Abstract
Purpose
Effects of fetal, perinatal and childhood environment on the health of children at birth and during later life have become a topic of concern. The Aichi regional sub-cohort of the Japan Environment and Children’s Study (JECS-A) is an ongoing birth cohort of pregnant women and their children which has been used to provide unique data, as adjunct studies of JECS, on multifaceted potential factors affecting children’s health.
Participants
The JECS-A is part of the JECS which follows a total of 100 000 pairs of children and their mothers (fathers’ participation is optional) across 15 regions in Japan. In JECS-A, of the 8134 pregnant women living in Ichinomiya City and Nagoya City, Japan, a total of 5721 pregnant women and their 5554 children were included. Sociodemographic and psychological data as well as biological specimens were collected from the pregnant women and their spouses (if available) in the cohort during their pregnancy. Information on children included in the JECS-A was collected from their mothers and includes demographic, behavioural, childcare, psychological and psychiatric data. Urine extracted from disposable diapers and anthropometric data were also obtained from the children.
Findings to date
A similar distribution trend for age at delivery was confirmed between the pregnant women enrolled in the JECS-A and the national statistics of the relevant areas. However, differences in education level and household income were observed. A total of 5502 children remained in the cohort at 18 months after delivery. Compared with the national statistics, the basic demographics of the children in the cohort represented the population in the study areas.
Future plans
The enrolled children in the JECS-A will be followed until the age of 13 years. The studies that come from JECS-A will complement JECS and bring novel results with a high level of generalisability.
Keywords: JECS, birth cohort, epidemiology, JECS-A
Strengths and limitations of this study.
The main strength of the Aichi regional sub-cohort of the Japan Environment and Children’s Study (JECS-A) of children is its large sample size, reflecting a representative population.
Another strength can be found in considering multifaceted potential factors affecting children’s health in the prospective birth cohort over two generations.
The main limitation of the cohort is that the population with low-income households is not included.
Introduction
The effects of fetal, perinatal and childhood environmental factors on the health of children at birth and during later life are a topic of concern. A number of birth cohort studies have thus been conducted worldwide to address this issue, and the Japan Environment and Children’s Study (JECS), which focuses on the effects of environmental chemical pollutants, is one of the largest. The JECS was launched in 2011 by the Ministry of the Environment, Japan, after 3 years of planning.1 The JECS consists of nationwide regional sub-cohorts that can be used to conduct studies, so called as adjunct studies of JECS, which are independent of the main study of the JECS. This article describes the cohort profile of a regional sub-cohort of the JECS, the Aichi regional sub-cohort of the JECS (JECS-A), containing 5721 pregnant women and their 5554 children. The following research themes are the main focuses of the adjunct studies conducted in JECS-A.
The first one is neurodevelopmental disorders2 including intellectual disability, communication disorders, autism spectrum disorder (ASD), attention deficit hyperactivity disorder, specific learning disorder and motor disorders. A review has indicated that the estimated prevalence of ASD in Asia before 1980 was around 1.9/10 000, but this figure has recently increased by 10 times.3 Although a previous study conducted in Denmark claimed that 33% of the increase in the prevalence of ASD in recent years can be accounted for by changes in the diagnostic criteria and reporting methods,4 this increased prevalence remains a complex and highly controversial issue that needs to be addressed. Recent epidemiological studies have linked neurodevelopmental outcomes to prenatal exposure to environmental toxicants such as heavy metals,5 6 prenatal tobacco exposure,7 8 environmental tobacco exposure,9 10 phthalates,11 persistent organic pollutants12 13 and organophosphate pesticides.14–16 Furthermore, interest is growing regarding the potential role of social stressors in modifying the relationship between the above early childhood exposure and the neurodevelopmental outcomes. A cohort study of 7-year-old predominantly Mexican American children in California’s Salinas Valley suggested that social adversities including a poor learning environment and parent–child interactions were significantly associated with a moderate decrease in the IQ score and its subset scores.17 Several recent studies have also discussed the effects of parent–child interactions, focusing on the effects of alexithymia in mothers18 and of depressive symptoms on parenting stress.19 Thus, there is the need to investigate postnatal influences, such as the effects of interactions between mothers and their children, as well as prenatal and postnatal toxicant exposures on the neurodevelopment of children. The above research questions will be addressed to complement those of the main study of the JECS.
The second theme of the adjunct studies conducted in JECS-A is the exposure assessments using urine collected during early childhood when the central nervous system develops rapidly. So far, the amount of exposure to environmental chemical substances taken into the bodies of infants has rarely been investigated because most infants wear diapers that fully absorb all urine. Non-invasive biomonitoring using urine samples is thus required especially for exposure assessments of chemicals with short biological half-lives, and a methodology for extracting urine from used disposable diapers has been investigated in our previous studies.20 21 Since the urine during early childhood was not collected in the main study of the JECS, the adjunct studies using such urine in JECS-A will address research questions regarding environmental exposure in that period.
The third theme of the adjunct studies is the investigation of objective screening tools to detect neurodevelopmental disorders at earlier stages. Public health services in local communities must be capable of providing early intervention support for children with such disorders. Preventive screening during early childhood can help to minimise later difficulties and to improve the trajectory of subjects with neurodevelopmental disorders in later life. For example, a validated screening checklist for autism at the age of 2 years, called M-CHAT-R/F,22 is available as part of early medical checkups, but this screening protocol requires a two-step screening test that must be conducted by a physician. As a secondary tool allowing easy and objective screening, the second to fourth digit (2D:4D) ratio, which is defined as the proportion between the lengths of the index and ring fingers, might be useful. A previous study suggested that autistic children aged 2 to 14 years have a smaller 2D:4D ratio than normal children of the same generation.23 Recent studies24 25 have also investigated whether the 2D:4D ratio, a controversial but commonly used proxy marker of prenatal androgen concentrations based on the extreme male brain theory, is associated with ASD. However, this hypothetical screening method needs further scientific evidence in different epidemiological settings in different part of the globe. Another recent trial aiming to early intervention for neurodevelopmental problems can be found in neonatal oral–motor assessments, such as sucking behaviour. While contradictory findings have been obtained,26 a recent longitudinal study also showed interesting evidence that some sucking behaviours in preterm infants are associated with later abnormal neurodevelopmental outcomes at the age of 2 years.27 As such, further research on the development of screening tools for early intervention is needed.
