Abstract
Background
Previous glycophylogenetic comparisons of dipteran and lepidopteran species revealed variations in the anionic and zwitterionic modifications of their N-glycans; therefore, we wished to explore whether species- and order-specific glycomic variations would extend to the hymenoptera, which include the honeybee Apis mellifera, an agriculturally- and allergologically-significant social species.
Methods
In this study, we employed an off-line liquid chromatography/mass spectrometry approach, in combination with enzymatic and chemical treatments, to analyse the N-glycans of male honeybee larvae and honeybee venom in order to facilitate definition of isomeric structures.
Results
The neutral larval N-glycome was dominated by oligomannosidic and paucimannosidic structures, while the neutral venom N-glycome displayed more processed hybrid and complex forms with antennal N-acetylgalactosamine, galactose and fucose residues including Lewis-like epitopes; the anionic pools from both larvae and venom contained a wide variety of glucuronylated, sulphated and phosphoethanolamine-modified N-glycans with up to three antennae. In comparison to honeybee royal jelly, there were more fucosylated and fewer Man4/5-based hybrid glycans in the larvae and venom samples as well as contrasting antennal lengths
Conclusions
Combining the current data on venom and larvae with that we previously published on royal jelly, a total honeybee N-glycomic repertoire of some 150 compositions can be proposed in addition to the 20 previously identified on specific venom glycoproteins.
Significance
Our data are indicative of tissue-specific modification of the core and antennal regions of N-glycans in Apis mellifera and reinforce the concept that insects are capable of extensive processing to result in rather complex anionic oligosaccharide structures.
Keywords: insect, glycomics, glucuronic acid, sulphate, fucose, phosphoethanolamine
Introduction
Honeybees, members of the order Hymenoptera, are not just providers of honey and royal jelly, but are important pollinators; they have been subject in recent years to multiple environmental stresses such as mites, pathogens and pesticides resulting in abrupt reductions in bee populations (1). Bees have compared to many other insects a sophisticated social lifestyle. Generally, each colony has one diploid queen, thousands of sterile diploid female worker bees (whereby the supply of royal jelly determines caste differentiation) and, seasonally, also thousands of fertile haploid male drones (2). While the role of the queen and the drones is solely reproductive, worker bees have a variety of non-reproductive tasks including acting as nurses, guards or foragers. The sting of a worker is used as defence and the injected venom contains various peptides and enzymes, which are primarily directed at either lysing cells, stimulating mast cell degranulation or blocking ion channels (3); some of the active components such as honeybee phospholipase A2 and hyaluronidase are glycoproteins which can cause allergy in humans and are referred to as allergens Api m 1 and Api m 2 (4).
In glycomic terms, only a few orders of insects have been investigated, but recently it has become obvious that the range of glycan structures they synthesise are indeed more complex than previously supposed. In the first studies, only pauci- and oligomannosidic glycans were detected (5), lacking entirely anionic or zwitterionic modifications. It is indeed 35 years since the glycoprotein nature of components of bee venom was first investigated by März and colleagues (6); this work culminated not only in finding the difucosylation (then novel) of an N-glycan core as supported by NMR and GC-MS analyses (7–9), but also in definition of the relevant enzyme activity in venom glands (10) and of the immunogenicity of the oligosaccharides themselves (11–13). The double α1,3/α1,6-fucosyl substitution of the core GlcNAc was originally controversial, but was confirmed in later independent studies on bee venom (14–16) and has since then been identified in a range of invertebrate species (17). Thereby, for many years, the most ‘complex’ structure known from insects was the trifucosylated structure from bee venom (7), in addition to ones modified with aminoethylphosphonate from a locust (18). In the following decades, most reports on insect N-glycan structures were either on species of the Hymenoptera, Diptera and Lepidoptera; the latter two orders are probably, as either model organisms (fruit flies), disease vectors (mosquitoes) or biotechnological factories (moths and moth cell lines), the most studied amongst the thirty or so within the class Insecta.
Recent studies from our own and other groups demonstrate that dipteran and lepidopteran insect species can synthesise N-glycans with up to three antennae modified with β1,3-galactose, α- and β-linked N-acetylgalactosamine, glucuronic acid and sulphate (19–22); additionally, one or two sialylated structures were detected in the N-glycomes of Drosophila embryos and adult heads (23,24). In lepidopterans we detected phosphorylcholine-modified antennae whereas the studied dipteran species were lacking zwitterionic moieties. The newest data on hymenopteran glycans are on royal jelly, a product of the hypopharyngeal glands of worker honeybees, in which N-glycans with up to three antennae were also detected, but the zwitterionic modification was phosphoethanolamine (25). In the current study, we have compared the N-glycans isolated from honeybee larvae with those from the venom and again reveal unusual multiantennary structures with antennal fucose, glucuronic acid and phosphoethanolamine residues; thus, these data reinforce the view that invertebrate species express highly variable forms of glycosylation, even within the same phylogenetic class as well as display tissue or stage-specific modifications.
Experimental Procedures
Enzymatic release and purification of N-glycans
Male honeybee larvae (Apis mellifera; collected from a bee hive in Schiltern, Lower Austria) were homogenised with a mortar and pestle, prior to suspension in water and heat-treated before proteolysis with thermolysin (26); lyophilised honeybee venom (200 mg, Bee Venom, LLC, Tbilisi, Georgia) was suspended in water prior to heat treatment and proteolysis with thermolysin. Proteolysis was followed by cation exchange (Dowex AG50, Bio-Rad; elution with 0.5 M ammonium acetate, pH 6) and gel filtration (Sephadex G25) chromatography of the proteolysate. Thereafter, N-glycans were either released from glycopeptides using peptide:N-glycosidase F (PNGase F, 3 U; Roche) at pH 8 as previously described (26), with a subsequent digestion of the remaining glycopeptides using PNGase A (recombinant; prepared in house in insect cells) or PNGase Ar (recombinantly produced in Pichia; New England Biolabs, 15 U) after adjusting to pH 5; the result was a combined PNGase F/A digest as in the case of one larval and one venom preparation. Alternatively, the PNGase F-released glycans were separated from remaining glycopeptides by another round of cation-exchange chromatography (Dowex AG50; flow through), while the bound fraction was gel filtrated prior to PNGase A treatment and a further cation-exchange chromatography step. Free glycans were subject to solid-phase extraction on non-porous graphitised carbon (SupelClean ENVICarb, Sigma-Aldrich) as described (26); the ‘neutral’ and ‘anionic-enriched’ fractions were then eluted with 40% acetonitrile and 40% acetonitrile containing 0.1% trifluoroacetic acid respectively. The pools of glycans were subject to MALDI-TOF MS before fluorescent labelling by reductive amination using 2-aminopyridine (PA; Sigma-Aldrich) (26). Similar results were obtained for different preparations of honeybee larvae from the same hive. For the workflow in schematic form, refer to the Scheme in the Supplement.
HPLC fractionation
Pyridylaminated N-glycomes (i.e., aliquots of the neutral and anionic pools) were fractionated by reversed-phase HPLC (Ascentis Express RP-amide; 150 x 4.6 mm, 2.7 μm; Sigma-Aldrich) and a gradient of 30% (v/v) methanol (buffer B) in 100 mM ammonium acetate, pH 4 (buffer A) was applied at a flow rate of 0.8 ml/min as follows: 0-4 min, 0% B; 4-14 min, 0-5% B; 14-24 min, 5-15% B; 24-34 min, 15-35% B; 34-35 min, return to starting conditions (26,27). For the larval anionic pool, RP-HPLC was also performed using a Kinetex XB-C18 column (250 x 4.60 mm, 5 μm; Phenomenex) with a gradient of 30% (v/v) methanol (buffer B) in 100 mM ammonium formate, pH 6 (buffer C) also at a flow rate of 0.8 ml/min: 0-30 min, 0-30% C; 30-35 min, 30-40% C; 35-40 mins, 40-55% C; 40-44 min, hold at 55% C; 44-46 min, return to starting conditions. The RP-HPLC columns were calibrated daily in terms of glucose units using a pyridylaminated dextran hydrolysate and the degree of polymerisation of single standards was verified by MALDI-TOF MS. Detection was by fluorescence (excitation/emission at 320/400 nm). All manually-collected HPLC glycan fractions were analysed by MALDI-TOF MS and MS/MS.
Glycan mass spectrometry
Monoisotopic MALDI-TOF MS was performed using an Autoflex Speed (Bruker Daltonics, Bremen) instrument in either positive or negative reflectron modes with 6-aza-2-thiothymine (ATT) as matrix. MS/MS was in general performed by laser-induced dissociation of the [M+H]+ or [M-H]- pseudomolecular ions; typically 2000 shots were summed for MS (reflector voltage, lens voltage, gain, pulsed ion extraction and Smartbeam parameter typically set respectively to 21 kV, 9 kV, 2116 V, 130 ns and 4_large with suppression up to 700 Da) and 4000 for MS/MS (reflector voltage, lift voltage and gain respectively 27 kV, 19 kV and 2174 V). Spectra were processed with the Bruker Flexanalysis software using the SNAP algorithm with a signal/noise threshold of 6 for MS (unsmoothed) and 3 for MS/MS (four-times smoothed). Glycan spectra were manually interpreted on the basis of the masses of the predicted component monosaccharides, differences of mass in glycan series, comparison with co-eluting structures from other insects and nematodes and fragmentation patterns before and after chemical treatments or exoglycosidase digestions. A list of theoretical m/z values for each glycan composition is presented in the Supplementary Table 1. Full details of MS/MS and chemical/enzymatic treatments of N-glycan structures which were well documented in other insect samples are not given here; readers are referred to our previous work on lepidopteran and royal jelly glycomes, e.g., for analyses of oligomannosidic isomers or of simple sulphated N-glycans (20,25), as also summarised in Supplementary Figure 2.
