addiction treatment services.
Eligibility
Participants will be adults ≥16 years of age who may benefit from OEND using criteria adapted from the 2015 American Heart Association Guidelines on Cardiopulmonary Resuscitation in Special Circumstances and WHO Guidelines.19 20 (See table 1 for detailed inclusion and exclusion criteria.)
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria: participants are eligible by meeting any one or more of the following: | Exclusion criteria: participants are ineligible by meeting any one or more of the following: |
|
|
Sixteen was chosen as the minimum participant age to (1) recognise that opioid use is a growing concern among adolescents, (2) affirm the importance of including youth in low-risk research where this population stands to benefit, (3) recognise that other basic life support studies have been conducted in children, while avoiding perceptions that the study extends to research with highly vulnerable populations if a lower minimum age were chosen.21-22 23
Since the study concerns resuscitation and first aid training, people with a ‘Do Not Resuscitate’ order or directive are excluded because such an order may reasonably alter a participant’s interest in or desire to learn resuscitative skills. For candidates who decline to participate in the study, we will retain the data collected in the recruitment questionnaire and request consent to collect demographic data to compare the characteristics of study participants and non-participants.
Sample size
Based on our budget and timelines for the proposed randomised control trial, we determined that a minimum recruitment rate of one participant per day of active recruitment and a minimum retention rate of 50% are required for the underlying RCT to be logistically feasible and scientifically acceptable. For logistical and budgetary reasons, we prepared to operate 28 days of participant recruitment. Therefore, we plan to recruit approximately 28 participants to the feasibility study, composed of between 8 and 12 participants from each of emergency department, family medicine and addictions medicine sites. Study investigators working at each of the study sites confirmed that many more than one eligible patient candidate present to each of the recruitment sites per day. Given that both patients and visitors to the study sites are eligible for recruitment, we therefore conclude that a recruitment rate of one participant per day is a viable target.
To exclude retention rates <50%, we computed a CI for the binomial distribution based on 28 participants. With a sample of 28 enrolled participants, we will be able to estimate a retention rate of 65% with a one-sided 95% CI of 14.8%:
Therefore, if at least 19 participants are retained we will be able to assert that any retention rate <50% falls outside a 95% CI for the retention rate point estimate. In a worst-case-yet-feasible scenario, the feasibility trial would therefore require 28 days of recruitment and would observe a retention rate of 65%.
Allocation
Participants will be assigned to either control or intervention group with 1:2 allocation by computerised randomisation schedule. Unbalanced allocation was selected for the feasibility study to gather additional information about study processes in the intervention arm, since the control arm incorporates existing processes of care.
In instances where eligible and consenting participants present as a part of a single clinical encounter (eg, a patient at risk of overdose presenting to the emergency department with his/her spouse), both participants will be randomised to the same study arm to avoid overt contamination between intervention and control arms. Randomisation will be stratified by site, using permuted blocks of random sizes. Block sizes will not be disclosed to ensure concealment.
Interventions
Study participants randomised to the treatment arm of the study will receive brief overdose first aid training and a naloxone kit. The intervention will involve the following:
-
Abbreviated point-of-care OEND training according to the training programme adapted from the Toronto Public Health Prevention Overdose in Toronto (POINT) programme.24 25 The key aspects of this training are:
Identify life-threatening overdose.
Activate 911 services.
Prepare and administer intranasal naloxone.
Perform chest compressions.
Reassess and repeat naloxone administration.
Continue chest compressions until paramedics arrive.
A naloxone kit containing two doses of Narcan naloxone hydrochloride, each 4 mg intranasal (Adapt Pharmaceuticals) and administration instructions.
Training will be provided at the three recruitment settings in the clinical environment in which the participant is receiving care (clinic room, emergency department room or hallway bed and so on). A dedicated research staff person trained in basic cardiopulmonary resuscitation, first aid and overdose education, and anti-oppression techniques will provide training and naloxone kits to the participant. Clinicians will not provide training for the participant.
If the purpose-designed point-of-care OEND toolkit from phase I of the SOONER project is available before or during the feasibility study, participants randomised to the intervention arm will receive the purpose-designed intervention instead. This will contain all of the elements of the intervention described above, but physically designed to facilitative brief training and distribution in clinical settings.
