Abstract
Introduction
Hospital stays are critical events as they often disrupt continuity of care. This process evaluation aims to describe and explore the implementation of the VESPEERA programme (Improving continuity of patient care across sectors: An admission and discharge model in general practices and hospitals, Versorgungskontinuitaet sichern: Patientenorientiertes Einweisungs- und Entlassmanagement in Hausarztpraxen und Krankenhauesern). The evaluation concerns the intervention fidelity, reach in targeted populations, perceived effects, working mechanisms, feasibility, determinants for implementation, including contextual factors, and associations with the outcomes evaluation. The aim of the VESPEERA programme is the development, implementation and evaluation of a structured admission and discharge programme in general practices and hospitals.
Methods and analysis
The process evaluation is linked to the VESPEERA outcomes evaluation, which has a quasi-experimental multi-centre design with four study arms and is conducted in hospitals and general practices in Germany. The VESPEERA programme comprises several components: an assessment before admission, an admission letter, a telephonic discharge conversation between hospital and general practice before discharge, discharge information for patients, structured planning of follow-up care after discharge in the general practice and a telephone monitoring for patients with a risk of rehospitalisation. The process evaluation has a mixed-methods design, incorporating interviews (patients, both care providers who do and do not participate in the VESPEERA programme, total n=75), questionnaires (patients and care providers who participate in the VESPEERA programme, total n=475), implementation plans of hospitals, data documented in general practices, claims-based data and hospital process data. Data analysis is descriptive and explorative. Qualitative data will be transcribed and analysed using framework analysis based on the Consolidated Framework for Implementation Research. Associations between the outcomes of the program and measures in the process evaluation will be explored in regression models.
Ethics and dissemination
Ethics approval has been obtained by the ethics committee of the Medical Faculty Heidelberg prior to the start of the study (S-352/2018). Results will be disseminated through a final report to the funding agency, articles in peer-reviewed journals and conferences.
Trial registration number
Trial status
The study protocol on hand is the protocol V.1.1 from 18 June 2018. Recruitment for interviews started on 3 September 2018 and will approximately be completed by the end of May 2019.
Keywords: process evaluation, CFIR, determinants for implementation, admission management, discharge management, continuity of care
Strengths and limitations of this study.
The process evaluation will help to interpret the findings of the outcomes evaluation of a hospital admission and discharge programme.
The perspectives of a broad range of stakeholders are considered, including care providers, patients and other stakeholders.
This mixed-methods process evaluation addresses a broad range of aspects, which are associated with implementation and outcomes of the VESPEERA programme (Improving continuity of patient care across sectors: An admission and discharge model in general practices and hospitals, Versorgungskontinuitaet sichern: Patientenorientiertes Einweisungs- und Entlassmanagement in Hausarztpraxen und Krankenhauesern).
Linkage of interview and questionnaire data with data sources of the outcome evaluation is not possible at individual level.
Introduction
Insufficient communication between hospitals and physicians in the outpatient sector may jeopardise the recovery process, lead to avoidable rehospitalisations1 2 and induce adverse events.3 These outcomes also affect health-related patient satisfaction and healthcare costs.4 The legislator in Germany responded to this care problem by obligating hospitals to offer discharge management measures to all patients (Rahmenvertrag über ein Entlassmanagement beim Übergang in die Versorgung nach Krankenhausbehandlung nach § 39 Abs. 1 s.9 SGB V). The VESPEERA programme aims to support the implementation of this regulation. It develops, implements and evaluates a structured hospital admission and discharge programme between general practices and hospitals to avoid interruptions in the hospital admission and discharge process. An overview on the intervention components and the outcomes evaluation is given down below and is described in detail elsewhere.5 Subsequently, we first summarise the patient-directed interventions in the VESPEERA programme (Improving continuity of patient care across sectors: An admission and discharge model in general practices and hospitals, Versorgungskontinuitaet sichern: Patientenorientiertes Einweisungs- und Entlassmanagement in Hausarztpraxen und Krankenhauesern), the VESPEERA outcomes evaluation and the implementation strategies. Then we elaborate on the process evaluation in the remaining of this paper.