Enzymatic and chemical treatments
Glycans were treated, prior to re-analysis by MALDI-TOF MS, with α-fucosidase (α1,3/4-specific from almond, Prozyme), α-mannosidases (jack bean from Sigma, Aspergillus α1,2-specific from Prozyme and Xanthomonas α1,2/3-specific from New England Biolabs; data not shown), β-galactosidase (β1,3-specific from New England Biolabs), β-glucuronidases (E. coli from Megazyme and Helix pomatia from Sigma-Aldrich; desalted and concentrated before use with a 10 kDa molecular weight cut-off ultrafiltration device), β-N-acetylhexosaminidases (jack bean from Sigma-Aldrich, Xanthomonas β1,2-specific N-acetylglucosaminidase from New England Biolabs, or in-house Pichia-expressed recombinant forms of Caenorhabditis elegans HEX-4 specific for β1,4-linked GalNAc residues or Apis mellifera FDL specific for the product of GlcNAc-transferase I (28)) in 50 mM ammonium acetate, pH 5, at 37 °C overnight (except for pH 6.5 in the case of HEX-4, pH 7 in the case of E. coli β-glucuronidase or an incubation time of only 3 hours in the case of FDL); these incubations were performed in 250 μl PCR tubes with a final volume of 3 μl (for further details about conditions and specificities, refer to the supplement). Hydrofluoric acid was used for removal of phosphoethanolamine or α1,3-linked fucose (26). As appropriate, treated glycans were re-chromatographed by RP-HPLC to ascertain retention time shifts prior to MALDI-TOF MS; otherwise, an aliquot (generally one-fifth) of any digest was analysed by MALDI-TOF MS without further purification.
Results
Neutral N-glycomic profile of honeybee larvae
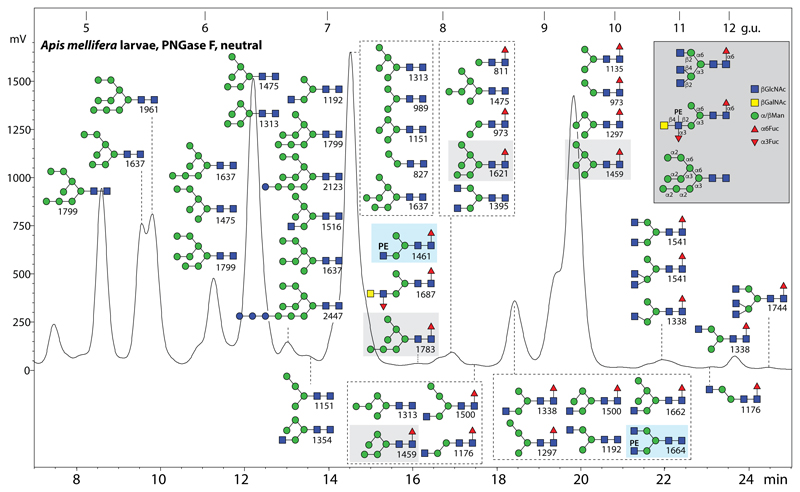
N-glycans were released enzymatically from glycopeptides derived from male honeybee larvae or from honeybee venom prior to fractionation on graphitised carbon to yield neutral and anionic pools, followed by fluorescent labelling, RP-HPLC and MALDI-TOF MS. All glycan annotations are based on MS/MS backed up with selected fucosidase, mannosidase, galactosidase and N-acetylhexosaminidase digests; however, as many of the neutral glycans have been previously detected and analysed in a number of insect samples using the same RP-amide column (20,25) (see also Supplementary Table 2), only the definition of a few of these is discussed in more detail. The neutral PNGase F-released pool from larvae displays a typical range of structures for an insect, especially oligomannosidic glycans as well as paucimannosidic and hybrid forms with or without core α1,6-linked fucose (Figure 1). Noteworthily, some oligomannosidic glycan isomers lacking an α1,2-mannose on the upper B and C-arms were, as in lepidopteran species (20), also found to be core α1,6-fucosylated resulting in Man5-7GlcNAc2Fuc1 structures (m/z 1459, 1621 and 1783 eluting at 7-10 g.u.; Figure 1), indicative of a relaxed α1,6-fucosyltransferase specificity capable of transfer to glycans with one or two α1,2-mannose residues, rather than GlcNAc, on the lower A-arm.
Figure 1. RP-HPLC of neutral N-glycans of male honeybee larvae.
PNGase F-released N-glycans were subject to solid phase extraction, whereby the neutral-enriched fraction was eluted with 40% acetonitrile, prior to fluorescent labelling and chromatography on an RP-amide column; each fraction was collected and subject to MALDI-TOF MS (m/z values for [M+H]+ being indicated); the glycans in each fraction are shown in order of occurrence with the most dominant glycan uppermost. The annotations in the Symbolic Nomenclature for Glycans (see also key in grey box) are based on elution time, MS/MS and digestion data in comparison to recently-published data on royal jelly, mosquito and moth N-glycans; for some simple glycans, a table of elution times in comparison to previous studies is given in Supplementary Table 2, whereby the order of retention is generally consistent with that tabulated by Tomiya (49). The glycome is dominated by typical insect glycans (oligomannosidic, paucimannosidic and hybrid), but also some complex bi-/tri-antennary forms are present; two phosphoethanolamine (PE)-modified glycans in this pool are highlighted in the blue boxes and fucosylated oligomannosidic structures are in light grey boxes. The column was calibrated in terms of glucose units (g.u.). For the core α1,3-fucosylated N-glycans released with PNGase A from larval glycopeptides, refer to Supplementary Figure 1.
Subsequent to the PNGase F digest, the remaining glycopeptides were then further treated with PNGase A. In addition to some residual oligomannosidic glycans, various mono- and difucosylated structures were released. Some of these were shown by their sensitivity to hydrofluoric acid treatment to be core α1,3-fucosylated as expected on the basis of previous data, whereby the RP-HPLC retention time is increased (Supplementary Figure 1 A-E). Also, comparison of the MS/MS patterns before and after treatment show loss of only one fucose from either singly and doubly fucosylated cores (shifts from m/z 446 to 300 or m/z 592 to 446 Y-fragment ions) compatible also with the resistance of α1,6-linked fucose to HF (Supplementary Figure 1 F-I).
Neutral N-glycomic profile of complete honeybee venom
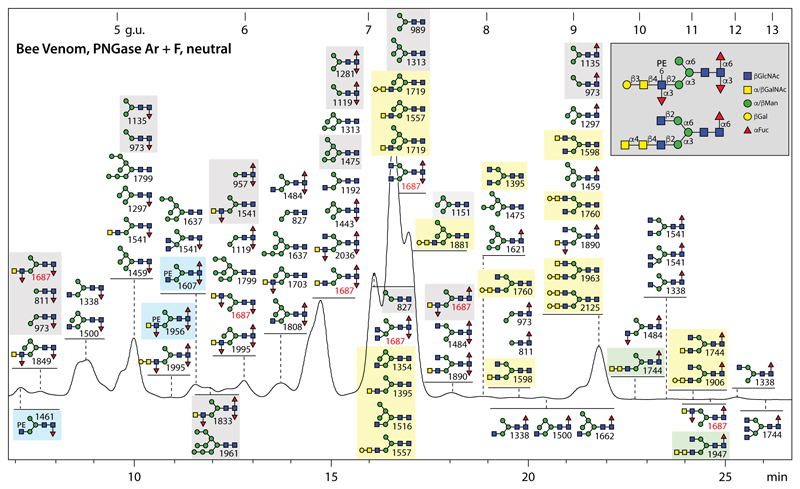
Due to the known presence of difucosylated glycans on honeybee venom phospholipase A2 and hyaluronidase (7,9), both PNGase F and Ar were used to release the N-glycans from the complete honeybee venom. The neutral fraction was subject to RP-HPLC (Figure 2) and MS/MS (for examples, see Supplementary Figure 2). In accordance with the aforementioned studies on single venom glycoproteins, core mono- and difucosylation is very dominant in the overall venom glycome, but with a higher isomeric variance than previously detected. For instance, there are, e.g., three isomers of Hex3HexNAc3Fuc2 (m/z 1484) and seven of Hex3HexNAc4Fuc2 (m/z 1687) separated by retention time and displaying distinct MS/MS spectra indicative of different combinations of α1,3-fucosylation and α1,6-fucosylation on the core or α1,3-fucosylation on ‘upper’ or ‘lower’ antennae (see Supplementary Figure 2 A-E and Supplementary Figure 3H for examples).
Figure 2. RP-HPLC of neutral N-glycans of honeybee venom.
N-glycans released by the combined use of PNGase F and Ar were subject to solid phase extraction, whereby the neutral-enriched fraction was eluted with 40% acetonitrile, prior to fluorescent labelling and chromatography on an RP-amide column; each fraction was collected and subject to MALDI-TOF MS. The annotations in the Symbolic Nomenclature for Glycans (see also key in grey box) are based on elution time, MS/MS and digestion data (see examples in Supplementary Figures 2 and 3). The column was calibrated in terms of glucose units. Phosphoethanolamine (PE)- or α-GalNAc-modified glycans in this pool are highlighted respectively in blue or green boxes, structures previously found on honeybee venom phospholipase A2 and hyaluronidase are in light grey boxes and those hybrid or biantennary forms reported by us in royal jelly in light yellow boxes; seven different isomers of Hex3HexNAc4Fuc2 are indicated by the m/z 1687 values in red. Due to their low abundance, neither the biantennary and Man4-5GlcNAc2-based hybrid glycans nor the PE-, β1,3-Gal and α1·4· alNAc-modified antennae were previously detected in honeybee venom.