Study participants randomised to the control arm will receive the present best available standard of care. Control group instructions will recommend that clinicians proceed with care exactly as they would outside of the trial. Dedicated research staff will provide participants randomised to the control arm with a referral to (1) the Toronto Public Health ‘The POINT’ programme, where intranasal naloxone and associated training is provided and (2) retail pharmacies where the Ontario Ministry of Health and Long-Term Care provides OEND with intranasal devices. Both of these OEND programme are available to the general public free of charge. If clinic or hospital-based naloxone distribution programme are in effect, control arm participants may also be referred or included in those programme at the attending clinician’s discretion.
Study procedures
Study procedures are shown in detail in table 2. Study visits will involve (1) the initial enrolment session and training for participants randomised to the intervention arm, (2) a follow-up between 3 and 14 days postenrolment to participate in the simulated overdose event and administer the knowledge and attitudes questionnaire and (3) a follow-up at 3 months (±14 days) to repeat knowledge and attitudes questionnaires by telephone or in-person. This latter 3-month follow-up is included to allow comparison of our results and study population with other studies using a 3-month follow-up with the same questionnaires.26–28
Table 2.
Study procedures timetable
| Assessment/activiy | Enrolment visit | Outcome simulation, knowledge and attitudes questionnaire, interview (3–14 days) | Knowledge and attitudes questionnaires (3 months)* |
| Eligibility Questionnaire | × | ||
| Informed Consent | × | ||
| Demographic Data Collection | × | ||
| Tertiary Clinical Outcome Baseline Questionnaire | × | ||
| Randomisation | × | ||
| Intervention Training or Control Referral | × | ||
| Outcome Simulation and Assessment | × | ||
| Knowledge and Attitudes Questionnaire | × | × | × |
| Follow-Up Interview | × |
*Three-month Knowledge and Attitudes Questionnaire completed only by participants who (a) inject drugs or (b) are friends or family members of people who inject drugs (see Outcomes of the underlying RCT section).
Participants who miss a scheduled visit may reschedule their visit at a mutually convenient time. Out-of-window visits will be permitted and noted in the final report. Study staff will also collect informal feedback from personnel at all study sites throughout recruitment to describe challenges, and opportunities for quality improvement of study processes.
Recruitment and retention strategies
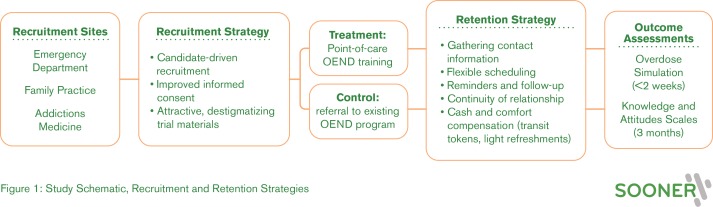
We expect our underlying study could be affected by attrition, as many participants experience unstable housing or incarceration, overdose or other health problems, may be difficult to reach by phone, and may experience stigma associated with opioid use. The recruitment and retention strategies under investigation in this feasibility study build on existing research on incentivising and improving clinical trial participation among people who use opioids.29 30 (See figure 1: study schematic, recruitment and retention strategies.)
Figure 1.
Study schematic, recruitment and retention strategies.
Recruitment
Candidate-driven recruitment
Candidates will be identified and recruited to the study according to a uniform general procedure, with site-specific modifications according to the practice patterns and operational needs of the three different clinical settings. All patients will be given an ultra-brief information card asking (1) if they take opioids or have a friend or family member who uses opioids, and (2) if they would be interested in participating in a study concerning OEND training. The card will indicate that patients should notify any of the clinical staff if they answer ‘yes’ to both of these questions. The clinician or administrative staff will then notify study personnel, who will approach the candidate to determine eligibility, obtain informed consent, randomise the participant and implement the intervention.