VESPEERA programme
Legislation in Germany is focused on hospital discharge and does not address admission management. The VESPEERA programme supports the implementation of structured discharge management and, among others, adds admission management procedures and further outpatient care after discharge in general practices. If admitted to the hospital electively, the general practitioner (GP) will conduct an assessment with the patient in order to generate an admission letter for the hospital, providing medical and social information on the patient before hospital admission. Intervention components in the hospital include a telephonic discharge conversation for defined high-risk patients between the hospital and the general practice as well as a patient discharge information. After discharge, another assessment will be conducted in the general practice to facilitate planning of follow-up care (such as medication plans, referrals to specialists, prescriptions for medication and medical products and devices) and to identify patients with an increased risk for rehospitalisation based on the HOSPITAL Score (a score to determine risk of 30-day rehospitalisation6). These patients will be enrolled in a 3-month telephone monitoring. Patients who had an emergency admission will receive the assessment for planning of follow-up care and, if eligible, the telephone monitoring. Table 1 gives an overview on the intervention components and study arms.
Table 1.
VESPEERA intervention components for all study arms
| Interventions | Study arm 1: planned admission into a participating hospital | Study arm 2: planned admission into a non-participating hospital | Study arm 3: unplanned admission into a participating hospital | Study arm 4: unplanned admission into a non-participating hospital | Study arm 5: control group, not participating in VESPEERA | |
| General practice |
Interventions in the general practice before admission:
(A) assessment for admission (B) admission letter and patient brochure |
X | X | |||
| Hospital |
Interventions in the hospital:
(C) telephonic discharge conversation (D) determination of HOSPITAL Score and patient discharge information |
X | ||||
| General practice |
Interventions in the general practice after discharge:
(E) assessment for planning of follow-up care (F) telephone monitoring, depending on the risk for rehospitalisation |
X | X | X | X | |
VESPEERA outcomes evaluation
The VESPEERA programme is ‘expected to reduce the number of avoidable rehospitalisations and emergency care contacts, to improve patient safety and patient involvement, to reduce overuse, underuse and misuse of health care, to improve the continuity of care and to improve interprofessional and cross-sectoral communication between patients, hospitals, general practices and the sickness fund “Allgemeine Ortskrankenkasse (AOK) Baden-Wurttemberg”’.5
The intervention is evaluated in a quantitative outcomes evaluation with a quasi-experimental design. The primary outcome is the number of rehospitalisations due to the same indication (three-digit ICD-10-GM code (International Classification of Diseases, German Modification)) within a time frame of 3 months (90 days) to the outpatient sector. The following indicators have been defined as secondary outcomes: rehospitalisation due to the same indication within 30 days; hospitalisations due to ambulatory care-sensitive conditions; delayed prescription of medication and medical products/devices and referral to other health practitioner/s after discharge; utilisation of emergency or rescue services within 3 months; average care cost per year and patient participating in the VESPEERA programme.
Using AOK claims data, patient data from the CareCockpit and data collected in a questionnaire-based patient survey, a difference-in-difference model is applied for the primary analysis. The change of the primary outcome (before vs after the intervention) of each intervention group will be pairwise compared with the control group. A detailed description of the outcomes evaluation can be found in the corresponding study protocol.5
Implementation strategies
Several strategies were applied to support the implementation of structured hospital admission and discharge management. The strategies are named according to the ERIC compilation (Expert Recommendations for Implementing Change) by Powell et al 7 and are reported using the recommendations by Proctor et al 8 as follows:
First, consensus discussions with representatives of all stakeholders, thus physicians, GPs, patients, sickness funds and researchers, have been conducted. All intervention components were thoroughly discussed in the developmental period concerning the relevance of items, wording of items and design of documents such as the patient discharge information. By involving users in the development of the intervention, acceptance and attractiveness of the programme are expected to increase.
Second, formal commitments are obtained by participating hospitals. Adaptability is promoted in order to facilitate the integration of study components into clinical processes. Therefore, each hospital will provide information on how they will ensure the identification of study patients, the use of the admission letter, the execution of the telephonic discharge conversation, the dissemination of the patient discharge information and the transmission data to calculate the HOSPITAL Score. These formal commitments are obtained within 4 weeks after signing the participation agreement. Thereby, intervention fidelity as well as acceptance and attractiveness of the VESPEERA programme is expected to increase.