The JECS-A was established in 2011 as a sub-cohort of the JECS and has three main objectives: (1) to clarify the effects of prenatal and postnatal chemical exposures and social stressors on pregnant women and/or their children, especially neurodevelopmental outcomes of the children; (2) to develop a biomonitoring method using urine samples extracted from diapers for measuring internal exposure to chemical substances and (3) to develop secondary tools such as the 2D:4D ratio or neonatal oral–motor assessments to screen future neurodevelopmental problems at earlier stages of childhood. This article outlines the JECS-A and its baseline data to date.
Cohort description
Setting
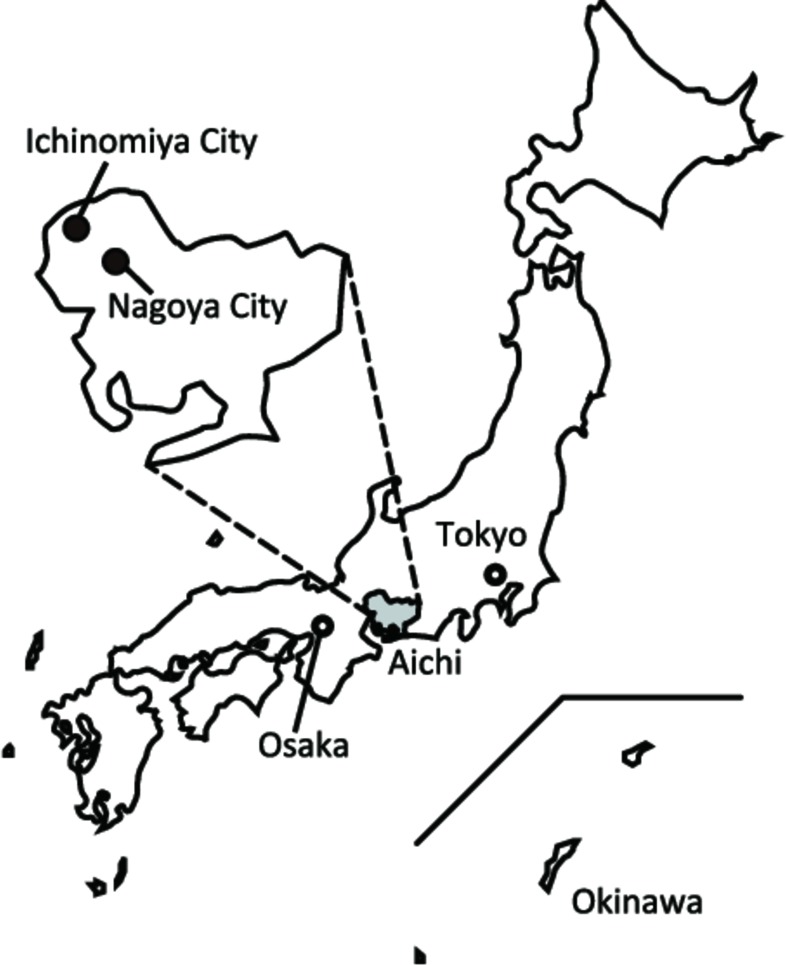
The JECS is an ongoing nationwide birth cohort study with a total of 100 000 children and their parents (father’s participation is optional, but suggested) across 15 regions in Japan.28–30 To identify risk factors in the environment affecting children’s growth and health, the participating children are being followed from their fetal stage until the age of 13 years. The JECS has 15 regional sub-cohorts. At Aichi Regional Center of the JECS, an interdisciplinary team composed of toxicologists, psychologists, epidemiologists, public health specialists, paediatricians, obstetricians and gynaecologists, biostatisticians and ergonomists designed the JECS-A as part of the JECS cohort. The study areas (figure 1) covered by the Aichi Regional Center of the JECS consist of Ichinomiya City (population of 387 000 in 2012) and Kita-ward in Nagoya City (population of 165 000 in 2012). Nagoya City consists of 16 wards with a population of over 2 million and is Japan’s third largest industrial metropolis, next to Tokyo and Osaka. Kita-ward is in the northern part of Nagoya City. Ichinomiya City neighbours Nagoya City and traditionally was known as an area involved in textile production but is now a regional commercial and residential area with a mixed economy of manufacturing and agriculture. Both areas are relatively urban and widely known in the automobile and ceramics industries.
Figure 1.
Study areas covered by the JECS-A.