This ‘candidate-driven’ recruitment procedure was designed to reduce recruitment biases and the stigma of study recruitment by informing all patients of the study and allowing people in clinical settings to self-identify as interested and potentially eligible for study enrolment. By distributing the informational card to all patients, this recruitment method reduces the effect of clinician biases and prejudices regarding which patients are at risk of opioid overdose because all patients are alerted to the study. Candidates may self-identify their potential eligibility in circumstances where clinicians are unaware of their eligibility. By using an information card rather than the more conventional practice of recruitment posters, patients and study candidates can be alerted to the study and discuss their interest in participating with staff discreetly, without having to point at or read a poster placed in a public area. This serves to protect confidentiality, normalise the information given to all patients, and positions patients as the initiators of the recruitment process. The candidate-driven approach is further enhanced by encouraging clinicians to discuss the study with potentially eligible patients or visitors directly.
Improved informed consent
Lengthy written consent forms may deter study candidates from participating in research, without improving the quality of informed consent or the knowledge of participants.31 Written signed consent may be perceived as an attempt to legalise the consent process and may itself deter participants in this study. We therefore favour oral or verbal consent for this trial.32 A systematic review on strategies to improve informed consent processes in trials found that having a study team member spend more time talking one-on-one with trial candidates was the most effective available way of improving research participants’ understanding.33 Informed consent will be obtained through a verbal process, assisted with a visual map of study procedures and a brief script, with ample time for participants to ask questions and discuss each phase of the study.
Attractive, destigmatising trial materials
Study informational materials, consent forms and consent processes have also been designed to reduce barriers to trial recruitment. Developed through the SOONER Project’s collaboration with design researchers and drawing on participatory co-design methods, study handouts have been written in plain, destigmatising and inviting language, and graphically designed to avoid stigmatising imagery associated with opioid use and overdose.
Retention
Gathering contact information
At enrolment, participants will know that they will be followed over time, and we will specify the timetable and methods that will be used to contact them. We will collect multiple points of contact based on participant preferences, including phone numbers (for phoning and text messages), email addresses and mailing addresses. As an alternate means of contact, we will ask to collect the names of two friends, relatives, case managers, clinics, community centres or shelters with whom participants have regular contact. Participants will be provided with multiple methods to contact study personnel, including dropping in at recruitment sites, phoning, emailing or speaking to any of the staff associated with the study.
Flexible scheduling
Outcome assessment simulations will be scheduled flexibly and outside business hours if required. We will offer to meet participants at their recruitment location and walk with them to the simulation centre if needed. The short (maximum 2 weeks) interval for the primary outcome evaluation will reduce attrition for our primary outcome. Research staff will schedule participants’ follow-up simulation for between 4 days and 1 week after randomisation. This leaves at least 1 week for rescheduling before the 2-week maximum follow-up time. Participants will be able to select a time for the simulation outcome assessment that meets their scheduling needs. Participants will also receive a study card with the simulation time and location as well as contact information of the research coordinator.
Reminders and follow-up
Based on advice from community representatives on the study steering committee, we developed a communication and reminder strategy to suit the participants’ diverse needs and contexts. Many members of the target population face tenuous housing and limited financial resources, but many do have cellular phones. For many, communication by letter mail will be untimely and ineffective. Limited financial resources mean that many participants may not have daytime telephone ‘minutes’ and do not take incoming calls, preferring instead to communicate by text message. We emphasise the use of text message reminders, drawing on research demonstrating the feasibility and effectiveness of text messaging for participant retention in randomised trials.34
Participants will be asked to choose their preferred and secondary method of contact from phone call, text message or email. Participants will be contacted by their preferred and secondary method 5, 3 and 1 day(s) before their scheduled simulation and on the morning of their simulation to confirm attendance or reschedule, with up to three attempts on each of the days of contact. In addition, consenting participants will receive a letter prior to the outcome assessment. Participants will be contacted 1 week before the 3-month assessment via their preferred method of contact. Communication scripts will be used when contacting participants and messages will not refer to details of the study or to opioid use.
Continuity of relationships
We will strive for consistency in research staff-participant pairing to enhance rapport and build trust, which has been shown to improve patient recruitment and retention.30 All staff have received anti-oppression training. Wherever possible, the same SOONER staff person who conducts informed consent and recruits a study participant will serve as the point of contact for a given study participant. Study staff will welcome participants at the research institute lobby for outcome visits and accompany them to the assessment centre.