Third, the record system is changed by enhancing the PraCMan-Cockpit software that is routinely used in Baden-Wuerttemberg within the PracMan case management programme.9 The resulting CareCockpit includes the additional VESPEERA module, which assists general practices with organising patient information, conducting the assessments and care planning, generating the admission letter and other documents, and administrating telephone calls within the telephone monitoring. The CareCockpit is software that works independently from the practice information system and is used by the Care Assistant in General Practice (Versorgungsassistentin in der Hausarztpraxis, VERAH) and the GP. Furthermore, the CareCockpit works as an electronical case report form for data analysis within the outcomes evaluation.
Fourth, train-the-trainer strategies are used in order to instruct GPs and VERAHs in software utilisation and study processes. Trainers are teams of two (GP and VERAH) who are experienced in training the PraCMan-Cockpit and who were instructed in handling the CareCockpit by the study central office. GPs and VERAHs who are interested in participating in the VESPEERA programme sign up for a one-time 2.5 hour training. GPs and VERAHs learn the handling of the software in a role-play format.
Fifth, in order to support GPs and VERAHs with implementation of all intervention components, educational materials are developed. Investigator site files are provided after participation in the training by the study central office. Investigator site files contain instructions and background information on the following: obtaining informed consent by patients, installation of the CareCockpit-software, an overview on frequently asked questions concerning the handling of the software, conduction of the intervention components and conduction of the patient survey. Furthermore, general practices are continuously provided with instructional video tutorials on handling the software by the study central office. Along with the trainings, educational materials are expected to increase intervention fidelity.
Sixth, both participating general practices and hospitals are provided ongoing consultation with the study central office and other consortium partners to support implementation. General practices and hospitals are repeatedly called by employees of the study central office and asked for the status of implementation and any problems that arise within the implementation process. General practices are offered refreshers on topics of the training such as the procedure for obtaining informed consent by patients, handling of the software and instruction of the intervention components. Thus, intervention fidelity is expected to increase.
Seventh, hospitals and general practices are provided feedback in the form of three benchmarking reports in September 2018, June 2019 and December 2019. The feedback reports are based on structured, quantified data sources (claims data, patient data from the CareCockpit and patient survey data), and are aggregated on a hospital or general practice level. These will be discussed in three moderated feedback meetings during the intervention period with care providers, where options for potential improvement will be developed. Feedback meetings are planned for September 2018, September 2019 and March 2020. Feedback meetings are moderated by the study central office with support by the other project partners. Care providers will have an active role in the meetings in a workshop format and report their perspective and experiences. Audit and feedback is a strategy to improve professional practice, which has mixed and overall moderate impacts on professional performance.10 11 In this context, feedback provided is expected to enhance intervention fidelity.
In addition, hospitals and general practices will receive fee-for-service for conducting patient-related care services as well as lump sum reimbursement for study organisation and participation in workshops and feedback meetings. General practices can invoice the care services as part of their usual invoice process, which is carried out at the end of each quarter year. Hospitals invoice the sickness fund ‘Allgemeine Ortskrankenkasse’ (AOK) Baden-Wurttemberg at the end of each quarter year. Lump sums are paid after participating in the feedback meetings. Fee-for-service gives an incentive to provide the different interventions components and thereby is expected to increase intervention fidelity.12
VESPEERA process evaluation
The VESPEERA programme is a complex intervention which intends to impact on a range of outcomes. The impact on outcomes depends not only on the effectiveness of planned interventions but also on the degree of implementation of these interventions, the reach in relevant healthcare providers and patient populations and the moderating impacts of the organisational and societal context in which the interventions are applied. As described by the Medical Research Council, complex interventions are characterised by multiple, mutually interacting intervention components; multiple targeted groups of individuals and organisations; multiple outcomes and mediating factors; high impact of the organisational and societal context on outcomes; and a ‘degree of flexibility or tailoring of the interventions’.13 These features largely apply to VESPEERA. A large number of interventions are applied; various organisations in different care sectors are involved, each with structural conditions specific to the sector (e.g. remuneration systems). The effects of the interventions cover a range of domains.5 Furthermore, hospitals are involved in the implementation within their organisation to tailor it to their local processes and structures.