Amongst the historically characteristic N-glycan modifications in venom is the fucosylated LacdiNAc motif (7) as exemplified by the Hex3HexNAc4Fuc3 glycan (m/z 1833; Supplementary Figure 3 and Supplementary Table 3): the pattern of sensitivity to a C. elegans β1,4-N-acetylgalactosaminidase (HEX-4) and almond α1,3/4-fucosidase as followed by MS and MS/MS not only is a verification of the structure, but also is informative in terms of the specificity of these two enzymes. While HEX-4 will remove a single GalNAc regardless of whether the antennal fucose is present, the almond fucosidase will only remove the fucose from a LacdiNAc unit and not from a single GlcNAc residue. Examples of other structures carrying the fucosylated LacdiNAc motif are biantennary or galactosylated hybrid versions (m/z 1687, 1890, 1995 and 2036; Supplementary Figure 2 D, H-J).
Previously undetected in venom are a variety of more complicated N-glycan structures of low abundance. While the phosphoethanolamine-modified forms are discussed below in the context of the anionic glycans (see annotations on Figure 2), glycans with either elongated or multiple antennae were also identified. Due to the use of RP-HPLC, it was possible to resolve multiple isomers for some compositions, as exemplified by two isomers of Hex3HexNAc5Fuc1 (m/z 1744; Supplementary Figure 2 F and G). One displays an m/z 610 (HexNAc3) B-fragment suggestive of a GalNAcα1,4GalNAcβ1,4GlcNAc motif such as found on some insect glycolipids (29) and L. dispar N-glycans (20), while the other is a triantennary structure with three unsubstituted non-reducing terminal GlcNAc residues. The disubstitution of the α1,3-mannose by β1,2- and β1,4-linked GlcNAc on the triantennary m/z 1744 isomer, also observed on some hybrid glycans (Supplementary Figure 2 E), is indicative of the action of β1,4GlcNAc-TIV.
Overall, it is interesting to compare the neutral PNGase F digest of the larvae with that from royal jelly as well as the combined PNGase Ar/F digest of bee venom in terms of the chromatograms and the glycans present in specific fractions. Based on comparisons of HPLC and MS peak heights (Supplementary Figure 4), it can be concluded that in the larvae and royal jelly Man5GlcNAc2 is relatively dominant, while Man3GlcNAc2 is a major glycan in venom (i.e., these are respectively yielding highest MS signals in the most dominant HPLC fractions in the three samples). Also, the degree of core fucosylation (higher in larvae and venom) and the occurrence of hybrid/complex structures (more in royal jelly) is different. Thereby, it is obvious that RP-HPLC and MS/MS are required to resolve in-depth the fine details of the structural possibilities even within one species.
Anionic glycomic profile of honeybee larvae
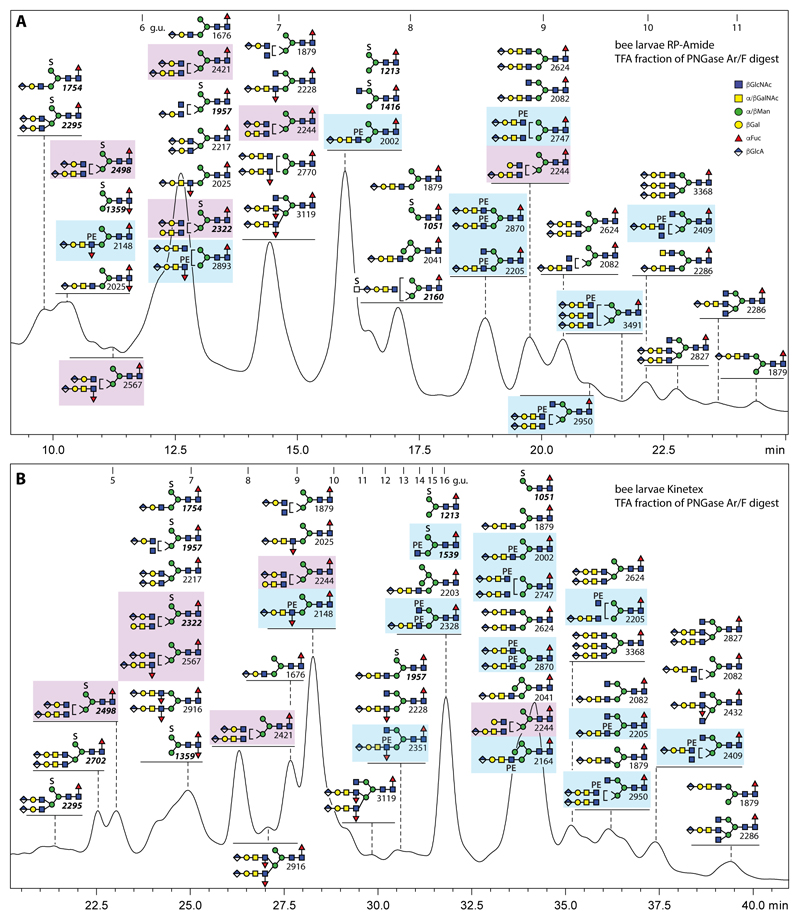
As is now standard in our glycomic workflow (see the Scheme in the supplement), we separated the pools of anionic N-glycans from the neutral ones by elution with 40% acetonitrile containing 0.1% trifluoroacetic acid from non-porous graphitised carbon prior to labelling. We then performed RP-HPLC on two different fused core columns (RP-amide at pH 4, as for the neutral glycome, or Kinetex XB-C18 at pH 6; Figure 3). The results are subtly different in terms of the order of elution; the Kinetex gradient is longer and extends beyond the normal glucose unit range, but also results in detection of a few more structures than with the RP-amide. From both runs, it is obvious that honeybee larvae express a range of sulphated, glucuronylated and phosphoethanolaminylated glycans accounting for an estimated 2-3% of the larval N-glycome based on fluorescence intensity. There are some similarities to the anionic glycomes of mosquitoes, moths and royal jelly in terms of the occurrence of sulphated mannose and of short or long glucuronylated (i.e., GlcAβ1,3Galβ1,3GlcNAc or GlcAβ1,3Galβ1,3GalNAcβ1,4GlcNAc) antennae, but also differences. For instance, whereas GlcNAc-linked phosphoethanolamine on N-glycans was, until now, only found in royal jelly as compared to the phosphorylcholine found on moth N-glycans (20,25), zwitterionic modifications were absent from the N-glycomes of larvae of two mosquito species (19). On the other hand, unlike royal jelly, the ‘charged’ N-glycans from male honeybee larvae tend to be core α1,6-fucosylated, while the occurrence of Man4-5GlcNAc2-based hybrid structures is less pronounced in the larvae (indeed, few structures are shared between royal jelly and larvae; see also the Supplementary Information).
Figure 3. RP-HPLC of anionic N-glycans of male honeybee larvae.
PNGase Ar/F-released N-glycans were subject to solid phase extraction, whereby the anionic-enriched fraction was eluted with 40% acetonitrile/0.1% trifluoroacetic acid, prior to fluorescent labelling and chromatography on either an RP-amide column (A; at pH 4) or a Kinetex XB-C18 column (B; at pH 6); each fraction was collected and subject to MALDI-TOF MS with m/z values in bold italics for [M-H]- ions of sulphated structures. The annotations in the Symbolic Nomenclature for Glycans are based on elution time, MS/MS and digestion data (see Figures 4-6 and Supplementary Figures 5-8). The columns were calibrated in terms of glucose units. Note that on both columns sulphation (S) and antennal fucosylation lead to large shifts to lower retention time, whereas this effect is only seen on the RP-amide column for phosphoethanolamine (PE)-modified structures (highlighted in blue boxes); structures with two different β1,2/β1,4-antennae, which are a special feature of larvae, are highlighted in purple boxes.