Cash and other compensation
Participants will receive cash per study visit: (1) Can$15 after consenting to trial activities, (2) Can$40 on arrival for the simulation and (3) Can$20 on completion of a follow-up interview. Participants will be offered public transit tokens for travel to and from each study visit, snacks and light refreshments at each study visit. The amount of remuneration proposed in our study for the time required from our participants is consistent with amounts provided in other studies with this population, and payments like those proposed here are effective to improve retention without demonstrating a coercive effect on participants nor precipitating drug use behaviours.35 36
Feasibility outcomes
Primary feasibility outcome
The primary outcome is the feasibility of recruitment and retention. The recruitment and retention strategy will be deemed ‘feasible’ if approximately 28 eligible participants are recruited in 28 days of recruitment and if attrition is <50% for the underlying study’s primary outcome assessment (overdose simulation) at 3–14 days (see table 2). We will optimise our recruitment strategy at each site before setting ‘time zero’ for recruitment days at each site. These outcomes will be recorded using data from the recruitment and retention log. We will compute the attrition at the outcome simulation and 95% binomial proportion CI.
Secondary feasibility outcomes
The secondary outcomes will be:
Proportion of eligible participants (people who meet enrolment criteria) who do not consent to the study, as reported in the study log.
Proportion of participants who drop out at the outcome assessment simulation. Dropout at the outcome assessment will be defined as people who attend the outcome simulation but do not complete the simulation or withdraw from the study, as reported in the study log.
Tertiary feasibility outcomes
The perspectives of participants will be gathered through a 15 minute individual semi-structured interview conducted at the outcome simulation visit. The interviews will be audio recorded and transcribed and the acceptability of study processes and opportunities for quality improvement will be analysed thematically and reported by theme, and with representative quotes. The tertiary outcomes will be acceptability of recruitment, retention and outcome assessment procedures for study participants, and for staff at recruitment sites and the simulation centre.
The recruitment and retention strategy will undergo basic quality improvement throughout the study based on the observations of research personnel and their interactions with recruitment site staff. Research personnel will gather informal feedback from recruitment site staff and simulation centre staff regarding quality improvement of the study procedures, and difficulties encountered with respect to recruitment and retention of study participants. The insights gained will be used to make minor changes to improve the quality of study processes. Study staff will keep a quality improvement log to record the feedback received from site staff and discuss feedback at weekly team meetings.
Outcomes of the underlying randomised control trial
Outcome measures of the underlying RCT will be gathered but will not be analysed or reported within the feasibility study.
Primary outcome of the underlying randomised control trial
The primary outcome will be the proportion of resuscitation failures in a standardised high-fidelity overdose simulation conducted at the simulation centre. Simulations will be conducted with individual participants privately, and not in a group or with other participants observing.
The simulation itself is adapted from analogous studies and, refined to mimic a realistic and imminently fatal overdose situation.10 37 38 The resuscitation sequence checklist is based on the 2015 American Heart Association bystander resuscitation recommendations.19 The scenario is intended to simulate a critically life-threatening opioid overdose, where the victim will be found with no signs of life and deteriorate rapidly to opioid-related cardiac arrest. A telephone in the simulation room will be available to simulate a phone call to 911 dispatch. Study participants will be briefed and oriented to the room using a standardised script and instructed to perform as if the simulation were real. The simulation will end with the announcement of paramedics’ arrival, after approximately10 minutes. Dedicated staff will provide a standardised semi-structured debrief for participants using a standardised framework.39 This debrief can include direct feedback and opportunities to correct techniques, affirm positive behaviours, as well as set the stage for reflection.39 40
Simulations will be video recorded, and performance assessments will be conducted based on the video recordings. Data collection will occur using a combination of a simple checklist and resuscitation simulator manikin, arranged to create a high-fidelity simulated overdose situation similar to the simulation described by Kobayashi et al.10 Assignment of the global assessment score will be based on a consensus of two assessors. Any discrepancy in the assessments of the simulation evaluators will be adjudicated by a lead investigator.