We planned a process evaluation to provide insight into how well the intervention was implemented, why it did or did not work (ie, did or did not have an effect on outcomes),13–15 what context factors had an influence on the implementation and outcomes and thereby allow to improve ‘transferability of potentially effective programs to other settings’.16 Investigation of implementation outcomes such as reach (whether the targeted population participated as intended/the degree to which the targeted population participated) or intervention fidelity (whether the intervention was delivered as planned) can help to better understand the results of the outcomes evaluation.17
Objectives
This process evaluation aims to examine the intervention fidelity, reach in targeted populations, perceived effects, working mechanisms, feasibility and determinants for implementation, including contextual factors, as well as associations with the outcomes evaluation, so that programme outcomes can be better interpreted. The research questions that are of interest within this process evaluation are illustrated in Box 1.
Box 1. Research questions.
-
Reach and intervention fidelity
Was the intervention implemented as planned (‘intervention fidelity’) in targeted populations (‘reach’)?
To what extent have the planned components been offered to care providers and patients?
To what extent have these been utilised by care providers and patients?
What was the adherence concerning the recommended practices of hospital admission and discharge?
Has the targeted patient population been reached?
-
Perceived effects
Which results, from the view point of care providers and patients, were:
Achieved as intended?
Not achieved although intended?
Achieved although not intended (positive or negative)?
-
Working mechanisms
Which components and aspects of the intervention programme contributed to achieving the results from the view point of care providers?
-
Feasibility
What were acceptability and attractiveness of the programme from the point of view of care providers?
-
Contextual factors
What are determinants for implementing the programprogramme?
Which contextual factors on system, hospital and practice level influenced the adoption of intervention components and outcomes of the programme?
Which practices concerning admission and discharge management have been implemented in non-participating hospitals during the intervention period (for example in consequence of the new regulation on hospital discharge management)?
-
Dose-response associations
Which associations exist between the outcomes (as disclosed by the outcomes evaluation) and findings of the process evaluation?
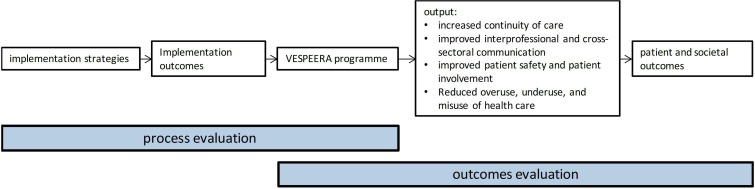
Figure 1 shows the hypothesised working mechanisms of the VESPEERA programme and the primary areas of interest of the outcomes and the process evaluation, respectively. The planned procedures for the process evaluation will be described in detail below.
Figure 1.
Logic model of the working mechanisms in the VESPEERA programme.
Methods of process evaluation
Study design
The process evaluation has an observational mixed-methods design, incorporating qualitative data from interviews and implementation plans with a description of the implementation in participating hospitals as well as quantitative data from questionnaires that are filled in for each patient in hospital, surveys and data collected through the CareCockpit software in general practices. This process evaluation is part of the VESPEERA study that lasts from October 2017 until March 2021. The planned time frame for the process evaluation started in July 2018; evaluations will be complete by the end of March 2021.
Study setting
The VESPEERA programme is implemented in 25 hospital departments and 115 general practices in a defined region in southern Germany. The process evaluation is carried out by the Department of General Practice and Health Services Research at the Heidelberg University Hospital.
Eligibility criteria
Patients who take part and gave their informed consent to the VESPEERA study participation and outcomes evaluation can participate in the process evaluation. GPs and VERAHs who participate in the VESPEERA study can participate in the process evaluation. Hospital staff from participating hospitals has to work in one of the departments selected for VESPEERA implementation or have to be involved in the implementation process of the VESPEERA intervention components on a higher hierarchical level (such as hospital management). Physicians, nursing staff and hospital management from non-participating hospitals as well as GPs and VERAHs from non-participating general practices are included if they can provide insight into their regular admission and discharge processes and the implementation of the new legislation on hospital discharge management.
Above that, all participants have to be 18 years and older, have written and spoken German language skills and have to be able to give their informed consent into study participation in the process evaluation. Persons who are unable to give their consent are excluded from study participation.