Characterisation of glucuronylated N-glycans in bee larvae
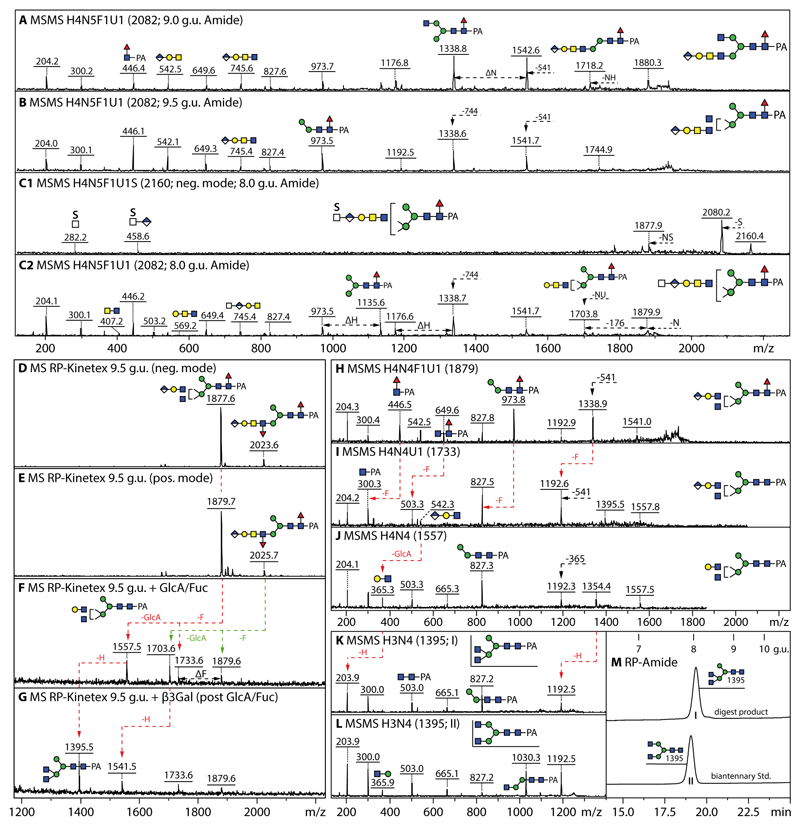
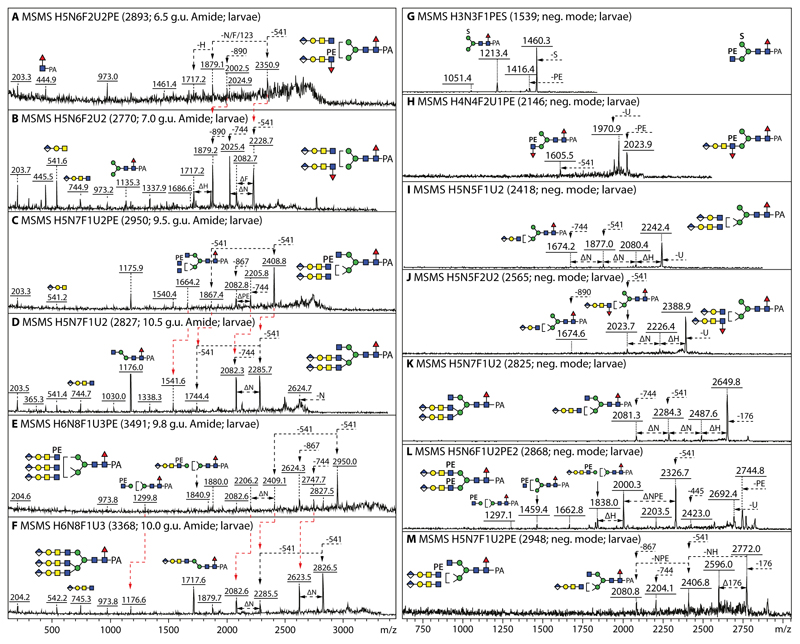
In the anionic pool of honeybee larvae, all but five of the over forty annotated N-glycans are concluded to carry glucuronic acid. Hallmarks of glucuronylated glycans are the ability to detect these in both positive and negative ion modes as well as the positive mode B-fragments of m/z 542 and 745 (Hex1HexNAc1-2HexA1 for short and long glucuronylated antennae; e.g., Figure 4 A, B and H). When the glycans were also sulphated, the molecular ions were only detected in the negative mode. In the case of HexA1Hex4HexNAc5Fuc1S1 (m/z 2160), negative mode MS/MS resulted in sulphated B-fragments of m/z 282 or 458 (HexA0-1HexNAc1S1) or terminal loss of HexNAc0-1S1 (Figure 4C1). More information regarding the underlying structure of the sulphated glycan (i.e., core fucosylation and the presence of a putative galactosylated LacdiNAc motif) was offered by fragmentation of the [M-SO3]+ ion (m/z 2082; Figure 4C2) which results from ‘in source’ loss of sulphate in positive mode; thus, we conclude that HexA1Hex4HexNAc5Fuc1S1 possesses a glucuronylated antenna elongated by a further sulphated N-acetylhexosamine resulting in a terminal motif reminiscent of glycosaminoglycans as observed on N-glycans in the larvae of other insect species (19).
Figure 4. Analysis of example monoglucuronylated biantennary and hybrid honeybee N-glycans.
(A and B) MALDI-TOF MS/MS data of two isomers of HexA1Hex4HexNAc5Fuc1 (m/z 2082, eluting at either 9.0 or 9.5 g.u.) analysed in positive mode. (C1 and C2) MALDI-TOF MS/MS data of HexA1Hex4HexNAc5Fuc1S1 analysed in both negative and positive modes (m/z 2160 and 2082 as [M-H]- and [M-SO3]+), whereby the m/z 458 B-fragment would indicate the presence of a sulphated HexNAcHexA terminal modification, while the underlying structure is revealed by fragmentation of the pseudomolecular ion resulting from ‘in source’ loss of sulphate. Losses of 541 or 744 Da (Hex1HexNAc1-2HexA1) or of NH (HexNAc1Hex1), NU (HexNAc1HexA1) or sulphate (S) from the molecular ion are noted. Compositions are of the form HxNyF1U1S whereby H is hexose, N N-acetylhexosamine, F fucose, U glucuronic acid and S sulphate. (D-G) Negative and positive mode MALDI-TOF MS of the larval anionic 9.5 g.u. fraction, also after subsequent Helix β-glucuronidase/α-fucosidase and Xanthomonas β1,3-galactosidase digestion. (H-J) MS/MS of the m/z 1879 glycan and its glucuronidase/fucosidase digestion products showing shifts in the fragmentation pattern due to loss of core fucose and antennal glucuronic acid. (K and L) Comparison of the MS/MS of the final galactosidase digestion product (I) with that of a standard biantennary N-glycan (II); also the RP-HPLC retention time (M) of the m/z 1395 digestion product is slightly different as that of the biantennary isomer, indicative that the original glycan is a hybrid structure with a β1,2/β1,4-disubstituted α1,3-linked mannose. The tendency for β1,4-GlcNAc to result in later retention times has been previously noted by Tomiya et al (50) and was also observed for Dirofilaria immitis glycans (51).
Proof for the presence of glucuronic acid as the terminus for the anionic glycans came from digests with two different β-glucuronidases (Figure 4F and Supplementary Figure 5). After this treatment, the glycans eluted 2-3 g.u. later on RP-HPLC and lost 176 Da, whereby the m/z 542 or 745 B-fragments were replaced by ones at m/z 366 and 569 (Hex1HexNAc1-2); the underlying hexose was sensitive to β1,3-galactosidase (Figure 4G). Relatively intense m/z 973 MS/MS Y-fragments (m/z 827 upon defucosylation) as well as the subtle differences in retention time and fragmentation pattern of the Hex3HexNAc4 ‘basic’ structure were observed as compared to a standard biantennary glycan (m/z 1395; Figure 4 K-M). Therefore, we concluded that some glucuronylated glycans carried two antennal motifs on the α1,3-mannose, which could be of varying lengths.
For instance, glycans in the 6.5 g.u. fraction were detected with ‘short’ GlcAβGalβGlcNAc and/or ‘long’ GlcAβGalβGalNAcβGlcNAc as indicated by the respective fragment ions at m/z 542 and 745, while the modification of lower arm is compatible with the strong m/z 973 Y-fragment (Supplementary Figure 5). In the case of the hybrid HexA1Hex5HexNAc5Fuc1 (m/z 2244), a mix of variations in terms of the antennae on the β1,2- and β1,4-GlcNAc residues could be demonstrated: i.e., either HexA1Hex1HexNAc1 and Hex1HexNAc2 or HexA1Hex1HexNAc2 and Hex1HexNAc1 as judged by the differential occurrence of m/z 569 and 745 B-fragments (Supplementary Figure 6 A and B). The long antennae could also be modified with fucose as shown by loss of 890 in various MS/MS spectra (Figure 5B and Supplementary Figure 6 C-G), whereby either β2,4-disubstitution of the α1,3-mannose or β2-substitution of both α1,3- and α1,6-mannose residues was possible as judged by the varying intensity of the m/z 973 Y3-fragment for two isomers of HexA2Hex5HexNAc6Fuc3 (m/z 2916; Supplementary Figure 6 E and F).
Figure 5. Positive and negative ion mode MALDI-TOF MS/MS of glucuronylated and zwitterionic N-glycans.
(A-F) Positive mode MALDI-TOF MS of larval bi- and tri-antennary glucuronylated glycans modified with or without phosphoethanolamine, whereby changes in fragmentation due to its absence or presence are indicated with red lines (Δm/z 123). (G-M) Negative mode MALDI-TOF MS/MS of glycans carrying either sulphate, phosphoethanolamine, glucuronic acid and/or fucose; in the negative mode, Y-ions resulting from serial losses are observed as long as they contain an anionic (GlcA or sulphate) or zwitterionic (PE) moiety. Panels D and K show the positive and negative mode MS/MS spectra of the same HexA2Hex5HexNAc7Fuc1 glycan (m/z 2827/2825). Losses of 541 (HexA1Hex1HexNAc1), 744 (HexA1Hex1HexNAc2), 890 (HexA1Hex1HexNAc2Fuc1) and 868 (HexA1Hex1HexNAc2PE1) from the multiple glucuronylated antennae are indicated; the annotations were aided by the pattern of losses (all panels) as well as the earlier RP-HPLC elution of the phosphoethanolamine-modified glycans (compare A and B, C and D, or E and F).