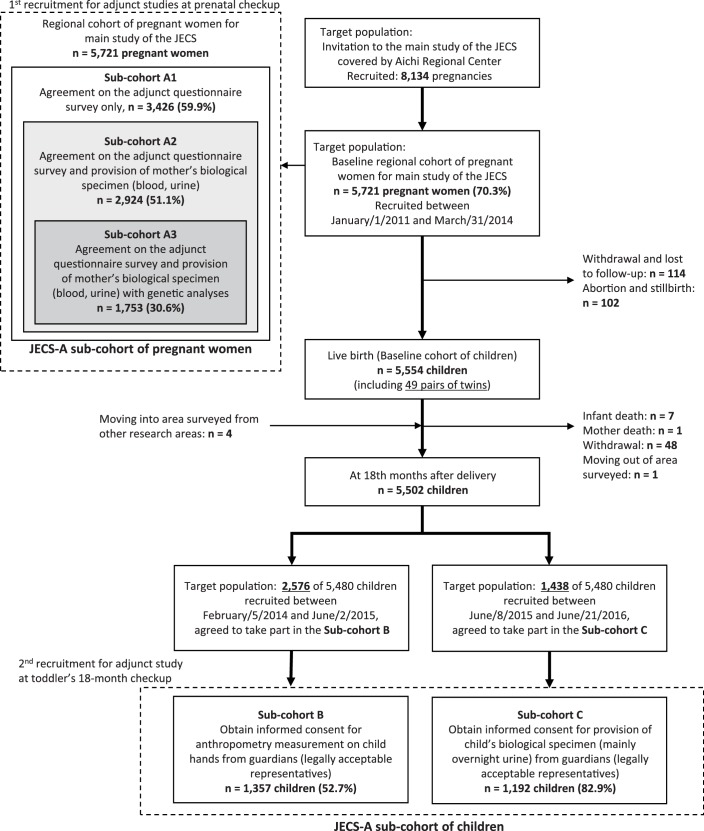
Enrolment strategy
Figure 2 shows a flow chart depicting each stage of study recruitment. A community-based recruitment strategy was adopted at designated 32 obstetric facilities including hospitals, clinics or midwifery units providing medical care for pregnant women in the study areas. The participants living in the areas can reach any of the 32 facilities within 30 min by car or public transportation such as subway or train. Women in the early stage of pregnancy who visited an obstetrics facility for prenatal healthcare were invited to participate in the JECS if they met the following criteria: (1) residence within the study areas, (2) an estimated delivery date after August 2011 and (3) an ability to read and write the Japanese language so as to complete the self-administered questionnaire. In addition to this recruitment, we asked public health centres of the local governments to help us approach hard-to-reach groups such as low socioeconomic status (SES) or reluctant pregnant women. All pregnant women receive the Maternal and Child Health Handbook at the public health centres to get complimentary municipal maternal care for pregnancy, delivery and childcare under the Maternal and Child Health Law in Japan. Taking advantages of the occasions enabling face-to-face communication with all pregnant women in the study area, our staffs carefully explained the importance of the JECS to them and requested their cooperation for the study at the obstetric facilities.
Figure 2.
Flow chart showing each stage of study recruitment for adjunct studies in the JECS-A.
Of the 8134 pregnant women who were invited as eligible recruits during the recruitment period from January 2011 to March 2014, a total of 5721 (70.3%) participants from co-operating 32 obstetrics facilities were enrolled as the baseline regional cohort of pregnant women for main study of the JECS. The study area has 4400 pregnant women annually on average, suggesting that 5721 participants accounted for about 40% of the target population in the area.
After the recruitment to the main study of the JECS, the registered 5721 JECS participants were also invited to participate in adjunct studies conducted solely in the JECS-A and asked if they could provide any of the following three types of consents: (1) agreement to complete the adjunct questionnaire survey only (sub-cohort A1, n=3426), (2) agreement to provide maternal biological specimens in addition to agreeing to participate in sub-cohort A1 (sub-cohort A2, n=2924) and (3) agreement to undergo genetic analyses in addition to agreeing to participate in sub-cohorts A1 and A2 (sub-cohort A3, n=1753). Sub-cohorts A1, A2 and A3 were designed as a hierarchy, that is, the participants in sub-cohort A3 agreed to participate in studies conducted in sub-cohorts A1 and A2. Each participant in the adjunct studies gave written informed consent before any surveys began.
Adjunct questionnaires for sub-cohort A1 were distributed three times at the obstetric facilities during first and second/third trimesters, in principle, and 1 month after the delivery, and mailed thereafter. Selective attrition caused by low SES may result in the estimates of findings being biased,31 so that enrolment and retention strategies play an important role in longitudinal cohort studies. We sent 1000 Japanese yen (JPY, 110 JPY=1 US$ as of 2019) worth of a prepaid card for every adjunct mail survey as monetary incentive to respondents. The prepaid card called ‘Quo card’ is familiar and can be used at all kinds of restaurants, convenience stores, gas stations, bookstores and so on in Japan. In addition to the monetary incentives to participate, we also conducted reminder calls or letters to retain participants who were likely to drop out of the study, focusing on non-respondents of the postal surveys.
In sub-cohorts A2 and A3, the parental blood (up to 1.5 mL) and urine (up to 50 mL, mothers only) during first and second/third trimesters in principle and the cord blood (up to 1.5 mL) were collected and stored at around −80°C. Participants in the JECS-A were then followed regardless of whether or not they took part in the above adjunct studies. Subsequently, of the 5554 children, including 49 pairs of twins, born from the enrolled mothers, 57 children dropped out of the JECS-A because of infant death (n=7), mother death (n=1), withdrawal of consent (n=48) or a change in residence to an area outside the surveyed area (n=1). On the other hand, four children who moved into the surveyed area from another one were included. As of 18 months after delivery, a total of 5502 children remained within the JECS-A, accounting for approximately 40% of the children born in the study area.
A second recruitment for the adjunct studies in the JECS-A was also conducted during the follow-up of the study participants of the main study of the JECS, focusing on children. We approached the guardians of targeted children at legal check-ups for 18-month-olds provided at regional public health centres and health consultation centres. The recruitment period was divided into two phases. Of the 5502 children remaining in the JECS-A at 18 months of age, a total of 2576 children had reached the age until June 2015. We conducted a survey of the anthropometric measurements of the 2D:4D ratio of the children on both hands (sub-cohort B). A total of 1357 children (coverage of target samples: 52.7%, consent rate: 99.0%) were enrolled in sub-cohort B. Subsequent to this recruitment, another recruitment was also set for 18-month-old children born from mothers registered during the last year of the pregnant women recruitment (sub-cohort C). This cohort was specifically planned to collect biological samples (mainly overnight urine) from diapers, and written informed consent for participation of 1192 children (82.9%) of the adjunct survey was obtained from their guardians. All the enrolled children had reached the age of 1.5 years as of June 2016. The follow-up schedule for children beyond the age of 3 years has yet to be finalised, but these sub-cohorts within the JECS-A will be followed until the children reach the age of 13 years. Informed parental consent from the legally authorised representative was obtained for all the enrolment of children in the studies. Note that the above figures were based on the data set jecs-ag-20160424, which was released in June 2016, and on the provisional data set determined as of 1 August 2018.