Outcomes of the process evaluation and data sources
The process evaluation uses data from a mix of sources, which in the following are described in detail (an overview on the research questions phrased, outcomes and data sources used can be found as a online supplementary file).
bmjopen-2019-031245supp001.pdf (52.2KB, pdf)
Interviews
Qualitative interviews will be conducted with nursing staff, physicians and management staff from participating and non-participating hospitals, GPs and VERAHs from participating and non-participating general practices as well as participating patients after hospital stay. The interview guide addresses the intervention fidelity, perceived effects and factors influencing implementation (barriers, facilitators, contextual factors) as well as acceptance and attractiveness of the intervention.
Questionnaires
In addition, quantitative data result from structured surveys with participating GPs, VERAHs, physicians, nursing staff, management staff and patients after a hospital stay. The questionnaire will be designed based on the results of the qualitative interviews as well as other studies on process evaluations and will be piloted before use. This pseudonymised questionnaire will not contain any data that allows identification of participants’ identity. Concepts addressed in the questionnaires will be, among others, reach (see research question 1), unintended effects (see research question 2), added value (see research question 3) and barriers and facilitators for implementation (see research question 5).
Hospital process data survey
As part of the VESPEERA programme, hospitals are asked to collect the HOSPITAL Score for patients to determine their risk of rehospitalisation. This questionnaire is expanded by questions used for the process evaluation. These include sociodemographic questions and questions on processes that are part of the study interventions that are implemented within hospitals (identification of VESPEERA patients, utilisation of the VESPEERA admission letter, telephonic discharge conversation with the general practice). Data from the hospital process data survey will be used to analyse intervention fidelity for intervention components within hospitals.
Hospital implementation plans
In order to facilitate the integration of study components into clinical processes, different approaches are suitable for different hospitals. Therefore, each hospital will provide information on how they will ensure the identification of study patients, the use of the admission letter, the execution of the telephonic discharge conversation, the dissemination of the patient discharge information and determination of the HOSPITAL Score. Hospital implementation plans will be used to analyse intervention fidelity for intervention components within hospitals.
Patient data
For the outcomes evaluation, patient data from the CareCockpit are linked with claims-based data from AOK Baden-Wurttemberg and data from the hospital process data survey. This data set will be provided for the process evaluation. These data provide information on the study arm that the patient belongs to as well as patient characteristics, the pseudonym generated in the CareCockpit for data linkage, diagnoses, the medical question for admission, information on previous antibiotic prescriptions, living situation, long-term care-related items (such as scales for activities of daily living and instrumental activities of daily living), medical information (such as pain, wounds, alarming symptoms for medical emergencies, PHQ-2 (Patient Health Questionnaire) instrument for mental disorders screening), compliance to medicinal therapy, the items of the HOSPITAL Score as well as process data (provision of information to patients, information on whether any follow-up care has been initiated and successfully executed). The patient data set will be used for the analysis of reach and intervention fidelity as well as dose-response associations. The following indicators are used as outcomes for the analysis of reach and intervention fidelity:
Proportion and description of patients who participated in VESPEERA compared with all targeted persons who meet the inclusion criteria.
Proportion of persons enrolled in the GP centred-care programme (HZV, hausarztzentrierte Versorgung) who have been admitted to a participating hospital by a participating practice, for whom a new patient account has been created in the CareCockpit and for whom a complete admission letter including a medication plan was generated and was given to the patient to take along, compared with all participating HZV-insured persons in participating practices with planned hospital admissions.
Proportion of participating patients who have been discharged from a participating hospital to their GP, for whom at the time of discharge the HOSPITAL Score has been determined, compared with all participating patients who have been discharged from a participating hospital.
Proportion of participating patients for whom the assessment for planning of follow-up treatment has been conducted compared with all participating patients.
Proportion of participating patients who have been enrolled in the follow-up telephone monitoring due to an intermediate or high risk for rehospitalisation and for whom at least two phone calls have been conducted within the given timeframe of 3 months, per all participating patients.
The degree to which the intervention components in hospitals have been implemented and offered as compared with the intention.
Sample size
The sample for the qualitative study is planned to reach saturation of data; the planned numbers are expected to be sufficient. The study sample for interviews on a hospital level consists of management staff, physicians and nursing staff and will be stratified by region and hospital size. On a practice level, GPs, VERAHs and patients will be recruited from participating practices, stratified by practice size, region and gender. In addition, staff from non-participating hospitals and general practices will be interviewed. This is important as interventions on a systems level can influence the effects of the evaluated care model. Table 2 gives an overview on the planned sample size for interviews.