Secondary outcome of the underlying randomised control trial
The secondary clinical outcome will be performance on eight skills: (1) recognise the emergency, (2) position the victim, (3) activate emergency medical services, (4) administer naloxone (prepare device, administer correctly), (5) hand placement, (6) chest compressions (rate and depth), (7) continue compressions until end of simulation and (8) order of operations and organisation.
These eight indicators were adapted from previous cardiopulmonary resuscitation and first response training intervention studies, and include both objective measures recorded by the resuscitation manikin and subjective measures assessed by the simulation assessor.37 38 Assessors will rate each skill as satisfactory or unsatisfactory. Data collection will occur using a validated basic life support checklist and resuscitation manikin data, modified for OEND.41 Non-indicated resuscitative actions will also be documented. These include rescue breathing, incorrect naloxone administration, or any other medication administration. For rescue breathing, we will collect the ventilation data automatically recorded by the resuscitation manikin.
Tertiary outcome of the underlying randomised control trial
An interviewer-administered questionnaire will be used at enrolment, at the simulation and at 3 months to measure tertiary clinical outcomes related to participants’ knowledge about overdose, confidence and willingness to intervene in overdose, reported responses to witnessing overdose events, self-assessed barriers to responding to an overdose and self-reported drug overdose risk behaviours. The questionnaire will be scripted to reduce variability between interviewers.
The questionnaires contain both close-ended questions and open-ended questions, developed for the Toronto Public Health OEND programme evaluation.24 Questionnaire items were developed using data points from other OEND programme and from the validated Opioid Overdose Knowledge Scale (OOKS) and Opioid Overdose Attitudes Scale (OOAS).26 Although the OOKS and OOAS are validated tools, they have been validated only among people who inject drugs and the family members of people who inject drugs, especially heroin.27 28 These studies have assessed the effectiveness of OEND programme using comparisons of OOKS and OOAS scores before and 3 months after training. Therefore, to permit comparison with these studies, participants who inject drugs or who are friends or family members of people who inject drugs will be asked to return to complete the OOKS and OOAS at 3 months after enrolment.
Data sharing
As stipulated in the study informed consent documents, data will not be shared directly with researchers outside the study investigator group. Investigators wishing to undertake further analyses of quantitative study data should contact the corresponding author.
Discussion
The proposed study will test study procedures and the feasibility of an integrated recruitment and retention strategy for people likely to witness opioid overdose in the context of an OEND trial using a simulated opioid overdose event for outcome assessment.
Published strategies to improve participant retention include the involvement of community members in study design and implementation, cash and comfort compensation for study participation, regular follow-up through multiple communications media, building trust and improving communication around trial methods, and flexible hours and scheduling for follow-up assessments.29 30 34 36 Attention to patient-centred, destigmatising and participatory language in research processes may also enhance participant recruitment and retention.42 The integration of these elements is the primary strength of our proposed recruitment and retention strategy. Additional strengths include our use of participatory co-design methods for the development of study interventions and materials, and ongoing engagement with people with lived experience of opioid use for study implementation. Recruitment and retention for this study might be further strengthened by engaging peer workers directly in participant recruitment and retention. Although this approach has been successful in other studies with similar populations, in the context of a study designed to test the effectiveness of OEND in a broad variety of clinical settings, we felt that the introduction of peer workers would act as a cointervention and reduce the scalability of the intervention.18 43–45
Since the study is occurring in an urban population and a randomised trial, our results may not be generalisable to other settings, clinical contexts or study designs. However, if the study demonstrates feasibility, this recruitment and retention strategy will be ready for deployment in a full-scale trial, and potentially for adaptation to other settings.
Ethics and dissemination
The study has been reviewed by ethics boards at St. Michael’s Hospital and the Toronto Academic Health Sciences Network, Toronto Public Health and the University of Toronto. Protocol amendments will also be managed through these research ethics boards. Results will be disseminated through peer-reviewed publication and scholarly presentations, and through the SOONER roject’s network of community agencies and people with lived experience of opioid use and overdose. Participants and study recruitment sites will be sent a lay summary of study results.