Patient and public involvement
To develop the JECS-A cohort, we have established a system for public involvement in research, organising an annual advisory committee consisting of the representatives from local government, medical association, nursing association, women’s group, lawyers and mass media. The role of the committee offers advice and research questions from public concern, as members of an external project supporting group. Moreover, the committee has great contribution not only to sharing knowledge or engaging and creating a dialogue with the public, but also to playing a valuable role in advising on recruitment of participants and suggesting ideas for conducting the research. We also have held an open lecture for the participants every year since 2012, as an opportunity in which information and knowledge about the research are provided and disseminated.
Data collection and measurements for adjunct studies of the JECS-A
The data collected for the adjunct studies in the JECS-A are summarised in table 1. Data acquisition for some sociodemographics and neurodevelopmental outcomes was planned within the main study of the JECS.
Table 1.
Summary of measurements collected for the adjunct studies conducted in JECS-A
| Main items | Sub-cohort covered | Timing of measurement | |||||||||||
| A1 | A2 | A3 | B | C | Prenatal (trimester) | Postnatal (months after birth) | |||||||
| First | Second/third | 1 | 6 | 18 | 24 | 36 | |||||||
| Pregnant women | |||||||||||||
| Risk and confounding factors | |||||||||||||
| Sociodemographic | Age, maternal age, marital status, household income, education level | ○ | ○ | ○ | ○ | ○ | ○ | ||||||
| Lifestyle | Smoking, secondhand smoking, drinking habits, dietary status, sleeping habits | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| Psychological and psychiatric | TAS-20 | ● | ● | ● | |||||||||
| POMS-SF | ● | ● | |||||||||||
| Readiness of Parenthood Scale | ● | ● | ● | ● | ● | ||||||||
| Obstetric | History of pregnancy, infertility treatment, prenatal diagnosis and gestational duration | ○ | |||||||||||
| Biomonitoring data | Urine (up to 50 mL, mothers only), parental blood (up to 1.5 mL), cord blood (up to 1.5 mL) | ● | ● | ● | ● | ● | |||||||
| Children | |||||||||||||
| Risk and confounding factors | |||||||||||||
| Demographic | Height, weight, BMI, sex | ○ | ○ | ○ | ○ | ○ | |||||||
| Behavioural | Neonatal oral–motor assessment | ● | ● | ||||||||||
| DCDQ | ● | ● | ● | ||||||||||
| Childcare | Breast feeding and weaning food status | ● | ● | ||||||||||
| Psychological and psychiatric | Mary Rothbart's Temperament Questionnaires | ● | |||||||||||
| IBQ-R-SF | ● | ||||||||||||
| ECBQ-SF | ● | ||||||||||||
| Biomonitoring data | Overnight urine extracted from disposable diapers (up to 30 mL) | ● | ● | ● | |||||||||
| Anthropometric data | 2D:4D | ● | ● | ● | |||||||||
| Outcome examples to be analysed | |||||||||||||
| LBW | ○ | ○ | ○ | ||||||||||
| ASD | ○ | ○ | ○ | ○ | ○ | ○ | |||||||
| ADHD | ○ | ○ | ○ | ○ | ○ | ||||||||
Sub - cohort A1, agreement on the adjunct questionnaire survey only; s ub - cohort A2, agreement on the adjunct questionnaire survey and provision of mother’s biological specimen (blood and urine); s ub - cohort A3, agreement on the adjunct questionnaire survey and provision of mother’s biological specimen (blood and urine) with genetic analyses; s ub - cohort B, informed consent obtained from guardians (legally acceptable representatives) for anthropometry measurement on child hands; s ub - cohort C, informed consent obtained from guardians (legally acceptable representatives) for provision of child’s biological specimen.
○: Refer to the main study of the JECS.
●: Measurements conducted solely in the JECS-A.
ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder;BMI, body mass index; DCDQ, Developmental Coordination Disorder Questionnaire; 2D:4D, the second to fourth digit ratio; ECBQ-SF, Early Childhood Behavior Questionnaire Short Form; IBQ-R-SF, The Infant Behavior Questionnaire Revised Short Form;JECS, Japan Environment and Children’s Study; JECS-A, The Aichi regional sub-cohort of the Japan Environment and Children’s Study; LBW, low birth weight; POMS-SF, Short form of the Profile of Mood States; TAS-20, the 20-item Toronto Alexithymia Scale.
Each of sub-cohorts in JECS-A was designed for the three main objectives as stated in the Introduction. First, to challenge the multifaceted problems surrounding children’s development, sub-cohort A1 was formed with the intention of collecting data on longitudinal changes in prenatal and postnatal exposures considered to affect neurodevelopmental outcomes. One of our special concerns is the effect of alexithymia in mothers on early child development. The 20-item Toronto Alexithymia Scale (TAS-20)32 will be used to measure this trait as a primary factor, and studies will investigate the pregnancy and perinatal outcomes and/or the developmental outcomes of postnatal infants. As there are many potential determinants extending from the prenatal to early childhood period, the risks will be estimated using the mother’s and child’s demographic variables or other related factors as confounding factors. Sub-cohorts A2 and A3 will mainly focus on studies using biological specimens (urine and/or blood) obtained from pregnant women and their spouses when available. These biological samples will be used for exposure assessments, genetic and epigenetic analyses, activity determinations of chemical metabolism-related enzymes and so on. Data on the levels of urinary exposure biomarkers including chemical metabolites will be combined with those in sub-cohort C and, eventually, the longitudinal exposure trajectory and the relationships between exposure and health outcomes will also be analysed. Second, sub-cohort C was designed to clarify the chemical exposure levels in toddlers. To estimate their amount of exposure to environmental chemicals, a noninvasive biomonitoring approach was adopted in which a paper diaper worn overnight by the infants at the age of 18 and 36 months was collected under refrigerated conditions. This cohort will also be used to derive human biomonitoring reference values (eg, RV95) of urinary chemical concentrations in the Japanese children.