Table 2.
Planned sample size for interviews
| Planned number of participants (n) | ||
| Hospitals | Nursing staff | 10 |
| Management staff | 10 | |
| Physicians | 10 | |
| Non-participating hospitals | Nursing staff | 5 |
| Management staff | 5 | |
| Physicians | 5 | |
| General practices | GPs | 10 |
| VERAHs | 10 | |
| Non-participating general practices | GPs | 10 |
| VERAHs | 10 | |
| Patients | Patient | 10 |
| Total number | 75 | |
GP, general practitioner; VERAH, Versorgungsassistentin in der Hausarztpraxis.
The sample for the quantitative survey study comprises of all participating practices and hospitals (full study population) and a sample of n=200 patients for explorative data analysis (see table 3). The sample size of patients was restricted out of feasibility reasons.
Table 3.
Planned sample size for questionnaires
| Planned number of participants (n) | ||
| Hospitals | Nursing staff | 25 |
| Management staff | 25 | |
| Physicians | 25 | |
| General practices | GPs | 100 |
| VERAHs | 100 | |
| Patients | Patient | 200 |
| Total number | 475 | |
GP, general practitioner; VERAH, Versorgungsassistentin in der Hausarztpraxis.
Recruitment
Within the process evaluation, participants will be recruited for interviews and written surveys.
Recruitment for qualitative interviews
Personnel from non-participating hospitals will be recruited by contacting the hospital management. A purposeful sample of hospitals will be selected, among others based on region, top-level versus basic care and previous interest to participate in VESPEERA. GPs and VERAHs from non-participating general practices will be recruited based on a list of all GPs who participate in GP-based care outside of the intervention region. A purposeful sample will be selected based on region, practice size and gender. All participating general practices are asked to recruit eligible patients, as they are not known to the study central office.
By using a response coupon eligible interview participants from all stakeholder groups can declare their interest in participating in an interview. They will then be contacted by the study central office, be provided with an information letter and the written consent form.
Recruitment for the survey
Personnel from participating hospitals will be recruited by the contact person at the hospitals. The contact persons will be provided with information letters, written informed consent forms and paper-based questionnaires and will be asked to hand it out to eligible personnel as defined by the study central office. All participating general practices will be sent the information letters, informed consent forms and paper-based questionnaires for GPs and VERAHs and will be asked to fill it in. Patients will be recruited by the general practices, as they are not known to the study central office. GPs will be provided with information letters, informed consent forms and paper-based questionnaires and will be asked to hand it out to eligible patients.
Data collection and management
Interviews
Interviews will be conducted as face-to-face or telephone interviews by researchers of the study central office. Interviews will last 30 min maximum and will be conducted using a semi-structured interview guide. In exceptional cases, for instance if problems within the recruitment process arise, written qualitative interviews consisting of open-end questions might be used. All interviews will be audio-recorded, transcribed verbatim and stored on a secured server of the study central office. Transcripts will contain pseudonymised data only.
Questionnaires
Paper-based questionnaires are mailed to physicians, VERAHs, nursing staff and management staff from participating hospitals, GPs and patients. The filled in questionnaires will be sent by mail using an enclosed post-paid envelope to the study central office, where they will be scanned and digitally stored on a secured server. Reminders for data collection of both interviews and questionnaires will be sent out to all potential participants one to two times via fax, mail or post.
Hospital process data survey
Hospitals fill in the hospital process data survey on the conduction of all intervention components for each case at the time of the patients’ discharge, using the form they use to collect data for the HOSPITAL score used in the VESPEERA study.
The hospitals can either integrate the questionnaire into their hospital information system as an electronic questionnaire (transfer to the aQua-Institut via secure file transfer protocol servers) or fill in paper-based questionnaires that are sent to the aQua-Institut via mail using enclosed post-paid envelopes.
Hospital implementation plans
Participating hospitals will hand in a description of their individual implementation plan to the study central office.
Patient data
During the intervention period, patient data from the CareCockpit are continuously collected for the purpose of data analysis. Data from the CareCockpit are transferred along with claims-based data each quarter year.