Supplementary Material
Footnotes
Twitter: @DougCam8672
Presented at: Ontario Node Canadian Research Initiative in Substance Misuse (CRISM) Summit, 10 Sept 2019, Toronto; Resuscitation in Motion, 2 May 2018, Toronto; University of Toronto Department of Family and Community Medicine Research Rounds, 21 Mar 2019, Toronto.
Collaborators: In addition to the authors listed, the SOONER Investigators also includes: Leigh Chapman; Nick Goso; Richard Hunt; Peter Jüni. Vicky Stergiopoulos; Suzanne Turner; Daniel Werb.
Contributors: All authors and contributors conceptualised the study and acquired funding (AO, DC, CH, SH, MK, PL, JAP, RSh, CS, KT, KS, GM, AW, MC, RSn, LM, LC, NG, RH, PJ, VS, ST and DW). RS, MC and AO undertook project administration, and LM and CS supervised the project. All authors contributed to the design and methodology of the study. AO prepared the first drafted the manuscript. All authors contributed to revising and approving the final manuscript.
Funding: This project was funded by a project grant from the Canadian Institutes of Health Research (CIHR) grant #148817. The Canadian Centre on Substance Use and Addiction (CCSA) also contributed to project workshops and community engagement.
Competing interests: AO: evidence reviewer for ILCOR 2015, and writer for AHA/HSFC Guidelines on CPR and Resuscitation in 2015. Receives salary support from the Canadian Institutes of Health Research, the Schwartz/Reisman Emergency Medicine Institute, and the University of Toronto Department of Family and Community Medicine. AO is an evidence reviewer for the International Liaison Committee on Resuscitation and a coauthor for the 2015 American Heart Association Guidelines for CPR and ECC concerning opioid overdose and 2019 American Heart Association Guidelines updates on First Aid. CH: Contract with Toronto Central Local Health Integration Network as primary care clinical lead for the mid-east Toronto sub-region. MK: speaker honoraria from the Ontario Pharmacists Association continuing education programs on the topic of opioid use disorder. I do not address take home naloxone beyond mentioning that the programme exists (Major >$5000). Advisory Committee Member, Health Quality Ontario, Quality Standard on Opioid Use Disorder, but recused from voting on standards that involved take-home naloxone. PL: Advisory Committee Member, Health Quality Ontario, Quality Standard: Opioid Use Disorder; Quality Statement 6 pertains to access to naloxone and overdose education. LM: contributor to the 2015 guidelines and ILCOR consensus on science where opioid management was reviewed and updated.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
the SOONER Investigators:
Leigh Chapman, Nick Goso, Richard Hunt, Peter Jüni, Vicky Stergiopoulos, Suzanne Turner, and Daniel Werb
Collaborators: the SOONER Investigators
References
- 1. United Nations Office on Drugs and Crime, World Health Organization Discussion paper UNODC/WHO 2013: opioid overdose: preventing and reducing opioid overdose mortality, 2013. Available: http://www.unodc.org/docs/treatment/overdose.pdf [Accessed 04/23, 2014].