Third, sub-cohort B was designed to develop secondary tools such as the 2D:4D ratio or neonatal oral–motor assessments to screen future neurodevelopmental problems at earlier stages of childhood. One of our major interest is the lengths of digits on both hands in children especially the longitudinal changes in 2D:4D ratios between 18 and 36-month-old infants. As for the anthropometric measurements of the palms and digits of the children’s hands, we have established a specialised protocol involving photographic records and digit measurements which has a high reliability (intra/inter-class correlations: ICC1=0.97 (95% CI, 0.87 to 0.99), ICC3=0.93 (0.83 to 0.98)). In short, our easy-to-use photocopying method, which involves placing the child’s palm on a box composed of transparent acrylic thin plates and photographing it with a digital camera fixed inside the box, was devised for application in health check-up settings enforced by law. All the 2D:4D ratio data will be obtained using this protocol. Sub-cohort A1 will also be used to examine the relationship between neonatal oral–motor assessments and subsequent abnormal neurodevelopmental outcomes. Using the short version of Infant Behavior Questionnaire (IBQ) and Early Childhood Behavior Questionnaire (ECBQ) as factors reflecting temperamental self-regulation, the utility of such assessments as early screening tools for predicting subsequent neurodevelopmental outcomes will be verified.
The JECS-A do not include any information regarding medical diagnoses affecting neurodevelopmental outcomes at this time, but such information will be available in the future through the main study of the JECS, which is being conducted under the same protocol across 15 regions in Japan.
Findings to date
Participant characteristics of pregnant women
Table 2 shows the main sociodemographic characteristics of the pregnant women. To ascertain whether the JECS-A baseline profiles are representative of the pregnant women in the study areas in general, the national statistics for Aichi prefecture including Nagoya City and Ichinomiya City (since data for each city were not available) were included in the table. Similar distribution trends for variables such as ‘age at delivery’ were confirmed when the JECS-A baseline cohort of pregnant women and the national statistics were compared; however, differences in ‘education level”’and ’household income’ were observed. Regarding education level, the percentage of JECS participants who had graduated from high school was relatively low compared with the national statistics (25.1% vs 39.7%), while the percentage of college/junior college/technology college students was higher among the JECS participants compared with the national statistics (35.3% vs 20.8%). Moreover, the number of JECS-A participants with a household income of 2 million yen or less was about 1/8 of the national statistics. Likewise, unemployed participants (mainly housewives) accounted for 45% of the JECS-A cohort. These results suggest a possible selection bias. We recruited pregnant women who had visited the obstetric facilities during the daytime; this might have resulted in a lower study participation rate among pregnant employees.
Table 2.
Comparison of the JECS-A baseline data with national statistics in Aichi Prefecture for pregnant women: verification of representativeness of the sample
| JECS-A baseline cohort of pregnant women (95% CI) | National statistics (Aichi pref.) | |
| Pregnant women * (n) | 5721 | 65 218 |
| Age at delivery* | ||
| 15–19 (%) | 0.7 (0.5 to 0.9) | 1.3 |
| 20–24 | 6.7 (6.0 to 7.3) | 8.2 |
| 25–29 | 25.3 (24.1 to 26.4) | 28.3 |
| 30–34 | 36.4 (35.1 to 37.6) | 36.6 |
| 35–39 | 23.2 (22.1 to 24.3) | 21.4 |
| 40–44 | 4.9 (4.4 to 5.5) | 4.2 |
| 45–49 | 0.1 (0.0 to 0.2) | 0.1 |
| 50– | 0.0 | 0.0 |
| Missing (n) | 157 | |
| Education level† (n) | 2 397 000 | |
| Junior high school/high school (%) | 29.9 (28.7 to 31.1) | 47.2 |
| College/junior college/technology college | 35.3 (34.1 to 36.6) | 20.8 |
| University | 27.6 (26.4 to 28.8) | 28.4 |
| Graduate school | 1.6 (1.3 to 2.0) | 3.6 |
| Missing (n) | 315 | |
| Household income† ‡ § (n) | 3 018 900 | |
| <2000 (%) | 2.4 (2.0 to 2.8) | 18.1 |
| 2000 to 4000 | 24.4 (23.3 to 25.5) | 26.7 |
| 4000 to <6000 | 33.1 (31.9 to 34.3) | 20.2 |
| 6000 to <8000 | 17.2 (16.2 to 18.2) | 13.4 |
| 8000 to <10 000 | 7.4 (6.7 to 8.1) | 8.3 |
| ≥10 000 | 4.3 (3.7 to 4.8) | 5.0 |
| Missing (n) | 643 |
*Data of Aichi prefecture as reference, provided by national vital statistics, Ministry of Health, Labour and Welfare in 2014.
†Data of Aichi prefecture provided by Ministry of Internal Affairs and Communications in 2012.
‡Population of Aichi Prefecture including single-person households and families.
§Household income shows 1000 Japanese yen (JPY), 110 JPY=1US$ as of 2019.
JECS-A, The Aichi regional sub-cohort of the Japan Environment and Children’s Study.
Participant characteristics of children
As for the baseline cohort of children, a total of 5502 (99.1%) of the 5554 children remained in the JECS-A at 18 months after delivery (provisional figures as of 1 August 2018). Table 3 compares the baseline data of the newborns with the national statistics. Basic demographics including sex, birth weight and birth height had similar distributions, indicating the representativeness of the sample.
Table 3.
Comparison of the JECS-A baseline data with national statistics in Aichi Prefecture for children: verification of representativeness of the sample
| JECS-A baseline cohort of children (n=5456) | National statistics (Aichi pref.)* | |||||
| Total | Male | Female | Total | Male | Female | |
| Children (n)† | 5456 | 2793 | 2660 | 65 218 | 33 649 | 31 569 |
| Sex (singleton births) | ||||||
| (%) | 51.2 | 48.8 | 51.6 | 48.4 | ||
| 95% CI | (49.9 to 52.5) | (47.4 to 50.1) | ||||
| Missing (n) | 3 | |||||
| Birth weight, g (singleton births) | ||||||
| Mean | 3034.7 | 3076.9 | 2990.7 | 3000.0 | 3040.0 | 2950.0 |
| 95% CI | (3023.3 to 3046.0) | (3060.6 to 3093.2) | (2975.1 to 3006.3) | – | – | – |
| Missing (n) | 43 | 24 | 17 | |||
| Low birth weight (singleton births) | ||||||
| <2500 g (%) | 7.8 | 6.6 | 9.0 | 9.8 | 8.5 | 11.1 |
| 95% CI | (7.1 to 8.5) | (5.7 to 7.5) | (7.9 to 10.1) | |||
| Missing (n) | 43 | 24 | 17 | |||
| Birth length, cm (singleton births) | ||||||
| Mean | 49.7 | 49.9 | 49.5 | 49.3 | 49.6 | 49.1 |
| 95% CI | (49.6 to 49.7) | (49.8 to 50.0) | (49.4 to 49.5) | – | – | – |
| Missing (n) | 51 | 29 | 20 | |||
–, no available data provided; 95% CI.