In the case of some triantennary structures, the occurrence of intense Y fragments at m/z 1176 suggested the presence of non-reducing terminal HexNAc residues and so hexosaminidase treatments were performed. For instance, a two-step treatment of an 11 g.u. triantennary monoglucuronylated glycan (m/z 2284 and 2286 as [M-H]- and [M+H]+) with first β1,2-specific Xanthomonas N-acetylglucosaminidase followed by the linkage-unspecific jack bean enzyme resulted in loss of the β1,2-GlcNAc on the upper arm followed by that on the lower arm. Due to the resultant forward shift in RP-HPLC retention to 9.3 g.u., rather than to the 8 g.u. typical for an m/z 1879 glycan with a β1,2-GlcNAc-based antenna, we concluded that the remaining (glucuronylated) antenna was attached via the β1,4-GlcNAc (Supplementary Figure 7 A-G). A diglucuronylated triantennary m/z 2827/2825 glycan (see Figure 5 D and K and Supplementary Figure 7I for MS/MS in positive and negative modes) lost one residue by jack bean N-acetylhexosaminidase digestion, thereby shifting from 10.5 to 9.5 g.u. on the RP-HPLC column (Supplementary Figure 7 H); this retention time corresponded to the hybrid isomer of HexA2Hex5HexNAc6Fuc1 (m/z 2622/2624 as [M-H]- or [M+H]+, which can be distinguished from the slightly-earlier eluting biantennary form by its fragmentation pattern (Supplementary Figure 7 J and K). As also triantennary glycans with three glucuronic acid residues were present (Figure 5 E and F), it can be concluded that β1,2GlcNAc-TI, β1,2GlcNAc-TII and β1,4GlcNAc-TIV products are represented in the anionic honeybee larval glycome.
Characterisation of phosphoethanolamine-modified and sulphated N-glycans in bee larvae
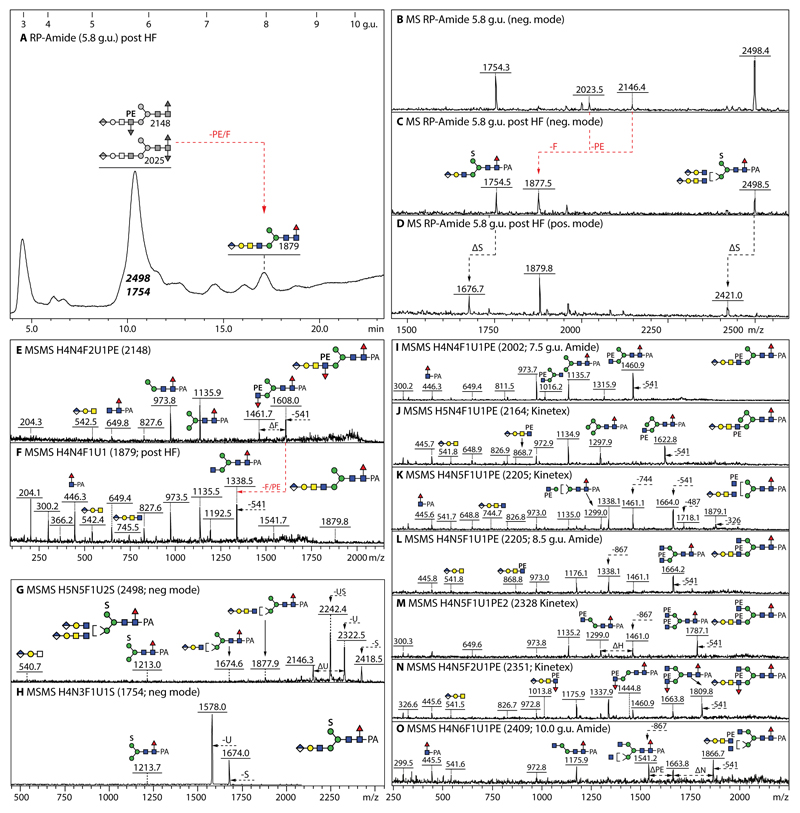
As judged by the glycans detected in both positive and negative modes with predicted compositions consistent with the presence of a 123 Da modification (Figure 5), about one quarter of the structures in the larval anionic N-glycome carried up to two phosphoethanolamine residues (Figure 3), but two zwitterionic forms were also detected in the neutral pool (Figure 1). Hydrofluoric acid treatment of phosphoethanolamine-modified glycans results in a shift to later retention time on RP-HPLC (Figure 6A and Supplementary Figure 8 A) and a loss of the zwitterionic moiety as shown by comparing MALDI-TOF MS data before and afterwards (Figure 6 B-D, Supplementary Figure 8 F-Q). Antennal α1,3-fucose is also lost at the same time as seen for the glycan of m/z 2148 which is converted by hydrofluoric acid to a simple glucuronylated structure of m/z 1879 (Figure 6 E and F), while co-eluting sulphated structures are resistant (Figure 6 C).
Figure 6. Analysis of example glucuronylated N-glycans modified with fucose, phosphoethanolamine or sulphate residues.
(A-D) RP-HPLC and MALDI-TOF MS of the 5.8 g.u. fraction upon hydrofluoric acid treatment, showing the shift in retention time (+2 g.u.) due to loss of either one core fucose residue from m/z 2025 or of one phosphoethanolamine and an antennal fucose from m/z 2148 resulting in the same m/z 1877/1879 product; the sulphate modifications of the co-eluting m/z 1754 and 2498 glycans are only lost ‘in source’ when performing positive mode MALDI-TOF MS, while the greyscale structures indicate the original elution times of the PE/α3Fuc-containing glycans before HF treatment. (E-H) MALDI-TOF MS/MS of the m/z 2148 glycan, the m/z 1879 product and the two sulphated m/z 1754 and 2498 glycans. (I-O) Example positive mode MALDI-TOF MS of N-glycans carrying one glucuronic acid and one or two phosphoethanolamine residues; phosphoethanolamine-containing B-fragments, e.g., at m/z 868 or 1014 (Hex1HexNAc2HexA1Fuc0-1PE1), are not always observed, but loss of whole or partial antennae results in phosphoethanolamine-containing Y-fragments, e.g., at m/z 1664 or 1460, indicative for substitution of a β1,2-linked GlcNAc by the zwitterionic moiety.
As the phosphoethanolamine residue is generally relatively ‘deep’ within the structure, relevant B-fragments are rare: examples, though, are the fragments at m/z 327/489 (Hex0-1HexNAc1PE1; Supplementary Figure 8 B and D), m/z 868 (HexA1Hex1HexNAc2PE1; Figure 6J and L, Supplementary Figure 8 R and T) and m/z 1014 (HexA1Hex1HexNAc2Fuc1PE1; Figure 6N). Generally, however, the information from the B-fragments is more limited (i.e., will only confirm the presence of antennal fucose, glucuronic acid or LacdiNAc); in this case, some of the larger Y-fragments (from which only a part of the antennae has been lost) indicate the position of the N-acetylglucosamine-bound phosphoethanolamine (e.g., positive mode ones at m/z 1299, 1445, 1461, 1623, 1664 and 1787 or negative mode ones at m/z 1297, 1459 and 1605 in Figure 5 C, E, H and L, Figure 6 I-O and Supplementary Figure 8 R and T).
While glycans modified with glucuronic acid and/or phosphoethanolamine can be observed in both positive and negative ion modes, those with sulphate are, as mentioned above, detected either as [M-SO3]+ or [M-H]- species due to the lability of the sulphate in positive mode. The MS/MS fragmentation patterns of all these types of glycans in negative mode is dominated by losses from the parent (e.g., loss of one or more anionic moieties), rather than by low m/z B-fragments. In the case of the m/z 1539 glycan carrying both phosphoethanolamine and sulphate, loss of either moiety and of PE and HexNAc in series facilitates the structural annotation as Hex3HexNAc3Fuc1PE1S1 (Figure 5G). Similarly, the MS/MS of m/z 2498 and 1754 glycans carrying glucuronic acid and sulphate are dominated by fragment ions resulting from loss of either anionic residue (Figure 6 G and H). The simple sulphated structures such as those of m/z 1051, 1213 and 1416 have the same elution times on the RP-amide column (respectively 8.0, 7.2 and 7.5 g.u.) and fragmentation pattern as those previously found in lepidopteran samples (20) (see also Supplementary Table 2); thus, no further characterisation of these glycans is presented here. In contrast to lepidopteran and mosquito glycomes, sulphation of core fucose was not detected on any honeybee glycan and no structure was doubly-sulphated.
Anionic and zwitterionic glycans in bee venom
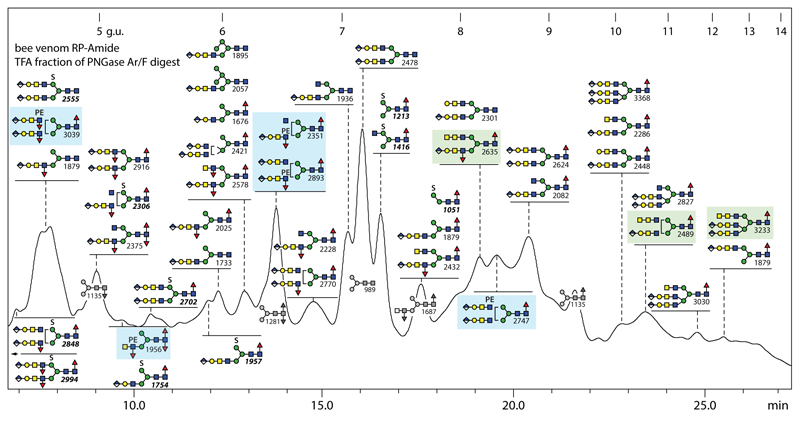
Off-line LC-MS of the anionic pool of honeybee venom glycans revealed the presence of over forty structures, which is similar to the number in the larval anionic N-glycome (Figure 7); there is a large degree of overlap also with royal jelly, but in keeping with the trend in the venom neutral sample, there are more glycans with antennal or core α1,3-fucose in the venom anionic pool, which overall is estimated as <1% of the total venom N-glycome. As mentioned above, there are phosphoethanolamine-modified forms of the fucosylated ‘venom-specific’ glycans partly in the neutral pool, such as Hex3HexNAc4Fuc3PE1 (m/z 1956); this structure was shown to contain an antennal fucose and N-acetylgalactosamine by use of specific glycosidases (Supplementary Figure 3 K-P), thus showing that it is the zwitterionic relative of the aforementioned and previously defined m/z 1833 structure.