Data analysis
Data analysis for the process evaluation is descriptive and explorative. Qualitative data will be transcribed according to established standards and will be analysed with regard to the research questions with framework analysis using the software MAXQDA.18 The framework used for data analysis is the Consolidated Framework for Implementation Research (CFIR).19 A deductive approach is chosen to assign paraphrases from the interviews to the themes and subthemes of the CFIR. Then, inductive coding within the CFIR themes is carried out and subthemes specific to the project are generated. The CFIR was chosen as it is a comprehensive framework that takes into account many of the aspects that need to be considered when evaluating the implementation of a complex intervention in healthcare organisations.
Quantitative survey data and the indicators for the intervention fidelity will be analysed descriptively. Correlations between the outcomes of the process evaluation and the outcomes evaluation will further be analysed using multilevel regression models. Using patient data, response (eg, rehospitalisations within 30 days after discharge) will be related to dose of the implementation interventions (eg, transmission of an admission letter to the hospital), taking clustering of patients in primary care practices into account. As the analysis is explorative, we refrain from a detailed pre-specified analysis plan.
Patient and public involvement
Patients were actively involved in the conduction of all intervention components, as described in the ‘Implementation Strategies’ section. With the ‘Gesundheitstreffpunkt Mannheim e.V.’ as consortium partner, an organisation representing patient interests is involved in all stages of the study (funding application, design of the study, conduction of intervention components, interpretation of results, dissemination of results).
Discussion
This process evaluation aims to provide insight into the implementation process of the VESPEERA programme in the participating general practices and hospital departments as well as the determinants influencing the degree of implementation. The results will contribute to adjusting the VESPEERA programme after the completion of all evaluations for a possible implementation into routine care. By relying on the GP as a gatekeeper to further healthcare and by proposing communication structures, the VESPEERA programme is expected to improve continuity of care.
Continuity of care is a complex concept with no clear definition.20 However, recurring components of continuity of care include the first contact with a primary care provider, ie gatekeeping, information continuity (‘the capacity of that information to travel with the patient and throughout the health system, between providers and over time’21) and longitudinal care provider continuity.2 20 By improving continuity of care patient outcomes are supposedly improved. In a systematic review, Huntley et al found that continuity of care, ie seeing the same GP, reduced utilisation of emergency departments and emergency hospital admissions.22 Furthermore, in another systematic review by an Loenen et al the authors showed that aspects of primary care such as a gatekeeping role and provider continuity are associated with a lower risk of avoidable hospitalisations due to ambulant care sensitive conditions.2
Huntley et al 22 and van Loenen et al 2 included mostly observational studies in their reviews on the effects of organisational features of primary care on hospitalisations and emergency care use. With a quasi-experimental approach and a thorough process evaluation, the VESPEERA programme is expected to contribute to the literature on the effects of continuity of care and care coordination on several patient outcomes.
Within this process evaluation, perspectives of a broad range of stakeholders are considered. Furthermore, interviews allow for gaining in-depth understanding of experiences with the VESPEERA programme and communication processes, whereas questionnaires allow for a higher sample size. Thus, this serves to understand the broad implementation of a complex intervention.
However, no linkage between interview and questionnaire data with data sources of the outcome evaluation is intended. The intervention fidelity and barriers and facilitators to implementing the intervention therefore cannot be linked with patient-individual outcomes.
Ethics, data protection and security, and dissemination
A data protection concept is part of the VESPEERA contractual agreement between consortium partners and has been approved by a data security officer. The regulations of the European General Data Protection Regulation are met.
Dissemination of the results of this study is planned through the final report to the funding agency, articles in peer-reviewed journals as well as relevant national and, if relevant, international conferences.
Supplementary Material
Acknowledgments
Furthermore, we thank all consortium partners of the VESPEERA study ‘AOK Baden-Württemberg’ for overall project organisation and consortium leadership, ‘University Hospital Heidelberg, Department for General Practice and Health Services Research’ for project coordination, execution of the study and all study central office-related issues, ’aQua-Institut’ for data management and preparation and execution of the patient survey, ‘HÄVG Hausärztliche Vertragsgemeinschaft AG’ for organisation of train-the-trainer events, ‘University Hospital Heidelberg, Institute for Medical Biometry and Informatics, Dept. for Medical Biometry’ for statistical expertise and statistical analyses and ‘Gesundheitstreffpunkt Mannheim e.V.’ for involvement of patients in the development of intervention components. Moreover, we thank participating hospitals, general practitioners and patients. We would like to thank Annika Baldauf and Marion Kiel for organisation and support of all study central office-related issues.