*Data of Aichi prefecture as reference, provided by national vital statistics, Ministry of Health, Labour and Welfare in 2014.
†Multiple births (49 pairs of twins) were excluded.
JECS-A, The Aichi regional sub-cohort of the Japan Environment and Children’s Study.
Similarly, table 4 shows the fundamental characteristics of the pregnant women and their children in each sub-cohort of the JECS-A. No notable differences in the descriptive statistics were observed among the sub-cohorts. Furthermore, the drop-out rates for each sub-cohort, to date, have been maintained at less than 10% relative to the baseline. However, 10% of the sub-cohort values for household income are missing because many participants did not provide information on the income. Therefore, caution is needed when using this variable as a confounding factor in multiple regression analyses.
Table 4.
Baseline characteristics of paired data of pregnant women and their children in the JECS-A cohort
| Sub-cohort | |||||
| A1 | A2 | A3 | B | C | |
| Pregnant women (n) | 3426 | 2924 | 1753 | 1352 | 1187 |
| Age at delivery | |||||
| Mean | 31.6 | 31.7 | 31.8 | 31.8 | 32.0 |
| SD | 4.9 | 4.9 | 5.0 | 4.8 | 4.7 |
| Missing (n) | 44 | 14 | 7 | 0 | 0 |
| Smoking habits | |||||
| Never smoked (%) | 62.8 | 63.7 | 63.7 | 64.2 | 64.6 |
| Ex-smokers who quit before pregnancy | 21.6 | 22.0 | 23.1 | 21.8 | 23.1 |
| Smokers during early pregnancy | 9.0 | 9.0 | 8.3 | 8.4 | 7.8 |
| Smokers | 2.5 | 2.5 | 2.1 | 2.8 | 2.1 |
| Missing (n) | 144 | 82 | 51 | 38 | 29 |
| Secondhand smoking | |||||
| Rarely (%) | 67.5 | 69.1 | 69.6 | 68.6 | 71.9 |
| A few days a week | 18.6 | 18.6 | 18.0 | 19.1 | 17.1 |
| Daily | 10.4 | 10.1 | 10.3 | 10.1 | 9.6 |
| Missing (n) | 124 | 66 | 36 | 30 | 17 |
| Household income (JPY) | |||||
| <2000 (%) | 2.3 | 2.1 | 2.5 | 1.8 | 2.1 |
| 2000 to <4000 | 25.8 | 26.0 | 25.6 | 24.4 | 24.3 |
| 4000 to <6000 | 33.4 | 33.7 | 34.2 | 35.1 | 35.9 |
| 6000 to <8000 | 17.5 | 18.1 | 17.5 | 18.6 | 18.6 |
| 8000 to <10 000 | 7.3 | 7.5 | 7.8 | 7.5 | 8.3 |
| ≥10 000 | 4.5 | 4.7 | 4.5 | 3.9 | 4.5 |
| Missing (n) | 314 | 233 | 141 | 116 | 76 |
| Children (n)* | 3336 | 2884 | 1726 | 1346 | 1182 |
| Sex (singleton births) | |||||
| Male (%) | 50.5 | 50.4 | 50.1 | 49.9 | 50.4 |
| Female | 49.5 | 49.6 | 49.9 | 50.1 | 49.6 |
| Missing (n) | 0 | 0 | 0 | 0 | 0 |
| Birth weight, g (singleton births) | |||||
| Mean | 3041.6 | 3052.3 | 3054.1 | 3039.1 | 3062.7 |
| SD | 432.8 | 419.9 | 430.4 | 410.0 | 416.7 |
| Missing (n) | 22 | 15 | 9 | 4 | 6 |
| Birth height, cm (singleton births) | |||||
| Mean | 49.6 | 49.7 | 49.7 | 49.7 | 49.7 |
| SD | 2.3 | 2.2 | 2.2 | 2.1 | 2.1 |
| Missing (n) | 28 | 18 | 11 | 6 | 7 |
Sub - cohort A1, agreement on the adjunct questionnaire survey only; sub-cohort A2, agreement on the adjunct questionnaire survey and provision of mother’s biological specimen (blood and urine); sub-cohort A3, agreement on the adjunct questionnaire survey and provision of mother’s biological specimen (blood and urine) with genetic analysis; sub-cohort B, informed consent obtained for anthropometry measurement on child hands from guardians (legally acceptable representatives); sub-cohort C, informed consent obtained for provision of child’s biological specimen from guardian (legally acceptable representatives). JPY: Japanese yen, 110 JPY=1 US$ as of 2019.
*Multiple births (twin pairs) were excluded; A1: 20 (n=40), A2: 16 (n=32), A3: 12 (n=24), B: 5 (n=10) and C: 6 (n=12).
JECS-A, The Aichi regional sub-cohort of the Japan Environment and Children’s Study.
Strengths and limitations
The main strength of the JECS-A of children is its large sample size, reflecting a representative population. Approximately 40% of the children born in the study area have been included in the study to date. The participating children will be followed until they reach the age of 13 years. The study protocol33 of the JECS decided on a target retention rate of 80% or higher at the age of 13 years. The linkage between the JECS-A sub-cohort and the main data of the JECS study will further allow novel and challenging studies with a high generalisability.