Figure 7. RP-HPLC of anionic N-glycans of honeybee venom.
PNGase F/Ar-released N-glycans were subject to solid phase extraction, whereby the anionic-enriched fraction was eluted with 40% acetonitrile/0.1% trifluoroacetic acid, prior to fluorescent labelling and chromatography on an RP-amide column; each fraction was collected and subject to MALDI-TOF MS. The annotations are based on elution time, MS/MS and digestion data (see Supplementary Figure 6); greyscale structures indicate the elution times of co-fractionating neutral glycans. The column was calibrated in terms of glucose units. Glycans with HexNAc3- or glucuronylated/phosphoethanolamine-modified antennae are highlighted respectively in green and blue boxes.
Similar to some of the more complex structures in larvae, some N-glycans in honeybee venom carried a fucosylated LacdiNAc motif capped with a glucuronylated galactose (B-fragment of m/z 891 or loss of 890 Da, corresponding to HexA1Hex1HexNAc2Fuc1; Supplementary Figure 6 H-K). Based on the m/z 1583 Y-fragment present when fragmenting two late-eluting structures of m/z 2489 and 3233, it was concluded that the rare antennal HexNAc3 motif (putatively GalNAcα1,4GalNAcβ1,4GlcNAc) was also present as in the neutral glycome (Supplementary Figure 6 L and M). In contrast to royal jelly, MS/MS of most anionic/zwitterionic venom N-glycans resulted in m/z 446 or (as for a m/z 2375 glycan) 592 Y1-fragments and so are shown to carry one or two core fucose residues.
Discussion
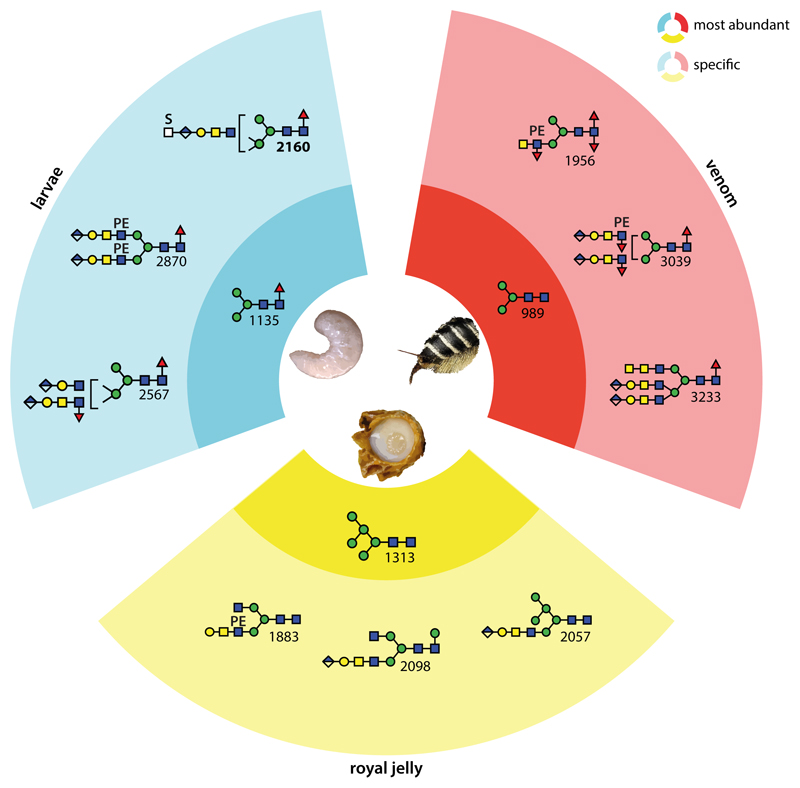
Glycomic studies of insects until now have normally analysed either whole organisms (e.g., whole embryos, larvae or adults) or individual natural or recombinant proteins, but tissue-specific studies are more difficult due to the amounts of material to hand or the ability to dissect individuals. Here, comparative analyses of the neutral and anionic N-glycans of honeybee venom and male larvae, in combination with the previous study on royal jelly (25), offer new insights into the variability of glycomes between and within insect species. Of the 170 N-glycan compositions detected in the three honeybee samples (a number not considering the isomers), 50 were found solely in royal jelly, yet 56 are absent from this natural product (see Supplementary Table 1): this is indicative of a large degree of tissue or stage specific glycomic variability in Apis mellifera with a tendency for fewer core fucosylated glycans in royal jelly as compared to the samples analysed in the present study (see also Figure 8 and Supplementary Figure 10). Retrospectively, the original analyses of honeybee venom glycoproteins still represent a tour de force and the concept of using HPLC before and after exoglycosidase digestion remains a basis for our own studies, whereby the increased possibilities offered by solid phase extraction, fused core columns and mass spectrometry mean that we can go deeper into the glycomes than previously possible. Thus, in comparison to the 20 or so N-glycan structures (all neutral) described in previous analyses of honeybee venom and royal jelly glycoproteins (7,9,30), our studies were able to greatly increase the number of demonstrated N-glycan structures in the honeybee; however, N-glycans of even lower abundance may still be present. Due to the presence of multiple glycans, partly with different ionisation potential (e.g., with sulphate or phosphoethanolamine) in each HPLC fraction, we refrain from an exact quantification, but have focussed on the lower abundance glycans with potential biological function; nevertheless, based on integration of overall MALDI-TOF MS we show relative occurrences for the major glycans in Supplementary Figure 9 and Supplementary Table 4.
Figure 8. Summary of tissue-specific and overlapping N-glycan types in the honeybee.
The blue, yellow and red segments indicate structures specific to larvae, royal jelly and venom respectively; the darker sub-segments show the most abundant glycans in each sample, while the lighter sub-segments show three example sample-specific glycans. In the royal jelly, there is a dominance of hybrid structures and, as compared to larvae and venom, the glucuronylated antennae are ‘long’ (i.e., GlcAGalGalNAcGlcNAc rather than GlcAGalGlcNAc); processed venom glycans tend to be fucosylated on the core and antennae; in larvae, there is a bias towards β1,2- and β1,4-antennae, sometimes of different lengths. Interesting is that overall larvae and royal jelly share more structures with venom than royal jelly does with larvae. See also Supplementary Figures 9 & 10 and Supplementary Table 4 for an alternative summary of structures and for comparisons of MALDI-TOF MS profiles showing the major structures in each sample.
It is clear that the male larvae and the hypopharyngeal and venom glands of the worker bees (the sources of the royal jelly and venom secretions) express a wide range of glycosyltransferases activities including β1,3-glucuronyltransferase, β1,3-galactosyltransferase, α1,4- and β1,4-N-acetylgalactosaminyltransferase, phosphoethanol-aminyltransferases, core and antennal α1,3-fucosyltransferases, core α1,6-fucosyltransferase, mannose-modifying sulphotransferase, branching β1,2-N-acetylglucosaminyltransferases I and II and β1,4-N-acetylglucosaminyltransferase IV, partly in a tissue specific manner; in some cases, such enzymes have been characterised from the honeybee (31,32) or from other insects (33–38). However, gaps in our knowledge include the identity of insect phosphoethanolaminyltransferases and sulphotransferases or the proof of activity of an insect β1,4-N-acetylglucosaminyltransferase IV. Some venom structures modified with LacdiNAc were also elongated by galactose or an extra N-acetylgalactosamine on one or both antennae as previously found by us on mono-, bi- and triantennary N-glycan scaffolds from moths or royal jelly (20,25). In the larvae, most LacdiNAc-based antennae are also galactosylated and glucuronylated, suggestive that complex biosynthetic modifications have a tendency to go to completion; thus, few variants of complex N-glycans are found in the neutral glycan pool, but rather in the anionic one. In venom, fewer ‘short’ antennae (GlcAβ1,3Galβ1,3GlcNAc as compared to the longer GlcAβ1,3Galβ1,3GalNAcβ1,4GlcNAc) are observed.
As mentioned above, royal jelly glycans are more likely to lack fucose and this is presumably due to a lower fucosylation in the hypopharyngeal glands producing this natural product; certainly the levels of core α1,3-fucosyltransferase (FucTA) transcripts is lower in hypopharyngeal than in venom glands as judged by RT-PCR (32). The branching and sulphation/glucuronylation potential may be less in the venom glands, while glucuronylated antennae were exclusively ‘long’ (i.e., GlcAβ3Galβ3GalNAcβ4GlcNAc) in royal jelly, but triantennary and anionic glycans are found in all three sources of honeybee N-glycans. The functional relevance of the different glycosylation variants in larvae, venom and royal jelly is currently only a matter of speculation; it is interesting that in royal jelly, N-glycans are on the outer surface of the oligomeric MRJP1 structure (39) and that these might mediate a trafficking function of the apisimin- and 24-methylenecholesterol-containing complex. On the other hand and at least in the context of O-glycans, glucuronylation is important for neuromuscular junction organisation in Drosophila (40). The tendency towards difucosylation in venom is shared with neural tissue of insects (41).