Footnotes
Contributors: JF, AK and MW drafted the original manuscript. CS, MW, JF, AK and JS have planned the study, planned the data collection and have designed all instruments for data collection. LU provided statistical expertise. SK is involved in data collection of patient data. All authors read and approved the final manuscript.
Funding: This work was supported by the Federal Joint Committee (G-BA), Innovation Fund, grant number 01NVF17024.
Competing interests: JS holds stocks of the aQua-Institut.
Patient consent for publication: Not required.
Ethics approval: The study protocol has been submitted to and approved by the ethics committee of the Medical Faculty Heidelberg prior to the start of the study (S-352/2018).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med 2012;157:417–28. 10.7326/0003-4819-157-6-201209180-00006 [DOI] [PubMed] [Google Scholar]
- 2. van Loenen T, van den Berg MJ, Westert GP, et al. Organizational aspects of primary care related to avoidable hospitalization: a systematic review. Fam Pract 2014;31:502–16. 10.1093/fampra/cmu053 [DOI] [PubMed] [Google Scholar]
- 3. Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003;138:161–7. 10.7326/0003-4819-138-3-200302040-00007 [DOI] [PubMed] [Google Scholar]
- 4. Goncalves-Bradley DC, Lannin NA, Clemson LM, et al. Shepperd S: discharge planning from hospital. Cochrane Database Syst Rev 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forstner J, Straßner C, Kunz A, et al. Improving continuity of patient care across sectors: study protocol of a quasi-experimental multi-centre study regarding an admission and discharge model in Germany (VESPEERA). BMC Health Serv Res 2019;19:206 10.1186/s12913-019-4022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the hospital score to predict 30-day potentially avoidable Hospital readmissions. JAMA Intern Med 2016;176:496–502. 10.1001/jamainternmed.2015.8462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implementation Science 2015;10 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implementation Science 2013;8 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freund T, Peters-Klimm F, Boyd CM, et al. Medical assistant–based care management for high-risk patients in small primary care practices: a cluster randomized clinical trial. Ann Intern Med 2016;164:323–30. [DOI] [PubMed] [Google Scholar]
- 10. Ivers NM, Sales A, Colquhoun H, et al. No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implementation Science 2014;9 10.1186/1748-5908-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012;154 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flodgren G, Eccles MP, Shepperd S, et al. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database of Systematic Reviews 2011;6 10.1002/14651858.CD009255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. BMJ 2008;337 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oakley A, Strange V, Bonell C, et al. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413–6. 10.1136/bmj.332.7538.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dobson D, Cook TJ. Avoiding type III error in program evaluation. Eval Program Plann 1980;3:269–76. 10.1016/0149-7189(80)90042-7 [DOI] [Google Scholar]
- 16. Bradley F, Wiles R, Kinmonth A-L, et al. Development and evaluation of complex interventions in health services research: case study of the Southampton heart integrated care project (SHIP). BMJ 1999;318:711–5. 10.1136/bmj.318.7185.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linnan L, Steckler A. An Overview : Linnan L, Steckler A, Process evaluation for public health interventions and research. San Francisco, CA: Jossey-Bass, 2002. [Google Scholar]
- 18. Mayring P. Qualitative Inhaltsanalyse. Grundlagen und Techniken, vol. 11. Weinheim, Basel: Beltz, 2016. [Google Scholar]
- 19. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009;4 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salisbury C, Sampson F, Ridd M, et al. How should continuity of care in primary health care be assessed? Br J Gen Pract 2009;59:e134–41. 10.3399/bjgp09X420257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardner K, Banfield M, McRae I, et al. Improving coordination through information continuity: a framework for translational research. BMC Health Serv Res 2014;14:590 10.1186/s12913-014-0590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huntley A, Lasserson D, Wye L, et al. Which features of primary care affect unscheduled secondary care use? A systematic review. BMJ Open 2014;4:e004746 10.1136/bmjopen-2013-004746 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031245supp001.pdf (52.2KB, pdf)