- 2. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med 2014;8:153–63. 10.1097/ADM.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 3. European Monitoring Centre for Drugs and Drug Addiction Preventing fatal overdoses: a systematic review of the effectiveness of take-home naloxone, EMCDDA Papers, Publications Office of the European Union. Luxembourg, 2015. [Google Scholar]
- 4. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. Clin Toxicol 2014:1–4. [DOI] [PubMed] [Google Scholar]
- 5. Beletsky L, Ruthazer R, Macalino GE, et al. . Physicians' knowledge of and willingness to prescribe naloxone to reverse accidental opiate overdose: challenges and opportunities. J Urban Health 2007;84:126–36. 10.1007/s11524-006-9120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coffin PO, Fuller C, Vadnai L, et al. . Preliminary evidence of health care provider support for naloxone prescription as overdose fatality prevention strategy in New York City. J Urban Health 2003;80:288–90. 10.1093/jurban/jtg031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacroix L, Thurgur L, Orkin AM, et al. . Emergency physicians' attitudes and perceived barriers to the implementation of take-home naloxone programs in Canadian emergency departments. CJEM 2017;18:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Leece P, Orkin A, Shahin R, et al. . Can naloxone prescription and overdose training for opioid users work in family practice? perspectives of family physicians. Canadian Family Physician 2015;61:538–43. [PMC free article] [PubMed] [Google Scholar]
- 9. Meyers K, Webb A, Frantz J. What does it take to retain substance-abusing adolescents in research protocols? delineation of effort required, strategies undertaken, costs incurred, and 6-month post-treatment differences by retention difficulty. Drug Alcohol Depend 2003;69:73–85. 10.1016/S0376-8716(02)00252-1 [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi L, Green TC, Bowman SE, et al. . Patient simulation for assessment of layperson management of opioid overdose with intranasal naloxone in a recently released prisoner cohort. Simul Healthc 2017;12. doi: 10.1097/SIH.0000000000000182 . [Epub ahead of print: 9 Jan 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orkin AM, Bingham K, Buick JE, et al. . Quality Assessment Errors and Study Misclassification Threaten Systematic Review Validity: Community Opioid Overdose Prevention and Naloxone Distribution Programs Review: Re: Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs.”. J Addict Med 2014. [DOI] [PubMed] [Google Scholar]
- 12. Friere K, Sangiorgi D. Service design and healthcare innovation: from consumption to co-production and co-creation. InService design and service innovation conference 2010 (PP. 39-50). Linköping electronic conference proceedings. [Google Scholar]
- 13. Donetto S, Pierri P, Tsianakas V, et al. . Experience-Based Co-design and healthcare improvement: Realizing participatory design in the public sector. The Design Journal 2015;18:227–48. 10.2752/175630615X14212498964312 [DOI] [Google Scholar]
- 14. Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, et al. . Patient and service user engagement in research: a systematic review and synthesized framework. Health Expectations 2015;18:1151–66. 10.1111/hex.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallerstein NB, Duran B. Using Community-Based Participatory Research to Address Health Disparities. : Health promotion practice. 2nd ed Sage Publications: Thousand Oaks, CA, 2016: 312–23. [DOI] [PubMed] [Google Scholar]
- 16. Eldridge SM, Lancaster GA, Campbell MJ, et al. . Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One 2016;11:e0150205 10.1371/journal.pone.0150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan A-W, Tetzlaff JM, Altman DG, et al. . Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thorpe KE, Zwarenstein M, Oxman AD, et al. . A pragmatic–explanatory continuum indicator summary (Precis): a tool to help trial designers. J Clin Epidemiol 2009;62:464–75. 10.1016/j.jclinepi.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 19. Lavonas EJ, Drennan IR, Gabrielli A, et al. . Part 10: special circumstances of resuscitation: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. United States 2015;132:S501–18. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization Community management of opioid overdose, 2014. Available: https://apps.who.int/iris/bitstream/handle/10665/137462/9789241548816_eng.pdf [Accessed 19 July 2019]. [PubMed]
- 21. Madadi P, Hildebrandt D, Lauwers AE, et al. . Characteristics of opioid-users whose death was related to opioid-toxicity: a population-based study in Ontario, Canada. PLoS One 2013;8:e60600 10.1371/journal.pone.0060600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada and Social Sciences and Humanities Research Council of Canada Tri-Council policy statement: ethical conduct for research involving humans, 2010. Available: www.pre.ethics.gc.ca [Accessed 1 December 2014].