The various anionic and zwitterionic glycans represent a class of oligosaccharide previously not found in venom. The present study also underlines the presence of phosphoethanolamine in the honeybee. Indeed, variations in the type and extent of zwitterionic moieties occur within the Insecta with phosphorylcholine being found on lepidopteran N-glycans (20) and aminoethylphosphonate on a locust glycoprotein (42), but phosphoethanolamine is found only on glycolipids in the diptera (43) and on hymenopteran O-glycans (44). Interestingly, phosphoethanolamine is a potential ligand for pentraxins such as mammalian serum amyloid P (45) and royal jelly glycans are recognised by this protein (25). On the other hand, it is obvious that glucuronic acid and sulphate are widespread anionic modifications of insect N-glycans (19,20), for whose detection the glycomic approach must be adjusted as these tend to be overlooked in complete spectra; on the other hand, despite the occurrence of sialyltransferase homologues in a number of species (46,47), there still is no evidence for natural sialylation in insects except in Drosophila (23). Indeed, no sialic acid was detected in the present study on honeybee glycans, although our workflows are compatible with its observation in glycomes of non-insect species.
As anionic and/or zwitterionic glycans have been detected in all insect species analysed by in-depth off-line LC-MS (19,20,25) as well as in more recent studies using special post-permethylation procedures (22,25,48), it is to be expected that also those organisms whose glycomes have been less intensively studied will also express such structures and that even revisiting glycomes, as done here with venom, can yield surprises. Thus, we conclude that adequate and comparable workflows are key to performing ‘evo-devo-glycomics’ (i.e., glycomics based on consideration of evolutionary and developmental aspects) in the Insecta and beyond.
Supplementary Material
Acknowledgements
We thank Dr. Erika Staudacher for her input regarding the history of honeybee venom glycosylation research. Angelika Paschinger for the photo of a honeybee (see graphical abstract) and Dr. Iain Wilson for help with preparation of the manuscript and mentoring. The larvae were collected from the hives of the late Dr. Hubert Paschinger in Schiltern fifteen years ago; noticeable is the continuous decline in local bee populations with zero colonies in the village at the time of submission of this paper, thereby resulting in a silent spring in 2019.
Funding Sources
This work was supported by the Austrian Science Fund (FWF); A.H. and K.P. are FWF fellows (grants P26662 and P25058) and B.E. a student within the FWF-funded BioTOP doctoral program W1224. The funders had no role in study design or the collection, analysis and interpretation of data.
Footnotes
Author Contributions
Alba Hykollari (experimental design, glycan preparation and analysis, data interpretation and selection), Daniel Malzl (glycan preparation and analysis, figure preparation), Rhiannon Stanton (glycan preparation and analysis), Barbara Eckmair (glycan analysis) and Katharina Paschinger (experimental design, data interpretation/selection and writing).
Conflicts of Interest: The authors have no conflicts of interest.
References
- 1.Vanengelsdorp D, Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol. 2010;103(Suppl 1):S80–95. doi: 10.1016/j.jip.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Rembold H, Lackner B, Geistbeck I. The chemical basis of honeybee, Apis mellifera, caste formation. Partial purification of queen bee determinator from royal jelly. Journal of Insect Physiology. 1974;20:307–314. [Google Scholar]
- 3.Hider RC. Honeybee venom: a rich source of pharmacologically active peptides. Endeavour. 1988;12:60–65. doi: 10.1016/0160-9327(88)90082-8. [DOI] [PubMed] [Google Scholar]
- 4.Brown TC, Tankersley MS. The sting of the honeybee: an allergic perspective. Ann Allergy Asthma Immunol. 2011;107:463–470. doi: 10.1016/j.anai.2011.09.015. quiz 471. [DOI] [PubMed] [Google Scholar]
- 5.Butters TD, Hughes RC. Isolation and characterisation of mosquito cell membrane glycoproteins. Biochim Biophys Acta. 1981;640:655–671. doi: 10.1016/0005-2736(81)90096-1. [DOI] [PubMed] [Google Scholar]
- 6.März L, Kuhne C, Michl H. The glycoprotein nature of phospholipase A2, hyaluronidase and acid phosphatase from honey-bee venom. Toxicon. 1983;21:893–896. doi: 10.1016/0041-0101(83)90080-6. [DOI] [PubMed] [Google Scholar]
- 7.Kubelka V, Altmann F, Staudacher E, Tretter V, März L, Hård K, Kamerling JP, Vliegenthart JFG. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur J Biochem. 1993;213:1193–1204. doi: 10.1111/j.1432-1033.1993.tb17870.x. [DOI] [PubMed] [Google Scholar]
- 8.Staudacher E, Altmann F, März L, Hård K, Kamerling JP, Vliegenthart JFG. α1-6(α1-3)-Difucosylation of the asparagine-bound N-acetylglucosamine in honeybee venom phospholipase A2. Glycoconjugate J. 1992;9:82–85. doi: 10.1007/BF00731703. [DOI] [PubMed] [Google Scholar]
- 9.Kubelka V, Altmann F, März L. The asparagine-linked carbohydrate of honeybee venom hyaluronidase. Glycoconjugate J. 1995;12:77–83. doi: 10.1007/BF00731872. [DOI] [PubMed] [Google Scholar]
- 10.Staudacher E, Altmann F, Glössl J, März L, Schachter H, Kamerling JP, Hård K, Vliegenthart JFG. GDP-fucose: β-N-acetylglucosamine (Fuc to (Fuc α1→6GlcNAc)-Asn-peptide) α1→3-fucosyltransferase activity in honeybee (Apis mellifica) venom glands. The difucosylation of asparagine-bound N-acetylglucosamine. Eur J Biochem. 1991;199:745–751. doi: 10.1111/j.1432-1033.1991.tb16179.x. [DOI] [PubMed] [Google Scholar]
- 11.Weber A, Schröder H, Thalberg K, März L. Specific interaction of IgE antibodies with a carbohydrate epitope of honey bee venom phospholipase A2. Allergy. 1987;42:464–470. doi: 10.1111/j.1398-9995.1987.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 12.Prenner C, Mach L, Glössl J, März L. The antigenicity of the carbohydrate moiety of an insect glycoprotein, honey-bee (Apis mellifera) venom phospholipase A2. The role of α1,3-fucosylation of the asparagine-bound N-acetylglucosamine. Biochem J. 1992;284:377–380. doi: 10.1042/bj2840377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tretter V, Altmann F, Kubelka V, März L, Becker WM. Fucose α1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int Arch Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- 14.Haslam SM, Reason AJ, Morris HR, Dell A. Core fucosylation of honeybee venom phospholipase A2. Glycobiology. 1994;4:105–106. doi: 10.1093/glycob/4.2.105. [DOI] [PubMed] [Google Scholar]
- 15.Wing DR. Core fucosylation in honeybee venom. Glycobiology. 1994;4:548–550. doi: 10.1093/glycob/4.5.548. [DOI] [PubMed] [Google Scholar]
- 16.Lai CC, Her GR. Analysis of N-glycosylation of phospholipase A2 from venom of individual bees by microbore high-performance liquid chromatography-electrospray mass spectrometry using an ion trap mass spectrometer. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;766:243–250. doi: 10.1016/s0378-4347(01)00479-0. [DOI] [PubMed] [Google Scholar]
- 17.Paschinger K, Rendić D, Wilson IBH. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj J. 2009;26:385–395. doi: 10.1007/s10719-008-9155-3. [DOI] [PubMed] [Google Scholar]
- 18.Hård K, van Doorn JM, Thomas-Oates JE, et al. Structure of the Asn-linked oliogsaccharides of apolipophorin III from the insect Locusta migratoria. Carbohydrate-linked 2-aminoethylphosphonate as a constituent of a glycoprotein. Biochemistry. 1993;32:766–775. doi: 10.1021/bi00054a005. [DOI] [PubMed] [Google Scholar]
- 19.Kurz S, Aoki K, Jin C, Karlsson NG, Tiemeyer M, Wilson IBH, Paschinger K. Targetted release and fractionation reveal glucuronylated and sulphated N- and O-glycans in larvae of dipteran insects. J Proteomics. 2015;126:172–188. doi: 10.1016/j.jprot.2015.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton R, Hykollari A, Eckmair B, Malzl D, Dragosits M, Palmberger D, Wang P, Wilson IBH, Paschinger K. The underestimated N-glycomes of lepidopteran species. Biochim Biophys Acta. 2017;1861:699–714. doi: 10.1016/j.bbagen.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera G, Salazar V, Montesino R, Tambara Y, Struwe WB, Lugo EL, Harvey DJ, Antoine L, Rincon M, Domon B, Mendez Martinez MD, et al. Structural characterization and biological implications of sulfated N-glycans in a serine protease from the neotropical moth Hylesia metabus (Cramer [1775]) (Lepidoptera: Saturniidae) Glycobiology. 2015;26:230–250. doi: 10.1093/glycob/cwv096. [DOI] [PubMed] [Google Scholar]
- 22.Aoki K, Tiemeyer M. The glycomics of glycan glucuronylation in Drosophila melanogaster. Methods Enzymol. 2010;480:297–321. doi: 10.1016/S0076-6879(10)80014-X. [DOI] [PubMed] [Google Scholar]