The main weakness of the JECS-A cohort concerns the pregnant women participants related to a selective attrition. A relatively large proportion of single-income, middle-class households were included in the JECS-A; many low-income households refused to participate in the surveys, and some participants did not provide information concerning their household income. As enrolment strategies, though we set about recruiting from the first trimester of pregnancy (<12-week gestation), it was hard to get their co-operation in earlier weeks of a pregnancy, when the risk of miscarriage is higher. Most of pregnant women registered were actually after 10th week of pregnancy. Furthermore, we had no choice but to call on pregnant women for participation who had visited the obstetric facilities mainly during the daytime, owing to limited number of staffs during the evening shift at hospitals. Employed participants including all non-regular employees (part-time, temporary or contract employees) only accounted for 33% in the group of annual household income with 2 million Japanese yen or less. This means a selective attrition that working pregnant women of low SES might have been excluded in the cohorts. Such family SES will have possibilities affecting several outcomes related to child development such as breastfeeding duration,34 obesity in children35 and child maltreatment.36 A British study,37 however, suggests that cognitive and behavioural development has a weak or absent direct effect of income inequality after controlling potential confounders. Thus, possible biases as a result of selective attrition and direct SES effects for child should be carefully examined in future studies when using the JECS-A cohort data. The impact of the missing values can be adjusted to some extent by comparing estimates resulting from the multiple imputation and from complete case analysis, using the data of main study of the JECS as reference. However, researchers must take into account the effect of response biases when conducting statistical analyses using paired data for pregnant women and their children and when interpreting these results.
Collaboration
The original data and specimens will be made available to investigators and stakeholders working within the JECS-A project. The study must adhere to the JECS policy on the availability of research results, publications, intellectual property rights and data sharing. At the moment, the Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare restrict the open sharing of epidemiological data. This means that researchers interested in using the data must collaborate with and participate in the JECS-A project. This project was recently started and is still ongoing. We are open to new proposals that fall within the nature of the JECS-A cohort.
Supplementary Material
Acknowledgments
We would like to express our gratitude to all the JECS-A cohort participants. We also express our sincere appreciation to the collaborating hospitals, clinics, local health care centres, local government agencies in Nagoya City and Ichinomiya City and the JECS-A staff members for their continuous assistance in promoting this study. The authors also specially thank Takaaki Hiraiwa, Hiroki Yoshizoe, Maiko Miyata, Hirohisa Kano and Takahiro Kurihara for the data management and their assistance in the data analysis.
Footnotes
Contributors: TE designed the JECS-A cohort architecture, developed the protocol, analysed the data and wrote the first draft of the manuscript. YY and MT organised the study team, obtained approvals and contributed to the development of the protocol, design and data collection tools. TOm was in charge of co-ordination with relevant organisations and organised the JECS-A members. NS, SK, TMa and TOg performed the analysis and interpreted the data. YI, HS, NO and JU designed and developed the protocol for the JECS-A sub-cohorts A2, A3 and B and edited and analysed the data. AN, MKo, YO, TMi, SSu, MSO and SSa designed each adjunct study, supervised the data collection and drafted the manuscript. MKa was a member of the JECS Steering Committee, was responsible for the study design and protocol, supervised the data collection and edited and drafted the manuscript. All the authors interpreted the data, contributed to the writing of the manuscript, revised it critically for important intellectual content and agreed with the final version and the findings.
Funding: The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. This work was also supported by grants from the Environment Research and Technology Development Fund (ERTDF, No 5-1551 and 5-1851), Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16H03733, 25293151, 25285185, 19H01078 and Grants-in-aid for research of Nagoya City University (2011-6, 2012-4, 2013-2, 2013-10, 2014-4, 2015-10, 2016-4).
Disclaimer: The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Japanese government.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Review Board of the Ministry of the Environment, by the Institutional Review Board of Nagoya City University Graduate School of Medical Sciences (No. 70-00-0134, 00000544-6, 00000574-2).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Ishitsuka K, Nakayama SF, Kishi R, et al. . Japan Environment and Children’s Study: backgrounds, activities, and future directions in global perspectives. Environ Health Prev Med 2017;22:61 10.1186/s12199-017-0667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association Diagnostic and statistical manual of mental disorders (DSM–5). American Psychiatric Association Publishing, 2013. [Google Scholar]
- 3. Xiang S, Carrie A. A review of the prevalence of autism spectrum disorder in Asia, research in autism spectrum disorders. Autism Spectrum Disorders 2010;4:156–67. [Google Scholar]
- 4. Hansen SN, Schendel DE, Parner ET. Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr 2015;169:56–62. [DOI] [PubMed] [Google Scholar]
- 5. Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, et al. . Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 2013;454-455:562–77. 10.1016/j.scitotenv.2013.03.047 [DOI] [PubMed] [Google Scholar]
- 6. Rodrigues EG, Bellinger DC, Valeri L, et al. . Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health 2016;15 10.1186/s12940-016-0127-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melchior M, Hersi R, van der Waerden J, et al. . Maternal tobacco smoking in pregnancy and children's socio-emotional development at age 5: the EDEN mother–child birth cohort study. Eur Psychiatry 2015;30:562–8. 10.1016/j.eurpsy.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 8. Joelsson P, Chudal R, Talati A, et al. . Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: a Finnish nationwide population-based cohort study. BMC Psychiatry 2016;16:306 10.1186/s12888-016-1007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han J-Y, Kwon H-J, Ha M, et al. . The effects of prenatal exposure to alcohol and environmental tobacco smoke on risk for ADHD: a large population-based study. Psychiatry Res 2015;225:164–8. 10.1016/j.psychres.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 10. Padrón A, Galán I, García-Esquinas E, et al. . Exposure to secondhand smoke in the home and mental health in children: a population-based study. Tob Control 2016;25:307–12. 10.1136/tobaccocontrol-2014-052077 [DOI] [PubMed] [Google Scholar]