- 23. Hurst B, Buick J, Cote J, et al. . Cpr Anytime any school: a randomized trial of strategies to teach CPR and use of AED to high school students. Can J Emerg Med 2012;14. [Google Scholar]
- 24. Leece PN, Hopkins S, Marshall C, et al. . Development and implementation of an opioid overdose prevention and response program in Toronto, Ontario. Can J Public Health 2013;104:e200–4. 10.17269/cjph.104.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leece P, Gassanov M, Hopkins S, et al. . Process evaluation of the prevent overdose in Toronto (point) program. Can J Public Health 2016;107:e224–30. 10.17269/CJPH.107.5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams AV, Strang J, Marsden J. Development of opioid overdose knowledge (OOKS) and attitudes (OOAS) scales for take-home naloxone training evaluation. Drug Alcohol Depend 2013;132:383–6. 10.1016/j.drugalcdep.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 27. Williams AV, Marsden J, Strang J. Training family members to manage heroin overdose and administer naloxone: randomized trial of effects on knowledge and attitudes. Addiction 2014;109:250–9. 10.1111/add.12360 [DOI] [PubMed] [Google Scholar]
- 28. Lott DC, Rhodes J. Opioid overdose and naloxone education in a substance use disorder treatment program. Am J Addict 2016;25:221–6. 10.1111/ajad.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neale J, Tompkins CNE, McDonald R, et al. . Improving recruitment to pharmacological trials for illicit opioid use: findings from a qualitative focus group study. Addiction 2018;113:1066–76. 10.1111/add.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scott CK. A replicable model for achieving over 90% follow-up rates in longitudinal studies of substance abusers. Drug Alcohol Depend 2004;74:21–36. 10.1016/j.drugalcdep.2003.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? Journal of Clinical Oncology 2007;25:e13–14. 10.1200/JCO.2006.10.3341 [DOI] [PubMed] [Google Scholar]
- 32. Health Canada Tri-Council policy statement 2, chapter 3: the consent process. Available: http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/chapter3-chapitre3/#toc03-1d [Accessed 17 February 2018].
- 33. Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research. JAMA 2004;292:1593–601. 10.1001/jama.292.13.1593 [DOI] [PubMed] [Google Scholar]
- 34. Varner C, McLeod S, Nahiddi N, et al. . Text messaging research participants as a follow-up strategy to decrease emergency department study attrition. Canadian journal of emergency medicine 2017:1–6. [DOI] [PubMed] [Google Scholar]
- 35. Festinger DS, Marlowe DB, Dugosh KL, et al. . Higher magnitude cash payments improve research follow-up rates without increasing drug use or perceived coercion. Drug Alcohol Depend 2008;96:128–35. 10.1016/j.drugalcdep.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Festinger DS, Marlowe DB, Croft JR, et al. . Do research payments precipitate drug use or coerce participation? Drug Alcohol Depend 2005;78:275–81. 10.1016/j.drugalcdep.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 37. Lynch B, Einspruch EL, Nichol G, et al. . Effectiveness of a 30-min CPR self-instruction program for lay responders: a controlled randomized study. Resuscitation 2005;67:31–43. 10.1016/j.resuscitation.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 38. Neset A, Birkenes TS, Furunes T, et al. . A randomized trial on elderly laypersons' CPR performance in a realistic cardiac arrest simulation. Acta Anaesthesiol Scand 2012;56:124–31. 10.1111/j.1399-6576.2011.02566.x [DOI] [PubMed] [Google Scholar]
- 39. Eppich W, Cheng A. Promoting excellence and reflective learning in simulation (pearls): development and rationale for a blended approach to health care simulation Debriefing. Simulation in Healthcare 2015;10. [DOI] [PubMed] [Google Scholar]
- 40. Dreifuerst KT. The essentials of Debriefing in simulation Learning…. Nurs Edu Perspec 2009;30:109–14. [PubMed] [Google Scholar]
- 41. Whitfield RH etal. Reliability of the Cardiff Test… Resuscitation 2003;59:291–314. [DOI] [PubMed] [Google Scholar]
- 42. van Boekel LC, Brouwers EPM, van Weeghel J, et al. . Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131:23–35. 10.1016/j.drugalcdep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 43. Valente TW, Zogg JB, Christensen S, et al. . Using social networks to recruit an HIV vaccine preparedness cohort. JAIDS Journal of Acquired Immune Deficiency Syndromes 2009;52:514–23. 10.1097/QAI.0b013e3181acff91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Young AM, Stephens DB, Khaleel HA, et al. . Hepatitis C vaccine clinical trials among people who use drugs: potential for participation and involvement in recruitment. Contemp Clin Trials 2015;41:9–16. 10.1016/j.cct.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sackett DL. Clinician-trialist rounds: 5. Cointervention bias – how to diagnose it in their trial and prevent it in yours. Clin Trials 2011;8:440–2. 10.1177/1740774511410995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.