- 23.Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- 24.Frappaolo A, Sechi S, Kumagai T, Robinson S, Fraschini R, Karimpour-Ghahnavieh A, Belloni G, Piergentili R, Tiemeyer KH, Tiemeyer M, Giansanti MG. COG7 deficiency in Drosophila generates multifaceted developmental, behavioral and protein glycosylation phenotypes. J Cell Sci. 2017;130:3637–3649. doi: 10.1242/jcs.209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hykollari A, Malzl D, Eckmair B, Vanbeselaere J, Scheidl P, Jin C, Karlsson NG, Wilson IBH, Paschinger K. Isomeric separation and recognition of anionic and zwitterionic N-glycans from royal jelly glycoproteins. Mol Cell Proteomics. 2018;17:2177–2196. doi: 10.1074/mcp.RA117.000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hykollari A, Paschinger K, Eckmair B, Wilson IBH. Analysis of Invertebrate and Protist N-Glycans. Methods Mol Biol. 2017;1503:167–184. doi: 10.1007/978-1-4939-6493-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan S, Wilson IBH, Paschinger K. Comparison of RP-HPLC modes to analyse the N-glycome of the free-living nematode Pristionchus pacificus. Electrophoresis. 2015;36:1314–1329. doi: 10.1002/elps.201400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dragosits M, Yan S, Razzazi-Fazeli E, Wilson IBH, Rendić D. Enzymatic properties and subtle differences in the substrate specificity of phylogenetically distinct invertebrate N-glycan processing hexosaminidases. Glycobiology. 2015;25:448–464. doi: 10.1093/glycob/cwu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennis RD, Geyer R, Egge H, Peter-Katalinic J, Li SC, Stirm S, Wiegandt H. Glycosphingolipids in insects. Chemical structures of ceramide tetra-, penta-, hexa-, and heptasaccharides from Calliphora vicina pupae (Insecta: Diptera) J Biol Chem. 1985;260:5370–5375. [PubMed] [Google Scholar]
- 30.Kimura Y, Miyagi C, Kimura M, Nitoda T, Kawai N, Sugimoto H. Structural features of N-glycans linked to royal jelly glycoproteins: structures of high-mannose type, hybrid type, and biantennary type glycans. Biosci Biotechnol Biochem. 2000;64:2109–2120. doi: 10.1271/bbb.64.2109. [DOI] [PubMed] [Google Scholar]
- 31.Ichimiya T, Maeda M, Sakamura S, Kanazawa M, Nishihara S, Kimura Y. Identification of β1,3-galactosyltransferases responsible for biosynthesis of insect complex-type N-glycans containing a T-antigen unit in the honeybee. Glycoconj J. 2015;32:141–151. doi: 10.1007/s10719-015-9585-7. [DOI] [PubMed] [Google Scholar]
- 32.Rendić D, Klaudiny J, Stemmer U, Schmidt J, Paschinger K, Wilson IBH. Towards abolition of immunogenic structures in insect cells: characterization of a honey-bee (Apis mellifera) multi-gene family reveals both an allergy-related core α1,3-fucosyltransferase and the first insect Lewis-histo-blood-group-related antigen-synthesizing enzyme. Biochem J. 2007;402:105–115. doi: 10.1042/BJ20060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim B-T, Tsuchida K, Lincecum J, Kitagawa H, Bernfield M, Sugahara K. Identification and characterization of three Drosophila melanogaster glucuronyltransferases responsible for the synthesis of the conserved glycosaminoglycan-protein linkage region of proteoglycans. Two novel homologs exhibit broad specificity toward oligosaccharides from proteoglycans, glycoproteins, and glycosphingolipids. J Biol Chem. 2003;278:9116–9124. doi: 10.1074/jbc.M209344200. [DOI] [PubMed] [Google Scholar]
- 34.Vadaie N, Jarvis DL. Molecular cloning and functional characterization of a Lepidopteran insect β4-N-acetylgalactosaminyltransferase with broad substrate specificity, a functional role in glycoprotein biosynthesis, and a potential functional role in glycolipid biosynthesis. J Biol Chem. 2004;279:33501–33508. doi: 10.1074/jbc.M404925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisler C, Jarvis DL. Substrate specificities and intracellular distributions of three N-glycan processing enzymes functioning at a key branch point in the insect N-glycosylation pathway. J Biol Chem. 2012;287:7084–7097. doi: 10.1074/jbc.M111.296814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurz S, King JG, Dinglasan RR, Paschinger K, Wilson IBH. The fucomic potential of mosquitoes: Fucosylated N-glycan epitopes and their cognate fucosyltransferases. Insect Biochem Mol Biol. 2016;68:52–63. doi: 10.1016/j.ibmb.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breloy I, Schwientek T, Althoff D, Holz M, Koppen T, Krupa A, Hanisch FG. Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen. Biomolecules. 2016;6:8. doi: 10.3390/biom6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mucha J, Domlatil J, Lochnit G, Rendić D, Paschinger K, Hinterkörner G, Hofinger A, Kosma P, Wilson IBH. The Drosophila melanogaster homologue of the humna histo-blood group Pk gene encodes a glycolipid-modifying α1,4-N-acetylgalactosaminyltransferase. Biochem J. 2004;382:67–74. doi: 10.1042/BJ20040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian W, Li M, Guo H, Peng W, Xue X, Hu Y, Liu Y, Zhao Y, Fang X, Wang K, Li X, et al. Architecture of the native major royal jelly protein 1 oligomer. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05619-1. 3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh K, Akimoto Y, Kondo S, Ichimiya T, Aoki K, Tiemeyer M, Nishihara S. Glucuronylated core 1 glycans are required for precise localization of neuromuscular junctions and normal formation of basement membranes on Drosophila muscles. Dev Biol. 2018;436:108–124. doi: 10.1016/j.ydbio.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabini G, Freilinger A, Altmann F, Wilson IBH. Identification of core α1,3-fucosylated glycans and the requisite fucosyltransferase in Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J Biol Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- 42.Hård K, Van Doorn JM, Thomas-Oates JE, Kamerling JP, Van der Horst DJ. Structure of the Asn-linked oligosaccharides of apolipophorin III from the insect Locusta migratoria. Carbohydrate-linked 2-aminoethylphosphonate as a constituent of a glycoprotein. Biochemistry. 1993;32:766–775. doi: 10.1021/bi00054a005. [DOI] [PubMed] [Google Scholar]
- 43.Helling F, Dennis RD, Weske B, Nores G, Peter-Katalinic J, Dabrowski U, Egge H, Wiegandt H. Glycosphingolipids in insects. The amphoteric moiety, N-acetylglucosamine-linked phosphoethanolamine, distinguishes a group of ceramide oligosaccharides from the pupae of Calliphora vicina (Insecta: Diptera) Eur J Biochem. 1991;200:409–421. doi: 10.1111/j.1432-1033.1991.tb16199.x. [DOI] [PubMed] [Google Scholar]
- 44.Maes E, Garenaux E, Strecker G, Leroy Y, Wieruszeski JM, Brassart C, Guerardel Y. Major O-glycans from the nest of Vespula germanica contain phospho-ethanolamine. Carbohydr Res. 2005;340:1852–1858. doi: 10.1016/j.carres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Mikolajek H, Kolstoe SE, Pye VE, Mangione P, Pepys MB, Wood SP. Structural basis of ligand specificity in the human pentraxins, C-reactive protein and serum amyloid P component. J Mol Recognit. 2011;24:371–377. doi: 10.1002/jmr.1090. [DOI] [PubMed] [Google Scholar]
- 46.Koles K, Irvine KD, Panin VM. Functional characterization of Drosophila sialyltransferase. J Biol Chem. 2004;279:4346–4357. doi: 10.1074/jbc.M309912200. [DOI] [PubMed] [Google Scholar]
- 47.Kajiura H, Hamaguchi Y, Mizushima H, Misaki R, Fujiyama K. Sialylation potentials of the silkworm, Bombyx mori; B. mori possesses an active α2,6-sialyltransferase. Glycobiology. 2015;25:1441–1453. doi: 10.1093/glycob/cwv060. [DOI] [PubMed] [Google Scholar]
- 48.Mabashi-Asazuma H, Kuo CW, Khoo KH, Jarvis DL. Modifying an Insect Cell N-Glycan Processing Pathway Using CRISPR-Cas Technology. ACS Chem Biol. 2015;10:2199–2208. doi: 10.1021/acschembio.5b00340. [DOI] [PubMed] [Google Scholar]
- 49.Tomiya N, Lee YC, Yoshida T, Wada Y, Awaya J, Kurono M, Takahashi N. Calculated two-dimensional sugar map of pyridylaminated oligosaccharides: Elucidation of the jack bean α-mannosidase digestion pathway of Man9GlcNAc2. Analytical Biochemistry. 1991;193:90–100. doi: 10.1016/0003-2697(91)90047-w. [DOI] [PubMed] [Google Scholar]
- 50.Tomiya N, Awaya J, Kurono M, Endo S, Arata Y, Takahashi N. Analyses of N-linked oligosaccharides using a two-dimensional mapping technique. Anal Biochem. 1988;171:73–90. doi: 10.1016/0003-2697(88)90126-1. [DOI] [PubMed] [Google Scholar]
- 51.Martini F, Eckmair B, Neupert C, Štefanić S, Jin C, Garg M, Jiménez-Castells C, Hykollari A, Yan S, Venco L, Varón Silva D, et al. Highly modified and immunoactive N-glycans of the canine heartworm. Nat Commun. 2019;10 doi: 10.1038/s41467-018-07948-7. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.