- 11. Polanska K, Ligocka D, Sobala W, et al. . Phthalate exposure and child development: the Polish Mother and Child Cohort Study. Early Hum Dev 2014;90:477–85. 10.1016/j.earlhumdev.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 12. Kyriklaki A, Vafeiadi M, Kampouri M, et al. . Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4years of age: the Rhea mother–child cohort, Crete, Greece. Environ Int 2016;97:204–11. 10.1016/j.envint.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 13. Goudarzi H, Nakajima S, Ikeno T, et al. . Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: the Hokkaido Study. Sci Total Environ 2016;541:1002–10. 10.1016/j.scitotenv.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 14. Yolton K, Cornelius M, Ornoy A, et al. . Exposure to neurotoxicants and the development of attention deficit hyperactivity disorder and its related behaviors in childhood. Neurotoxicol Teratol 2014;44:30–45. 10.1016/j.ntt.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 15. Engel SM, Wetmur J, Chen J, et al. . Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 2011;119:1182–8. 10.1289/ehp.1003183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouchard MF, Chevrier J, Harley KG, et al. . Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 2011;119:1189–95. 10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stein LJ, Gunier RB, Harley K, et al. . Early childhood adversity potentiates the adverse association between prenatal organophosphate pesticide exposure and child IQ: the CHAMACOS cohort. Neurotoxicology 2016;56:180–7. 10.1016/j.neuro.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yürümez E, Akça Ömer Faruk, Uğur Çağatay, et al. . Mothers' alexithymia, depression and anxiety levels and their association with the quality of mother-infant relationship: a preliminary study. Int J Psychiatry Clin Pract 2014;18:190–6. 10.3109/13651501.2014.940055 [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Jiang W-Q, Du Y-S, et al. . Relationships between behavioral symptoms of non-medicated Chinese children with attention deficit hyperactivity disorder and parenting stress: comparison of different subtypes and comorbidities. Asia Pac Psychiatry 2016;8:127–35. 10.1111/appy.12213 [DOI] [PubMed] [Google Scholar]
- 20. Oya N, Ito Y, Hioki K, et al. . Quantitative analysis of organophosphate insecticide metabolites in urine extracted from disposable diapers of toddlers in Japan. Int J Hyg Environ Health 2017;220:209–16. 10.1016/j.ijheh.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 21. Saito S, Ueyama J, Kondo T, et al. . A non-invasive biomonitoring method for assessing levels of urinary pyrethroid metabolites in diapered children by gas chromatography–mass spectrometry. J Expo Sci Environ Epidemiol 2014;24:200–7. 10.1038/jes.2013.31 [DOI] [PubMed] [Google Scholar]
- 22. Robins DL, Casagrande K, Barton M, et al. . Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics 2014;133:37–45. 10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manning JT, Baron-Cohen S, Wheelwright S, et al. . The 2nd to 4th digit ratio and autism. Dev Med Child Neurol 2001;43:160–4. 10.1111/j.1469-8749.2001.tb00181.x [DOI] [PubMed] [Google Scholar]
- 24. Teatero ML, Netley C. A critical review of the research on the extreme male brain theory and digit ratio (2D:4D). J Autism Dev Disord 2013;43:2664–76. 10.1007/s10803-013-1819-6 [DOI] [PubMed] [Google Scholar]
- 25. Guyatt AL, Heron J, Knight BLC, et al. . Digit ratio and autism spectrum disorders in the Avon Longitudinal Study of Parents and Children: a birth cohort study. BMJ Open 2015;5:e007433 10.1136/bmjopen-2014-007433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slattery J, Morgan A, Douglas J. Early sucking and swallowing problems as predictors of neurodevelopmental outcome in children with neonatal brain injury: a systematic review. Dev Med Child Neurol 2012;54:796–806. 10.1111/j.1469-8749.2012.04318.x [DOI] [PubMed] [Google Scholar]
- 27. Wolthuis-Stigter MI, Luinge MR, da Costa SP, et al. . The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J Pediatr 2015;166:26–30. 10.1016/j.jpeds.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 28. Kawamoto T, Nitta H, Murata K, et al. . Rationale and study design of the Japan Environment and Children’s Study (JECS). BMC Public Health 2014;14:25 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michikawa T, Nitta H, Nakayama SF, et al. . The Japan Environment and Children's Study (JECS): a preliminary report on selected characteristics of approximately 10 000 pregnant women recruited during the first year of the study. J Epidemiol 2015;25:452–8. 10.2188/jea.JE20140186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Michikawa T, Nitta H, Nakayama SF, et al. . Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J Epidemiol 2018;28:99–104. 10.2188/jea.JE20170018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Booker CL, Harding S, Benzeval M. A systematic review of the effect of retention methods in population-based cohort studies. BMC Public Health 2011;11:249 10.1186/1471-2458-11-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moriguchi Y, Maeda M, Igarashi T, et al. . Age and gender effect on alexithymia in large, Japanese community and clinical samples: a cross-validation study of the Toronto Alexithymia Scale (TAS-20). Biopsychosoc Med 2007;1:7 10.1186/1751-0759-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Centre for Japan Environment and Children's study Japan Environment and Children's study (JECS), Study Protocol (ver. 1.4) [Available from. Available: https://www.env.go.jp/en/chemi/hs/jecs/data/about/jecs-study_protocol_14_en.pdf
- 34. Flacking R, Nyqvist KH, Ewald U. Effects of socioeconomic status on breastfeeding duration in mothers of preterm and term infants. Eur J Public Health 2007;17:579–84. 10.1093/eurpub/ckm019 [DOI] [PubMed] [Google Scholar]
- 35. Ogden CL, Lamb MM, Carroll MD, et al. . Obesity and socioeconomic status in children and adolescents: United States, 2005-2008. NCHS Data Brief 2010;51:1–8. [PubMed] [Google Scholar]
- 36. Eckenrode J, Smith EG, McCarthy ME, et al. . Income inequality and child maltreatment in the United States. Pediatrics 2014;133:454–61. 10.1542/peds.2013-1707 [DOI] [PubMed] [Google Scholar]
- 37. Violato M, Petrou S, Gray R, et al. . Family income and child cognitive and behavioural development in the United Kingdom: does money matter? Health Econ 2011;20:1201–25. 10.1002/hec.1665 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.